ABSTRACT
Objective: To examine the efficacy of lisdexamfetamine (LDX) versus placebo on behavioral attributes of sluggish cognitive tempo (SCT) in adults with attention-deficit/hyperactivity disorder (ADHD) and SCT.
Methods: In a randomized crossover trial conducted January 2016–April 2018, 38 adults with DSM-5 ADHD (via the Adult ADHD Clinical Diagnostic Scale v1.2) and SCT were recruited at 2 academic medical centers and assessed for symptoms of ADHD, SCT, executive function deficits, and functional impairment at baseline and weekly during treatment. Participants received 4 weeks of treatment with either LDX (30–70 mg/d; mean = 59.1 ± 14.8 mg/d) or matching placebo (mean = 66.6 ± 9.1 mg/d) with a 2-week washout before switching to the other arm. The ADHD Rating Scale and Barkley Adult ADHD Rating Scale-IV SCT subscale were coprimary outcome measures.
Results: There were moderately large treatment effects of LDX vs placebo on SCT ratings in both treatment periods (block 1 effect size = 0.68; block 2 effect size = 0.61), which reached significance only in block 1 owing to carryover effects of the first treatment epoch into the second. Significant effects were also seen for LDX over placebo in ADHD, executive function deficit, and functional impairment ratings, without order effects; no site differences were seen except on the Global Executive Complex score of the Behavior Rating Inventory of Executive Function—Adult Version. No moderating effects of sex, age, race, and ethnicity were seen.
Conclusions: Adults with ADHD and comorbid SCT had significant improvement after LDX vs placebo in ratings of SCT, ADHD, executive function deficits, and functional impairment. This is the first study to show improvement in SCT after stimulant therapy in adults with ADHD.
Trial Registration: ClinicalTrials.gov identifier: NCT02635035
J Clin Psychiatry 2021;82(4):20m13687
To cite: Adler LA, Leon TL, Sardoff TM, et al. A placebo-controlled trial of lisdexamfetamine in the treatment of comorbid sluggish cognitive tempo and adult ADHD. J Clin Psychiatry. 2021;82(4):20m13687.
To share: https://doi.org/10.4088/JCP.20m13687
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, NYU Grossman School of Medicine, New York, New York
bDepartment of Child and Adolescent Psychiatry, NYU Grossman School of Medicine, New York, New York
cDepartment of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York
dDepartment of Psychiatry and Behavioral Sciences, SUNY Upstate Medical University, Syracuse, New York
eDepartment of Psychology, Drexel University, Philadelphia, Pennsylvania
*Corresponding author: Lenard A. Adler, MD, NYU School of Medicine, One Park Ave, 8th Fl, New York, NY 10016 ([email protected]).
Sluggish cognitive tempo (SCT) describes individuals who are “dreamy,” “spacey,” slow moving, hypoactive, have difficulty initiating tasks, and often seem undermotivated and underaroused. Barkley has identified 9 cardinal symptoms of SCT: (1) prone to daydreaming, instead of concentrating; (2) trouble staying alert/awake in boring situations; (3) being easily confused; (4) being easily bored; (5) feeling spacey/in a fog; (6) frequently feeling lethargic; (7) being underactive/having less energy than others; (8) being slow moving; and (9) not processing information quickly/accurately.1 In a study of 1,249 individuals with and without attention-deficit/hyperactivity disorder (ADHD), Barkley identified individuals as having SCT if they had at least 5 of 9 symptoms rated often or very often on the 9-item SCT subscale from the Barkley Adult ADHD Rating Scale-IV: Self-Report (BAARS-IV; the Barkley SCT Scale).2 The prevalence of SCT in this subgroup was 5.8%, approximately half of whom had ADHD. Additionally, having SCT added to the impairment seen with ADHD, such that the cohort with ADHD and SCT was significantly more impaired overall and in 15 domains of impairment (including home, social, occupational, and educational).2 The results of this study suggest that SCT may be present and highly impairing in a large subgroup of adults with ADHD in a community sample, but SCT is not necessarily restricted to individuals with ADHD. In a meta-analysis by Becker et al,3 considering 23 independent studies including 19,000 participants, SCT was more strongly associated with the inattentive (IA) symptoms of ADHD than the hyperactive-impulsive symptoms (HI), in both children and adults. Other studies have found elevated levels of SCT in children with ADHD-combined type.4,5 Garner and coworkers evaluated parent and teacher reports in 322 children and adolescents for behavioral, emotional, and/or learning problems and found that SCT symptoms were greatest in youth with ADHD inattentive type, though they were also found in non-ADHD clinical groups.6 SCT symptoms have been thought to separate into cognitive (ie, daydreaming) and behavioral (ie, slowness, drowsiness) dimensions, with the behavioral dimension uniquely associated with learning/organization problems. Furthermore, studies have found a negative correlation between SCT and quality of life, an effect likely mediated by the adverse consequences of SCT on executive function (EF).7 Becker and Langberg8 assessed parent and teacher ratings for 52 adolescents with ADHD and found that ADHD and SCT symptoms were highly correlated with ratings of EF. ADHD-HI symptoms were strongly associated with EF deficits in behavioral regulation, while ADHD-IA and SCT symptoms were unrelated to EF. As recent studies in community samples of adults and children have found high SCT in the absence of ADHD, the current thinking is that SCT might be a clinically meaningful condition not restricted to ADHD, with distinct underlying pathophysiology and treatment response.1,4,9 Of note, although SCT is highly prevalent and associated with substantial impairment, it is not a primary driver of clinical treatment and is often not inquired about in the clinical evaluation, highlighting the need for appropriate identification and treatment.
The few treatment studies of SCT in patients with ADHD to date have focused on children, rather than adults. Milich et al10 investigated the use of methylphenidate for treatment of youth with ADHD-IA and did not find the medication to be effective for symptoms of inattention linked to SCT. One trial of methylphenidate in children with ADHD and SCT found that the response for SCT behaviors was lower than the response for core ADHD symptoms.11 A more recent study (Froehlich et al12) examined whether SCT behavioral attributes rated by teachers moderate the dose response to long acting methylphenidate and, specifically, whether the moderating effect of SCT on medication response is distinct from that of ADHD subtype. Certain SCT behavioral attributes—eg, sluggish and sleepy—were associated with methylphenidate nonresponse, while daydreaming was not. Finally, Firat et al13 assessed the effects of SCT and pretreatment ADHD severity among other variables on 1 month of open-label methylphenidate treatment response in children (n = 185); they found that parent- and teacher-rated ADHD-HI and SCT scores decreased after treatment. Older age positively affected the methylphenidate-SCT treatment response in both ratings. Like the results of Froehlich et al,12 increased SCT scores were associated with decreased methylphenidate response in teacher ratings of ADHD.
A study of atomoxetine, a nonstimulant selective norepinephrine reuptake inhibitor, versus placebo in children with ADHD, dyslexia, and ADHD + dyslexia found that atomoxetine reduced SCT behavioral attributes in all groups, with the most significant change in the ADHD + dyslexia group.14 Post hoc analyses revealed that controlling for changes in ADHD scores did not significantly influence changes in SCT scores.
No treatment trials have been conducted investigating treatment of SCT in adults with ADHD, and there have been no treatment trials of amphetamines in children or adults with SCT and ADHD. This is particularly important as a recent meta-analysis showed the amphetamine stimulant class to have the largest effect of all treatments for adults with ADHD.15,16
The current study is a part of a 2-phase (phenotypic and treatment) examination of SCT and ADHD at 2 academic centers (NYU Grossman School of Medicine and Icahn School of Medicine at Mount Sinai). The phenotypic phase characterized SCT, ADHD, and EF symptoms in subjects who had ADHD and SCT versus those who had ADHD and were SCT negative.17 An interim17 report of the NYU cohort seen in that study found that, similar to prior studies noted above in children and adults, individuals with both SCT and ADHD, versus adults with ADHD alone, have higher levels of inattentive symptoms and impairment.
We are now reporting data from the second phase of this study—the treatment phase. Subjects at NYU Grossman School of Medicine and Icahn School of Medicine at Mount Sinai who had both ADHD and SCT in the phenotypic phase were recruited into this crossover trial of LDX versus placebo. The goal of this study is to determine the efficacy of LDX on the nature and severity of ADHD symptoms and SCT behavioral indicators in adults with ADHD and SCT.
METHODS
Study Design
This was a 10-week crossover trial, with 2 double-blind treatment periods consisting of 4 weeks each and an intervening 2-week single-blind placebo washout. During the first treatment period, subjects were randomized to receive either oral LDX or matching placebo before crossing over during the second treatment phase. During the first week of a treatment period, LDX or placebo was given at a starting dose of 30 mg/d. Based on weekly assessments of clinical response and adverse effects, doses were increased or decreased during weeks 2 and 3 by 20-mg/d increments to a minimum of 30 mg/d and maximum of 70 mg/d. After week 3, the highest effective dose was maintained until the end of the treatment period.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) man or woman between the ages of 18 and 60 years; (2) met DSM-5 criteria for a primary diagnosis of inattentive or combined type ADHD as diagnosed via Adult ADHD Clinical Diagnostic Scale (ACDS) v1.2; and (3) demonstrated significant impairment, determined based on the norms of the Barkley Functional Impairment Scale (BFIS). The subgroup with SCT was required to have 5 or more items on the Barkley SCT Scale rated clinically significant and a total SCT symptom score of 26 or higher. Additionally, the group with SCT must have had clinically significant impairment in executive function. Impairment was determined by a T score of 65 or higher on the Metacognition Index subscale of the Behavior Rating Inventory of Executive Function—Adult Version (BRIEF-A), a self-reported rating scale of executive function. The subgroup without SCT had fewer than 5 clinically significant items on the Barkley SCT Scale with a total symptom score of less than 26, in addition to a T score of less than 65 on the Metacognition Index of the BRIEF-A.
Participants were excluded if they (1) met DSM-5 criteria for a primary diagnosis of hyperactive-impulsive type ADHD as diagnosed via the ACDS v1.2; (2) had any other current psychiatric disorder, determined via the Mini International Neuropsychiatric Interview (MINI) v7.0, that required pharmacotherapy treatment; (3) had current suicidal ideation or a history of suicide attempts; (4) had a lifetime history of bipolar disorder or any psychotic disorder as per the MINI; (5) were pregnant, breast-feeding, or planning to become pregnant; (6) had a positive urine drug toxicology screen at baseline; (7) had any other clinical or contextual issues (including a history of sleep disorders) that, in the opinion of the investigator, would prevent the person from participating in the study or compromise the participant’s safety; and (8) had previously been treated with LDX for ADHD.
The study was approved by the Institutional Review Boards of New York University Grossman School of Medicine and Icahn School of Medicine at Mount Sinai and registered at ClinicalTrials.gov (identifier: NCT02635035). The trial was conducted January 2016 through April 2018.
Assessments
Study participants’ ADHD diagnoses were confirmed at baseline via the ACDS v1.2, and comorbid psychiatric disorders via the MINI v7.0; suicidality was assessed via Columbia-Suicide Severity Rating Scale. Medical and psychiatric histories, as well as demographics, were self-reported.
Prior to the first treatment period and at the end of the 2-week placebo washout, baseline measures of ADHD were assessed via the ADHD-RS with adult prompts, baseline SCT symptom severity was assessed via the Barkley SCT scale, and functional impairment was measured via the BRIEF-A, the BFIS, and the Clinical Global Impression-Severity (CGI-S) scale. During treatment periods, symptoms were rated on a weekly basis with ADHD-RS and Barkley SCT scales. Any adjustment to medication dosage was decided using clinician judgment regarding symptoms and adverse events. At the end of each treatment period, ADHD-RS, BRIEF-A, Barkley SCT, and BFIS scales were repeated to assess overall changes in symptom severity and functional impairment.
MINI v7.0. The MINI v7.018,19 is a structured clinical interview used to assess DSM-5 psychiatric disorders and has been widely used to evaluate psychiatric comorbidity in adult ADHD studies.20,21
ACDS v1.2. ADHD diagnosis was evaluated with the ACDS v1.2, a semistructured diagnostic interview widely used in adult ADHD studies.22,23 The clinical interview begins with an assessment of childhood symptoms of ADHD and then probes an expanded set of recent (past year) symptoms, including all DSM-5 A1 and A2 symptoms. The scale includes developmentally relevant prompts and stem questions designed to capture DSM symptoms of ADHD as they present in childhood and adulthood. Based on participant responses, the clinician rates the symptom severity as 1 (never), 2 (mild), 3 (moderate), or 4 (severe).
The ACDS v1.2 has been expanded to include an additional 16 clinical prompts intended to assess executive function deficits (EFDs; 9 items) and emotional dyscontrol (ED; 4 items). ED includes behavioral descriptors of mood lability, irritability, and emotional overreactivity. As with the DSM-5 items, developmentally relevant prompts have been written for the EFD and ED items.
ADHD-RS. ADHD-RS23,24 is a semistructured clinical interview to measure ADHD symptom severity in the last 1 to 2 weeks (and in this trial during the treatment phase, since the last rating). Adult interview prompts were incorporated to ensure adequate probing of symptoms; items are rated using a 4-point Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). The 9 inattentive and symptoms are summed and reported as the inattentive subscale (IA), and the 9 hyperactive-impulsive symptoms are also summed and reported as the hyperactive-impulsive (HI) subscale. The IA and HI subscales are added to obtain the ADHD-RS total score, which is the coprimary outcome measure in this trial, along with the total score on the Barkley SCT scale (see below).
Barkley SCT scale. The SCT subscale of the Barkley Adult ADHD Rating Scale1 is a 9-item self-report of SCT behavioral symptoms scored on a 4-point rating scale (1 = “never or rarely,” 2 = “sometimes,” 3 = “often,” and 4 = “very often,” with the latter two being the cutoff for clinical significance for each item). Subjects with SCT were required to have 5 or more items rated “often” or “very often,” with an additional study criterion requiring a total score of at least 26.
BRIEF-A. The Behavior Rating Inventory of Executive Function—Adult Version25 is a self-report composed of 9 clinical subscales designed to measure various aspects and deficits of executive function. The Metacognition Index (MI) is the sum of the initiate, working memory, plan/organize, task monitor, and organization of material subscales, and the Behavioral Regulation Index (BRI) is the sum of inhibit, shift, emotional control, and self-monitor subscales. The 2 indices, when combined, yield the Global Executive Complex (GEC) score. The GEC serves as a general measure of executive function. The GEC, BRI, MI, and individual subscales have been standardized, and T scores are used to describe severity. A T score of ≥ 65 is considered to represent clinically significant behavioral impairment in executive function.
BFIS. The Barkley Functional Impairment Rating Scale measures self-perceived, current impairment in 15 different major life activities (eg, completing chores, social interactions) using a 10-point Likert scale.26 Mean scores can be calculated to quantify impairment.
CGI-S. Overall impairment was also assessed by the CGI-S scale, a widely used clinician-rated measure of global ADHD impairment that uses a 7-point Likert rating (1 = normal, 7 = among the most severely ill patients).27
Participants
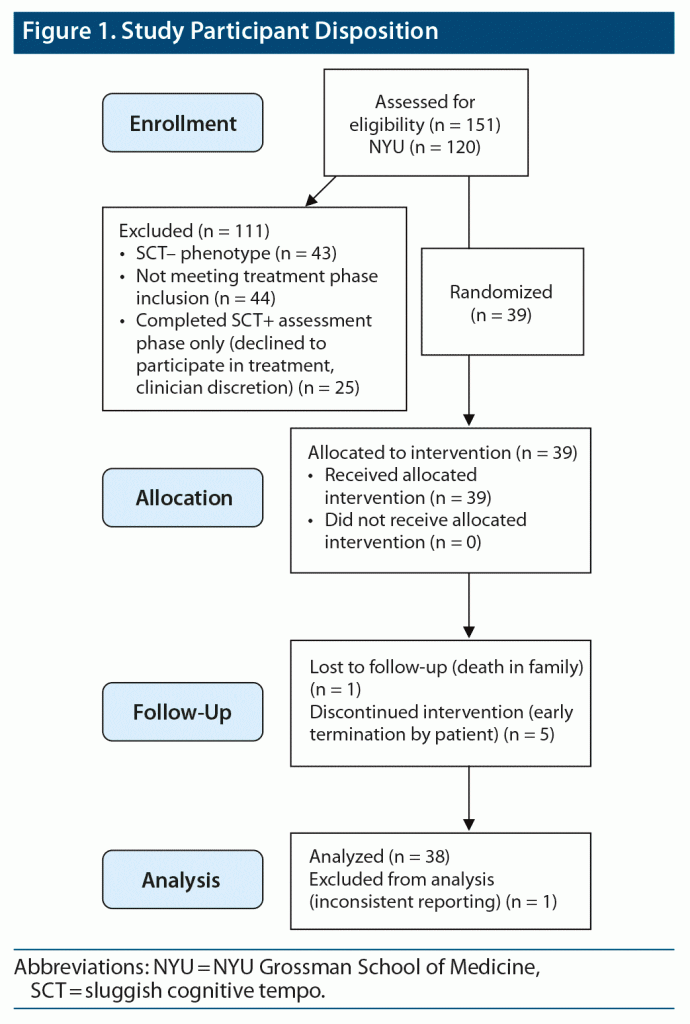
Thirty-nine participants with comorbid ADHD and SCT were recruited to participate in this study. Ratings for 1 subject were excluded secondary to significant adherence violations with treatment on multiple visits; data on 38 subjects were included in the analyses (see Figure 1). Participants were drawn from 2 academic medical centers, New York University Grossman School of Medicine (n = 23) and Icahn School of Medicine at Mount Sinai (n = 15). Thirty-four percent of the subjects had inattentive presentation of ADHD, while 66% had the combined presentation. There were no significant demographic differences between sites, except for a higher proportion of Latino subjects at NYU (Pearson χ2 = 6.6087, P = .010). Participants had a mean age of 34.6 ± 10.1 years, and 53% (n = 20) were Caucasian; 34% were male and 66% were female. Comorbid current psychiatric diagnoses included generalized anxiety disorder (GAD; n = 3), obsessive-compulsive disorder (n = 1), and GAD and social anxiety disorder (n = 1). All subjects gave written informed consent prior to participation.
Data Analysis
Demographic data were analyzed using logistic regression for continuous variables and Pearson χ2 tests for categorical variables. Clinical trial outcomes data were analyzed with negative binomial regression with generalized estimating equations and robust standard errors. A negative binomial model was chosen because our outcome measures were limited to positive integers. We used generalized estimating equations with robust standard errors to account for correlations between visits.
RESULTS
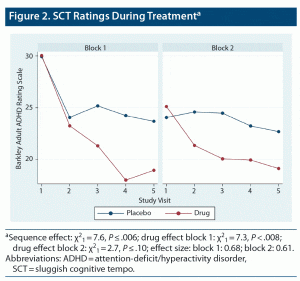
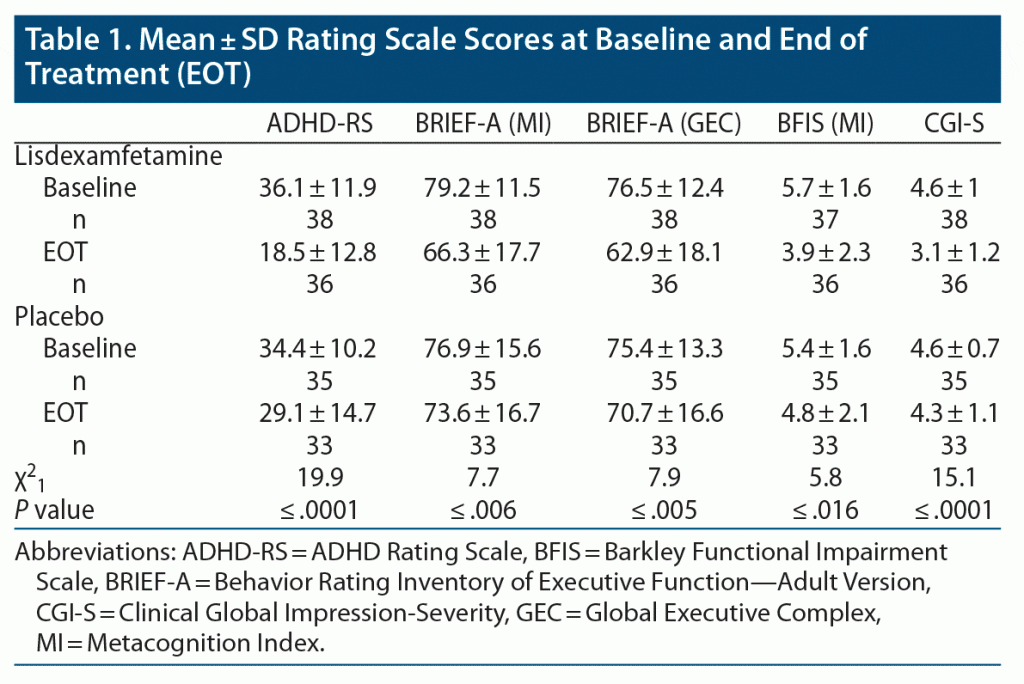
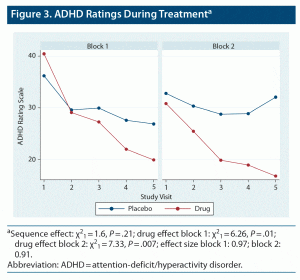
Titration patterns of LDX and placebo were similar in the 2 blocks, with slightly more subjects having their dose reduced by 1 step (20 mg) during treatment with LDX (n = 5) than placebo (n = 2). The mean (SD) overall titrated dose of LDX was 59.1 (14.8) mg/d, while the mean overall titrated dose of placebo was 66.6 (9.1) mg/d. The mean placebo dose was significantly higher than the mean LDX dose by 7.5 (15.4) mg/d (P = .0037). There were moderately large effects of LDX over placebo on SCT ratings in both treatment epochs (Figure 2) (block 1 effect size [ES; Cohen d] = 0.68, P = .008; block 2 ES = 0.61, P = .10), which reached significance only in block 1. There were significant carryover effects of both LDX and placebo treatment following block 1 such that SCT ratings did not return to baseline; therefore, SCT results are presented separately for the 2 treatment blocks (Figure 2). For all other measures, no significant order effects were seen, and results from the 2 blocks were combined and presented as LDX vs placebo (see Table 1). Significant effects were seen for LDX over placebo for total ADHD symptoms, EF, and functional impairment ratings (all P ≤ .05). Significant effects were also seen for LDX over placebo on IA and HI subscales of the ADHD-RS (IA: sequence effect: χ21 = 1.04, P = .31; drug effect: χ21 = 9.54, P ≤ .0001; ES: 0.92; HI: sequence effect: χ21 = 1.15, P = .28; drug effect: χ21 = 5.32, P ≤ .02; ES: 0.53). No site differences were seen except on the BRIEF GEC. No moderating effects of sex, age, race, and ethnicity were seen.
To examine ADHD total symptoms by treatment block, ADHD-RS total scores are shown for LDX and placebo in Figure 3 (by block); significant effects are seen for LDX over placebo in each block (ES—Cohen d: block 1: 0.97, P = .01; block 2: 0.91, P = .007).
To examine the relationship between changes in ADHD and SCT symptoms, we examined correlations between the 2 symptom sets; for the LDX treated group, the correlation between the change in ADHD total symptoms and the change in SCT symptoms was 0.49 (P = .003). In a multiple regression analysis predicting change in SCT symptoms from the changes in total ADHD and BRIEF GEC symptoms, the association between BRIEF GEC and SCT remained significant (t33 = 2.6, P = .01). In contrast, the association between the changes in total ADHD and SCT symptoms lost significance (t33 = 2.6, P = .01), which suggests that the univariate association between ADHD and SCT symptoms was mediated by changes in BRIEF GEC symptoms.
Safety
Overall, LDX was well tolerated, and no serious adverse events were reported. Adverse events reported were mild to moderate in severity. Two participants discontinued treatment early during the LDX phase, although neither discontinuation was due to adverse events. Only 1 participant withdrew due to an adverse event—anxiety, which is commonly reported during LDX treatment. This subject withdrew during placebo treatment, having already gone through the LDX phase. The most commonly reported adverse events during both LDX and/or placebo treatments were decreased appetite (LDX: 10.92%; placebo: 0.00%), headache (LDX: 10.08%; placebo: 7.56%), trouble sleeping or falling asleep (LDX: 10.08%; placebo: 3.36%), dry mouth (LDX: 7.56%; placebo: 0.84%), and anxiety/jitteriness (LDX: 4.20%; placebo: 2.52%). These adverse events are commonly reported with stimulants.28 Moreover, there was no reporting of suicidality or suicidal ideation throughout the study. There were minor, clinically insignificant changes in systolic and diastolic blood pressure (BP) (mm Hg) and pulse (beats/min [bpm]) in the LDX and placebo groups throughout the study. The mean changes (baseline to end of treatment) for systolic BP (SD) were LDX: −1.2 mm Hg (13.8); placebo: −5 mm Hg (12.8). Mean changes for diastolic BP were LDX: 4.3 mm Hg (8.5); placebo: −6.6 mm Hg (9.8). Mean changes for pulse were LDX: 6.7 bpm (11.6); placebo: −2.2 bpm (11.4). (Changes over treatment: systolic BP: χ21 = 3.93, P ≤ .05; diastolic BP: χ21 = 13.78, P ≤ .001; pulse: χ21 = 11.91, P ≤ .001.)
DISCUSSION
Adults with comorbid ADHD and SCT significantly improved in ratings of SCT, ADHD, EF, and impairment when treated with LDX vs placebo. The SCT findings were present in both treatment epochs, with a moderately large ES; however, an order effect was seen with larger placebo effects in block 1 than block 2 that limited statistical significance to block 1. In addition, there were also carryover effects, and SCT ratings failed to return to baseline for both treatments in block 2. Consequently, group differences in ADHD ratings only reached significance in block 1.
The magnitude of effects of LDX on ADHD symptoms in this trial in adults with ADHD and SCT is quite similar to that seen in other studies of LDX in adult ADHD, and specifically in patients with ADHD and EFD.17 This study extends the efficacy of LDX to subjects with ADHD and SCT. Although we found moderate to large ESs for both ADHD symptoms and SCT ratings, the baseline to endpoint change scores for the two types of ratings were only modestly correlated, sharing 24% of their variance; the regression analysis presented above suggests that the relationship between ADHD and SCT scores may be driven by changes in EFD scores. This provides further evidence that SCT is not simply another measure of ADHD and suggests the possibility that different patient characteristics and/or mechanisms (such as EFD) may moderate or mediate the response of ADHD and SCT symptoms to LDX and other medications. These findings highlight the importance of assessing for the presence of SCT and EFD at baseline in patients with ADHD, and to also monitor the potential response of ADHD, SCT, and EFD symptoms throughout treatment. As EFDs have been shown to be responsive to cognitive behavioral therapy in combination with pharmacotherapy in adults with ADHD, it remains to be seen whether such a combination would also be an effective treatment strategy to optimize symptom reduction in adults with ADHD and SCT.29,30
The finding of a small but significantly higher final dose of placebo versus LDX is not surprising, as clinicians could clinically titrate the dose, and the smaller response to placebo led to higher up-titration in the placebo group. Finally, the small but nonsignificant increases in blood pressure and pulse, along with the other adverse events we observed, were like those seen in prior trials of LDX in adult ADHD.31
Several studies described above have examined potential moderating effects of ADHD symptoms, or lack thereof, on SCT behavioral attributes in children treated with methylphenidate or atomoxetine. As noted above, we found that one-fourth of the effect of LDX on SCT ratings can be potentially be accounted for by changes in ADHD ratings—suggesting partial distinctiveness of the two constructs. However, it is difficult to fully examine potential moderating effects in our trial as all our subjects had ADHD and SCT. Differences in findings in this study from those studies that examined potential moderating factors in children could be due to (1) our studying adults, (2) our studying effects of amphetamine versus methylphenidate or atomoxetine, (3) differences in the rating instrument used or the raters (teacher/parent/SCT scale), (4) absence of a placebo control group (Firat and coworkers13 and the SCT cohort in McBurnett and colleagues14) and (5) inclusion of a comorbid diagnosis (dyslexia) (McBurnett et al14).
Several additional caveats should be noted. We specifically weighted our sample toward having EFD with the requirement for having a significant MI cutoff on the BRIEF, and also toward having more significant SCT symptoms by requiring a total score of ≥ 26 on the Barkley SCT scale in addition to the standard threshold of ≥ 5/9 symptoms. Furthermore, the finding of an order effect in both LDX and placebo treated subjects such that ratings did not fully return to baseline after washout of block 1 drug is of note. Such carryover effects are a potential liability of any crossover design trial and are not specific to trials of ADHD or SCT. However, a prior crossover design study of LDX versus mixed amphetamine salts did not see such carryover effects.32 Nevertheless, moderate to large effects were seen for LDX over placebo in both treatment epochs. Our sample was not large enough to fully examine potential differential effects of ADHD subtype or symptom subsets on the LDX treatment effect; however, treatment effects on IA symptoms were twice as large as on HI symptoms, which might be due to potential effects of the enrichment of the sample with SCT and EFD symptoms. Nor could we evaluate the extent to which enrollment in prior adult ADHD treatment trials or receiving treatment at a tertiary center, such as those conducting this study, may have affected the results. These issues may the foci of future investigations.
In summary, this is the first study to find treatment effects of stimulants on SCT behavioral attributes in adults with ADHD and SCT. Future trials should examine potential mitigating effects of EFD symptoms on response and should be conducted with parallel designs to mitigate any potential for carryover effect. Additionally, the responsiveness of SCT in other conditions or as stand-alone disorder to ADHD treatments remains a potential line of investigation.
Submitted: September 11, 2020; accepted March 15, 2021.
Published online: June 29, 2021.
Potential conflicts of interest: Dr Adler has received grant/research support from Sunovion Pharmaceuticals, Enzymotec, Shire/Takeda Pharmaceuticals, Otsuka, and Lundbeck; has served as a consultant to Bracket, Sunovion Pharmaceuticals, Shire/Takeda Pharmaceuticals, Otsuka Pharmaceuticals, SUNY, the National Football League, and Major League Baseball; and has received royalty payments (as inventor) since 2004 from NYU for license of adult ADHD scales and training materials. Dr Faraone has received income, potential income, travel expenses, continuing education support, and/or research support from Akili Interactive Laboratories, Arbor, Genomind, Ironshore, Ondosis, Otsuka, Rhodes, Shire/Takeda, Sunovion, Supernus, Tris, and Vallon. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. He also receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health; Oxford University Press: Schizophrenia: The Facts; and Elsevier: ADHD: Non-Pharmacologic Interventions. He is also Program Director of www.adhdinadults.com. Dr Newcorn has been an advisor and/or consultant for Adlon Therapeutics, Akili Interactive, Arbor, Cingulate Therapeutics, Eisai, Ironshore, Medice, Myriad Neuroscience, NLS, OnDosis, Rhodes, Shire/Takeda, and Supernus. He was a data and safety monitoring board member for Pfizer and Sunovion and received research funds from Adlon, Otsuka, Shire/Takeda, and Supernus. He also has received speaker fees from Shire/Takeda for disease-state presentations and served as a consultant for the US National Football League. Mss Leon and Sardoff, Dr Krone, and Mr Silverstein report no potential conflicts of interest relevant to the subject of this article.
Funding/support: Supported in part by an investigator-initiated grant (Dr Adler, principal investigator) from Shire/Takeda Pharmaceuticals.
Role of the sponsor: Shire/Takeda Pharmaceuticals paid for the collection and analysis of data.
Previous presentation: The data were previously presented in part as a poster at the Annual Meeting of the American Professional Society of ADHD and Related Disorders; January 17–19, 2020; Washington, DC.
Acknowledgments: The authors thank Ashley Chan, BS; Joshua Jiang, BA; and Tamara Kahan, BA, for their assistance in the examination of the data and in the preparation of the manuscript, all from NYU Grossman School of Medicine, New York, New York. Mss Chan and Kahan and Mr Jiang have no conflict of interests to declare.
Clinical Points
- Recognizing the symptoms of sluggish cognitive tempo (SCT) in adults with ADHD is complex, and there are no accepted treatments.
- Clinicians could consider lisdexamfetamine as a potential treatment option for adults with ADHD and SCT.
References (32)

- Barkley RA. Distinguishing sluggish cognitive tempo from attention-deficit/hyperactivity disorder in adults. J Abnorm Psychol. 2012;121(4):978–990. PubMed CrossRef
- Brown TE. ADD/ADHD and impaired executive function in clinical practice. Curr Psychiatry Rep. 2008;10(5):407–411. PubMed CrossRef
- Becker SP, Leopold DR, Burns GL, et al. The internal, external, and diagnostic validity of sluggish cognitive tempo: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 2016;55(3):163–178. PubMed CrossRef
- Barkley RA. Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: executive functioning, impairment, and comorbidity. J Clin Child Adolesc Psychol. 2013;42(2):161–173. PubMed CrossRef
- Marshall SA, Evans SW, Eiraldi RB, et al. Social and academic impairment in youth with ADHD, predominately inattentive type and sluggish cognitive tempo. J Abnorm Child Psychol. 2014;42(1):77–90. PubMed CrossRef
- Garner AA, Marceaux JC, Mrug S, et al. Dimensions and correlates of attention deficit/hyperactivity disorder and sluggish cognitive tempo. J Abnorm Child Psychol. 2010;38(8):1097–1107. PubMed CrossRef
- Combs MA, Canu WH, Broman-Fulks JJ, et al. Impact of sluggish cognitive tempo and attention deficit/hyperactivity disorder symptoms on adults’ quality of life. Appl Res Qual Life. 2014;9(4):981–995. CrossRef
- Becker SP, Langberg JM. Attention-deficit/hyperactivity disorder and sluggish cognitive tempo dimensions in relation to executive functioning in adolescents with ADHD. Child Psychiatry Hum Dev. 2014;45(1):1–11. PubMed CrossRef
- Becker SP, Barkley RA. Sluggish Cognitive Tempo. Oxford Medicine Online; 2018:147–153.
- Milich R, Balentine AC, Lynam DR. ADHD Combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clin Psychol Sci Pract. 2001;8(4):463–488. CrossRef
- Ludwig HT, Matte B, Katz B, et al. Do sluggish cognitive tempo symptoms predict response to methylphenidate in patients with attention-deficit/hyperactivity disorder-inattentive type? J Child Adolesc Psychopharmacol. 2009;19(4):461–465. PubMed CrossRef
- Froehlich TE, Becker SP, Nick TG, et al. Sluggish cognitive tempo as a possible predictor of methylphenidate response in children with ADHD: a randomized controlled trial. J Clin Psychiatry. 2018;79(2):17m11553. PubMed CrossRef
- Fırat S, Gul H, Aysev A. An open-label trial of methylphenidate treating sluggish cognitive tempo, inattention, and hyperactivity/impulsivity symptoms among 6- to 12-year-old ADHD children: what are the predictors of treatment response at home and school? [published online ahead of print February 17, 2020] J Atten Disord. PubMed CrossRef
- McBurnett K, Clemow D, Williams D, et al. Atomoxetine-related change in sluggish cognitive tempo is partially independent of change in attention-deficit/hyperactivity disorder inattentive symptoms. J Child Adolesc Psychopharmacol. 2017;27(1):38–42. PubMed CrossRef
- Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727–738. PubMed CrossRef
- Faraone SV, Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19(4):353–364. PubMed CrossRef
- Silverstein MJ, Leon TL, Krone B, et al. The characteristics and unique impairments of comorbid adult ADHD and sluggish cognitive tempo: an interim analysis. Psychiatr Ann. 2019;49(10):457–465. CrossRef
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33, quiz 34–57. PubMed
- Wigal TL, Newcorn JH, Handal N, et al. A double-blind, placebo-controlled, phase ii study to determine the efficacy, safety, tolerability and pharmacokinetics of a controlled release (CR) formulation of mazindol in adults with DSM-5 attention-deficit/hyperactivity disorder (ADHD). CNS Drugs. 2018;32(3):289–301. PubMed CrossRef
- Silverstein MJ, Faraone SV, Leon TL, et al. The relationship between executive function deficits and DSM-5-defined ADHD symptoms. J Atten Disord. 2020;24(1):41–51. PubMed CrossRef
- Bradley AJ, Webb-Mitchell R, Hazu A, et al. Sleep and circadian rhythm disturbance in bipolar disorder. Psychol Med. 2017;47(9):1678–1689. PubMed CrossRef
- Adler L, Cohen J. Diagnosis and evaluation of adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27(2):187–201. PubMed CrossRef
- Adler LA, Shaw DM, Alperin S. ADHD diagnostic and symptom assessment scales for adults. In: Adler LA, Spencer TJ, Wilens TE, eds. Attention-Deficit Hyperactivity Disorder in Adults and Children. Cambridge, UK: Cambridge University Press; 2015:224–232.
- Spencer T, Biederman J, Wilens T, et al. Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58(8):775–782. PubMed CrossRef
- Roth R, Isquith P, Gioia G. BRIEF-A Behavior Rating Inventory of Executive Function-Adult Version, Publication Manual. Lutz, FL: Psychological Assessment Resources, Inc; 2005.
- Barkley RA. Barkley Functional Impairment Scale (BFIS). New York, NY: Guilford Press; 2011.
- Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217–222.
- Adler LA, Goodman DW, Kollins SH, et al; 303 Study Group. Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69(9):1364–1373. PubMed CrossRef
- Knouse LE, Safren SA. Current status of cognitive behavioral therapy for adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33(3):497–509. PubMed CrossRef
- Cherkasova MV, French LR, Syer CA, et al. Efficacy of cognitive behavioral therapy with and without medication for adults with ADHD: a randomized clinical trial. J Atten Disord. 2020;24(6):889–903. PubMed CrossRef
- Adler LA, Dirks B, Deas PF, et al. Lisdexamfetamine dimesylate in adults with attention-deficit/ hyperactivity disorder who report clinically significant impairment in executive function: results from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(7):694–702. PubMed CrossRef
- Adler LA, Alperin S, Leon T, et al. Clinical effects of lisdexamfetamine and mixed amphetamine salts immediate release in adult ADHD: results of a crossover design clinical trial. Postgrad Med. 2014;126(5):17–24. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite