Abnormal Integrity of Long Association Fiber Tracts Is Associated With Cognitive Deficits in Patients With Remitted Geriatric Depression: A Cross-Sectional, Case-Control Study
Objective: The aim of this study was to explore the integrity of association fiber tracts of the interhemispheric and within-hemispheric communication in patients with remitted geriatric depression (RGD) by diffusion tensor imaging.
Method: A region of interest-based diffusion tensor imaging approach was applied to explore fiber tract differences between 37 patients with DSM-IV-diagnosed RGD and 33 well-matched, normal, aging healthy controls. Correlations were also sought between fractional anisotropy values and cognitive performance scores in the patients with RGD. The patients were recruited from January 2007 to December 2007.
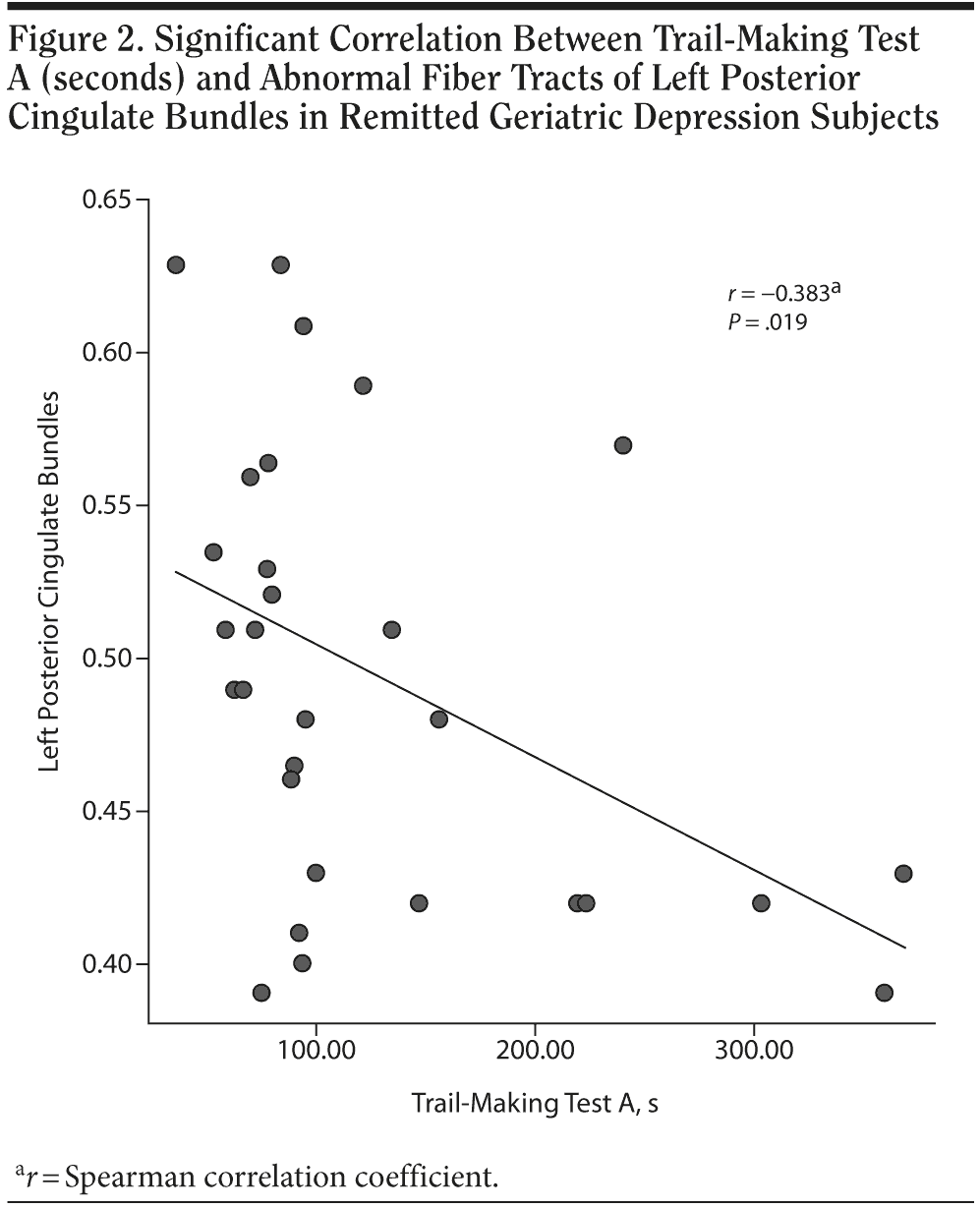
Results: Extensive impairment of the integrity of association fiber tracts was observed in patients with RGD, including in the bilateral inferior fronto-occipital fascicles, genu of corpus callosum, and posterior cingulate bundles. In addition, the fractional anisotropy value of left posterior cingulate bundles displayed a significantly negative correlation with the performance (time) of Trail-Making Test A (r = −0.383, P = .019).
Conclusions: This study suggested that association fiber tracts between remote cortexes may yield important new clues to predict whether a patient will eventually develop Alzheimer’s disease.
J Clin Psychiatry 2010;71(10):1386-1390
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: April 27, 2009; accepted September 8, 2009.
Online ahead of print: June 1, 2010 (doi:10.4088/JCP.09m05313gry).
Corresponding author: Zhijun Zhang, MD, PhD, School of Clinical Medicine, Southeast University, Nanjing 210009, China ([email protected]).
Cognitive impairment is common in late-life depression. There have been many follow-up studies of older adults with depression with varying degrees of cognitive impairment, although the findings have been mixed, including marked or mild improvement in cognitive function and persistence of cognitive impairment or deterioration after the remission of depressive symptoms.1 Late-life depression with cognitive impairment has increased risk of conversion to dementia, and it might be a preclinical stage of dementia.2
Increasing evidence showed that the abnormalities of microstructural white matter were associated with geriatric depression.3 Alexopoulos et al4 found that lower fractional anisotropy in distributed cerebral networks was associated with poor antidepressant response of geriatric depression. Some studies also reported that white matter hyperintensities of geriatric depression were related to cognitive decline in various domains, particularly in executive skills, attention, and mental speed.5-7
Diffusivity changes have been observed in the frontal, temporal, and parietal white matter regions in patients with remitted geriatric depression (RGD).8 Importantly, white matter regions are not isolated entities but instead form highly interconnected neural circuits encompassing often remote brain regions. The integrity of network level was believed to be important neuronal substrates of higher cognitive function.9,10 However, the state of long association fiber tracts between remote cortexes remains unclear in patients with RGD, and to our knowledge, no published study has investigated the relationship between long association fiber tracts integrity and cognitive performances in patients with RGD.
In this study, we hypothesize that the abnormalities of long association fiber tracts integrity are correlated with RGD, and the abnormalities might be one of the neuroimaging markers of cognitive deficits in the patients with RGD. Investigations of region of interest (ROI) in patients with mild cognitive impairment were conducted using the same method in our previous article11 and other studies.12,13 This investigation should contribute to our understanding of the position of association fiber tracts in the pathophysiology of RGD.
METHOD
Participants
A total of 37 patients (13 men and 24 women; average age of 69.98 ± 4.63 years) were recruited from the Affiliated Brain Hospital of Nanjing Medical University, China, from January 2007 to December 2007. All patients were interviewed in a semistructured interview included in the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version,14 by 2 trained and senior psychiatrists (Y.Y., J.Y.). They met the following inclusion criteria:
1. They once met DSM-IV criteria for major depressive disorder and had been in remission for more than 6 months before the enrollment.
2. The depressive episode had been their first, and the age at onset was over 60 years.
3. Their Hamilton Depression Rating Scale (HDRS)15 scores were lower than 7 and Mini Mental State Examination (MMSE)16 scores were higher than 24.
4. Their duration of illness was less than 5 years, and the medication-free period for all subjects was longer than 3 months prior to the assessment.
5. They had an absence of another major psychiatric illness, including substance abuse or dependence.
6. They had an absence of primary neurologic illness, including dementia or stroke.
7. They had an absence of medical illness impairing cognitive function.
8. They had no history of receiving electroconvulsive therapy.
9. Their T2-weighted magnetic resonance imaging showed no major white matter changes such as infarction or other vascular lesions.
All patients received antidepressant medication: 26 patients treated with selective serotonin reuptake inhibitors and 11 patients treated with serotonin and norepinephrine reuptake inhibitors. Thirty-three well-matched healthy controls had also been recruited from the community, including 15 men and 18 women with an average age of 70.51 ± 3.91 years. Healthy controls had no history of psychiatric disorder. They also met the inclusion criteria 3, 5, 6, 7, 8, and 9. All subjects gave written informed consent after the procedure had been carefully explained and after they had the opportunity to ask any questions they wanted. The research was approved by the Research Ethics Committee of Southeast University. All subjects were unequivocally and naturally right-handed and of Han Chinese ethnicity.
Neuropsychological Tests
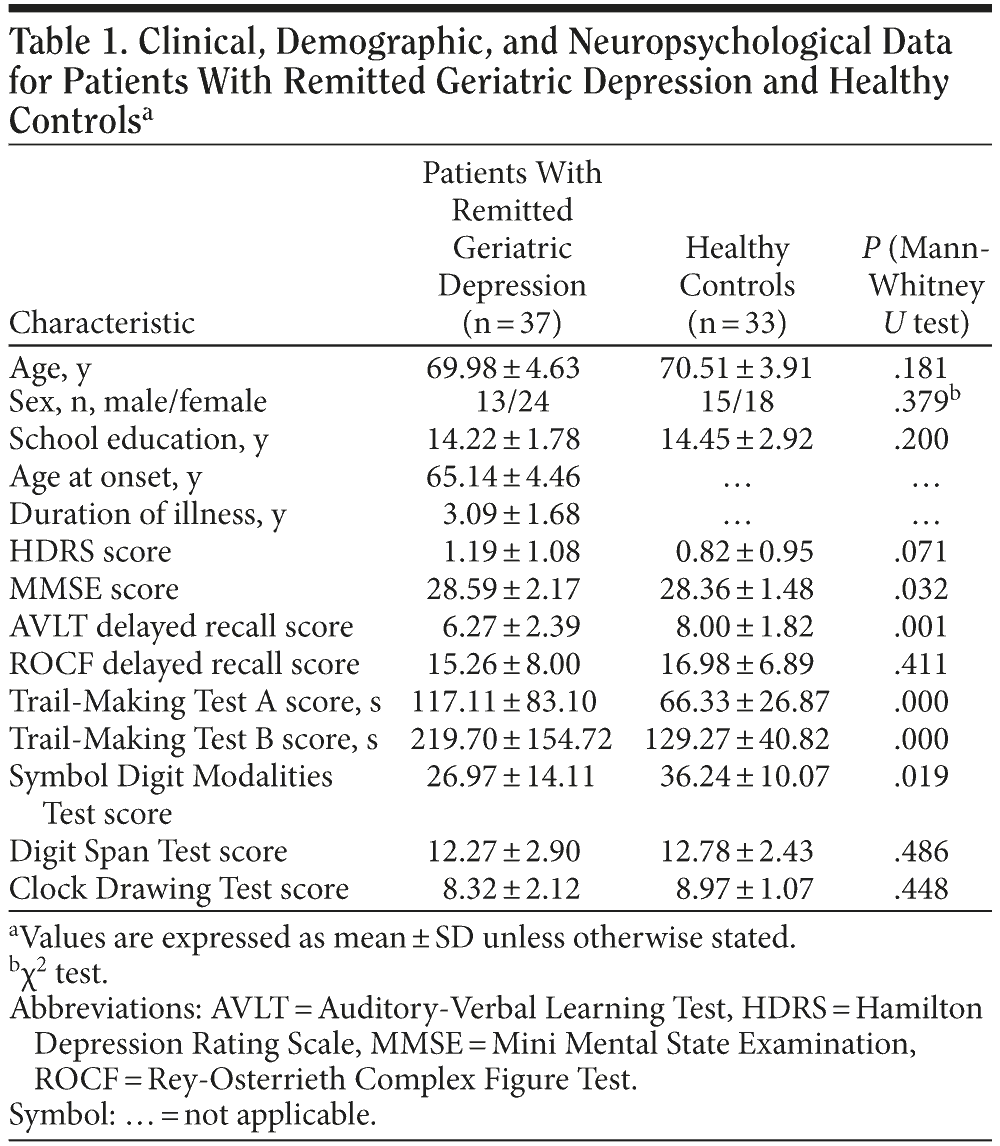
All subjects received a neuropsychological battery using standardized administration by 2 psychiatrists (Y.Y., J.Y.). The neuropsychological battery consisted of the Auditory Verbal Learning Test (AVLT), the Rey-Osterrieth Complex Figure Test (ROCF), the Trail-Making Tests A and B, the Digit Span Test, the Symbol Digit Modalities Test, and the Clock Drawing Test. Table 1 contains descriptive demographic and neuropsychological data for the 2 groups.
Magnetic Resonance Imaging Data Acquisition
Subjects were scanned using a General Electric 1.5 Tesla scanner (General Electric Medical Systems, Fairfield, Connecticut) with a homogeneous birdcage head coil. Conventional axial T2-weighted images were obtained previously to rule out cerebral infarction or other lesions. Diffusion-weighted imaging was acquired with a single shot echo planar imaging sequence in alignment with the anterior-posterior commissural plane. The diffusion sensitizing gradients were applied along 25 noncollinear directions (b = 1,000 s/mm2), together with an acquisition without diffusion weighting (b = 0 s/mm2). Thirty contiguous axial slices were acquired with a slice thickness of 4 mm and no gap. The acquisition parameters were as follows: repetition time = 10,000 ms; echo time = 81.2 ms; flip angle = 90°; acquisition matrix = 128 ×— 128; field of view = 240 mm ×— 240 mm; and number of excitations = 2.0.
Diffusion Tensor Imaging Data Postprocessing
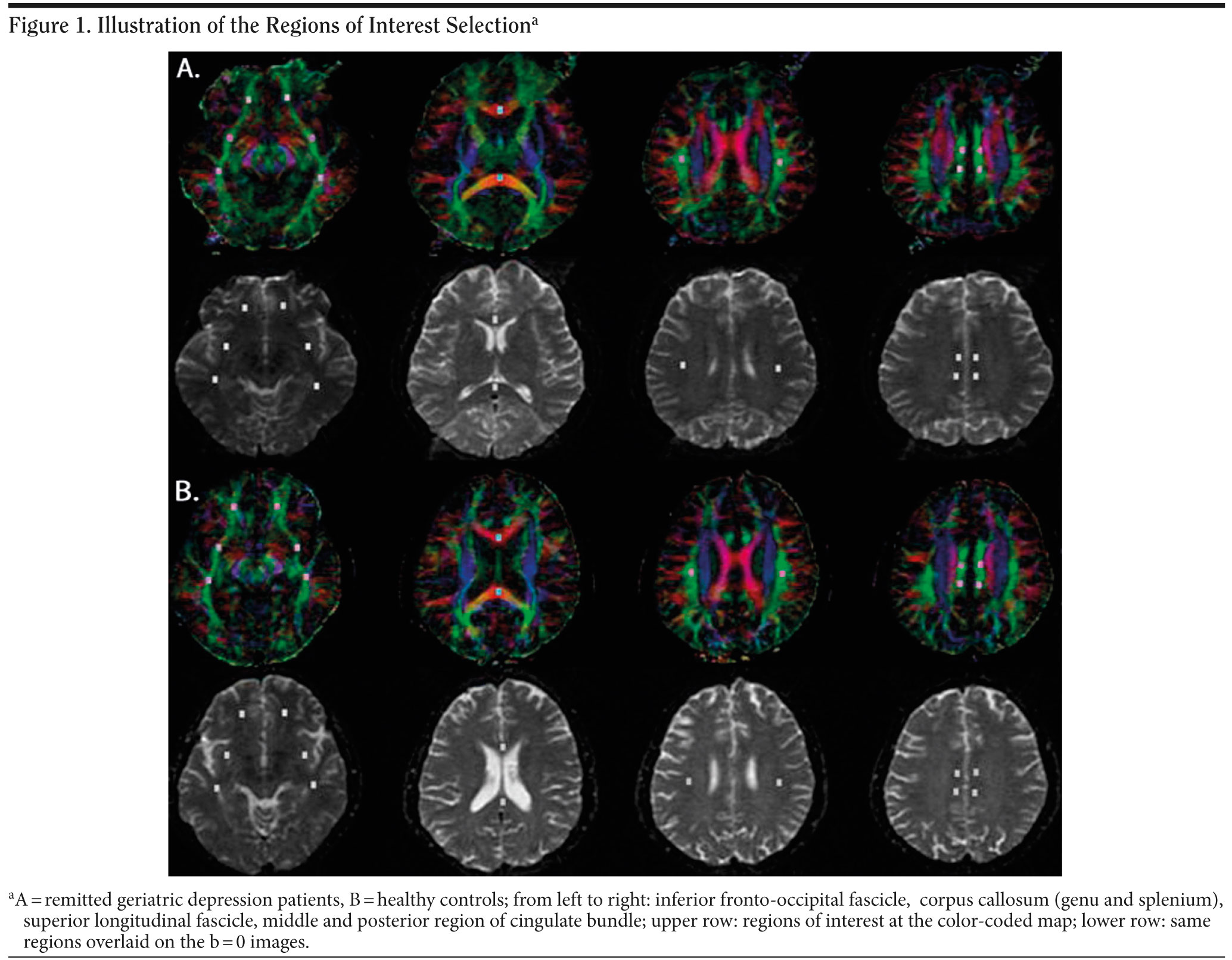
Diffusion tensor imaging data were calculated off-line with DTI Studio software, Version 2.40 (Johns Hopkins University, Baltimore, Maryland). First, fractional anisotropy maps and color-coded directionality maps of diffusion were created in individual images. The color-coded directional maps (red: left-to-right direction, green: anterior-to-posterior direction, blue: superior-to-inferior direction) provided easy visualization of the white matter fiber tracts. Brick-shaped ROIs were drawn based on the identification of white matter tracts on the color-coded maps. Then, fractional anisotropy maps were overlaid on these color-coded directional maps. A total of 8 pairs of ROIs were placed on axial slices to select the following white matter regions (Figure 1). For most regions, we used the ROI designations described by Bai et al.11 These ROIs were as follows: (1) inferior fronto-occipital fascicle (3 pairs ROIs: 1 frontal, 1 frontotemporal, 1 temporal); (2) genu and splenium ROIs at the center of anterior and posterior corpus callosum; (3) bilateral cingulate bundle (2 pairs ROIs: 1 midcingulate and 1 posterior cingulate region); (4) bilateral superior longitudinal fascicle (1 pair ROI at the segment II). Regions of interest were generally placed at an in-plane size of 4 ×— 6 mm2, with a 4 mm slice thickness. Fractional anisotropy values within each ROI were averaged. The same process was adopted for all subjects. To determine reliability of the ROI measurements, the same rater repeated ROI drawings on 10 randomly selected subjects, blinded to the previous evaluation. The correlation coefficient between previous and repeated measures was 0.91.
Statistical Analysis
Nonparametric Mann-Whitney U tests were used for group comparisons of clinical demographics, neuropsychological performances, and ROI value measurements (statistical significance was set at P < .05). Correlative analyses (Spearman) were performed to examine relationships between ROI value measurements and clinical neuropsychological performances. These statistical analyses were performed using SPSS 10.0 software (SPSS, Inc, Chicago, Illinois).
RESULTS
No significant differences in age, sex distribution, years of education, or scores for HDRS, ROCF delayed recall, Digit Span Test, or Clock Drawing Test were observed between the patients with RGD and healthy controls (all P > .05). However, the patients with RGD performed significantly worse in the MMSE, delayed recall of AVLT, Trail-Making Tests A and B (seconds), and Symbol Digit Modalities Test when compared with the control group (all P < .05) (Table 1).
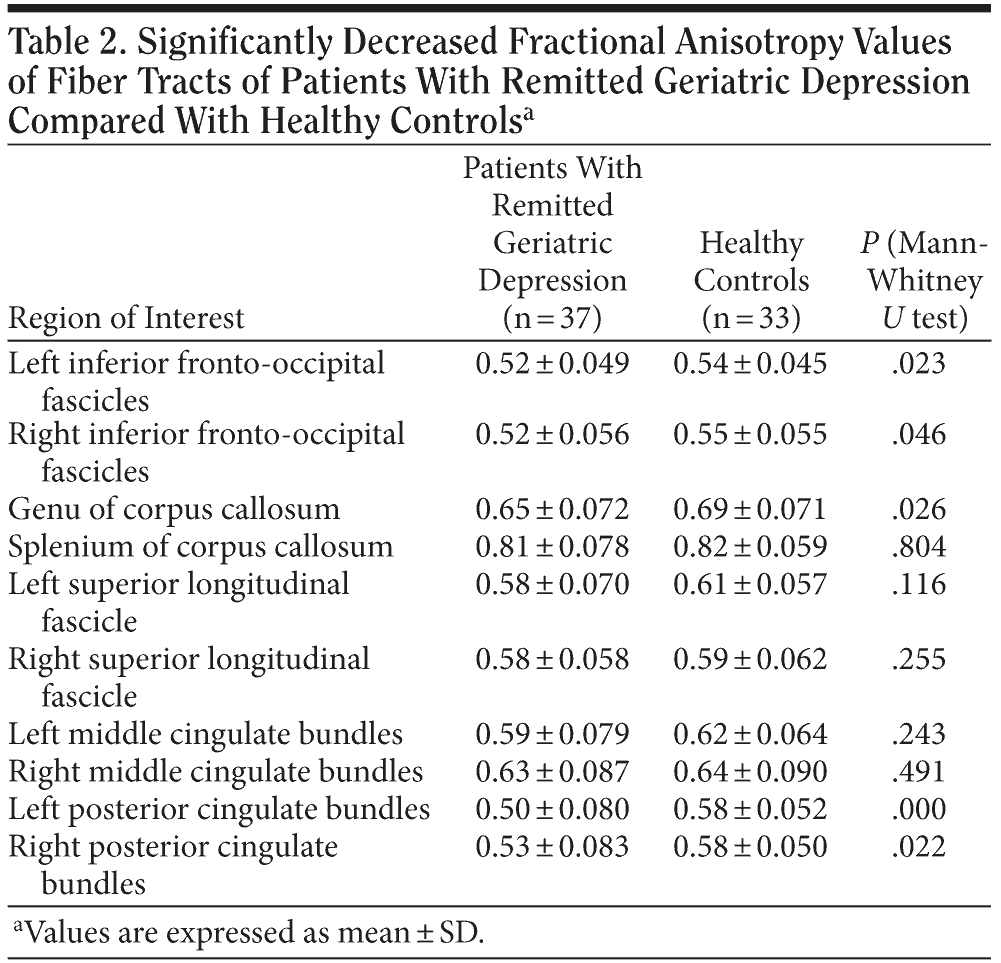
Fractional anisotropy values of bilateral inferior fronto-occipital fascicles, genu of corpus callosum, and bilateral posterior cingulate bundles were significantly decreased in patients with RGD compared to healthy controls (all P < .05). There were no significant differences in fractional anisotropy values of splenium of corpus callosum, bilateral middle cingulate bundles, or superior longitudinal fascicle between patients with RGD and healthy controls (all P > .05) (Table 2).
The fractional anisotropy value of left posterior cingulate bundles displayed a significantly negative correlation with the performance (time) of Trail-Making Test A (r = −0.383, P = .019) (Figure 2). Neuropsychological performance on all other tests did not significantly correlate with any other fractional anisotropy findings (P > .05).
DISCUSSION
In the present study, we first focused on the multiple long association fiber tracts of RGD because they represent important connections of those association cortico-cortical and limbic-association cortical networks, which are known to be neuronal substrates of cognitive function. The major finding is that fractional anisotropy values of bilateral inferior fronto-occipital fascicles, genu of corpus callosum, and bilateral posterior cingulate bundles are significantly decreased in patients with RGD compared to healthy controls. This finding was similar to those of previous diffusion tensor imaging-related studies, which showed hypo-integrity in these white matter regions in Alzheimer’s disease or patients with mild cognitive impairment.13,17,18 This finding further supported that late-onset depression might be a preclinical stage of dementia.2
Another major finding of the study was that the patients with RGD have significantly worse function of general cognition, episodic memory, executive function, and psychomotor speed (to be represented by MMSE, AVLT delayed recall, Trail-Making Tests A and B, and Symbol Digit Modalities Test, respectively) than healthy controls. The results are consistent with the previous reports.19-21 In addition, we found a significantly negative correlation of left posterior cingulate bundle integrity with the performance of Trail-Making Test A. The result suggested that not only was this structure affected early in disease progression, but it was also an important component of the network supporting high-level cognitive functions. As part of the Papez circuit, cingulum fibers connect the anterior thalamus; cortical cingulum; frontal, parietal, and temporal association cortices; and the hippocampus.13 Lesion study of the posterior cingulate found relatively mild memory deficits in rats.22 Importantly, current findings suggested that the posterior cingulate bundle is an important and clinically relevant site of degeneration in patients with RGD. Therefore, if these findings are replicated, a diffusion tensor imaging measure of fractional anisotropy in the left posterior cingulate bundle may be used as an early predictor of future cognitive decline and development of Alzheimer’s disease. Prospective studies are underway to determine if diffusion tensor imaging predicts cognitive decline and conversion to Alzheimer’s disease.
One further notable finding of the present study was the significant decrease of the inferior fronto-occipital fascicle integrity. Functionally, the inferior fronto-occipital fibers connecting the inferolateral and dorsolateral frontal cortex, the temporal cortex, and occipital lobe play a role in visual spatial processing, object recognition, and memory.23 Although we were able to detect significant structural changes within the inferior fronto-occipital fascicles, no significant correlation with the cognitive functional decline in the group with RGD could be obtained. The results are consistent with early Alzheimer’s disease.13 One reason for this lack of significant correlation might be that the ROI-based approach covered the small extension of these anatomically and functionally complex fiber bundles.13 Moreover, it is possible that neurodegenerative changes in these areas are not as strongly related to the disease-specific cognitive decline as in the cingulate bundles.13
Anatomically, the splenium of the corpus callosum connects the temporal and parietal cortices, while the genu of corpus callosum connects frontal cortices.24 We found fractional anisotropy reductions in the genu but not splenium of the corpus callosum in the patients with RGD. Thus, this finding supports the suggestion that the frontal connections are more impacted than temporoparietal connections by RGD.
However, the present study is a cross-sectional, case-control study but not a follow-up study. It remains unclear whether the diffusion tensor imaging findings in RGD are indeed relevant to progression to Alzheimer’s disease. Further work is needed to determine how these changes contribute to accomplish early detection of disease processes that presage the development of Alzheimer’s disease by follow-up study.
In conclusion, we find marked alterations of the long association fiber integrity, namely in the areas of the bilateral inferior fronto-occipital fascicles, genu of corpus callosum, and bilateral posterior cingulate bundles, in patients with RGD. Additionally, we demonstrated significantly negative correlation of left posterior cingulate bundle integrity with the executive function. This study suggested that investigation of association fiber tracts between remote cortexes may yield important new data to predict whether a patient will eventually develop Alzheimer’s disease.
Author affiliations: School of Clinical Medicine, Southeast University (Drs Yuan, Zhang, Bai, and Yu); Department of Psychiatry, Nanjing Brain Hospital, Nanjing Medical University (Drs Yuan, Hou, You, and Liu); and Department of Neurology, Affiliated ZhongDa Hospital of Southeast University (Drs Zhang and Shi), Nanjing, China; and National Laboratory of Pattern Recognition, Institute of Automation, Chinese Academy of Sciences (Dr Jiang), Beijing, China.
Potential conflicts of interest: None reported.
Funding/support: This study was partly supported by National Basic Research Program of China (973 Program) (No. 2007CB512308, Dr Zhang), National Hi-Tech Research and Development Program of China (863 Program) (No. 2007AA0200Z435, Dr Zhang), National Natural Science Foundation of China (No. 30825014, No. 30770779; Dr Zhang), Nature Science Foundation of JiangSu Province (No. BK2009050, Dr Yuan), and Key Program of Medical Development of Nanjing (No. ZKX07018, Dr Yuan).
Acknowledgment: The authors wish to express their appreciation to Zhu Wanlin, PhD, at Institute of Automation, Chinese Academy of Sciences, for providing a semi-automated imaging analysis program, and we thank all individuals who served as research participants. The acknowledged persons report no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
REFERENCES
1. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587-595. PubMed doi:10.1001/archpsyc.61.6.587
2. Kohn R, Epstein-Lubow G. Course and outcomes of depression in the elderly. Curr Psychiatry Rep. 2006;8(1):34-40. PubMed doi:10.1007/s11920-006-0079-y
3. Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79(6):619-624. PubMed doi:10.1136/jnnp.2007.124651
4. Alexopoulos GS, Murphy CF, Gunning-Dixon FM, et al. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165(2):238-244. PubMed doi:10.1176/appi.ajp.2007.07050744
5. Lesser IM, Boone KB, Mehringer CM, et al. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153(10):1280-1287. PubMed
6. Kramer-Ginsberg E, Greenwald BS, Krishnan KR, et al. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1999;156(3):438-444. PubMed
7. Murata T, Kimura H, Omori M, et al. MRI white matter hyperintensities, (1)H-MR spectroscopy and cognitive function in geriatric depression: a comparison of early- and late-onset cases. Int J Geriatr Psychiatry. 2001;16(12):1129-1135. PubMed doi:10.1002/gps.501
8. Yuan Y, Zhang Z, Bai F, et al. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. Neuroreport. 2007;18(17):1845-1849. PubMed doi:10.1097/WNR.0b013e3282f1939f
9. Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77-94. PubMed doi:10.1006/nimg.2002.1136
10. Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study [published online ahead of print December 8, 2004]. Cereb Cortex. 2005;15(6):854-869. PubMed doi:10.1093/cercor/bhh186
11. Bai F, Zhang Z, Watson DR, et al. Abnormal integrity of association fiber tracts in amnestic mild cognitive impairment. J Neurol Sci. 2009;278(1-2):102-106. PubMed doi:10.1016/j.jns.2008.12.009
12. Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13-19. PubMed doi:10.1212/01.wnl.0000250326.77323.01
13. Fellgiebel A, Schermuly I, Gerhard A, et al. Functional relevant loss of long association fibre tracts integrity in early Alzheimer’s disease. Neuropsychologia. 2008;46(6):1698-1706. PubMed doi:10.1016/j.neuropsychologia.2007.12.010
14. First MS, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I -Clinician Version). 4th ed. Washington, DC: American Psychiatric Press; 1997.
15. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62.
16. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Bartzokis G, Sultzer D, Lu PH, et al. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical "disconnection" in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25(7):843-851. PubMed doi:10.1016/j.neurobiolaging.2003.09.005
18. Xie S, Xiao JX, Gong GL, et al. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66(12):1845-1849. PubMed doi:10.1212/01.wnl.0000219625.77625.aa
19. Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37(2):99-108. PubMed doi:10.1016/S0022-3956(02)00085-7
20. Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14(5):419-427. PubMed doi:10.1097/01.JGP.0000203130.45421.69
21. Lee JS, Potter GG, Wagner HR, et al. Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr. 2007;19(1):125-135. PubMed doi:10.1017/S1041610206003607
22. Neave N, Nagle S, Sahgal A, et al. The effects of discrete cingulum bundle lesions in the rat on the acquisition and performance of two tests of spatial working memory. Behav Brain Res. 1996;80(1-2):75-85. PubMed doi:10.1016/0166-4328(96)00022-8
23. Catani M, Jones DK, Donato R, et al. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093-2107. PubMed doi:10.1093/brain/awg203
24. Huang H, Zhang J, Jiang H, et al. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26(1):195-205. PubMed doi:10.1016/j.neuroimage.2005.01.019