Objective: To compare the activity patterns of inpatients with unipolar depression, who had been divided into groups with and without motor retardation prior to actigraphy monitoring.
Method: Twenty-four-hour actigraphy recordings from 52 consecutively, acutely admitted inpatients with unipolar depression (ICD-10) were compared to recordings from 28 healthy controls. The patients, admitted between September 2011 and April 2012, were separated into 2 groups: 25 with motor retardation and 27 without motor retardation. Twenty-eight healthy controls were also included. Twenty-four-hour recordings, 9-hour daytime sequences, and 64-minute periods of continuous motor activity in the morning and evening were analyzed for mean activity, variability, and complexity.
Results: Patients with motor retardation had a reduced mean activity level (P = .04) and higher intraindividual variability, as shown by increased standard deviation (SD) (P = .003) and root mean square successive difference (RMSSD) (P = .025), during 24 hours compared to the patients without motor retardation. Both patient groups demonstrated significantly lower mean activity compared to healthy controls (P < .001) as well as higher SD (P < .02) and RMSSD (P < .001) and a higher RMSSD/SD ratio (P = .04). In the active morning peto be potencies of 2riod, the patients without motor retardation displayed significantly increased complexity compared to motor-retarded patients (P = .006).
Conclusions: The patients with and without motor retardation differ in activity patterns. Findings in depressed inpatients without motor retardation closely resemble those of inpatients with mania.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
ABSTRACT
Objective: To compare the activity patterns of inpatients with unipolar depression, who had been divided into groups with and without motor retardation prior to actigraphy monitoring.
Method: Twenty-four-hour actigraphy recordings from 52 consecutively, acutely admitted inpatients with unipolar depression (ICD-10) were compared to recordings from 28 healthy controls. The patients, admitted between September 2011 and April 2012, were separated into 2 groups: 25 with motor retardation and 27 without motor retardation. Twenty-eight healthy controls were also included. Twenty-four-hour recordings, 9-hour daytime sequences, and 64-minute periods of continuous motor activity in the morning and evening were analyzed for mean activity, variability, and complexity.
Results: Patients with motor retardation had a reduced mean activity level (P = .04) and higher intraindividual variability, as shown by increased standard deviation (SD) (P = .003) and root mean square successive difference (RMSSD) (P = .025), during 24 hours compared to the patients without motor retardation. Both patient groups demonstrated significantly lower mean activity compared to healthy controls (P < .001) as well as higher SD (P < .02) and RMSSD (P < .001) and a higher RMSSD/SD ratio (P = .04). In the active morning period, the patients without motor retardation displayed significantly increased complexity compared to motor-retarded patients (P = .006).
Conclusions: The patients with and without motor retardation differ in activity patterns. Findings in depressed inpatients without motor retardation closely resemble those of inpatients with mania.
J Clin Psychiatry 2015;76(9):1181-1187
dx.doi.org/10.4088/JCP.14m09106
© Copyright 2015 Physicians Postgraduate Press, Inc.
aDepartment of Neuroscience, the Norwegian University of Science and Technology, Trondheim, Norway
bDepartment of Psychiatry, St Olav’s University Hospital, Trondheim, Norway cDepartment of Clinical Medicine, Section for Psychiatry, Faculty of Medicine and Dentistry, University of Bergen, Bergen, Norway
dDivision of Mental Health Care, Valen Hospital, Fonna Regional Health Authority, Valen, Norway
eMoodNet Research Group, Division of Psychiatry, Haukeland University Hospital, Bergen, Norway
*Corresponding author: Karoline Krane-Gartiser, MD, Department of Neuroscience, the Norwegian University of Science and Technology (NTNU), PO Box 3008 Lade, N-7441 Trondheim, Norway ([email protected]).
In unipolar depression, the presence of psychomotor retardation and agitation is traditionally expressed by the patient’s subjective experience and observations by the clinician. Retardation is common in melancholic depression,1 while activated or agitated depressions have features of mixed states, in which the depressions include manic symptoms.2-6 It is important to recognize activated depressions, as there is an increased risk of potentially harmful behavior, including suicidality, and it has clinical consequences.5,7 Psychomotor symptoms might characterize subtypes of depression and be predictive of treatment response,8 and a scarce, but increasing, number of studies have used objective assessment methods to explore this phenomenon.9,10
Actigraphy is a wrist-worn device for activity monitoring, which has been implemented in the evaluation of sleep disorders.11,12 It has also been used to evaluate activity in psychiatric disorders, including major depressive disorder (MDD).13-20 Twenty-four-hour motor activity in the depressed state seems to be lower than in the euthymic state.8,21-23 Previous studies have failed to find correlations between total scores in depression rating scales and actigraphic daytime activity,24-27 and it has been suggested that motor retardation may be better assessed by specific retardation scales.26
The temporal resolution and capacity of actigraphs have improved in the past years.11,26,28 By employing adequate analytic methods, the full potential of actigraphy data can be captured. Actigraphy studies using mathematical techniques with a theoretical basis in nonlinear dynamics have described the complexity of motor patterns in patients with depression.29,30 In a previous study from our group, we used these nonlinear methods to compare actigraphically assessed motor activity in acutely admitted inpatients with bipolar disorder and found distinct differences in activity parameters and diurnal patterns between the patients with mania and the patients with bipolar depression.31
The purpose of the current study was to assess the mean activity level and variability and complexity of motor activity in 24-hour actigraphy recordings from acutely admitted inpatients with unipolar depression, who had been divided into those with and without motor retardation prior to actigraphy monitoring. Our a priori hypothesis was that the patient groups would display differences in activity variables, not only mean activity level. We also wanted to study differences between morning and evening sequences, as well as periods of continuous motor activity. Both patient groups would be compared to healthy controls.
METHOD
Patients
Consecutively, acutely admitted inpatients at the Department of Psychiatry, St Olav’s University Hospital, Trondheim, Norway, were asked to participate in a study assessing symptoms of agitation during admission.31 The only exclusion criterion was inability to give an informed consent. All Norwegian acute psychiatric services are public. All patients older than 18 years in the catchment area who suffer from any acute psychiatric condition in need of acute admittance are admitted to this department. At the hospital wards, the patients go to sleep and have their meals at the same time. Patients too disturbed to comply with such routines have individually based services.
A total of 424 admissions were included in the study. The patients with an inpatient stay of more than 1 day after inclusion were asked to wear an actigraph for 24 hours. In total, 280 actigraphy recordings were effectuated during admissions between September 1, 2011 and March 31, 2012. Diagnoses according to ICD-10 criteria for research32 were set at discharge in a department’s staff consensus meeting including the patient’s therapist and at least 2 psychiatrists, of whom at least one personally knew the patient. The largest diagnostic group was affective disorders, which included 110 admissions (39.3% of the 280 admissions). Of these, 62 admissions were due to a primary diagnosis of a depressive episode or recurrent depressive disorder (F32 and F33).

- Actigraphy can quantify motor activity and might be used to distinguish between motor-retarded and non-motor-retarded patients with depression.
- Further studies might reveal the use of actigraphy as a support for other diagnostic tools.
- Findings in patients with activated depression resemble previously studied mania.
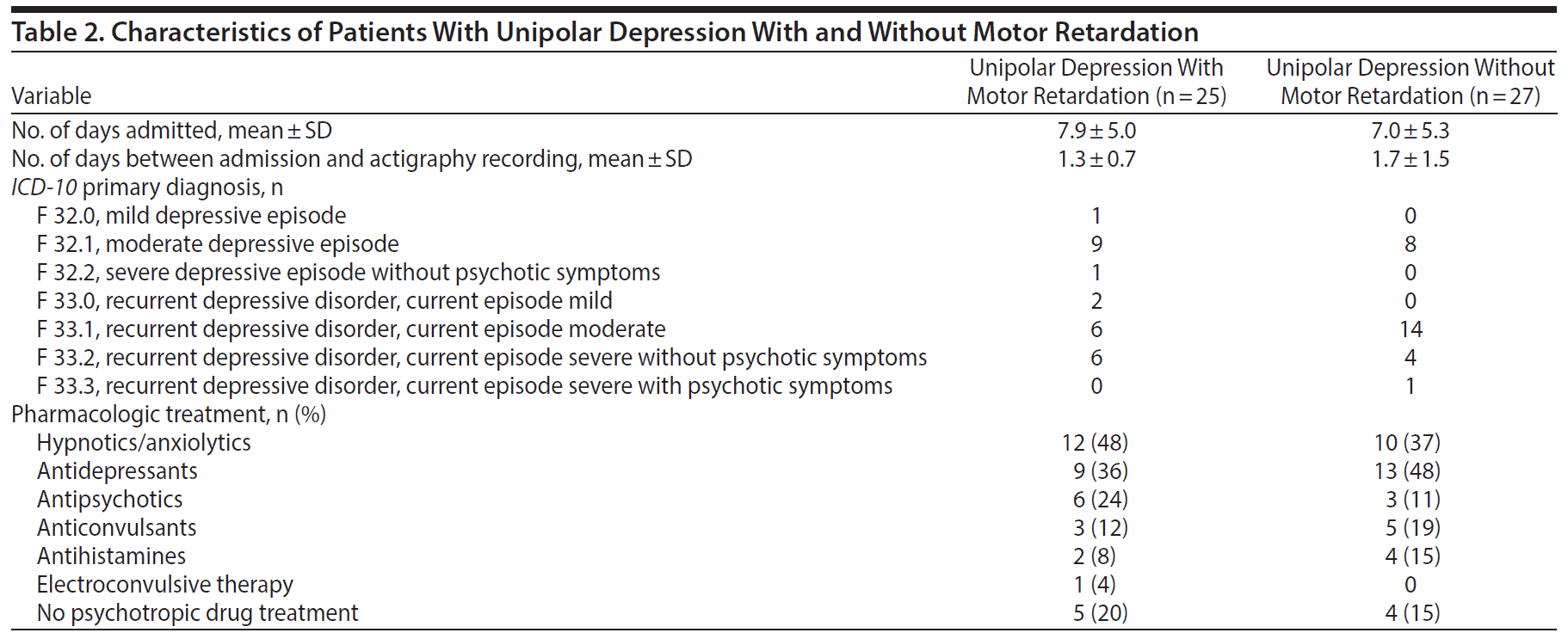
The 62 patients in a depressive episode with valid actigraphy recordings were separated into 2 groups based on the degree of motor retardation assessed by the Symptomatic Organic Mental Disorder Assessment Scale (SOMAS), item B: “Degree of motor retardation, rated during the period or periods of the previous 24 hours in which the patient was most depressed.”33(p6) SOMAS is a 5-item scale developed to measure the prevalence and degree of atypical depressive mood symptoms. Item B is modified from the Positive and Negative Syndrome Scale (PANSS) item “motor retardation” (general psychopathology scale), and item C, which rates the degree of increased motor activity during the period or periods the previous 24 hours when the patient was most depressed, is modified from the PANSS item “hyperactivity” (positive scale).34 Patients with any observable motor retardation according to SOMAS item B were classified as motor-retarded, whereas patients rated to have increased motor activity (SOMAS item C) or neither retardation nor increased motor activity were classified as non-motor-retarded. Three patients were not rated for motor activation and were excluded, as were 5 patients who had mixed ratings of both retardation and increased motor activity (SOMAS items B and C). Two of the patients in the non-motor-retardation group were admitted twice; only their first admissions were included in the analyses. Finally, a total of 52 patients were included; 25 in the motor retardation group and 27 in the non-motor-retardation group.
Healthy Controls
The comparison group of healthy controls (n = 28) primarily consists of employees at the Department of Psychiatry, Fonna Regional Health Authority in Western Norway. None of the controls reported to be diagnosed with an affective disorder or prescribed psychopharmacologic drugs. They wore an actigraph for at least 24 hours during the period from March 13, 2012 to June 6, 2013. Eighteen of them wore the actigraph in spring or summer months (April through August).
Recordings of Motor Activity
Motor activity was recorded using an actigraph (Actiwatch Spectrum, Philips Respironics Inc, Murrysville Pennsylvania), which contains a piezoelectric accelerometer programmed to record the integration of intensity, amount, and duration of movement in all directions. A corresponding voltage is produced and stored as an activity count in the memory unit of the actigraph. Patients and healthy controls wore the actigraph around the wrist of their choice and were instructed not to take it off during the next 24 hours. Which wrist was not noted, but previous studies have not found significant differences between the left and right wrist.35,36 Patient recordings contained a mean ± SD of 1,354 ± 195 minutes for analysis, ranging from 627 to 1,439 minutes. The median number of minutes was 1,437. Healthy control recordings contained 1,440 minutes from midnight to midnight, as controls were 100% compliant in wearing the actigraph for 24 hours.
Activity counts were recorded for 1-minute intervals during 24 hours. Data were analyzed for the total time of recording. The time period from 6 am to midnight was separated in morning epochs from 6 am to 3 pm and evening epochs between 3 pm and midnight. Because many of the recordings contained periods of inactivity, we searched each recording for periods of continuous motor activity in the morning and evening. The active morning period was searched from the start of the series and the active evening period from the end of the series. From each participant we selected the first period of 64 minutes not containing more than 2 consecutive minutes of zero activity counts. If there was no such period, we searched for sequences with no more than 3 consecutive minutes with zero activity, and if that was not found, sequences with at most 4 consecutive minutes with zero activity. In this way we were able to obtain 64-minute sequences from almost all of the participants. Sixty-four minutes was chosen due to the Fourier analysis, which requires sequence lengths to be potencies of 2 (32, 64, 128…).
Two patients in the retardation group lacked a 64-minute sequence with at most 4 consecutive minutes with zero activity in the morning. Both patients were omitted from active morning series analyses, reducing the group with retardation to 23 patients. Two patients in the retardation group and 1 healthy control lacked a 64-minute sequence with at most 4 consecutive minutes with zero activity in the evening and were therefore excluded from active evening series analyses.
Mathematical Analyses
From the activity counts in the actigraph software program (Actiware, version 5.70.1; Philips), we calculated means for the whole recording period and the 64-minute periods of continuous motor activity. We further calculated the standard deviation (SD) for each time series, which is an intraindividual measure of fluctuations in activity; the root mean square successive difference (RMSSD), which describes the difference in successive counts from minute to minute; and the RMSSD/SD ratio. For the 64-minute time periods, we additionally performed a Fourier analysis and used sample entropy, a measure of complexity. All techniques represent distinct means of characterizing a series of data in time. The software used for the estimation of sample entropy and for the Fourier analysis was obtained from the Physio Toolkit Research Resource for Complex Physiologic signals37 (see http://www.physionet.org).
Sample Entropy
For the analysis of sample entropy, the data were normalized by transforming the time series to have sample mean of 0 and sample variance of 1. Sample entropy is a nonlinear measure that indicates the degree of regularity (complexity) of a time series and is the negative natural logarithm of an estimate of the conditional probability that subseries of a certain length (m) that match pointwise, within a tolerance (r), also match at the next point. We chose the following values, m = 2 and r = 0.2. Sample entropy was used since it can be employed with comparatively short time series (> 50 points) and is robust with regard to outliers.38
Fourier Analysis
Data were normalized before analysis. No windows were applied. Results are presented as the relation between variance in the high frequency part of the spectrum (0.0021-0.0083 Hz, corresponding to the period of 2-8 minutes) and the low frequency part (0.00026-0.0021 Hz, corresponding to 8-64 minutes).
Statistics
Statistical analyses were carried out using SPSS version 20.0 (IBM). Means and standard deviations were calculated for the continuous variables and proportions for the categorical variables. For comparison of means between patients, we used t tests, and for comparison of counts of categorical data, χ2 tests. For comparison of subject characteristics and activity variables in all 3 groups, we used 1-way analyses of variance with least significant difference (LSD) post hoc tests to obtain differences between groups. If unequal variances were assumed, the Tamhane T2 post hoc test was used. A P value ≤ .05 was considered significant.
Ethics Review
The patient study has been approved by the Regional Committee for Medical and Health Research Ethics of Central Norway and the healthy control study by the Regional Committee for Medical and Health Research Ethics of Western Norway. All participants signed a written informed consent form prior to inclusion. The funding organizations had no role in the design or conduct of the study.
RESULTS
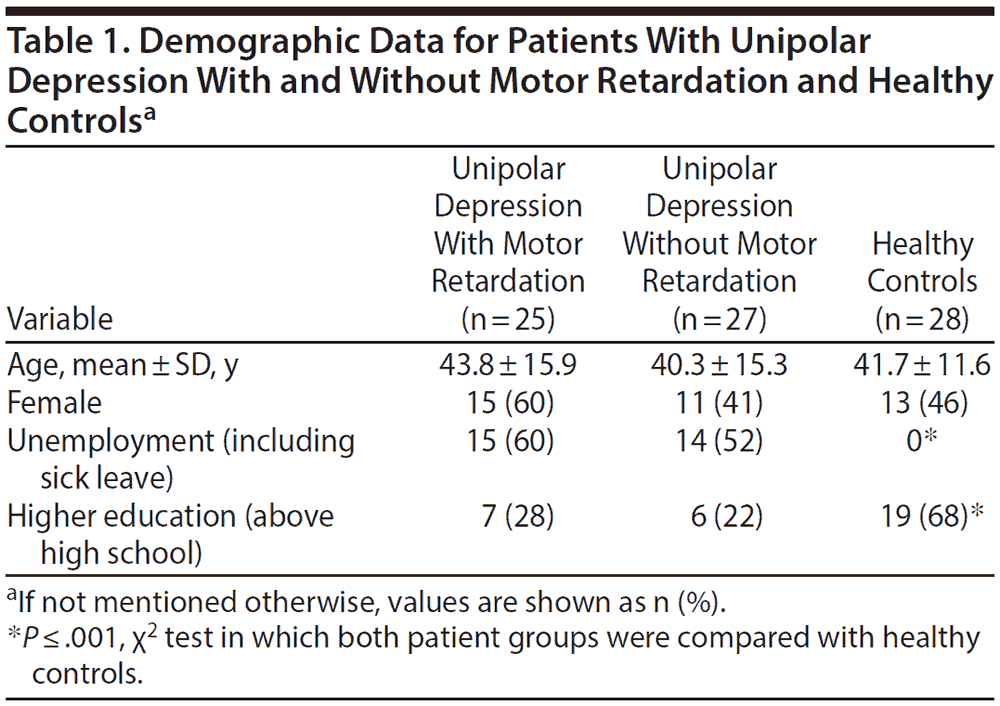
Demographic data for all 3 groups are shown in Table 1 and patient characteristics in Table 2.
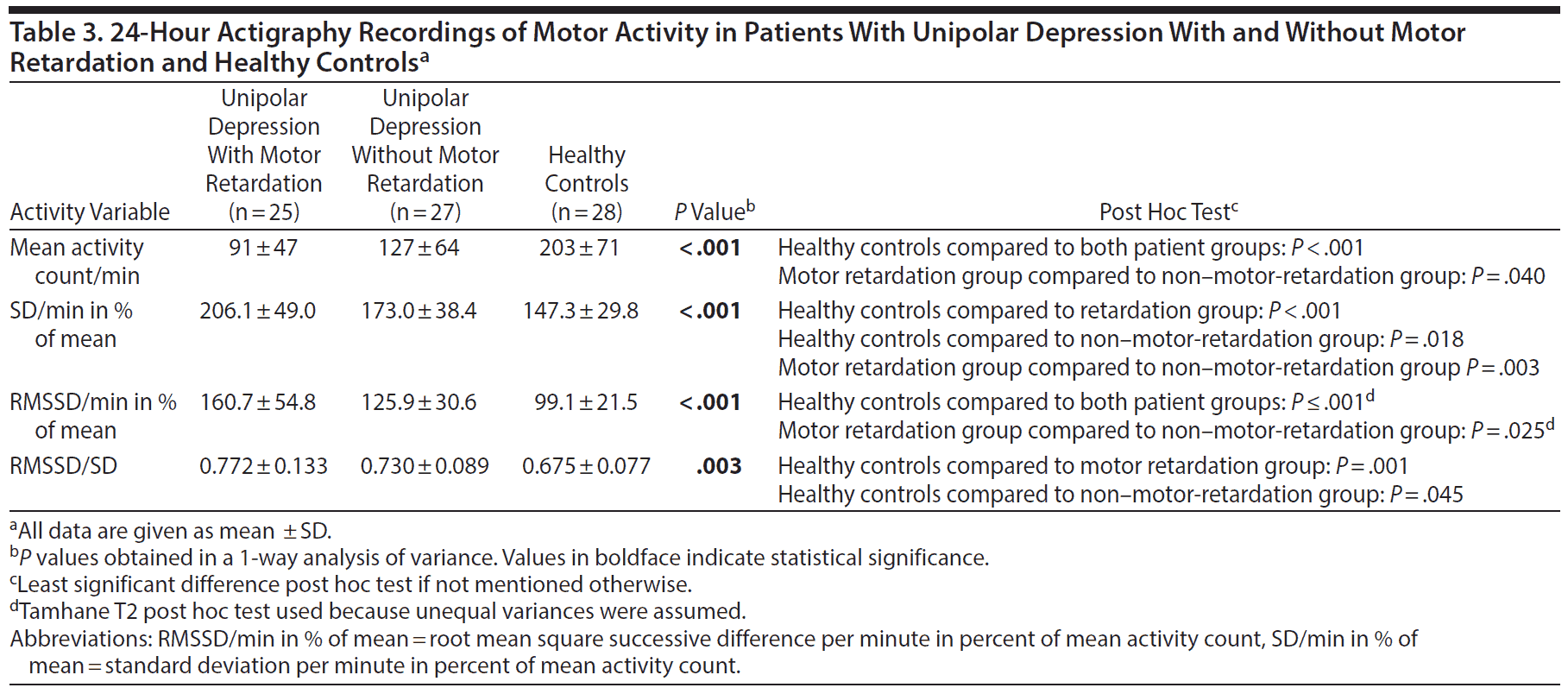
In the 24-hour recording, the patients with motor retardation displayed a reduced mean activity level and greater variability, as given by increased SD and RMSSD in percent of mean activity, compared to the patients without motor retardation. Both patient groups differed from healthy controls in all activity variables in the 24-hour recording (Table 3).
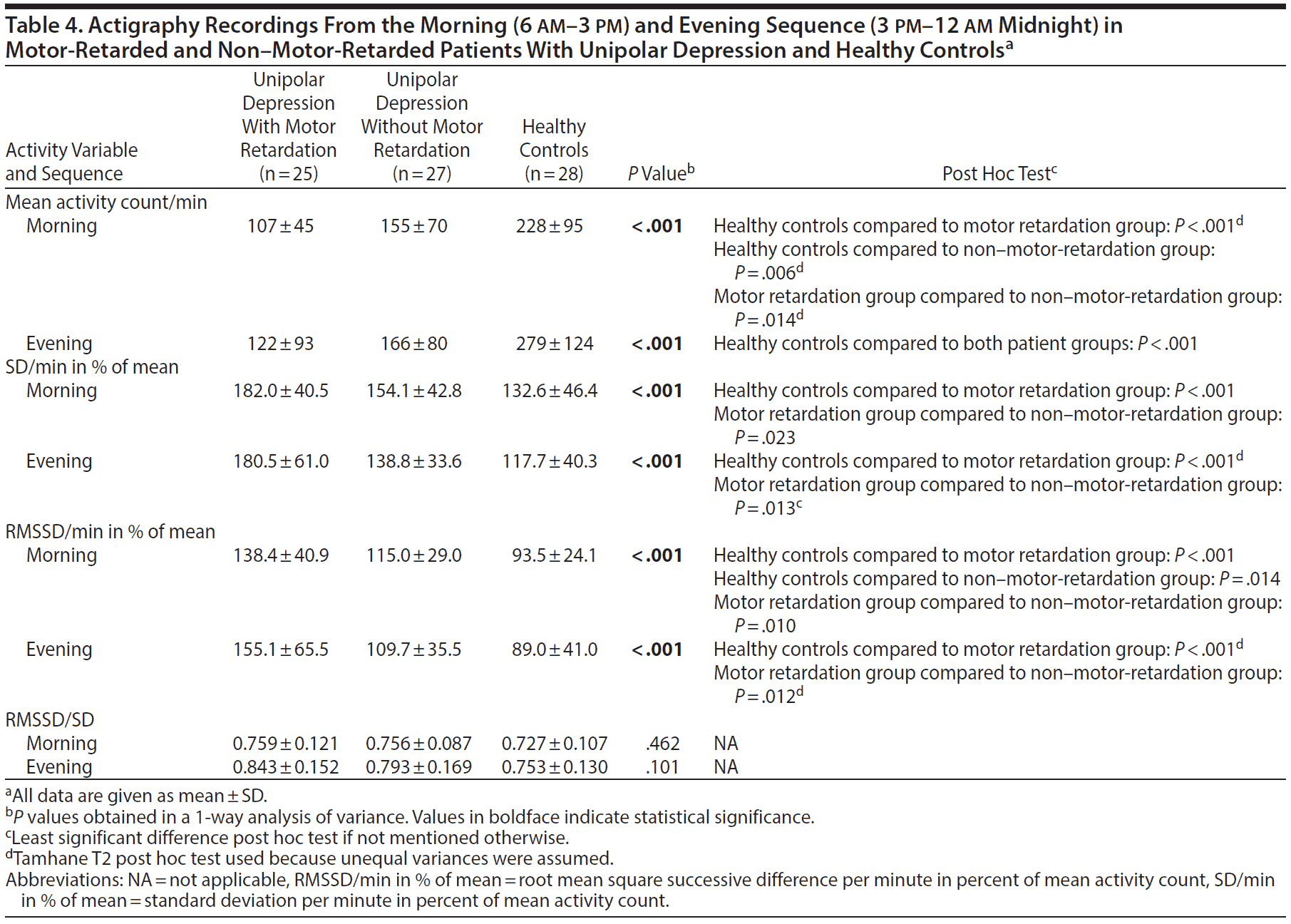
Analysis of the morning sequence (6 am-3 pm) produced many of the same findings as in the 24-hour recording (Table 4). In the evening (3 pm-12 am midnight), both patient groups were less active than the healthy controls (Table 4). The motor retardation group again had a higher SD, RMSSD, and RMSSD/SD ratio compared to the healthy controls. However, there was no difference in mean activity between patients in the evening, but the patients with motor retardation had both higher SD and RMSSD in percent of mean activity compared to the patients without motor retardation.
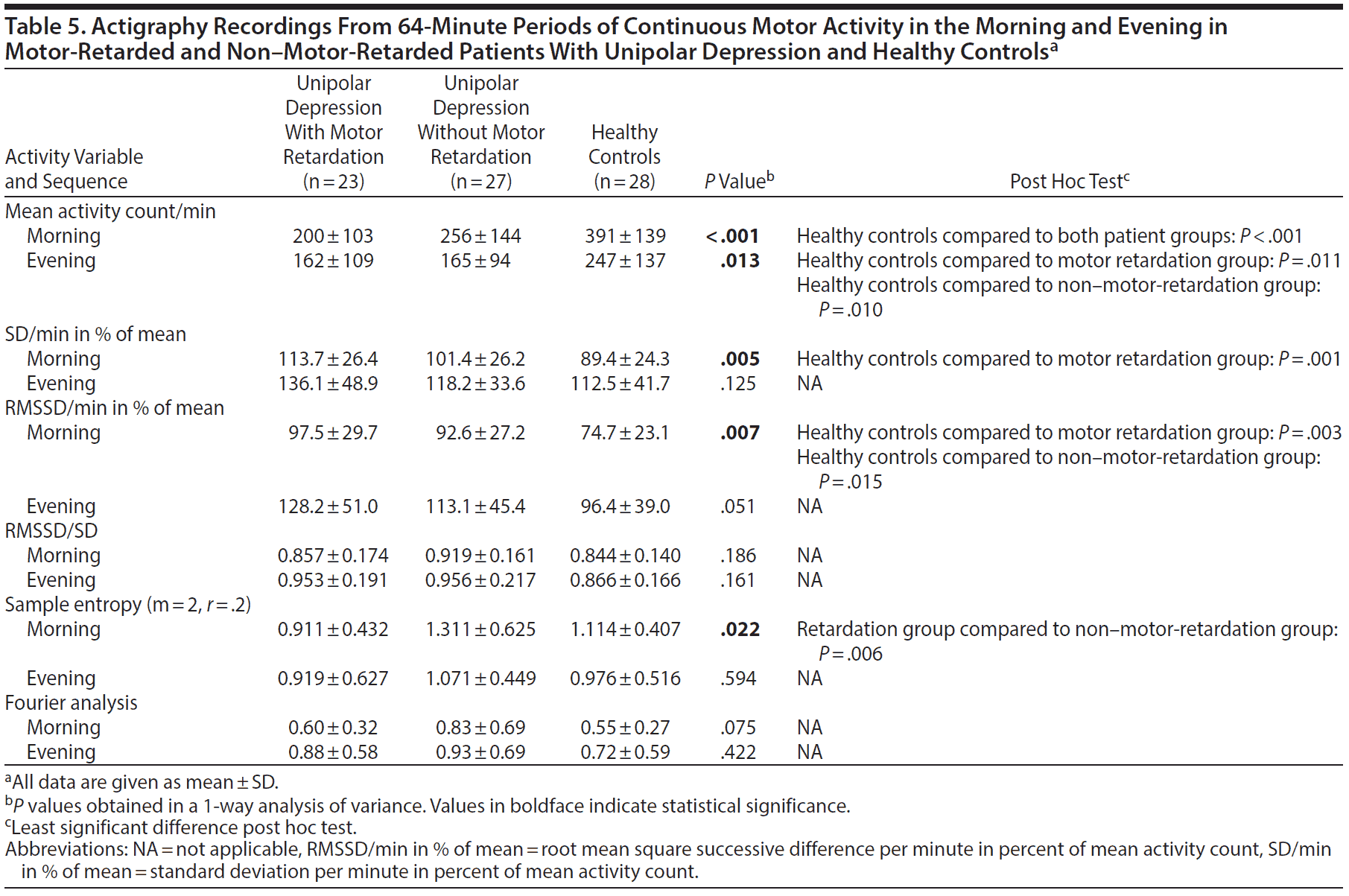
The only finding that separated patients with motor retardation from patients without motor retardation in the 64-minute period of continuous motor activity in the morning was significantly higher sample entropy in the patients without motor retardation (Table 5). The motor activity level was significantly lower and the RMSSD in percent of mean activity higher among patients than healthy controls. The patients with retardation additionally distinguished themselves from the healthy controls by a significantly increased SD in percent of mean activity.
In the active evening sequence (Table 5), the healthy controls were again more active than both patient groups. No other between-group differences were found in this sequence.
DISCUSSION
Actigraphy recordings support clinical assessments of motor activation in patients with depression. Distinct differences in activity variables between motor-retarded and non-motor-retarded patients were found in 24-hour recordings and sequences in the morning and evening. Patients without motor retardation closely resemble previously studied patients with mania in several activity parameters.31
The patients with motor retardation had a lower mean activity level than the patients without motor retardation in the 24-hour recordings and the morning sequence. Motor-retarded patients further displayed higher variability in activity throughout the 24 hours and in the morning and evening sequence compared to non-motor-retarded patients and healthy controls. The finding of increased intraindividual variability in patients with depression is in agreement with a previous study29 and our findings in a bipolar depression sample.31
Both patient groups were less active than the controls, which corroborates findings in a number of actigraphy studies.19 As Burton et al19 mention, such differences in mean activity between institutionalized patients and healthy individuals living in the community may be explained by living environment and hospital routines rather than by affective level. Yet, the patients with motor retardation in the current study differed from controls in other characteristics compared to how patients without motor retardation differed from controls, namely in having increased SD in percent of mean activity in the morning, evening, and active morning sequence.
As with our previously reported bipolar depression sample,31 the patients with unipolar depression and motor retardation showed an increased RMSSD/SD ratio in the 24-hour period compared to healthy controls, meaning that the alteration between successive activity counts increased relative to overall variability.
Selecting periods of continuous motor activity allows for more advanced analyses of complexity and variability. In such active sequences, the patients clearly separated themselves from the healthy controls. The differences between patients with and without retardation were fewer. Both patient groups were again significantly less active than the controls in the morning and the evening, and they displayed higher RMSSD in percent of mean activity in the active morning sequence. The patients with motor retardation also had increased SD in percent of mean activity in the morning and increased RMSSD in the evening.
The unipolar depression patients without motor retardation resemble patients with mania31 and schizophrenia29 in having significantly higher sample entropy compared to motor-retarded patients. An increase in entropy indicates a higher level of disorder and unpredictability in a time series and has been suggested to represent a partial breakdown in structured normal activities of everyday life.29
Although the Fourier analysis of the active morning sequence did not produce significant between-group differences, the non-motor-retarded patients demonstrated the highest ratio between variance in the high and low frequency part of the spectrum, corresponding to the period from 2 to 8 minutes. An increased ratio has previously been found in patients with mania31 and schizophrenia29 and healthy individuals treated with the glutamatergic N-methyl-d-aspartate (NMDA) antagonist memantine.30
In periods with continuous activity, healthy controls were more active in the morning than in the evening while patients with motor retardation had minimal changes in activity from the morning to the evening.
In summary, the patients with motor retardation have an activity pattern that is characterized by lower mean activity, higher intraindividual variability, and increased differences from minute to minute compared to both non-motor-retarded patients with depression and healthy controls over 24 hours and during the morning and evening periods. The activity pattern of the motor-retarded patients is furthermore represented by less complexity than that of the non-motor-retarded patients, and with the additional finding of increased successive difference relative to overall variability during 24 hours in patients compared to controls, the motor-retarded patients more closely resemble patients with bipolar depression.31 Regarding complexity and variability in a period of continuous motor activity in the morning, the activity characteristics of patients without retardation are similar to those found in patients with mania and schizophrenia and those induced by glutamatergic antagonism in healthy individuals.29-31
In the present study, we have not investigated immobility periods or minimum activity levels throughout the day, although they could be an important indicator of motor retardation. Royant-Parola et al22 suggested that lack of initiation of spontaneous movements in depression seemed to be a more fundamental deficiency than low activity level and that it could additionally be observed in non-retarded patients. In the current study, immobility is reflected only in the mean activity counts per minute as well as in periods with more than 4 consecutive activity counts of zero, which were excluded from active sequence analyses. Sequences with continuous motor activity are necessary for the nonlinear analyses, as sequences with many zero counts will substantially lower sample entropy values and thus give a misleading impression of increased order.
The study has some limitations. Psychotropic medication and comorbid disorders may bias the differences in activity found between patients. The study does not have the power to examine the impact of pharmacologic agents on activity parameters. No rating of affective symptom score was available, and a possible influence of depression severity on motor activity was not evaluated; the patients were separated into 2 groups based on a clinical assessment of retardation. Finally, it is possible that the activity characteristics found in patients compared to controls are affected by hospitalization.
Despite the limitations mentioned, the results support the application of actigraphy to quantify motor components of clinical depression, which could become useful in differentiating between motor-retarded and non-motor-retarded patients with depression. By using actigraphy recordings, a depressive group without motor retardation has demonstrated many of the same actigraphy findings as in patients with mania described in an earlier publication from the same study.31
The findings are potentially important clinical signatures of depression and might suggest phenotypic differences between depressive subgroups based on activity levels and patterns. Further research might reveal information to assist in choices of treatment.
Drug names: memantine (Namenda).
Submitted: March 5, 2014 2014; accepted July 14, 2014.
Online ahead of print: July 7, 2015.
Potential conflicts of interest: The authors declare no competing interests.
Funding/support: The patient study was financially supported by the Department of Neuroscience, the Norwegian University of Science and Technology (NTNU), Trondheim, Norway, and St. Olav’s University Hospital, Trondheim, Norway. The healthy control study was supported by Fonna Regional Health Authority, the Western Norway Regional Health Authority, and MoodNet, a regional research network on mood disorders located at Haukeland University Hospital, Bergen, Norway, also funded by the Western Norway Regional Health Authority.
Role of the sponsor: The funding organizations had no role in the design or conduct of the study.
Previous presentations: Oral presentation of the study at a chronobiology satellite symposium during The Norwegian Psychiatric Association Annual Congress; March 10, 2014; Bergen, Norway.
Acknowledgments: We are grateful to Kjetil S׸rensen, Master in Mental Health, St Olav’s University Hospital, for managing the data collection and to Erlend Fasmer, BEng, for making programs to automatically extract and analyze the active morning and evening sequences. These individuals report no relevant financial disclosures.
REFERENCES
1. Parker G, Fink M, Shorter E, et al. Issues for DSM-5: whither melancholia? the case for its classification as a distinct mood disorder. Am J Psychiatry. 2010;167(7):745-747. PubMed doi:10.1176/appi.ajp.2010.09101525
2. Maj M, Pirozzi R, Magliano L, et al. Agitated depression in bipolar I disorder: prevalence, phenomenology, and outcome. Am J Psychiatry. 2003;160(12):2134-2140. PubMed doi:10.1176/appi.ajp.160.12.2134
3. Koukopoulos A, Sani G, Koukopoulos AE, et al. Melancholia agitata and mixed depression. Acta Psychiatr Scand suppl. 2007;115(s433):50-57. PubMed doi:10.1111/j.1600-0447.2007.00963.x
4. Angst J, Gamma A, Benazzi F, et al. Does psychomotor agitation in major depressive episodes indicate bipolarity? evidence from the Zurich Study. Eur Arch Psychiatry Clin Neurosci. 2009;259(1):55-63. PubMed doi:10.1007/s00406-008-0834-7
5. Swann AC. Activated depression: mixed bipolar disorder or agitated unipolar depression? Curr Psychiatry Rep. 2013;15(8):376. PubMed doi:10.1007/s11920-013-0376-1
6. Koukopoulos A, Sani G. DSM-5 criteria for depression with mixed features: a farewell to mixed depression. Acta Psychiatr Scand. 2014;129(1):4-16. PubMed doi:10.1111/acps.12140
7. Akiskal HS, Benazzi F, Perugi G, et al. Agitated “unipolar” depression re-conceptualized as a depressive mixed state: implications for the antidepressant-suicide controversy. J Affect Disord. 2005;85(3):245-258. PubMed doi:10.1016/j.jad.2004.12.004
8. Todder D, Caliskan S, Baune BT. Longitudinal changes of day-time and night-time gross motor activity in clinical responders and non-responders of major depression. World J Biol Psychiatry. 2009;10(4):276-284. PubMed doi:10.3109/15622970701403081
9. Iverson GL. Objective assessment of psychomotor retardation in primary care patients with depression. J Behav Med. 2004;27(1):31-37. PubMed doi:10.1023/B:JOBM.0000013642.43978.f9
10. Bennabi D, Vandel P, Papaxanthis C, et al. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed Res Int. 2013;2013:158746. PubMed doi:10.1155/2013/158746
11. Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342-392. PubMed
12. Kaplan KA, Talbot LS, Gruber J, et al. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord. 2012;14(8):870-879. PubMed doi:10.1111/bdi.12021
13. Dantchev N, Allilaire JF, Raoux N. Significance of studies of motor activity in depression [article in French]. Ann Med Psychol (Paris). 1992;150(2-3):206-210. PubMed
14. Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3(1):18-35. PubMed doi:10.3109/10673229509017161
15. Faedda GL, Teicher MH. Objective measures of activity and attention in the differential diagnosis of psychiatric disorders of childhood. Essent Psychopharmacol. 2005;6(5):239-249. PubMed
16. Walther S, Horn H, Razavi N, et al. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology. 2009;60(2):80-86. PubMed doi:10.1159/000236448
17. Berle JO, Hauge ER, Oedegaard KJ, et al. Actigraphic registration of motor activity reveals a more structured behavioural pattern in schizophrenia than in major depression. BMC Res Notes. 2010;3(1):149. PubMed doi:10.1186/1756-0500-3-149
18. Faurholt-Jepsen M, Brage S, Vinberg M, et al. Differences in psychomotor activity in patients suffering from unipolar and bipolar affective disorder in the remitted or mild/moderate depressive state. J Affect Disord. 2012;141(2-3):457-463. PubMed doi:10.1016/j.jad.2012.02.020
19. Burton C, McKinstry B, Szentagotai Tătar A, et al. Activity monitoring in patients with depression: a systematic review. J Affect Disord. 2013;145(1):21-28. PubMed doi:10.1016/j.jad.2012.07.001
20. Gonzalez R, Tamminga CA, Tohen M, et al. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75(4):e317-e322. PubMed doi:10.4088/JCP.13m08506
21. Benoit O, Royant-Parola S, Borbely AA, et al. Circadian aspects of motor activity in depressed patients. Acta Psychiatr Belg. 1985;85(5):582-592. PubMed
22. Royant-Parola S, Borbely AA, Tobler I, et al. Monitoring of long-term motor activity in depressed patients. Br J Psychiatry. 1986;149(3):288-293. PubMed doi:10.1192/bjp.149.3.288
23. Wolff EA 3rd, Putnam FW, Post RM. Motor activity and affective illness: the relationship of amplitude and temporal distribution to changes in affective state. Arch Gen Psychiatry. 1985;42(3):288-294. PubMed doi:10.1001/archpsyc.1985.01790260086010
24. Kupfer DJ, Weiss BL, Foster G, et al. Psychomotor activity in affective states. Arch Gen Psychiatry. 1974;30(6):765-768. PubMed doi:10.1001/archpsyc.1974.01760120029005
25. Raoux N, Benoit O, Dantchev N, et al. Circadian pattern of motor activity in major depressed patients undergoing antidepressant therapy: relationship between actigraphic measures and clinical course. Psychiatry Res. 1994;52(1):85-98. PubMed doi:10.1016/0165-1781(94)90122-8
26. Razavi N, Horn H, Koschorke P, et al. Measuring motor activity in major depression: the association between the Hamilton Depression Rating Scale and actigraphy. Psychiatry Res. 2011;190(2-3):212-216. PubMed doi:10.1016/j.psychres.2011.05.028
27. Lemke MR, Puhl P, Broderick A. Motor activity and perception of sleep in depressed patients. J Psychiatr Res. 1999;33(3):215-224. PubMed doi:10.1016/S0022-3956(98)00067-3
28. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527. PubMed doi:10.1378/chest.10-1872
29. Hauge ER, Berle JO, Oedegaard KJ, et al. Nonlinear analysis of motor activity shows differences between schizophrenia and depression: a study using Fourier analysis and sample entropy. PLoS ONE. 2011;6(1):e16291. PubMed doi:10.1371/journal.pone.0016291
30. Johnsen E, Fasmer OB, van Wageningen H, et al. The influence of glutamatergic antagonism on motor variability, and comparison to findings in schizophrenia patients. Acta Neuropsychiatr. 2013;25(2):105-112. PubMed doi:10.1111/j.1601-5215.2012.00674.x
31. Krane-Gartiser K, Henriksen TEG, Morken G, et al. Actigraphic assessment of motor activity in acutely admitted inpatients with bipolar disorder. PLoS ONE. 2014;9(2):e89574. PubMed doi:10.1371/journal.pone.0089574
32. WHO. The ICD-10 Classification of Mental and Behavioural Disorders-Diagnostic Research Criteria. Geneva, Switzerland: World Health Organization; 1993.
33. Vaaler AE, Morken G, Iversen VC, et al. Acute Unstable Depressive Syndrome (AUDS) is associated more frequently with epilepsy than major depression. BMC Neurol. 2010;10(1):67. PubMed doi:10.1186/1471-2377-10-67
34. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
35. Van Hilten JJ, Middelkoop HA, Kuiper SI, et al. Where to record motor activity: an evaluation of commonly used sites of placement for activity monitors. Electroencephalogr Clin Neurophysiol. 1993;89(5):359-362. PubMed doi:10.1016/0168-5597(93)90076-2
36. Littner M, Kushida CA, Anderson WM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337-341. PubMed
37. Goldberger AL, Amaral LA, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215-E220. PubMed doi:10.1161/01.CIR.101.23.e215
38. Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278(6):H2039-H2049. PubMed
Save
Cite
Advertisement
GAM ID: sidebar-top