This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “Activating and Sedating Properties of Medications Used for the Treatment of Major Depressive Disorder and Their Effect on Patient Functioning,” which were held in December 2018 and January and February 2019.
The teleconferences were chaired by Leslie L. Citrome, MD, MPH, Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla. The faculty were Roger S. McIntyre, MD, FRCPC, Department of Psychiatry and Pharmacology, University of Toronto; Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada; J. Sloan Manning, MD, Department of Family Medicine at the University of North Carolina, Chapel Hill; and Diane McIntosh, MD, FRCPC, Department of Psychiatry, University of British Columbia, Vancouver, British Columbia, Canada.
Financial disclosures: Dr Citrome has been a consultant for Acadia, Alkermes, Allergan, Impel, Indivior, Intra-Cellular Therapeutics, Janssen, Lundbeck, Merck, Neurocrine, Noven, Osmotica, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, and Vanda; has served on speakers/advisory boards for Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva; is a stock shareholder in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (purchased >10 years ago); and has received royalties from Wiley (Editor-in-Chief, International Journal of Clinical Practice), UpToDate (reviewer), and Springer Healthcare (book). Dr McIntyre is an employee of University Health Network; has been a consultant for and and served on speakers/advisory boards for Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva; and has received grant/research support from Stanley Medical Research Institute and CIHR/GACD/Chinese National Natural Research Foundation. Dr Manning has been a consultant for Otsuka/Lundbeck, Allergan, and Sunovion and has served on speakers/advisory boards for Sunovion and Takeda/Lundbeck. Dr McIntosh has been a consultant for, received grant/research support from, received honoraria from, and served on speakers/advisory boards for Janssen, Shire, Purdue, Otsuka, Pfizer, Valeant, Lundbeck, Sunovion, Allergan, and AbbVie.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this Academic Highlights was provided by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, USA, and H. Lundbeck A/S, Valby, Denmark. The faculty acknowledge Sarah Brownd, MA, ELS, for editorial assistance in developing the manuscript. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc, the publisher, or the commercial supporters. This article is distributed by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S for educational purposes only.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “Activating and Sedating Properties of Medications Used for the Treatment of Major Depresive Disorder and Their Effect on Patient Functioning,” which were held in December 2018 and January and February 2019.
The teleconferences were chaired by Leslie L. Citrome, MD, MPH, Department of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla. The faculty were Roger S. McIntyre, MD, FRCPC, Department of Psychiatry and Pharmacology, University of Toronto; Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada; J. Sloan Manning, MD, Department of Family Medicine at the University of North Carolina, Chapel Hill; and Diane McIntosh, MD, FRCPC, Department of Psychiatry, University of British Columbia, Vancouver, British Columbia, Canada.
Financial disclosures: Dr Citrome has been a consultant for Acadia, Alkermes, Allergan, Impel, Indivior, Intra-Cellular Therapeutics, Janssen, Lundbeck, Merck, Neurocrine, Noven, Osmotica, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, and Vanda; has served on speakers/advisory boards for Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva; is a stock shareholder in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer (purchased >10 years ago); and has received royalties from Wiley (Editor-in-Chief, International Journal of Clinical Practice), UpToDate (reviewer), and Springer Healthcare (book). Dr McIntyre is an employee of University Health Network; has been a consultant for and and served on speakers/advisory boards for Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva; and has received grant/research support from Stanley Medical Research Institute and CIHR/GACD/Chinese National Natural Research Foundation. Dr Manning has been a consultant for Otsuka/Lundbeck, Allergan, and Sunovion and has served on speakers/advisory boards for Sunovion and Takeda/Lundbeck. Dr McIntosh has been a consultant for, received grant/research support from, received honoraria from, and served on speakers/advisory boards for Janssen, Shire, Purdue, Otsuka, Pfizer, Valeant, Lundbeck, Sunovion, Allergan, and AbbVie.
This evidence-based peer-reviewed Academic Highlights was prepared by Healthcare Global Village, Inc. Financial support for preparation and dissemination of this Academic Highlights was provided by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, USA, and H. Lundbeck A/S, Valby, Denmark. The faculty acknowledge Sarah Brownd, MA, ELS, for editorial assistance in developing the manuscript. The opinions expressed herein are those of the faculty and do not necessarily reflect the views of Healthcare Global Village, Inc, the publisher, or the commercial supporters. This article is distributed by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S for educational purposes only.
J Clin Psychiatry 2019;80(3):lu18052ah1
To cite: Activating and sedating properties of medications used for the treatment of major depressive disorder and their effect on patient functioning. J Clin Psychiatry. 2019;80(3):lu18052ah1
To share: https://doi.org/10.4088/JCP.lu18052ah1
© Copyright 2019 Physicians Postgraduate Press, Inc.
For patients with major depressive disorder (MDD) who do not achieve sustained remission with antidepressants, atypical antipsychotics have demonstrated efficacy as adjunctive therapy.1,2 Three atypical antipsychotics—aripiprazole, brexpiprazole, and quetiapine extended-release—have been approved by the US Food and Drug Administration (FDA) for adjunctive use in MDD, and a fourth, olanzapine, is approved for use in combination with fluoxetine. Although the sedative and extrapyramidal side effects associated with first-generation antipsychotics are well known, some second-generation antipsychotics are also associated with substantial sedation and activation effects. In this Academic Highlights, 4 experts on depression from the fields of psychiatry and primary care take a closer look at activation and sedation effects of atypical antipsychotics in patients with MDD. They examine the likelihood of each agent to cause these effects; the impact of these effects on patient functioning, quality of life, and treatment adherence; and the question of whether leveraging activation and sedation to address acute symptoms is ever advisable.
How Do Activation and Sedation Impact Choice of an Antipsychotic in MDD Patients?
The presentation by Roger S. McIntyre, MD, FRCPC, began by focusing on the reasons why some atypical antipsychotics cause sedation or activation effects and the factors that impact clinicians’ choices of these agents in patients with treatment-resistant MDD.
Pharmacology
Why are some agents used in MDD more likely to be activating, while others are more likely to be sedating? Although the answer is not entirely known, some hypotheses exist.
Antipsychotics act on multiple receptors, resulting in a response that can manifest as either therapeutic or as a side effect. The effect depends not only on the specific receptor subtype but also on the affinity with which the antipsychotic binds to it. For instance, dopamine-2 (D2) receptor antagonism produces a therapeutic effect that effectively treats psychosis. However, if the affinity of the antipsychotic for the D2 receptor is too high, side effects such as extrapyramidal symptoms (EPS) or elevated prolactin can occur.3
Dr McIntyre noted that D2 blocking agents are not known to significantly enhance motivation or reward experiences but that dopamine partial agonism may in fact do just that. The D2 partial agonists that are FDA approved for MDD are aripiprazole and brexpiprazole, and a key issue for these agents is how much intrinsic activity (relative efficacy) they have, as high D2 intrinsic activity can lead to hyperkinesias and restlessness.4 Compared with aripiprazole, brexpiprazole has less D2 intrinsic activity,4 which could therefore lessen the risk of those effects.
Quetiapine and olanzapine have high histaminergic activity,5 which generally speaking, causes sedation. Related to that is the α1 effect; α1 affinity is also linked to sedation and somnolence, as well as other well-known side effects such as postural hypotension.6 Aripiprazole and brexpiprazole have moderate but not high levels of affinity for the histaminergic system,4,7 and that appears to be related to why they are not particularly likely to cause sedation or somnolence.
What Influences Real-World Prescription of an Antipsychotic for an MDD Patient?
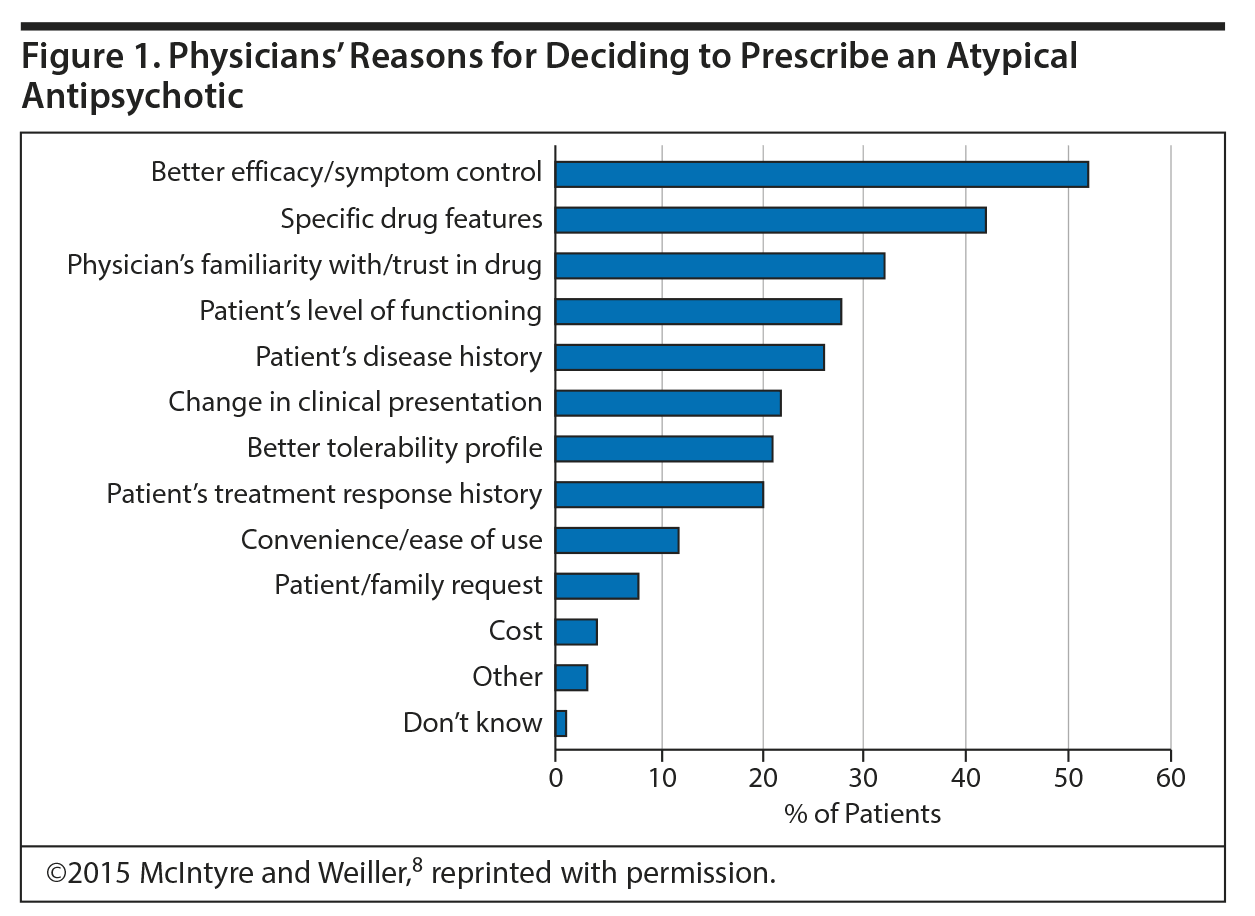
Dr McIntyre highlighted a survey8 that examined determinants driving clinician choice of an adjunctive antipsychotic for patients with MDD. Completed by 411 psychiatrists and primary care physicians, the survey reflected treatment choices for 4,018 MDD patients with inadequate response to their current treatment. Responses indicated that adjunctive antipsychotics were considered in 23.9% of the patients and prescribed in 12.8% of patients.8
The top reason for prescribing (52% of respondents) was to improve overall symptoms (Figure 1). Notably, the second most common motivating factor (42%) was “specific drug features,” implying that physicians are making distinctions based on the effects of specific medications to address particular symptoms. These effects included nonsedative calming (20%), sedation (16%), and activation (14%). Dr McIntyre observed that, rather than looking at depression in its totality, clinicians are looking at domains such as arousal and overactivation or, at the other end of the spectrum, fatigue and amotivation. Further, while prominent symptoms like psychosis would undoubtedly influence clinicians to choose an antipsychotic versus another course of treatment, symptoms like agitation, hostility, and irritability seem to then play a role in the choice of one antipsychotic over another.
Concern over specific side effects may limit use of adjunctive antipsychotics for some patients with MDD.8 Weight gain (60.0%) and metabolic side effects (57.6%) were of greatest concern for physicians, followed by extrapyramidal symptoms (43.2%), sedation (31.9%), and akathisia (25.6%). Dr McIntyre pointed out that mitigating anxiety and sleep problems with medication would be adjudicated as improving the person’s quality of life, if we assume the absence of significant safety concerns.
Anxiety and Sleep Problems in MDD
Next, Dr McIntyre discussed the burden of anxiety and sleep problems in MDD as well as the question of how best to address these symptoms in the long term.
The Sequenced Treatment Alternatives to Relieve Depression study demonstrated that 46% of MDD patients had high levels of anxiety at baseline,9 and research has indicated that about 40%-50% of MDD patients have an anxiety disorder.10,11 The addition of the anxious distress specifier to DSM-5 was an attempt to warn physicians of the hazards posed by anxiety. Anxious distress not only is a predictor of nonresponse12 to conventional antidepressants but also is highly associated with a more complex illness presentation, including higher rates of suicidality.13
Sleep disturbances (eg, insomnia, sleep deprivation, alterations in the circadian rhythm) are common chief complaints in people with depression and a frequent reason they see health care providers. Sleep impairment has also been shown to be linked to suicidality,14 as well as impaired cognition,15 which clearly adversely impacts daily functioning. Further, insomnia has been shown to predict future work disability.16
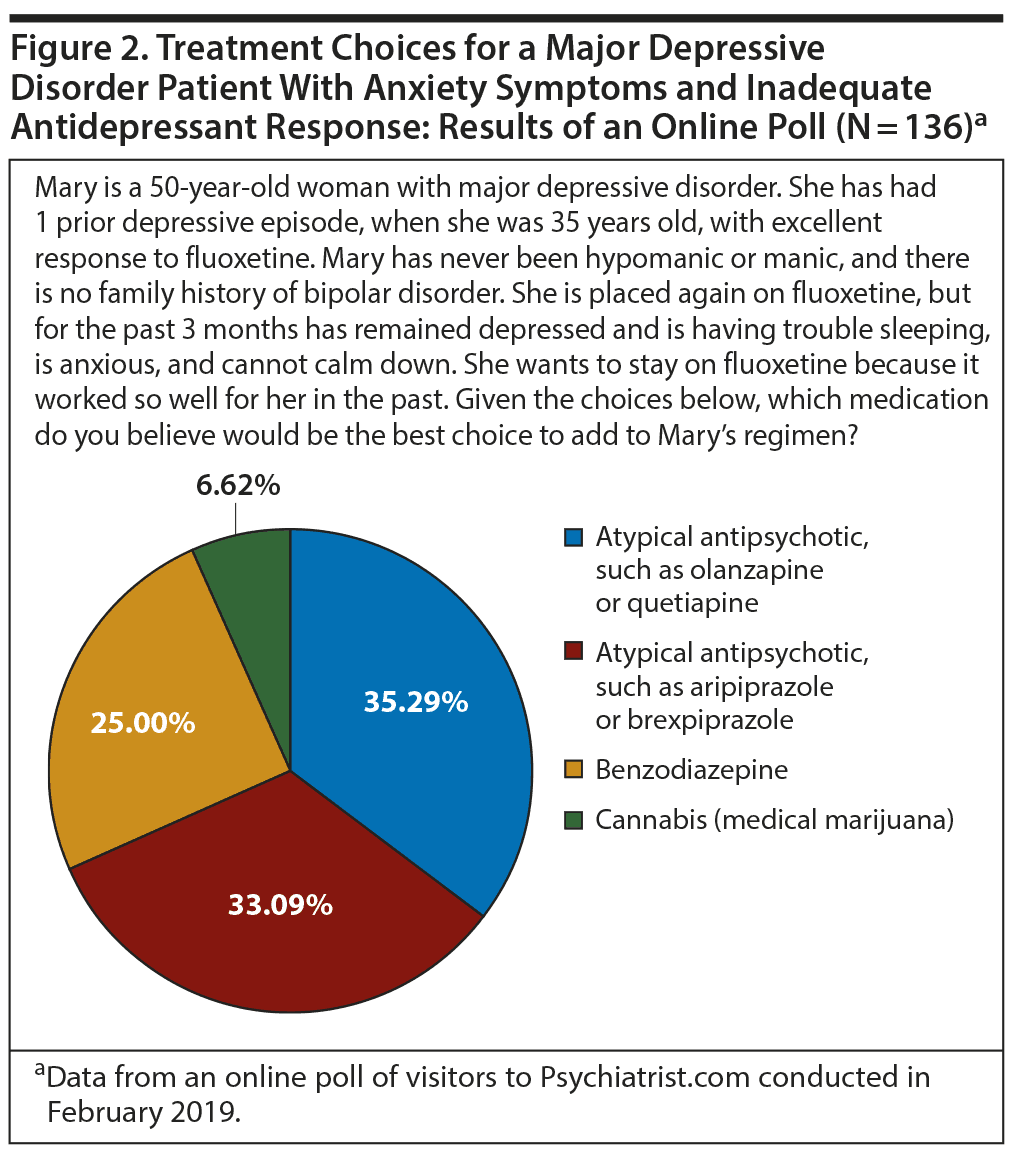
Addressing anxiety and insomnia symptoms. In February 2019, 2 online polls were conducted via the Psychiatrist.com website to assess the treatment choices clinicians are making when faced with a challenging scenario—an MDD patient struggling with insomnia, anxiety, or restlessness or, conversely, lethargy, fatigue, and hopelessness. Both polls indicated that clinicians are indeed considering atypical antipsychotics to address symptoms that remain after antidepressant treatment. The first poll described a patient with treatment-resistant depression and symptoms of anxiety and sleeplessness (Figure 2).
The most commonly selected answer was a sedating atypical antipsychotic: olanzapine or quetiapine. A close second was a less sedating atypical antipsychotic such as aripiprazole or brexpiprazole. A benzodiazepine was the third most popular choice, representing a quarter of the respondents.
Dr McIntyre explained that the objective of administering an antipsychotic in someone with high levels of arousal and agitation should be to shift their arousal not to the point where they are sedated and falling asleep during the day, but to the point where they are optimally functional. It is essential, for human function, that people have some degree of arousal and even anxiety; too much or too little, though, is associated with severe impairment.17 As he put it, “The art of selecting an antipsychotic is to choose one that is able to mitigate key distressing symptoms that contribute to functional impairment, such as anxiety or fatigue, without causing harm to the patient.”
As indicated by the online poll results, clinicians do indeed sometimes select a particular antipsychotic for its sedating effects. Quetiapine’s sedative effect is well known, and Hermes et al18 showed that clinicians frequently prescribe quetiapine in patients who have problems with sleep. In a double-blind study,19 quetiapine, administered once daily at bedtime, produced improvements in the quality of sleep as measured by the Pittsburgh Sleep Quality Index. Excessive sedation, though, may lead to nonacceptance of treatment: rates of patient withdrawal due to somnolence or sedation in that study were 12.2% for quetiapine 600 mg/d and 9.5% for quetiapine 300 mg/d. Most of these withdrawals occurred during the first week of the study.19
Benzodiazepines can also mitigate anxiety when prescribed adjunctively with an antidepressant,20 and according to Dr McIntyre, use of antidepressants with sedating qualities, such as trazodone, remains somewhat common. A caveat with benzodiazepines, especially when thinking beyond short-term use, is that they can cause cognitive impairment,21 worsen anhedonia,22 and increase the risk of fractures and falls. He noted that agents such as mirtazapine and anticonvulsants have also sometimes been used to improve sleep.
The more sedating antipsychotics such as quetiapine and olanzapine unfortunately often come with the trade-offs of daytime somnolence and excessive sedation. Dr McIntyre commented that, too often, patients for whom a sedating antipsychotic has been prescribed to improve sleep are left indefinitely on the medication without any monitoring of adverse effects that may develop. Clinicians should be mindful of the risk of moving patients too far in the direction of feeling sedated and somnolent the next day—and perhaps having those problems persist.
The question “Is there such a thing as ‘ helpful’ sedation?” shifts if we consider the possibility of ameliorating excessive activation in the absence of sedation or sleepiness. Evidence now indicates that some atypical antipsychotics can reduce anxiety and activation without making patients lethargic. Aripiprazole, an agent not generally associated with excessive sedation, has been shown to be efficacious in treating anxiety in patients who have major depressive disorder with anxious symptoms.23 Dr McIntyre further mentioned that a benzodiazepine is sometimes used in conjunction with nonsedating agents such as aripiprazole in an effort to address sleeplessness. He did express concern that, as an adjunct to selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors in depression, the dosing target for aripiprazole is not always apparent and requires significant titration.
Other caveats of the dopamine partial agonists approved for MDD (aripiprazole and brexpiprazole) include the risks of moderate weight gain and akathisia. It should be noted, though, that the risk of weight gain with these agents is lower than that associated with other atypical agents such as quetiapine or olanzapine.24 A recent study25 demonstrated efficacy of brexpiprazole specifically in patients with MDD with anxious distress; brexpiprazole was not associated with activating adverse events such as akathisia or restlessness. Another study26 indicated that brexpiprazole mitigated sleep disturbances in patients with MDD without daytime sedation. Dr McIntyre believed that the possibility of addressing sleep complaints in the absence of a separate medication such as a benzodiazepine would be ideal in that the medication regimen would be simpler and the likelihood of long-term benzodiazepine use would be lessened.
Fatigue and Amotivation Symptoms in MDD
Fatigue, hopelessness, and “flatness” are frequent complaints heard in clinical practice from MDD patients, and, as seen in the survey discussed earlier,8 clinicians seem to prioritize them as reasons to choose an antipsychotic. Essentially, these symptoms are related to amotivation, or the lack of desire to engage with the world, and they can threaten job performance and psychosocial functioning and impact patients’ lives substantially.
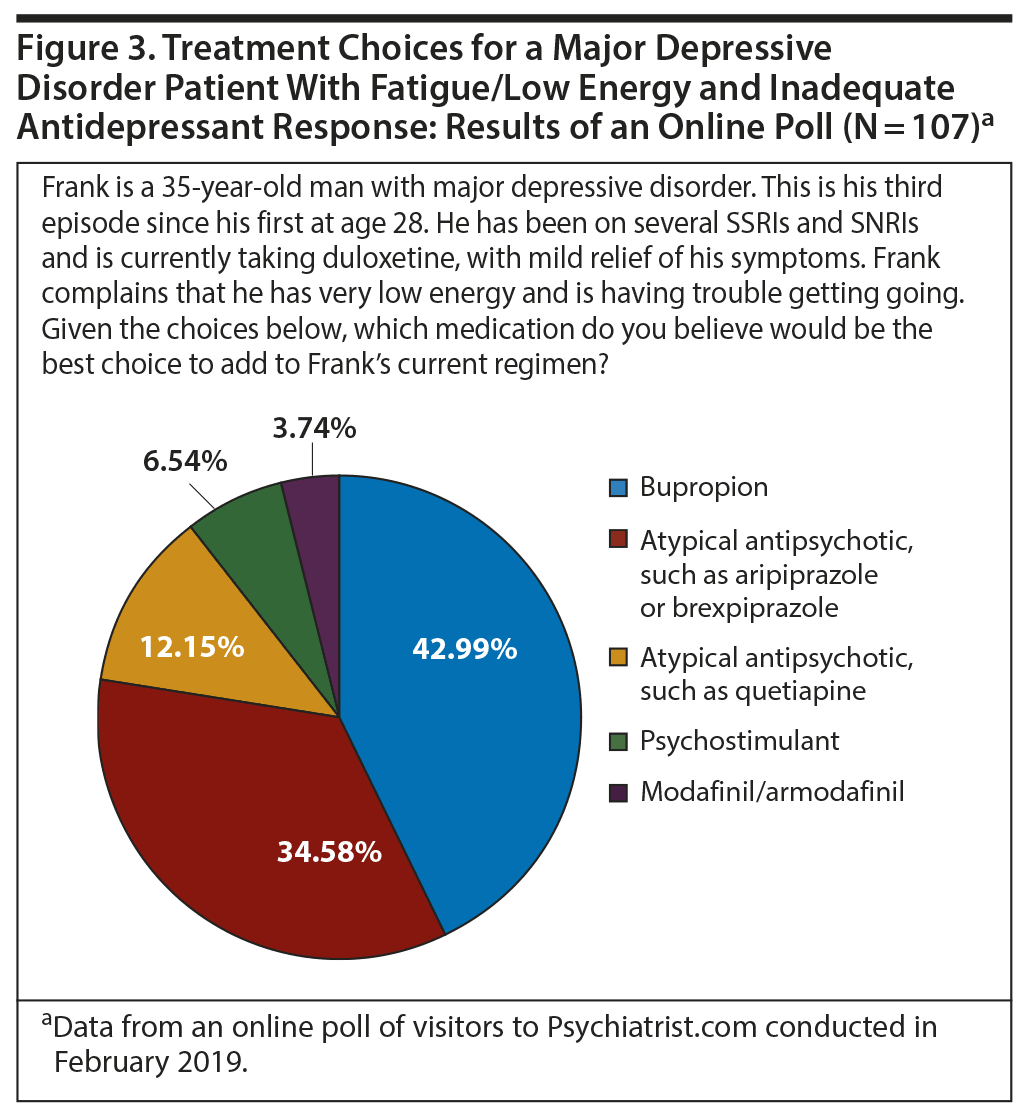
Addressing fatigue and amotivation symptoms. The second online poll (Figure 3) focused on medication choice in a patient with inadequate antidepressant response and low energy. The most common selection as a next step for this patient was bupropion. The second choice, interestingly, was an atypical antipsychotic such as aripiprazole or brexpiprazole. In distant third and fourth places were psychostimulants and modafinil.
The online polls suggested that choice of augmentation on the basis of tolerability profile seemed particularly likely if the symptom profile included fatigue and low energy; the first choice, bupropion, has a mild stimulant effect, while the next choices, aripiprazole or brexpiprazole, are dopamine partial agonists with relatively activating effects. Dr McIntyre observed that clinicians could be choosing these agents with the goal of conferring a “brightening” effect, that is, enhanced motivation, the experience of pleasurable rewards, increased energy, and less hopelessness and pessimism.
Indeed, one of the key questions when a patient has an inadequate outcome with an antidepressant is whether to combine antidepressants or add an atypical antipsychotic. There is now evidence suggesting that use of an atypical antipsychotic may, in fact, be superior to the use of an antidepressant such as bupropion in improving overall symptoms in these patients. In a randomized open-label trial, Cheon et al27 showed greater remission rates for aripiprazole versus bupropion at week 6 endpoint (55.4% vs 34.0%, respectively; P = .031). In addition to the positive results seen for aripiprazole, adjunctive brexpiprazole was shown to improve general and cognitive functioning as well as alertness in a 6-week study of patients with treatment-resistant MDD.28
As noted earlier, these agents avoid the problematic somnolence and next-day sedation that can occur with some atypical antipsychotics. It appears to be a rational decision, then, to select one of these agents to address symptoms of apathy, fatigue, and anhedonia. The trade-off that comes with this strategy, though, is an increased propensity for akathisia and restlessness. Both aripiprazole and brexpiprazole are associated with higher risks of akathisia versus quetiapine and olanzapine in number-needed-to-harm analyses, as will be discussed in the next section; however, the risk was lower with brexpiprazole versus aripiprazole.29
Conclusion
Clinicians do sometimes select antipsychotics specifically to leverage sedation and activation effects. However, it should be kept in mind that judicious antipsychotic selection requires familiarity with not just the short-term effects but also the potential long-term, possibly adverse, effects of the medication such as impairing sedation or akathisia/restlessness. Dr McIntyre summarized: “The aim should be to alleviate patients’ distress but not leave them cognitively impaired with decreased reaction time and decrease in function. Unfortunately, sedation is often interchangeable with these unwanted adverse events. Essentially, the goal is to provide distress alleviation and target symptom alleviation without burdening the patient.”
 Patient Perspectives
Patient Perspectives
“I have been taking 150 mg × 2 [of quetiapine] along with 10 mg of Lexapro to help with chronic depression. Due to divorce this summer, I told my doctor I was feeling more depressed and he raised my dose to 200 × 2 at bedtime. I am a teacher, and the last 2 days I have not been able to get up. I start to get dressed and wake up hours later standing at the mirror with a toothbrush in my hand. Lucky for me we don’ t have students yet, but I am terrified I will continue oversleeping and miss my classes.“30
Understanding Risk of Sedation and Activation Using Number Needed to Harm (NHH)
Leslie L. Citrome, MD, MPH, explained how clinicians can meaningfully assess and understand the risks of antipsychotic adverse events, specifically sedation and activation, using the number needed to harm (NNH) statistic. Incorporating NNH analyses in the assessment of adverse event risk represents one component of evidence-based medicine, a philosophy of care that exhorts clinicians to integrate the best available research evidence on efficacy and safety with individualized assessment of patients and their preferences, and then apply this insight to medical decision making.31 He emphasized that evidence-based medicine is not “cookbook medicine” and requires substantial clinical judgment.32 Physicians can use the concepts and tools of evidence-based medicine, such as calculating NNH, to appraise the value of adjunctive antipsychotic use in persons with MDD.
How Can We Best Understand Adverse Event Rates?
Although the 4 antipsychotics approved as adjunctive therapy in MDD have similar efficacy in reducing depressive symptoms, their adverse effect profiles differ widely.33,34 As also highlighted by Dr McIntyre, clinically relevant concerns have arisen regarding olanzapine’s propensity toward weight gain, quetiapine’s association with sedation, and reports of akathisia with aripiprazole,33 and it is within this context that brexpiprazole has been introduced as a potential alternative choice. But although adverse event rates in persons with MDD are described in the product label of each antipsychotic,35-38 the problem arises as to how clinicians can interpret a rate of akathisia reported as 9% for all brexpiprazole doses compared to a rate of 2% for placebo, or rates of somnolence of 5% versus 0.5%, respectively.38 From examining the relative differences between brexpiprazole versus placebo, it is easily calculated that rates of akathisia are 4.5 times higher and rates of somnolence are 10 times higher for brexpiprazole than for placebo. However, calculating relative differences is misleading for day-to-day clinical practice because the absolute rates, and absolute differences, are low, and thus the adverse effects would be encountered in a small minority of patients.
How Is NNH Calculated?
To address the issue of interpreting rates of adverse events for one medication compared to another (or to placebo), the “number needed to harm” concept can be helpful.39-42 NNH answers the question “How many patients would you need to treat with Medication A instead of Medication B before you would expect to encounter 1 additional outcome of interest that you would like to avoid?” The higher NNH value, the greater the advantage for Medication A, as it would take a larger number of patients to be treated with Medication A instead of Medication B before one would expect to see the adverse event being considered. If the rate of an adverse event for each drug is available, NNH is easy to calculate, as follows:
NNH = 1/[fa – fb], where
fa = rate of adverse event for Medication A
fb = rate of adverse event for Medication B
The denominator, fa – fb, is often called the attributable risk increase or absolute risk increase. Unfortunately for the purposes of comparison, product labels describe studies of medications compared with placebo, not with other drugs. Thus, we are left to make indirect comparisons: for example, what is the NNH for an adverse event for Medication A versus placebo, and is it bigger or smaller than the NNH for that same adverse event for Medication B versus placebo? The medication with the higher NNH value versus placebo will be the one less likely to be associated with that adverse event. In general, if the NNH is less than 10 when a medication is compared with placebo, the adverse event in question will be commonly encountered in day-to-day clinical practice.
What Is an Acceptable NNH?
When thinking about NNH, clinicians must consider the relevance of the adverse event for the individual patient being treated, and an acceptable NNH for drug versus placebo depends on the outcome in question. For example, a relatively minor adverse outcome such as transient mild nausea may be inconsequential even though the NNH value may be a single digit. In general, a single-digit NNH (ie, < 10) may be acceptable if the adverse event is mild or moderate, does not lead to discontinuation, is temporary or causes little distress, and does not pose a serious health risk or if the need for efficacy is so great that it mitigates the low NNH tolerability limitation.42 An NNH value of 10-100 may be acceptable for adverse events that may lead to discontinuation but are not associated with serious immediate health risks, or when alternative medications do not have a better profile; an example might be moderate weight gain.42 Even higher (> 100) NNH values are usually required for adverse events that pose a significant health risk.
Comparing NNH for Activation and Sedation Among Antipsychotics Approved for MDD
Activating and sedating properties of first-line oral second-generation antipsychotics were evaluated in a prior publication29 by examining the rates of adverse reactions as reported in product labeling for the indications of schizophrenia and adjunctive treatment of MDD. Activating adverse events included akathisia, restlessness, agitation, and insomnia. Sedating adverse events included somnolence, sedation, and fatigue.
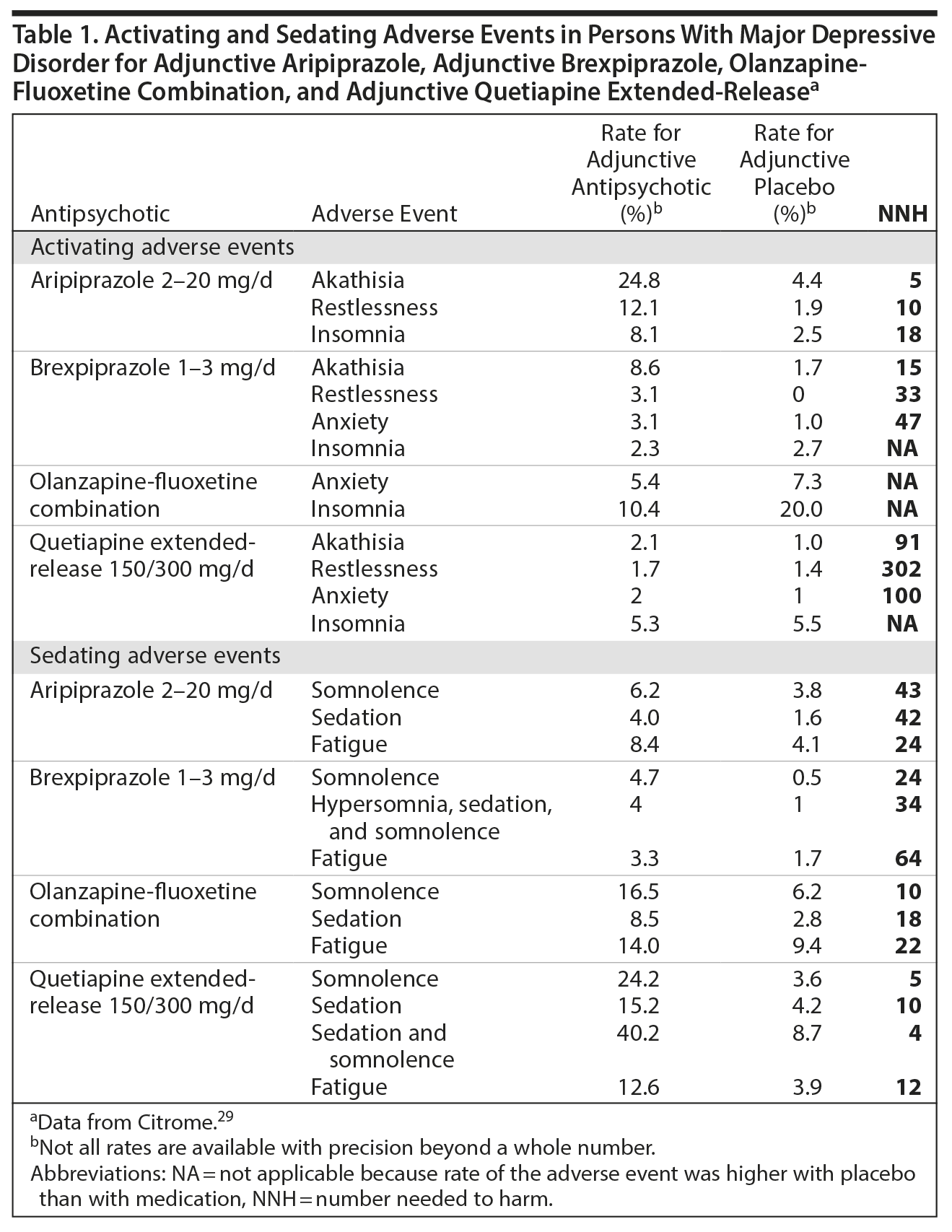
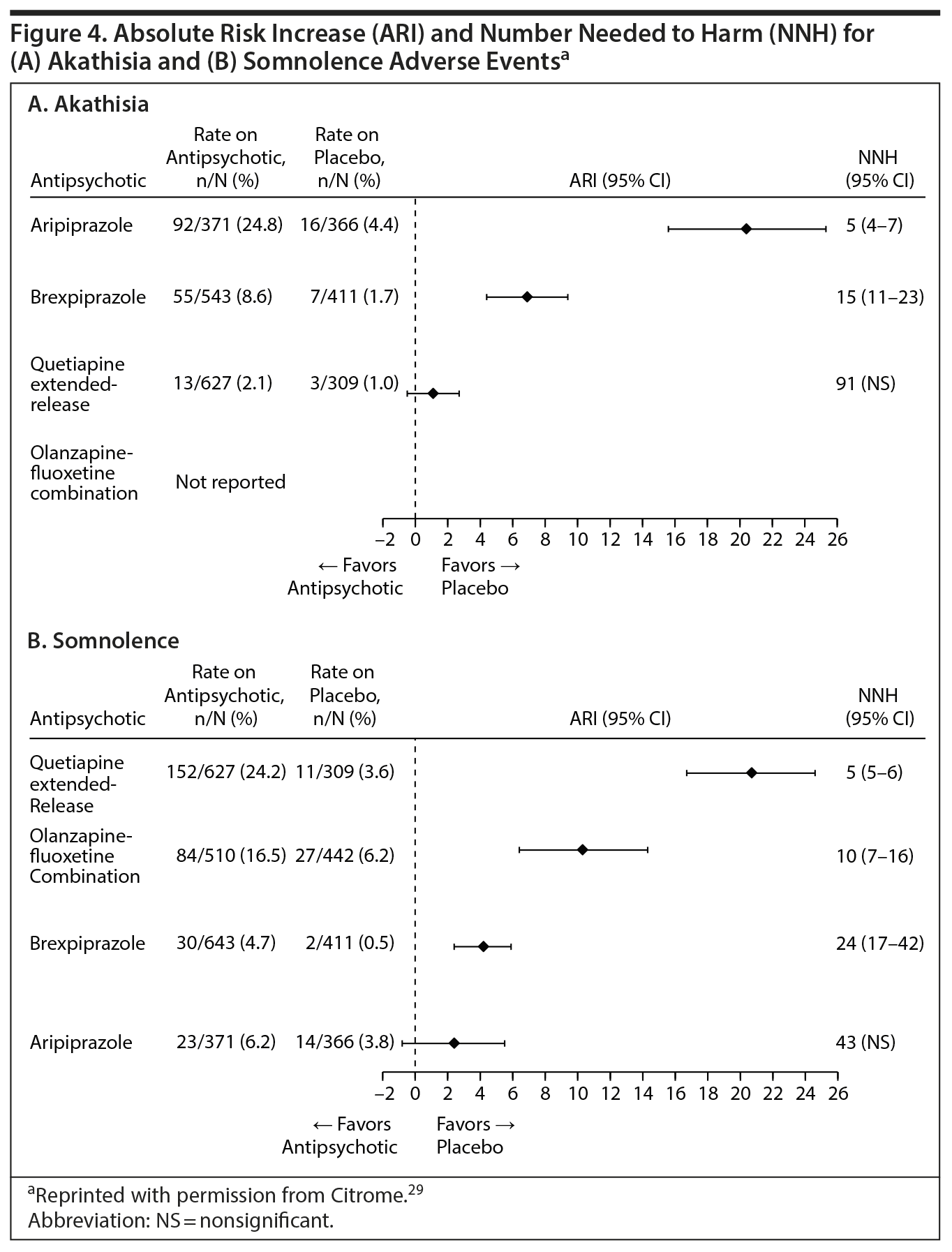
Table 1 provides the rates and NNH values for these adverse events for the 4 agents approved for adjunctive MDD treatment, as observed in the short-term acute placebo-controlled clinical trials used to obtain FDA approval. Not all activating or sedating adverse event rates are available, as some of the specific adverse event terms for some of the medications did not meet the minimum frequency threshold for reporting.
NNH values less than 10 were reported for aripiprazole for akathisia (NNH = 5) and for quetiapine extended-release for somnolence (NNH = 5) as well as the combined terms “sedation and somnolence” (NNH = 4). These values can be interpreted to mean that for every 5 patients on antidepressant therapy who were randomized to adjunctive aripiprazole instead of to adjunctive placebo, one can expect to encounter 1 additional patient with an adverse event of akathisia, and that for every 4 patients randomized to adjunctive quetiapine extended-release instead of adjunctive placebo, one can expect to encounter 1 additional patient with a complaint of sedation or somnolence. Importantly, the values predict that akathisia with aripiprazole and sedation/somnolence with quetiapine extended-release would occur commonly in day-to-day clinical practice. Forest plots for akathisia adverse events and for somnolence adverse events are shown in Figure 4. Although comparisons of adverse events associated with olanzapine-fluoxetine combination versus placebo-fluoxetine combination did not demonstrate any NNH values less than 10 for activating or sedating adverse events, somnolence adverse event reporting came close, with an NNH value of 10.
The NNH analysis29 further illustrates that, in contrast to the above agents, brexpiprazole does not appear to be either particularly activating or sedating, with none of the NNH values in either category being a single digit or close to it. Thus, these events would not be expected to occur as commonly with brexpiprazole compared to akathisia with aripiprazole or sedation/somnolence with quetiapine extended-release. Not included in Table 1, but of relevance for clinicians, is that the NNH value for the outcome of weight gain of at least 7% from baseline for olanzapine-fluoxetine combination versus placebo-fluoxetine combination was 3, compared with NNH values of greater than 20 for the alternatives.33,34 Therefore, clinically relevant weight gain would be anticipated to occur substantially more frequently with olanzapine-fluoxetine combination than with adjunctive aripiprazole, brexpiprazole, or quetiapine extended-release.
Additional Considerations
Quantifying the risk of encountering adverse events using NNH allows us to make indirect comparisons in a straightforward fashion. Clinicians should keep in mind, though, that there is substantial heterogeneity in how individual patients tolerate different medications. Although quetiapine extended-release is generally considered sedating, there are patients who tolerate this medication well and have no appreciable sedation, somnolence, hypersomnia, or fatigue. Likewise, some patients who receive aripiprazole do not exhibit akathisia, restlessness, agitation, or insomnia. In addition, there may be a dose-response relationship with these adverse effects, allowing mitigation by dose reduction provided that efficacy is maintained. Past history of tolerability, or lack thereof, to other medications may be helpful in determining an individual patient’s vulnerability to activating or sedating adverse events. Having this prior information enables the clinician to better predict potential medication effects and use NNH information to make an informed individualized treatment decision.
 Case Practice Question
Case Practice Question
Discussion of best response can be found at the end of the activity.
Case 1. Geno is a 28-year-old man who is unemployed. He is overweight and has hypertension and elevated cholesterol. He has had a diagnosis of MDD for the past 2 years, and both parents have a history of MDD. Geno has received multiple antidepressants, including combinations of these medications, but remains symptomatic. He struggles to remain alert during the day and in the past has complained of tiredness with paroxetine. He also reports that when he was taking a high dose of fluoxetine, he felt irritable. Currently, he takes escitalopram. What would be the most suitable adjunctive antipsychotic intervention for Geno?
a. Olanzapine-fluoxetine combination because the number needed to harm (NNH) versus placebo-fluoxetine combination for akathisia is ≥ </10 and for somnolence is ≥ 10
b. Adjunctive quetiapine extended-release because the NNH versus adjunctive placebo for akathisia is ≥ 10 and for somnolence is < 10
c. Adjunctive brexpiprazole because the NNH versus adjunctive placebo for akathisia is ≥ 10 and for somnolence is ≥ 10
d. Both a and c
CLINICAL PERSPECTIVES
J. Sloan Manning, MD, began by listing important points for clinicians to remember when thinking about sedation and activation effects of atypical antipsychotics in MDD patients. He then outlined the roles of motivational interviewing and collaborative care models in making treatment choices that minimize adverse effects and maximize the likelihood of remission.
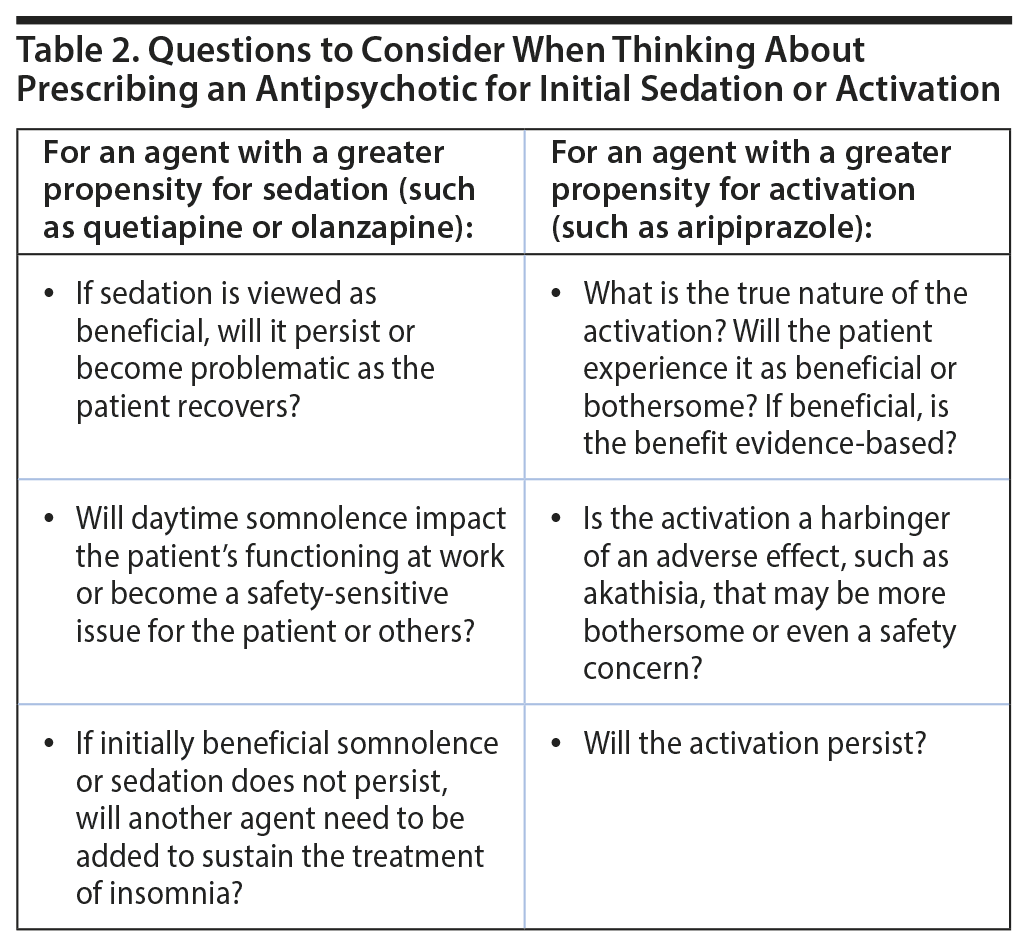
Table 2 shows a useful list of questions for clinicians who are considering leveraging a sedative or activating effect of an antipsychotic to treat a patient with MDD. As echoed by other presenters, Dr Manning cautioned that individual patients can vary unpredictably with regard to the intensity of an adverse effect, the way the effect is expressed, and the patient’s tolerance of it. He further pointed out that primary care physicians can use NNH comparisons, such as those discussed by Dr Citrome, to help guide discussions with patients regarding relative risk. Doing so can help set expectations and guide the selection of the best treatment choice for an individual.
Especially in primary care, physicians often hear complaints of poor sleep, and as many as 90% of patients with depression will have problems with sleep quality.43 Poor sleep predicts poorer clinical outcomes in MDD and may be a symptom of the illness itself or a side effect of another medication such as an antidepressant. Thus, these symptoms are thus frequently a priority for both patient and clinician with regard to treatment strategy. Dr Manning highlighted a study demonstrating that sedation may not be necessary in order to achieve sleep improvement. Krystal et al26 used polysomnography and sleep diaries to assess patients with inadequate antidepressant responses and poor sleep efficiencies who were given adjunctive brexpiprazole. In this sample, physiologic measures of sleep and daytime alertness were improved. In fact, insomnia decreased, and functioning assessed by the Massachusetts General Hospital-Cognitive and Physical Functioning Questionnaire improved as well. Furthermore, sleep architecture improvements were noted in sleep efficiency, total sleep time, sleep onset latency, wake-time after sleep onset, and latency to persistent sleep. These data, when combined with NNH measures of sedation, give clinicians a broader sense of the sleep benefits that may accrue beyond first-order patient reports of sedation or somnolence as adverse effects in clinical trials.
Using Motivational Interviewing to Minimize Adverse Effects and Optimize Treatment Choice
Patient-centered approaches, such as motivational interviewing (MI), intersect with the conversation around adverse events, as they can be used to inform treatment choice by pinpointing symptoms and adverse effects that may trouble the patient. Motivational interviewing has been introduced into the treatment of depression44 and can be used hand-in-hand with advanced pharmacologic strategies, such as augmenting with an atypical antipsychotic, to foster better outcomes in MDD patients. Originally developed in substance abuse settings,45 MI is based in empathy, patient autonomy, and recognizing that patients possess expertise in their illness. It encourages psychological attunement between patient and provider, which can increase likelihood of remission regardless of the treatment modality chosen.46,47 Patients who participate in treatment selection may be more adherent and persistent and therefore more successful in reaching treatment goals.
To implement MI, the clinician allows the MDD patient to set the agenda for their visits and indicate readiness to proceed with treatment by using “change talk” such as “I can,” “I would be willing,” and “I am ready.” Some open-ended questions could include “What do you hope to achieve in the treatment of your depression?” “How would your improvement impact others?” “How confident are you, on a scale of 1 to 10, that the treatment we are considering is likely to help you?” and “What adverse effects would you most want to avoid?”
Questions relating specifically to adverse events include:
- Is anything about your medication bothering you or causing you to miss doses? Do you wish your treatment could be changed?
- Is sedation a problem?
- Is restlessness a problem?
followed by…
- Tell me more about that.
- How do these effects interfere with your life?
In this way, clinicians can draw out which symptoms might be most bothersome, as well as which adverse effects might be tolerable—and which could lead to nonadherence and therefore treatment failure. For instance, sedation could interfere with a patient’s ability to care for their child. Akathisia may distract from a task or foster an irritable mood. The clinician should keep in mind that the overarching goal of treatment is long-term remission, and distressing adverse effects could threaten that goal. With the patient’s permission, the clinician can go on to advise about treatment options that are least likely to cause impairing side effects and most likely to bring about remission.
 Patient Perspectives
Patient Perspectives
“I cannot stay still—I feel like I have to be doing something constantly and even when I am doing something, I feel the need to lay down all the time. I get super bad shakes, super sweaty all the time, and bad insomnia. I can’ t even eat anymore. The medication [aripiprazole] makes me feel like not myself.”48
Addressing Symptoms and Side Effects in a Collaborative Care Model
Dr Manning described how the collaborative care model can provide a framework that supports adverse event monitoring and helps physicians make informed and responsive treatment choices. Managing treatment-resistant depression in primary care settings can be challenging due to time constraints, lack of resources, and even lack of provider training and experience. Integrating primary care and behavioral care could help close treatment gaps by augmenting clinic resources and offering opportunities for consultation to improve clinical skills. Collaborative care models have been shown to improve outcomes and provide medical care offsets.49,50
The primary care provider (PCP) remains the leader of the care team, with the assistance of a collaborative care manager for behavioral treatment and a consultation-liaison psychiatrist. The PCP administers treatment metrics and can also use open-ended questions such as those mentioned in the previous section. The care manager tracks patient progress using a registry or dashboard. Patients receive brief cognitive psychotherapies to create a multidisciplinary treatment milieu. The care manager meets with the psychiatrist at least weekly to discuss treatment issues for MDD patients who fail to respond, remit, or have other problems with pharmacologic management. Recommendations for medication adjustment are communicated to the PCP, who remains responsible for initiating the adjustment and may confer with the psychiatrist. The clinical care manager, as an advocate, can help monitor for important or bothersome adverse effects. If a patient experiences substantial sedation or akathisia that could lead to noncompliance, a clinical care manager can identify the problem early and inform the PCP or consultant psychiatrist, who can then initiate a treatment change, such as switching to another agent to achieve a better result. Patients who remain refractory to care, are diagnostic dilemmas, or are too ill to remain in the PCP setting may be transitioned to specialty care.
Pharmacologic interventions delivered in these care models may be more likely to be evidence based, optimized in dosing and duration, and successful in creating remission.51 In these settings, PCPs can acquire new clinical skills and gain experience with pharmacologic strategies for MDD, such as adjunctive use of atypical antipsychotics.
Summary
Primary care providers should ensure that they are familiar with the use of atypical antipsychotics as adjunctive agents for treatment-resistant MDD, as these agents represent tools for achieving remission and recovery. They should understand how to effectively inquire about bothersome symptoms and adverse events in patients with MDD, and they can use motivational interviewing to improve communication along these lines. Integration of primary care and behavioral care may help close gaps in MDD treatment and can offer opportunities for consultation to improve clinical skills and increase PCPs’ knowledge about MDD treatment options such as atypical antipsychotics.
Although clinicians may be tempted to use adverse or secondary effects of atypical antipsychotics to improve symptoms, efficacy should remain their central focus, with better tolerability and avoidance of effects such as sedation and akathisia supporting adherence. Maximum, sustained improvements in core depression symptoms should remain a main target of MDD treatment.52
 Case Practice Question
Case Practice Question
Discussion of best response can be found at the end of the activity.
Case 2. Lauren, a 35-year-old nurse, is experiencing her second MDD episode and has been referred by her psychotherapist for medication management. She has no significant medical conditions, and she uses a levonorgestrel-releasing intrauterine contraceptive device (IUD) for birth control. Her Patient Health Questionnaire-9 score is 17, with core symptoms of depressed mood and anhedonia on most days. She sleeps 9 to 10 hours per day and has gained 20 lb since the onset of MDD. In addition to cognitive therapy, she has been treated with fluoxetine 20 mg/d for 8 weeks, followed by escitalopram 10 mg/d for 6 weeks. She is concerned that these medications might be contributing to her weight gain. She says that she is too exhausted to exercise and as a working mother has no time to do so anyway. A patient-centered approach to treatment would include:
a. Completing a battery of symptom metrics and telling her that the best choice is antipsychotic augmentation
b. Writing a prescription for exercise and advising her to return for follow-up in 6 weeks
c. Telling her that her IUD is probably responsible for her treatment resistance and recommending its removal
d. Encouraging her to set an agenda for treatment, asking her permission to discuss treatment options, and outlining current MDD treatment strategies, including both benefits and possible adverse events
IMPACT ON PATIENT FUNCTIONING
Diane McIntosh, MD, FRCPC, focused on the impact of sedation and activation effects on patients’ lives as well as strategies for minimizing the risk of their occurrence. She presented 2 cautionary cases that illustrate how a clinician’s initial attempts to manage symptoms led to the development of intolerable sedation and akathisia side effects and explained how treatment might have been optimized instead.
Sedation and Its Implications: Short-Term vs Long-Term
Sedation may be experienced as desirable in the short term, especially for an individual who is enduring an acute exacerbation of their mental illness. Sedating medications can help ameliorate insomnia, anxiety, or agitation, but prolonged use of a sedating treatment can lead to unpleasant side effects: as Dr McIntosh related, “People don’ t like feeling drugged or, as my patients sometimes put it, ‘ like a zombie.’ ” Further, excessive sedation can be so functionally impairing that it results in “presenteeism”: the person is at work, but their brain is not. The patient may be too sedated or impaired to do their work properly or efficiently, which could put their job at risk. Dr McIntosh pointed out that excessive sedation could also increase the risk of accidents in multiple settings—whether in the context of safety-sensitive occupations, motor vehicle accidents, or falls. Significantly, fatigue can be a symptom of the primary illness, and if the medication provokes fatigue or is excessively sedating, it might lead both prescriber and patient to conclude that there are untreated symptoms. When medication side effects resemble symptoms of the disorder, the patient may get a sense that their illness is ongoing and that the medication is not effective.
Case Vignette
Marcus, a 52-year-old postal worker, was diagnosed with major depression, moderately severe, with a Patient Health Questionnaire-9 (PHQ-9) score of 17. He was first diagnosed with depression at age 40, after his father died unexpectedly. His first depression was difficult to treat. He was prescribed escitalopram 20 mg and mirtazapine 45 mg, which he stopped after 1 year. As he put it, “I was way too tired with those drugs. I could hardly finish my route. And even though I didn’ t change anything about my activity or diet, I gained a ton of weight.” Marcus’s symptoms now include depressed mood, anhedonia, restless sleep (initial and middle insomnia, and he never feels refreshed), and cognitive symptoms impacting his ability to work. He reports feeling fatigued all day. His doctor prescribed escitalopram, which helped his mood only modestly. He told his doctor, “If you can do just one thing, please get my sleep back on track. I’ m so tired and useless at work, I think I might lose my job.” Marcus refused to try adjunctive mirtazapine, given his past experience with weight gain, so his doctor prescribed quetiapine. While Marcus felt a rapid benefit from the quetiapine in terms of improved sleep and a brighter mood, he also felt excessively sedated: “I hoped sleeping better would help me at work, but now my sleep is too good. I’ ve slept through my alarm several times, so I’ ve been written up for being late. Not only that, I’ m tired all day. I have to drink tons of coffee to get myself going in the morning, and then I need more just to make it through my shift.”
Case discussion and clinical strategies. Once Marcus’s acute symptoms improved, the initially beneficial sedation associated with quetiapine turned into a liability.
So, what might Marcus’s doctor have done differently? Dr McIntosh noted that she tends to prescribe agents that are less sedating because, ultimately, sedation tends to be experienced as excessive and undesirable: “If I prescribe something highly sedating, such as quetiapine or olanzapine, I might make it difficult for my patient to get back to work and function in their daily lives.” Dr McIntosh emphasized rapid management of sedation if it arises. With rapid titration of some agents, such as quetiapine, the adverse effect can sometimes be overcome, but in her clinical opinion, the sedation often persists and is intolerable for many patients, leading to nonadherence or serious functional consequences. One might consider using a sedating antipsychotic only in the short term and quickly switching to a less sedating one, but that situation poses complications, such as requiring a drug switch or the simultaneous use of 2 medications.
Ideally, excessive sedation should be prevented in the first place. Dr McIntosh encouraged clinicians to think long term when prescribing for an acutely ill patient. This is especially important in hospital or acute treatment settings; patients are often discharged to an outpatient physician who may be reluctant to change the existing treatment, even if side effects arise that could jeopardize adherence. So, she prefers to prescribe a medication that treats the depression symptoms, with remission as the goal, and that is less likely to cause problems in the long run. If acute insomnia, anxiety, or agitation is an issue, simultaneous short-term use of a benzodiazepine could be prescribed and would then usually be discontinued within a few weeks, once the primary treatment is managing the core symptoms.
She cited aripiprazole and brexpiprazole as agents that are not usually sedating: “I can prescribe brexpiprazole in the morning, or at night; patients can choose for themselves when they prefer to take it, depending on their routine and when they are most likely to remember. Aripiprazole is usually prescribed in the morning because it can be activating.”
Activation: Not Necessarily a Good Thing?
Dr McIntosh noted that the terms sedation and activation can have different meanings for clinicians, depending on the clinical situation. She pointed out that the word activation is sometimes used with positive connotations, in the context of improved energy or helping patients “get going” again. However, the same term—activation—also describes adverse events such as restlessness, agitation, or akathisia that can be highly unpleasant, even frightening experiences for the patient and must be regarded as serious issues.
It is widely known that many first-generation antipsychotics pose a substantial risk for akathisia. Although the risk may be relatively lower with newer antipsychotics, they still pose a hazard,53,54 and clinicians should always be on the lookout for this side effect because it is easy to miss. Akathisia is considered an extrapyramidal side effect characterized by an intense feeling of restlessness or a need to move, rather than by prominent, visibly abnormal movements. Consensus about the relative importance of objective and subjective features for diagnosing akathisia has not been reached.55 It is most often associated with use of high-potency D2 blocking antipsychotics, rapid dose titration, and sudden large dose increases.55,56 Certain groups, including women, those with mood disorders, and those with greater depression severity and more cognitive symptoms, may be at higher risk.55,57,58 The consequences of unrecognized akathisia can be very serious. It is associated with a high rate of treatment nonadherence, resulting in illness exacerbation and recurrence. The akathisia symptoms themselves have also been associated with negative outcomes such as exacerbation of illness, aggression, violence, and suicide.53,59
Recognition of problematic activation effects. As suggested by Dr Citrome’s NNH analyses, akathisia is a common clinical outcome when prescribing aripiprazole. Dr McIntosh expressed that her general preference would be to choose an agent from the outset that is less likely to cause excessive activation or sedation effects in the long run. Clinicians should monitor their patients carefully for symptoms of excessive activation. They should know how to differentiate akathisia from anxiety, agitation, and tardive dyskinesia and know how best to proceed if they encounter a patient who is experiencing akathisia.
A main reason for failing to recognize activation-related symptoms is that patients often struggle to describe their experience and may say, “I’ m anxious” or “I feel agitated”53; or simply, “I can’ t get comfortable,” “I’ m jumping out of my skin.”60 Further, akathisia may be missed if it occurs in areas other than the legs, if other prominent psychiatric symptoms are present, or if there are no other apparent extrapyramidal signs.61 Clinicians sometimes fail to ask patients who are prescribed an antipsychotic about akathisia; they may limit their assessment to objective, obvious restlessness, or they may adhere too strictly to research diagnostic criteria regarding how akathisia will present.61
- Anxious patients experience worry or fear and physical manifestations (eg, sweating, palpitations, tremor, restlessness),53 but they don’ t experience the urgent “I need to move” sensation that defines akathisia.
- Agitation is an observable sign.53 It may include pacing, fidgeting, or foot-tapping and can be both an emotional and a physical experience, overlapping with anxiety. Dr McIntosh emphasized that both anxiety and agitation should be treated with a sense of urgency, but differentiated.
- Tardive dyskinesia, in contrast to akathisia, may or may not be distressing to the patient. The involuntary movements can be observed during an appointment. Akathisia tends to occur in the lower limbs; tardive dyskinesia, in the extremities and face. Tardive dyskinesia is chronic and potentially irreversible. Whereas tardive dyskinesia may actually worsen if the medication is stopped, at least in the short term, stopping the medication usually provides immediate relief of akathisia.55
Case Vignette
Leora, a 57-year-old high school counselor, was diagnosed with severe depression (PHQ-9 score of 21) and anxious distress. She had tried 3 different antidepressants, but none were fully effective. Her doctor added aripiprazole to sertraline, which was the antidepressant she tolerated best. The starting dose prescribed was 2 mg, but the pharmacy was short of supply, so they gave her 5-mg tablets and asked her to take half a tablet. Within a week, Leora’s mood was a little brighter, she was not as tearful, and she felt a glimmer of hope that she might recover. She thought that since the smaller dose helped so much, she would try doubling it. A week later, Leora called the doctor’s office in a panic, telling his assistant, “I think I’ m having a serious adverse reaction. I feel like I’ m crawling out of my skin. I’ ve never felt like this in my life. It’s like I have to move my legs or I’ m going to go out of my mind.” Leora said the day after the dose increase she felt an uncontrollable urge to move. “I’ ve been pacing for hours. I can’ t stop fidgeting. As soon as I sit down, I have to cross and uncross my legs. I can last about 2 minutes and I have to get up and move again.”
Case discussion and clinical strategies. Leora’s symptoms did not fully resolve, and so without consulting her doctor, she increased the dose herself. For Leora, the aripiprazole dose increase provoked distressing akathisia.
So, what steps might Leora’s clinician take? Options for managing akathisia include stopping the medication or lowering the dose, starting short-term adjunctive therapy (eg, benzodiazepines, β-adrenergic agonists),55 or switching to an antipsychotic with a lower liability for akathisia.54 Dr McIntosh pointed to the importance of “starting low and going slow” and monitoring the patient when dosing antipsychotics. The clinician should consider, though, that the patient is at risk for recurrence of the akathisia, so stopping aripiprazole and switching to a more balanced medication with a lower liability for the effect would be a reasonable choice that could potentially sidestep future problems.
Which Adverse Events Do Patients Find Most Distressing?
Dr McIntosh was in agreement with other presenters regarding the importance of valuing patients’ subjective experience of symptoms and adverse events when making treatment decisions. She noted that some patients express that the adverse effects they experience are worse than the symptoms being targeted. Unfortunately, clinicians’ assumptions and perceptions may not match up with their patients’ reality regarding which side effects trouble them most.
Llorca and colleagues60 interviewed both patients diagnosed with MDD or schizophrenia and psychiatrists regarding common treatment-emergent adverse effects of antipsychotics. Patients diagnosed with MDD were most likely to report cognitive issues as a treatment-emergent adverse event (92%) and also commonly reported somnolence (76%), weight gain (64%), low energy (56%), and EPS (52%). Cognitive issues (72%), weight gain (44%), and excessive sleepiness (36%) were most commonly characterized by MDD patients as bothersome. The study also assessed the top 3 events patients found most bothersome. Cognitive issues, weight gain, and excessive sleepiness were again cited most frequently; low energy (28%) and flat affect (32%) were also rated as among the most bothersome events.
Interestingly, physicians failed to include sedation among the events they thought most bothersome to patients, citing weight gain, reduced sexual desire/performance, EPS, akathisia, and hormonal issues among their top answers.60 One area of apparent discrepancy between physician and patient responses, akathisia, may partially be explained by the terminology confusion also mentioned by Dr McIntosh: although patients were not using the clinical term akathisia, they often described experiences consistent with akathisia, such as “jumping out of my skin.”
Clinical research tends to focus on more heavily on objective measures and on what doctors or researchers think is important than on patients’ lived experiences of, for example, how sedation may impact daily functioning or even safety. A review by Longden and Read62 systematically analyzed strategies used to report antipsychotic adverse events and then assessed the clarity and comprehensiveness of the reporting. Their study revealed that neurologic, metabolic, and sedation-related cognitive effects are most consistently measured and reported across the literature. However, they identified a significant research gap regarding assessment of the global impact of antipsychotics on patient well-being. The authors also cite a number of (currently underused) tools that can be used to measure the global subjective impact of antipsychotics, such as the Hogan Drug Attitude Inventory63 and the Subjective Well-being Under Neuroleptics scale.64 As discussed in depth by Dr Manning, skillful patient interviewing goes hand-in-hand with structured assessments.
Conclusion
Sedating or energizing effects of antipsychotics are sometimes viewed by clinicians as helpful in the short term. In the long term, however, if sedation becomes highly impairing or if activation-related adverse events such as akathisia develop, these events can negatively impact the patient’s quality of life and even increase suicide risk. Even when managing an acute illness, clinicians should consider the long-term implications of their treatment choices with regard to meeting the goal of full remission and functional recovery as well as avoiding unpleasant and impairing adverse effects.
 Case Practice Question
Case Practice Question
Discussion of best response can be found at the end of the activity.
Case 3. Anne, a 67-year-old retired accountant, has had an MDD diagnosis for 10 years and has been taking bupropion extended release 450 mg/d for the last 5 years. After years of stability, she has recently experienced what she describes as a “rapid downward mood spiral.” A car accident 6 weeks ago left her with a foot fracture that has been preventing her from enjoying her usual daily walks and gardening, which she feels were therapeutic for her depression. Four weeks ago, at a visit to her primary care physician, she asked about increasing her bupropion dose. The physician instead decided to add aripiprazole 2 mg as an adjunct to her current treatment. After a few days of taking aripiprazole, however, she experienced “through the roof” anxiety and stopped taking it. Fearing a return of her depression, she has scheduled a visit with you for a second opinion. Which of the following is your best next step?
a. Rule out akathisia because her age and sex mean that she is at low risk of extrapyramidal symptoms
b. Probe for more details about her mental and physical experience of the “anxiety” she encountered with aripiprazole before presenting the patient with treatment options
c. Prescribe a sedating antipsychotic such as quetiapine, since she is prone to anxiety
d. Tell her that early anxiety is common when starting aripiprazole and to resume taking it
 Clinical Points
Clinical Points
- Aripiprazole, brexpiprazole, quetiapine, and olanzapine (in combination with fluoxetine) are FDA-approved as augmentation therapy in major depressive disorder. Clinicians sometimes attempt to leverage sedating or activating effects of these medications to address certain symptom profiles.
- Number-needed-to-harm analyses indicate that quetiapine and olanzapine are associated with sedating events such as somnolence and fatigue, and aripiprazole is associated with activating events such as insomnia and akathisia.
- Skillful patient interviewing and, when applicable, collaboration with other health care providers can help ensure timely management of any adverse events that arise.
- Sedating or activating effects that seem helpful in the short term may, over time, become highly impairing and impact patient quality of life. Clinicians should think in the long term when prescribing an antipsychotic, with sustained remission and avoidance of impairing adverse effects as goals.
 Discussion of Case Practice Questions
Discussion of Case Practice Questions
Case 1: Preferred response is c. Adjunctive brexpiprazole because the NNH versus adjunctive placebo for akathisia is ≥ 10 and for somnolence is ≥ 10.
Geno has a prior history of not tolerating medications because of activation or sedation. Compared to the alternatives, brexpiprazole would be less likely to cause activation or sedation. NNH values ≥’ ‰10 versus placebo imply that the adverse event will not be as commonly encountered in day-to-day clinical practice than if the NNH value is < 10. The NNH value for olanzapine-fluoxetine combination is on the borderline for causing somnolence, but, more importantly, olanzapine-fluoxetine combination has a highly undesirable NNH value regarding clinically significant weight gain (the NNH is 3 for the outcome of weight gain ≥ 7% from baseline for olanzapine-fluoxetine combination versus placebo-fluoxetine combination).
Case 2: Preferred response is d. Encouraging her to set an agenda for treatment, asking her permission to discuss treatment options, and outlining current MDD treatment strategies, including both benefits and possible adverse events.
A motivational interviewing approach begins by allowing patients to exhaust their concerns in the initial agenda setting. A collaborative goal-oriented conversation focused on the language of change in an atmosphere of acceptance and compassion will help create a working therapeutic alliance. New information on advanced pharmacologic and nonpharmacologic strategies may be offered with the patient’s permission. The quality of the therapeutic alliance, including level of psychological attunement and patient contribution to the therapeutic alliance, is predictive of remission.
Case 3: Preferred response is b. Probe for more details about her mental and physical experience of the “anxiety” she encountered with aripiprazole before presenting the patient with treatment options.
Patients commonly struggle to describe the adverse effects they experience with atypical antipsychotics, particularly those related to activation. They might use words like anxiety or agitation when they are actually experiencing akathisia. Given that she is an older woman with an MDD diagnosis, she is at heightened risk for extrapyramidal symptoms. Prescribing quetiapine on the basis of possible anxiety does not take into account the fact that the “anxiety” was most likely caused by the previous medication in some regard, as opposed to being a symptom of the patient’s illness. An objective examination and in-depth discussion of her subjective experience of the “anxiety” she experienced with aripiprazole would be a good starting point in formulating an appropriate response, which may include augmentation with an antipsychotic agent with a lower risk of akathisia.
Published online: April 30, 2019.
REFERENCES
1. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Focus. 2010;8(4):570-582. CrossRef
2. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed CrossRef
3. Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385-3403. PubMed CrossRef
4. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. PubMed CrossRef
5. Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68(1):29-39. PubMed CrossRef
6. Kane JM, Sharif ZA. Atypical antipsychotics: sedation versus efficacy. J Clin Psychiatry. 2008;69(suppl 1):18-31. PubMed
7. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29(9):773-799. PubMed CrossRef
8. McIntyre RS, Weiller E. Real-world determinants of adjunctive antipsychotic prescribing for patients with major depressive disorder and inadequate response to antidepressants: a case review study. Adv Ther. 2015;32(5):429-444. PubMed CrossRef
9. Fava M, Alpert JE, Carmin CN, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34(7):1299-1308. PubMed CrossRef
10. Melartin TK, Rytsälä HJ, Leskelä US, et al. Current comorbidity of psychiatric disorders among DSM-IV major depressive disorder patients in psychiatric care in the Vantaa Depression Study. J Clin Psychiatry. 2002;63(2):126-134. PubMed CrossRef
11. Zimmerman M, Chelminski I, McDermut W. Major depressive disorder and Axis I diagnostic comorbidity. J Clin Psychiatry. 2002;63(3):187-193. PubMed CrossRef
12. Romera I, Pérez V, Ciudad A, et al. Residual symptoms and functioning in depression, does the type of residual symptom matter? a post-hoc analysis. BMC Psychiatry. 2013;13(1):51. PubMed CrossRef
13. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342-351. PubMed CrossRef
14. Bjørngaard JH, Bjerkeset O, Romundstad P, et al. Sleeping problems and suicide in 75,000 Norwegian adults: a 20 year follow-up of the HUNT I study. Sleep. 2011;34(9):1155-1159. PubMed CrossRef
15. Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, et al. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83-94. PubMed CrossRef
16. Sivertsen B, Overland S, Neckelmann D, et al. The long-term effect of insomnia on work disability: the HUNT-2 historical cohort study. Am J Epidemiol. 2006;163(11):1018-1024. PubMed CrossRef
17. Howells FM, Stein DJ, Russell VA. Synergistic tonic and phasic activity of the locus coeruleus norepinephrine (LC-NE) arousal system is required for optimal attentional performance. Metab Brain Dis. 2012;27(3):267-274. PubMed CrossRef
18. Hermes EDA, Sernyak M, Rosenheck R. Use of second-generation antipsychotic agents for sleep and sedation: a provider survey. Sleep. 2013;36(4):597-600. PubMed CrossRef
19. Calabrese J, MacFadden W, McCoy R. Double-blind, placebo controlled study of quetiapine in bipolar depression [poster]. 157th Annual Meeting of the American Psychiatric Association; May 1-6, 2004; New York, NY.
20. Furukawa TA, Streiner DL, Young LT. Antidepressant plus benzodiazepine for major depression. Cochrane Database Syst Rev. 2001;(2):CD001026. PubMed CrossRef
21. Barker MJ, Greenwood KM, Jackson M, et al. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18(1):37-48. PubMed CrossRef
22. Rizvi SJ, Sproule BA, Gallaugher L, et al. Correlates of benzodiazepine use in major depressive disorder: the effect of anhedonia. J Affect Disord. 2015;187:101-105. PubMed CrossRef
23. Trivedi MH, Thase ME, Fava M, et al. Adjunctive aripiprazole in major depressive disorder: analysis of efficacy and safety in patients with anxious and atypical features. J Clin Psychiatry. 2008;69(12):1928-1936. PubMed CrossRef
24. Weiss C, Weiller E, Baker RA, et al. The effects of brexpiprazole and aripiprazole on body weight as monotherapy in patients with schizophrenia and as adjunctive treatment in patients with major depressive disorder: an analysis of short-term and long-term studies. Int Clin Psychopharmacol. 2018;33(5):255-260. PubMed CrossRef
25. McIntyre RS, Weiller E, Zhang P, et al. Brexpiprazole as adjunctive treatment of major depressive disorder with anxious distress: results from a post-hoc analysis of two randomised controlled trials. J Affect Disord. 2016;201:116-123. PubMed CrossRef
26. Krystal AD, Mittoux A, Meisels P, et al. Effects of adjunctive brexpiprazole on sleep disturbances in patients with major depressive disorder: an open-label, flexible-dose, exploratory study. Prim Care Companion CNS Disord. 2016;18(5). PubMed CrossRef
27. Cheon E-J, Lee K-H, Park Y-W, et al. Comparison of the efficacy and safety of aripiprazole versus bupropion augmentation in patients with major depressive disorder unresponsive to selective serotonin reuptake inhibitors: a randomized, prospective, open-label study. J Clin Psychopharmacol. 2017;37(2):193-199. PubMed CrossRef
28. Fava M, Okame T, Matsushima Y, et al. Switching from inadequate adjunctive or combination treatment options to brexpiprazole adjunctive to antidepressant: an open-label study on the effects on depressive symptoms and cognitive and physical functioning. Int J Neuropsychopharmacol. 2017;20(1):22-30. PubMed CrossRef
29. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138-147. PubMed CrossRef
30. User Reviews for Quetiapine to Treat Depression. August 2012. https://www.drugs.com/comments/quetiapine/for-depression.html. Accessed February 27, 2019.
31. Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’ t. BMJ. 1996;312(7023):71-72. PubMed CrossRef
32. Citrome L, Ketter TA. Teaching the philosophy and tools of evidence-based medicine: misunderstandings and solutions. Int J Clin Pract. 2009;63(3):353-359. PubMed CrossRef
33. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder. https://www.mdedge.com/psychiatry/article/103114/schizophrenia-other-psychotic-disorders/brexpiprazole-schizophrenia-and. Accessed November 26, 2018.
34. Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39-48. PubMed CrossRef
35. Eli Lilly. Symbyax (Olanzapine and Fluoxetine) Capsules for Oral Use. Prescribing Information. March 2018. http://pi.lilly.com/us/symbyax-pi.pdf. Accessed January 7, 2019.
36. AstraZeneca. Seroquel XR (Quetiapine Fumarate) Extended-Release Tablets, for Oral Use. November 2018. https://www.azpicentral.com/seroquel-xr/seroquelxr.pdf. Accessed January 7, 2019.
37. Otsuka. Abilify (Aripiprazole) Tablets. Prescribing Information. February 2018. https://www.otsuka-us.com/media/static/Abilify-PI.pdf. Accessed January 7, 2019.
38. Otsuka. Rexulti (Brexpiprazole) Tablets, for Oral Use. Prescribing Information. February 2018. https://www.otsuka-us.com/media/static/Rexulti-PI.pdf. Accessed January 7, 2019.
39. Citrome L. Quantifying risk: the role of absolute and relative measures in interpreting risk of adverse reactions from product labels of antipsychotic medications. Curr Drug Saf. 2009;4(3):229-237. PubMed CrossRef
40. Citrome L. Compelling or irrelevant? using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419. PubMed CrossRef
41. Citrome L. Relative vs absolute measures of benefit and risk: what’s the difference? Acta Psychiatr Scand. 2010;121(2):94-102. PubMed CrossRef
42. Citrome L, Ketter TA. When does a difference make a difference? interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411. PubMed CrossRef
43. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66(10):1254-1269. PubMed
44. Holt C, Milgrom J, Gemmill AW. Improving help-seeking for postnatal depression and anxiety: a cluster randomised controlled trial of motivational interviewing. Arch Women Ment Health. 2017;20(6):791-801. PubMed CrossRef
45. Rollnick S, Miller WR, Butler C. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: Guilford Press; 2008.
46. Geerts E, Bouhuys N, Van den Hoofdakker RH. Nonverbal attunement between depressed patients and an interviewer predicts subsequent improvement. J Affect Disord. 1996;40(1-2):15-21. PubMed CrossRef
47. Krupnick JL, Sotsky SM, Simmens S, et al. The role of the therapeutic alliance in psychotherapy and pharmacotherapy outcome: findings in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol. 1996;64(3):532-539. PubMed CrossRef
48. User Reviews for Abilify to Treat Depression. November 2018. https://www.drugs.com/comments/aripiprazole/abilify-for-depression.html. Accessed February 27, 2019.
49. Unützer J, Powers D, Katon W, et al. From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatr Clin North Am. 2005;28(4):1079-1092. PubMed CrossRef
50. Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259-263. PubMed CrossRef
51. Grypma L, Haverkamp R, Little S, et al. Taking an evidence-based model of depression care from research to practice: making lemonade out of depression. Gen Hosp Psychiatry. 2006;28(2):101-107. PubMed CrossRef
52. Rush AJ, Kraemer HC, Sackeim HA, et al; ACNP Task Force. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31(9):1841-1853. PubMed CrossRef
53. Lohr JB, Eidt CA, Abdulrazzaq Alfaraj A, et al. The clinical challenges of akathisia. CNS Spectr. 2015;20(suppl 1):1-14, quiz 15-16. PubMed CrossRef
54. Kane JM, Fleischhacker WW, Hansen L, et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70(5):627-643. PubMed CrossRef
55. Bratti IM, Kane JM, Marder SR. Chronic restlessness with antipsychotics. Am J Psychiatry. 2007;164(11):1648-1654. PubMed CrossRef
56. Kumar R, Sachdev PS. Akathisia and second-generation antipsychotic drugs. Curr Opin Psychiatry. 2009;22(3):293-299. PubMed CrossRef
57. Sachdev P. The epidemiology of drug-induced akathisia, part II: chronic, tardive, and withdrawal akathisias. Schizophr Bull. 1995;21(3):451-461. PubMed CrossRef
58. Hsu JH, Mulsant BH, Lenze EJ, et al. Clinical predictors of extrapyramidal symptoms associated with aripiprazole augmentation for the treatment of late-life depression in a randomized controlled trial. J Clin Psychiatry. 2018;79(4):17m11764. PubMed CrossRef
59. Gualtieri CT. The problem of tardive akathisia. Brain Cogn. 1993;23(1):102-109. PubMed CrossRef
60. Llorca P-M, Lançon C, Hartry A, et al. Assessing the burden of treatment-emergent adverse events associated with atypical antipsychotic medications. BMC Psychiatry. 2017;17(1):67. PubMed CrossRef
61. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull. 2003;29(3):547-558. PubMed CrossRef
62. Longden E, Read J. Assessing and reporting the adverse effects of antipsychotic medication: a systematic review of clinical studies, and prospective, retrospective, and cross-sectional research. Clin Neuropharmacol. 2016;39(1):29-39. PubMed CrossRef
63. Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13(1):177-183. PubMed CrossRef
64. Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. Int Clin Psychopharmacol. 1995;10(suppl 3):133-138. PubMed
This PDF is free for all visitors!
Save
Cite