ABSTRACT
Between 0.3%–4.6% of women use antipsychotic (AP) drugs during pregnancy. Two large, retrospective, population-based cohort studies, conducted in Nordic countries and in the US, examined the risk of neurodevelopmental disorders (NDDs) following gestational exposure to APs. The Nordic study found that, in unadjusted analyses, exposure to APs during pregnancy was associated with increased risk of attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) in offspring; that the risk all but disappeared after adjusting for covariates; and that the risk appeared to be related to maternal major mental illness rather than to gestational exposure to APs. The US study also found that, in unadjusted analyses, gestational exposure to APs was associated with an increased risk of almost all of the study-specified NDDs in offspring; however, after adjusting for covariates, the risks were no longer meaningfully increased and, importantly, were no longer statistically significant for ADHD and ASD. Thus, these 2 studies suggest that gestational exposure to APs is a marker of NDD risk in offspring rather than a potential cause. Whereas a small but significantly increased risk was identified for aripiprazole in the US study, the signal was inconsistent across analyses, and confounding due to maternal mental illness was not ruled out. Previous studies have suggested that the use of APs during pregnancy is not associated with an increased risk of major congenital malformations and other adverse gestational outcomes. Considering the potential harm and suffering associated with major mental illness and the very low risks associated with AP use during pregnancy, initiation or continuation of APs appears to carry a favorable risk-benefit ratio in pregnant women who need these drugs; however, decision-making should be shared between patients, their caregivers, and the treating team.
J Clin Psychiatry 2022;83(3):22f14529
To cite: Andrade C. Attention-deficit/hyperactivity disorder, autism spectrum disorder, and other neurodevelopmental outcomes associated with antipsychotic drug exposure during pregnancy. J Clin Psychiatry. 2022;83(3):22f14529.
To share: https://doi.org/10.4088/JCP.22f14529
© Copyright 2022 Physicians Postgraduate Press, Inc.
Antipsychotic (AP) drugs are used as primary treatments and augmentation agents for many approved and off-label indications in psychiatry, including schizophrenia, bipolar disorder, depression, obsessive-compulsive disorder, anxiety, insomnia, tic disorder, and others. In a study of data from 8,394,343 pregnancies in 10 countries, Reutfors et al1 found that the prevalence of AP use during pregnancy ranged from a low of 0.28% in Germany (2006–2015) to a high of 4.64% in the UK (2006–2016); annual use data showed that, in later years of the survey, quetiapine and prochlorperazine were the most commonly prescribed drugs. The safety of APs during pregnancy in women with mental health disorders is therefore a matter of importance.
Earlier articles in this column examined major congenital malformations (MCMs), gestational outcomes, and neurodevelopmental outcomes associated with AP use during pregnancy; broad conclusions were that gestational exposure to APs does not appear to increase the risk of MCMs2 but that the data are not convincingly reassuring for neurodevelopmental outcomes.3 Two important new studies,4,5 examining neurodevelopmental disorders (NDDs) after gestational exposure to APs, have now been published, and these are briefly reviewed in the present article.
The Nordic Study4
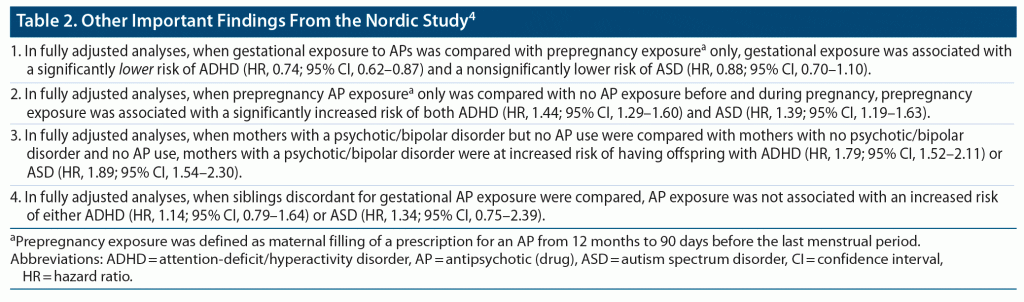
Hálfdánarson et al4 described a population-based cohort study with data extracted from linked nationwide registers in Denmark (1997–2017), Finland (1996–2016), Iceland (2004–2017), Norway (2004–2017), and Sweden (2006–2016). Children gestationally exposed to APs (n = 15,466) were those whose mothers filled at least 1 prescription for an AP between the last menstrual period (LMP) and childbirth. Unexposed children (n = 4,308,620) were those whose mothers did not fill a prescription for an AP from 90 days before the LMP until birth. Maternal psychosis or bipolar disorder contributed to 4,385 exposed and 8,356 unexposed pregnancies.
Across a median of 8.2 years of follow-up, 72,257 children with attention-deficit/hyperactivity disorder (ADHD) were identified, and across a median of 9.9 years of follow-up, 38,674 children with autism spectrum disorder (ASD) were identified. Cox proportional hazard regression was used to determine the risk of development of ADHD or ASD in gestationally AP-exposed relative to AP-unexposed children after adjusting for covariates that included maternal age, education, body mass index, smoking status, parity, comorbidities, other drug use, sex of the child, and other relevant variables. Important findings from the study are presented in Table 1 and Table 2.
In summary, the study4 found that gestational exposure to APs was associated with increased risk of ADHD and ASD in the offspring; that the risk all but disappeared after adjusting for covariates; and that maternal psychotic/bipolar illness rather than gestational exposure to APs was associated with the increased risk of ADHD and ASD. Readers may note that there were some isolated significant findings in the adjusted analyses, but these were not part of a pattern and could have been due to unmeasured confounding, or could have been false positives associated with multiple hypothesis testing (the authors did not correct for type 1 errors).
The findings of the Nordic study suggest that, in women who use APs during pregnancy, genetic factors and perhaps shared family environmental variables may drive the offspring risk of ADHD and ASD rather than the gestational exposure to APs. The authors of the study4 concluded that the findings are reassuring for women who need APs during pregnancy.
The US Study5
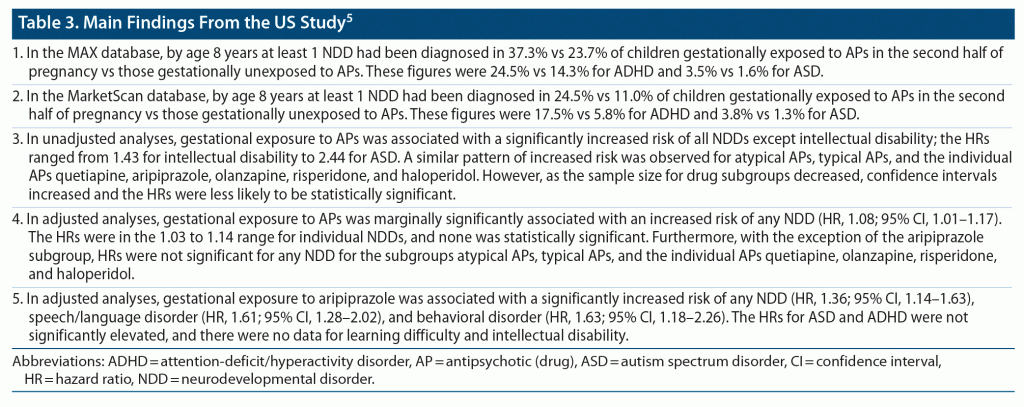
Straub et al5 identified cohorts of publicly and privately insured women in the nationwide Medicaid Analytic eXtract (MAX; 2000–2014) and the IBM Health MarketScan Research Database (MarketScan; 2003–2015), US. Children gestationally exposed to APs were those whose mothers had filled a prescription for any AP in the second half of pregnancy; that is, after 18 weeks of gestation, when synaptogenesis begins. Unexposed children were those whose mothers had not filled an AP prescription between 90 days before pregnancy and delivery. There were 9,551 exposed and 2,034,883 unexposed pregnancies in the MAX database and 1,214 exposed and 1,306,408 unexposed pregnancies in the MarketScan database. Children were followed for up to 14 years.
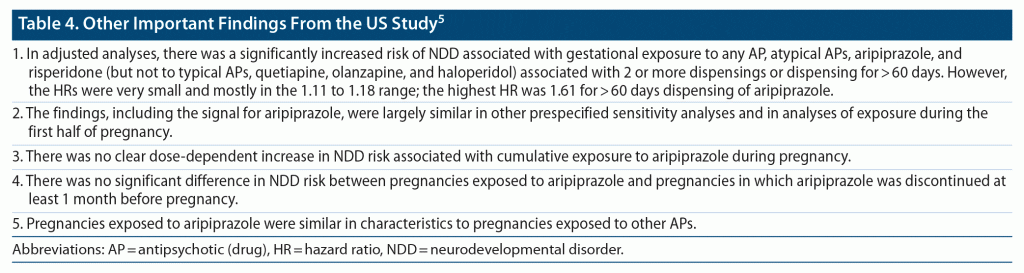
The association between gestational exposure to APs and NDDs was studied using Cox proportional hazard regression after adjustment for important covariates. The hazard ratios obtained from each cohort were combined using fixed effects meta-analysis. NDDs of interest in this study were ASD, ADHD, learning difficulty, developmental speech or language disorder, developmental coordination disorder, intellectual disability, and behavioral disorder. Covariates that were adjusted for included demographic variables, treatment indications, lifestyle behaviors, indices of severity of maternal psychiatric illness, maternal comorbidities, medication exposures, and others. Important findings from the study are presented in Table 3 and Table 4.
In summary, the study5 found that, in unadjusted analyses, gestational exposure to antipsychotic drugs was associated with an increased risk of almost all NDDs; however, after adjusting for demographic and confounding variables, the risks were no longer meaningfully increased, and were no longer statistically significant for ADHD and ASD. For some NDDs (speech/language disorder, behavioral disorder; Table 3), there appeared to be a signal for a small but statistically significantly increased risk with aripiprazole; however, the signal was inconsistent across analyses. The findings from the main analyses were broadly similar in sensitivity analyses.
The authors of the study5 concluded that the risk of NDDs identified in their study was perhaps driven by maternal characteristics rather than by the gestational AP exposure. They suggested that the signal for aripiprazole should be examined in future studies.
General Notes
Both studies4,5 reviewed in this article found that gestational exposure to APs was associated with an increased risk of NDDs in offspring and that the risk all but disappeared after adjustment for confounding variables. Thus, gestational exposure to APs is more a marker of NDD risk in offspring than a potential cause; maternal mental illness, the causes thereof, and behaviors related thereto, are likely to drive the observed risk.
In the Nordic study,4 gestational exposure to APs was associated with a significantly lower risk of offspring ADHD in comparison with prepregnancy but not gestational exposure. This advantage was observed for ASD, as well, but did not reach statistical significance. Speculatively, stopping APs before pregnancy, as opposed to continuing APs into pregnancy, may result in psychiatric instability during pregnancy, and such instability may be associated with behaviors that increase the risk of NDDs in offspring. This possibility, suggested by the study authors, merits examination in future studies.
Regrettably, the Nordic study4 analyses did not include women who had used prochlorperazine during pregnancy; the justification was that prochlorperazine is used almost exclusively as an antiemetic. The logic is baffling because the biologically mediated beneficial and adverse effects of a drug depend on its pharmacodynamics and not on its indication. Prochlorperazine could at least have been studied in a sensitivity analysis. This was clearly a missed opportunity to obtain information about the safety of prochlorperazine during pregnancy.
The authors of the US study5 speculated that because aripiprazole is a partial agonist and not an antagonist at D2 receptors, it may cause lactation failure; anecdotal data support this conjecture.6–8 They further speculated that lactation failure may mediate the association between aripiprazole and NDD. However, other explanations are possible for the observed association observed between aripiprazole and some NDDs (speech/language disorder, behavioral disorder; Table 3) in the adjusted analyses. One is that the US study5 examined a very large number of associations in primary, sensitivity, and post hoc analyses, and these results with aripiprazole may merely have been a type 1 (false positive) statistical error. The other is that the aripiprazole results may have been due to residual confounding, driven by an overlap in genes between major mental illness and NDDs.9 In this context, eTable 6 in the supplementary data provided the important finding that, when pregnancies exposed to aripiprazole were compared with pregnancies before which mothers discontinued aripiprazole, gestational exposure to aripiprazole was no longer associated with a significantly increased risk of NDD in the offspring (this implies that maternal characteristics rather than aripiprazole exposure was related to the risk). Regardless of the above, aripiprazole was not associated with an increased risk of ADHD and ASD in any of the adjusted analyses.
General Conclusions
An earlier study10 provided reassurance that gestational exposure to APs did not increase the risk of ADHD and ASD in offspring; however, there were many concerns about this study that weakened its conclusions.3 The 2 more recent studies4,5 reviewed in this article provide more convincing reassurance; the safety of aripiprazole, however, may need confirmation in future research in which maternal mental illness and shared family environment are controlled for through analyses such as those conducted in the Nordic study.4 Other studies indicate that exposure to APs during pregnancy is not associated with an increased risk of MCMs and other adverse gestational outcomes.2 Given the potential harm and suffering associated with major mental illness and the very low risks associated with AP use during pregnancy, it appears that the risk-benefit analysis favors initiation or continuation of APs in pregnant women who need these drugs. Nevertheless, decision-making should be shared on a case-by-case basis between patients and caregivers on the one hand and health care professionals on the other.
Parting Notes
Readers may also be interested in other recent reviews on AP use in pregnancy. For example, based on the limited literature available, Beex-Oosterhuis et al11 concluded that although clozapine crosses the placental barrier, there is no evidence, so far, that clozapine is teratogenic, or that it increases the risk of abortion, premature birth, stillbirth, fetal disorders, or complications of delivery. Reinstein et al12 provided many practical suggestions for the use of long-acting injectable antipsychotic drugs during pregnancy. Tosato et al13 provided a risk-benefit analysis of the use of antipsychotic drugs during pregnancy in patients with schizophrenia and bipolar disorder, concluding that, in consenting patients, the most reasonable and least harmful option is to continue antipsychotic treatment at the lowest effective dose.
Published online: May 30, 2022.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
References (13)

- Reutfors J, Cesta CE, Cohen JM, et al. Antipsychotic drug use in pregnancy: a multinational study from ten countries. Schizophr Res. 2020;220:106–115. PubMed CrossRef
- Andrade C. Major congenital malformations associated with exposure to second-generation antipsychotic drugs during pregnancy. J Clin Psychiatry. 2021;82(5):82. PubMed CrossRef
- Andrade C. Gestational and neurodevelopmental outcomes associated with antipsychotic drug exposure during pregnancy. J Clin Psychiatry. 2021;82(5):21f14265. PubMed CrossRef
- Hálfdánarson Ó, Cohen JM, Karlstad Ø, et al. Antipsychotic use in pregnancy and risk of attention/deficit-hyperactivity disorder and autism spectrum disorder: a Nordic cohort study. Evid Based Ment Health. 2022;25(2):54–62. PubMed
- Straub L, Hernández-Díaz S, Bateman BT, et al. Association of antipsychotic drug exposure in pregnancy with risk of neurodevelopmental disorders: a national birth cohort study. JAMA Intern Med. 2022;182(5):522–533. PubMed CrossRef
- Mendhekar DN, Sunder KR, Andrade C. Aripiprazole use in a pregnant schizoaffective woman. Bipolar Disord. 2006;8(3):299–300. PubMed CrossRef
- Yskes R, Thomas R, Nagalla M-L. A case of decreased milk production associated with aripiprazole. Prim Care Companion CNS Disord. 2018;20(6):18l02303. PubMed CrossRef
- Komaroff A. Aripiprazole and lactation failure: The importance of shared decision making. a case report. Case Rep Womens Health. 2021;30:e00308. PubMed CrossRef
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179(7):1469–1482.e11. PubMed CrossRef
- Wang Z, Chan AYL, Coghill D, et al. Association between prenatal exposure to antipsychotics and attention-deficit/hyperactivity disorder, autism spectrum disorder, preterm birth, and small for gestational age. JAMA Intern Med. 2021;181(10):1332–1340. PubMed CrossRef
- Beex-Oosterhuis MM, Van Gool AR, Heerdink ER, et al. Clozapine treatment during pregnancy and the postpartum period: a systematic literature review. J Clin Psychiatry. 2021;83(1):21r13952. PubMed CrossRef
- Reinstein SA, Cosgrove J, Malekshahi T, et al. Long-acting injectable antipsychotic use during pregnancy: a brief review and concise guide for clinicians. J Clin Psychiatry. 2020;81(6):20ac13597. PubMed CrossRef
- Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477–e489. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite