Objective: Attention-deficit/hyperactivity disorder (ADHD) medications are increasingly used in pregnancy. Studies on the pregnancy safety of these medications that are restricted to live births may underestimate severe teratogenic effects that cause fetal demise or termination of pregnancy. The present study addresses this limitation by including data from both prenatal and postnatal diagnoses of major malformations.
Methods: A nationwide registry-based study was conducted of 364,012 singleton pregnancies in Denmark from November 1, 2007, to February 1, 2014. Exposures to ADHD medication were obtained from redeemed prescriptions from the Danish Health Services Prescription Database. Outcome data included prenatally diagnosed malformations from the Danish Fetal Medicine Database and postnatally diagnosed malformations from the Danish National Patient Registry. The primary outcome was major malformations overall, and secondary outcomes were malformations of the central nervous system and cardiac malformations. The comparison group was pregnancies with no redeemed prescriptions for ADHD medication. We defined severe cardiac malformations (SCM) as concurrent diagnoses of a cardiac malformation with miscarriage, termination, stillbirth, postnatal death, or cardiac surgery within 1 year of birth.
Results: The prevalence of first-trimester exposure to ADHD medication increased during the study period from 0.05% in 2008 to 0.27% in 2013, with the majority (473/569) of the exposures being to methylphenidate. There were 5.1% malformations overall and 2.1% cardiac malformations among the exposed compared to 4.6% and 1.0%, respectively, among the unexposed. For methylphenidate, the adjusted prevalence ratios (PRs) were 1.04 (95% confidence interval [CI], 0.70-1.55) for malformations overall and 1.65 (95% CI, 0.89-3.05) for any cardiac malformations (number needed to harm [NNH] = 92), with septum defects in 10 out of 12 cases. The PR for ventricular septal defect was 2.74 (95% CI, 1.03-7.28) and for SCM, 2.59 (95% CI, 0.98-6.90).
Conclusions: Exposure to methylphenidate was not associated with an increased risk of malformations overall in data that included information from both prenatal and postnatal diagnoses of major malformations. There was an increased risk of cardiac malformations with NNH of 92 based on 12 cases among the exposed. More data are needed on other types of ADHD medication.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
• Provide current data on potential pregnancy risks to patients taking medication for attention-deficit/hyperactivity disorder
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in January 2021 and is eligible for AMA PRA Category 1 Credit™ through February 28, 2023. The latest review of this material was December 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; and has been a member of the Independent Data Safety and Monitoring Committee for Janssen. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Associations Between ADHD Medication Use in Pregnancy and Severe Malformations Based on Prenatal and Postnatal Diagnoses:
A Danish Registry-Based Study
ABSTRACT
Objective: Attention-deficit/hyperactivity disorder (ADHD) medications are increasingly used in pregnancy. Studies on the pregnancy safety of these medications that are restricted to live births may underestimate severe teratogenic effects that cause fetal demise or termination of pregnancy. The present study addresses this limitation by including data from both prenatal and postnatal diagnoses of major malformations.
Methods: A nationwide registry-based study was conducted of 364,012 singleton pregnancies in Denmark from November 1, 2007, to February 1, 2014. Exposures to ADHD medication were obtained from redeemed prescriptions from the Danish Health Services Prescription Database. Outcome data included prenatally diagnosed malformations from the Danish Fetal Medicine Database and postnatally diagnosed malformations from the Danish National Patient Registry. The primary outcome was major malformations overall, and secondary outcomes were malformations of the central nervous system and cardiac malformations. The comparison group was pregnancies with no redeemed prescriptions for ADHD medication. We defined severe cardiac malformations (SCM) as concurrent diagnoses of a cardiac malformation with miscarriage, termination, stillbirth, postnatal death, or cardiac surgery within 1 year of birth.
Results: The prevalence of first-trimester exposure to ADHD medication increased during the study period from 0.05% in 2008 to 0.27% in 2013, with the majority (473/569) of the exposures being to methylphenidate. There were 5.1% malformations overall and 2.1% cardiac malformations among the exposed compared to 4.6% and 1.0%, respectively, among the unexposed. For methylphenidate, the adjusted prevalence ratios (PRs) were 1.04 (95% confidence interval [CI], 0.70-1.55) for malformations overall and 1.65 (95% CI, 0.89-3.05) for any cardiac malformations (number needed to harm [NNH] = 92), with septum defects in 10 out of 12 cases. The PR for ventricular septal defect was 2.74 (95% CI, 1.03-7.28) and for SCM, 2.59 (95% CI, 0.98-6.90).
Conclusions: Exposure to methylphenidate was not associated with an increased risk of malformations overall in data that included information from both prenatal and postnatal diagnoses of major malformations. There was an increased risk of cardiac malformations with NNH of 92 based on 12 cases among the exposed. More data are needed on other types of ADHD medication.
J Clin Psychiatry 2021;82(1):20m13458
To cite: Kolding L, Ehrenstein V, Pedersen L, et al. Associations between ADHD medication use in pregnancy and severe malformations based on prenatal and postnatal diagnoses: a Danish registry-based study. J Clin Psychiatry. 2021;82(1):20m13458.
To share: https://doi.org/10.4088/JCP.20m13458
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Obstetrics and Gynecology, Aarhus University Hospital, Aarhus, Denmark
bDepartment of Clinical Medicine, Aarhus University, Aarhus, Denmark
cDepartment of Clinical Epidemiology, Aarhus University Hospital, Aarhus University, Aarhus, Denmark
dCentre for Fetal Diagnostics, Aarhus University Hospital, Aarhus University, Aarhus, Denmark
eDepartment of Obstetrics, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
fDepartment of Clinical Medicine, University of Copenhagen, Copenhagen, Copenhagen, Denmark
gDepartment of Clinical Pharmacology, Aarhus University Hospital, Aarhus, Denmark
hDepartment of Biomedicine, Aarhus University, Aarhus, Denmark
*Corresponding author: Line Kolding, MD, PhD, Department of Obstetrics and Gynecology, Aarhus University Hospital, Palle Juul-Jensens Blvd 99, 8200 Aarhus N, Denmark (e-mail: [email protected]).
Use of attention-deficit/hyperactivity disorder (ADHD) medication in pregnancy increased from 5 per 100,000 person-years in 2003 to 533 per 100,000 in 2010 in Denmark.1 Such dramatic increases have been seen in other populations1 even though there is a relative paucity of pregnancy safety data for ADHD medication.1 In fact, most ADHD medications are sympathomimetics with pharmacologic actions similar to those of known teratogens (amphetamine and cocaine),1,2 which further underscores the need for safety data on ADHD medication exposure in pregnancy.
Reassuringly, a 2018 meta-analysis of 8 cohort studies did not find an association of exposure to any ADHD medication during pregnancy and any major malformation (risk ratio [RR] = 1.05; 95% confidence interval [CI], 0.96-1.16) or cardiac malformations (RR = 1.08; 95% CI, 0.92-1.27).3 However, methylphenidate was associated with an increased risk of cardiac malformations (RR = 1.27; 95% CI, 0.99-1.63), with the result nearly solely based on data from a large study from the United States and the Nordic countries that included 3,474 methylphenidate-exposed pregnancies.4
The above evidence, similarly to most such studies, stems from data on pregnancies ending in live births. Such studies cannot detect associations for severe malformations that cause spontaneous or induced pregnancy loss. Lack of data on malformations ending in a pregnancy loss may consequently lead to an underestimation of the true occurrence of malformations and may result in survivor bias if only data on liveborn children are available.5
We assembled pregnancies with complete information on outcomes, ie, data on malformations detected by prenatal ultrasound, terminations, miscarriages, stillbirths, and malformations among liveborn children, and examined the potential association between first-trimester exposure to ADHD medication and major malformations, overall and specifically malformations of the central nervous system and heart.
METHODS
This prevalence study was set in Denmark, a prosperous country with universal access to health care.6 We used routinely collected data from 5 nationwide databases: the Danish Fetal Medicine Database,7,8 the Danish National Patient Registry,9,10 the Danish Medical Birth Registry,11 and the Danish Health Services Prescription Database,12 all linked on individual level (including mother-child linkage) via a unique identifier from the Danish Civil Registration System.8,13
The study population comprised all clinically recognized singleton pregnancies with a live fetus at the first-trimester scan from 11 gestational weeks, with conception dates from November 1, 2007, through February 1, 2014. Pregnancies terminated before gestational week 22 were identified in the Danish National Patient Registry, and pregnancies ending in live birth or stillbirth from week 22 onward were identified from the Danish Medical Birth Registry. Gestational age at pregnancy end was estimated from ultrasound measurements in early pregnancy or, if unavailable, calculated from the last menstrual period. Conception date was estimated by subtracting the gestational age from the date of pregnancy end and adding 14 days. Pregnancies with fetal chromosomal abnormalities were excluded regardless of presence of other malformations.
Exposure to ADHD medication was measured using redeemed prescriptions through linkage to the Danish Health Services Prescription Database.12 First-trimester exposure was defined as 1 or more redeemed prescriptions from 28 days before conception date through 70 days after conception date. We restricted the cohort by excluding preconception exposure to ADHD medication (former use), an adapted model previously suggested by Huybrechts et al4 and Jimenez-Solem et al.14 The unexposed group had no redemptions for an ADHD medication from 28 days before conception date through 70 days after conception date (first trimester) and from 365 to 183 days before conception date (former use). Medications were analyzed as any ADHD medication and as specific drugs (methylphenidate, modafinil, atomoxetine).
Major malformations were classified according to the European Surveillance of Congenital Anomalies (EUROCAT) classification, version 2014.15 Data on prenatally diagnosed major malformations came from the Danish Fetal Medicine Database, which contains data on malformations diagnosed prenatally including all first-trimester screenings and second-trimester malformation scans starting from gestational week 11. The database was 95% complete during its period of coverage.7,8 Data on postnatally diagnosed major malformations were obtained from the Danish National Patient Registry and ascertained up to 1 year of age.9 The primary outcome was major malformations overall, and secondary outcomes were malformations of the central nervous system or the heart. Cardiac malformations were classified by severity into severe cardiac malformations (SCM; malformations in pregnancies ending in a miscarriage or termination, stillbirth, death, or cardiac surgery during the first year of life, arranged in hierarchy of termination > miscarriage > death > surgery) and non-SCM (all other cardiac malformations). Indication for terminations after week 12 was either on fetal indication (eg, malformation) or non-fetal indication (eg, severe disease of the pregnant woman).
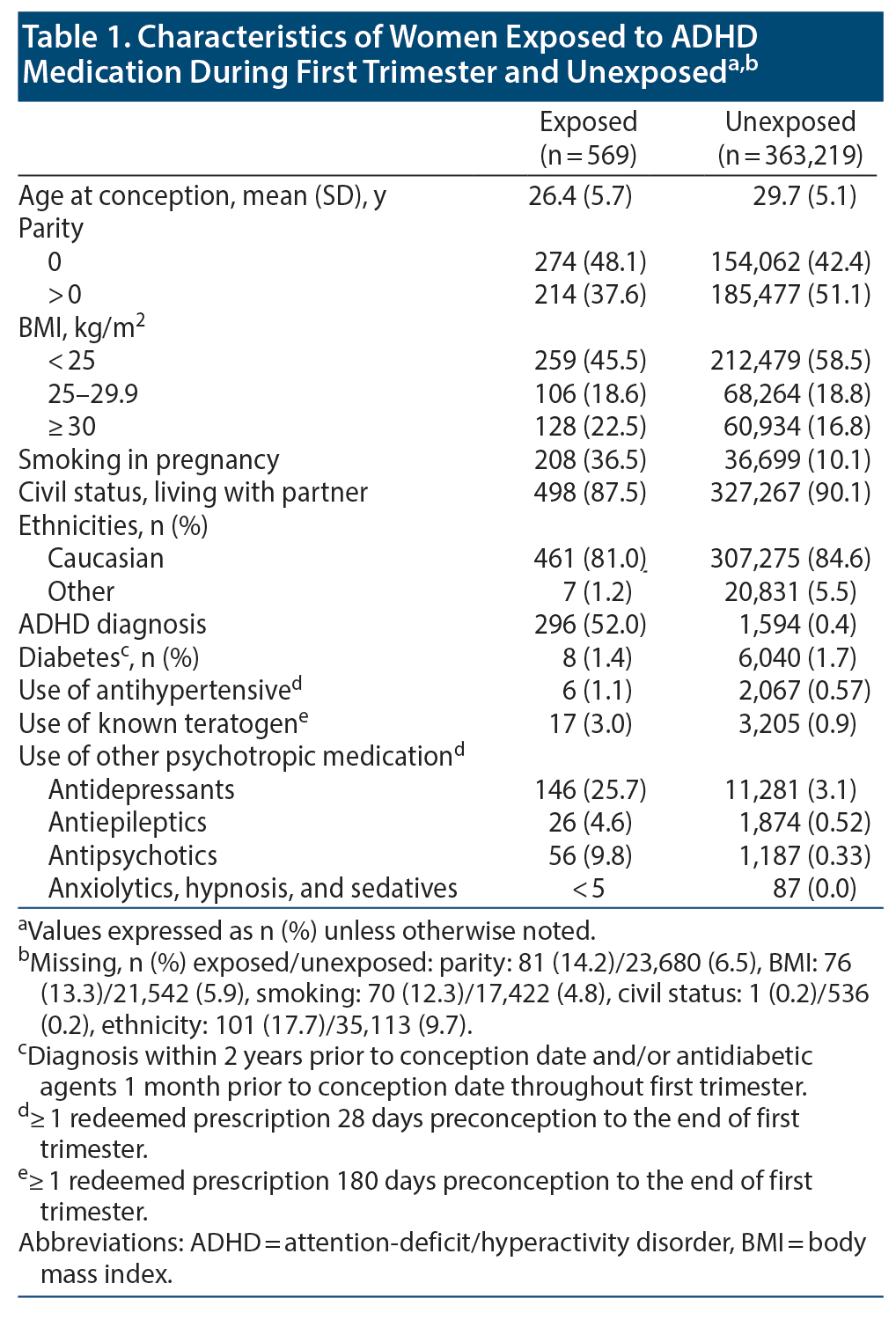
Data on covariates, obtained from the above-mentioned data sources, included maternal ethnicity (Caucasian, other), civil status (living with partner, yes/no), parity (nulliparous/multiparous), age at conception (≤ 35, > 35 years), prepregnant body mass index (BMI; < 25, 25 to < 30, ≥ 30 kg/m2), smoking during pregnancy, redeemed prescription of a known teratogen from 180 days preconception and through the first trimester, first-trimester and 2 years preconception dispensing of other medication (antihypertensives, antidiabetic agents, antiepileptics, antipsychotics, anxiolytics, hypnotics, and sedatives), and maternal hospital diagnoses of ADHD or diabetes 2 years pre-conception date.9,10
We tabulated maternal characteristics according to first-trimester exposure to ADHD medication. We used log-binomial regression16 to estimate prevalence ratios (PRs) with 95% CIs for the study outcomes. We presented crude PRs; PRs minimally adjusted, by including in a regression model, for age, smoking, and ADHD diagnosis (if > 5 cases); and PRs fully adjusted using propensity-score fine stratification (50 strata) for ethnicity, civil status, parity, age, BMI, smoking, exposure to teratogens, antihypertensives, antidiabetics, use of other psychotropic drugs, ADHD diagnosis, and diabetes diagnosis.17

- Use of ADHD medication increased 5-fold in the study period, underscoring the need for updated and unbiased information on pregnancy safety.
- There was no association between methylphenidate and malformations overall in a study with information on both prenatal and postnatal diagnoses.
- An increased risk of cardiac malformations (number needed to harm = 92) based on 12 cases among the exposed was found.
In sensitivity analyses, we redefined first-trimester exposure by 2 or more redeemed prescriptions to assess the effect of misclassification on the results. We repeated the analyses restricting the study population to pregnancies ending in live births to assess the impact of the resulting selection bias on the results.
Statistical analyses were performed with Stata (version 14, StataCorp, Texas) and SAS (version 9.4, SAS Inc, Cary, North Carolina). In all analyses, cell counts under 5 were masked to avoid identification of individuals.
RESULTS
We identified 366,489 eligible pregnancies during the study period. After excluding 354 pregnancies due to coding errors and 2,123 pregnancies with chromosome anomalies, the study population comprised 364,012 clinically recognized singleton pregnancies in week 11 onward, of which, from week 12 and onward, 96% had data from ultrasound examinations from the Danish Fetal Medicine Database.
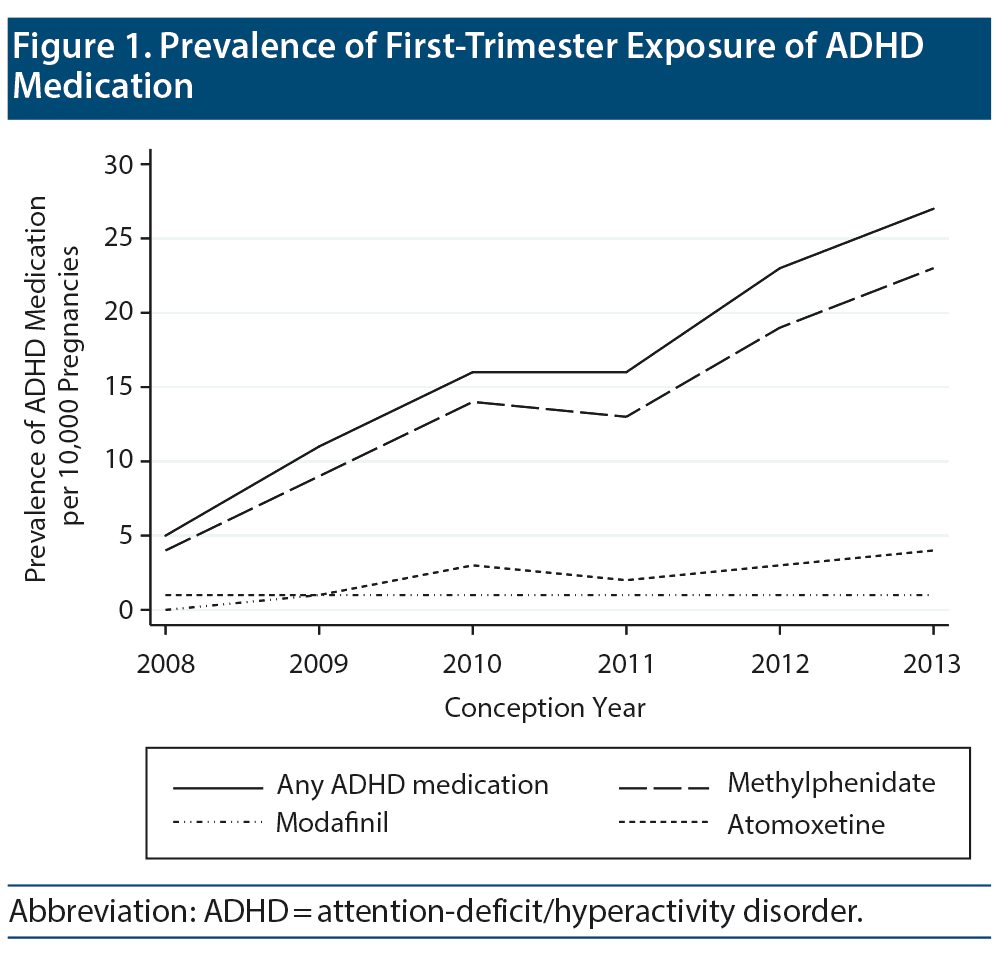
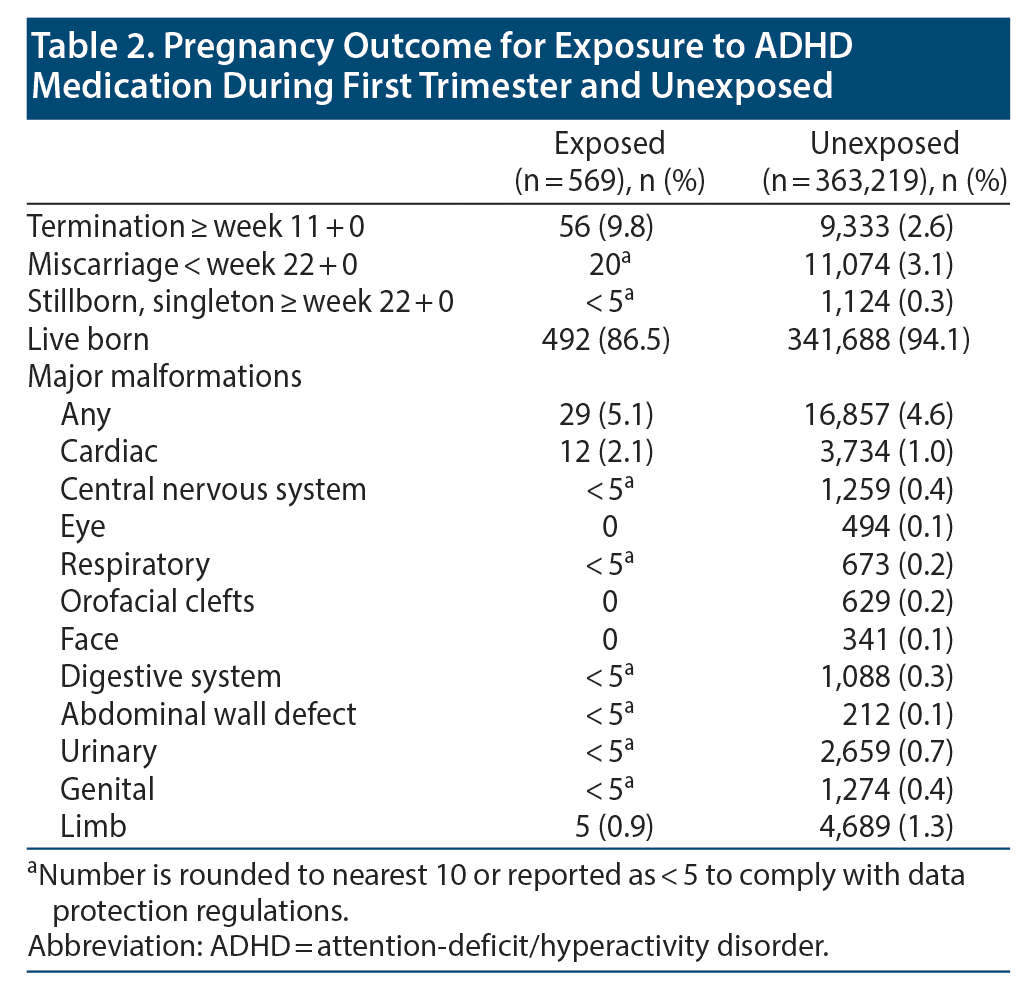
The prevalence of first-trimester exposure to ADHD medication was 0.16% (n = 569) on average. It increased more than 5-fold during the study period, from 0.05% in 2008 to 0.27% in 2013 (Figure 1). The majority of the exposed pregnancies (473/569, 83%) were exposed to methylphenidate. Only half of the women exposed to ADHD medication had a hospital diagnosis of ADHD 2 years prior to the conception date. Some of the remaining exposed pregnancies may be because the primary hospital diagnosis was set earlier than 2 years prior to conception, and the treatment was maintained by the general practitioner. Compared with unexposed women, women who used ADHD medication were younger and more likely to smoke and/or use other psychotropic medications (Table 1). Termination for any reason after gestational week 11 had a prevalence of 9.8% among pregnancies with first-trimester exposure to ADHD medicine and 2.6% among the unexposed (Table 2). The majority of terminations (after week 12) in the exposed group were due to nonfetal indications.
The overall prevalence of malformations was 5.1% among the methylphenidate-exposed pregnancies and 4.6% among the unexposed pregnancies. The prevalence of cardiac malformations was 2.1% among the methylphenidate exposed and 1.0% among the unexposed. Number needed to harm (NNH) was 92. For SCM, the prevalences were 0.9% among the methylphenidate exposed and 0.2% among the unexposed.
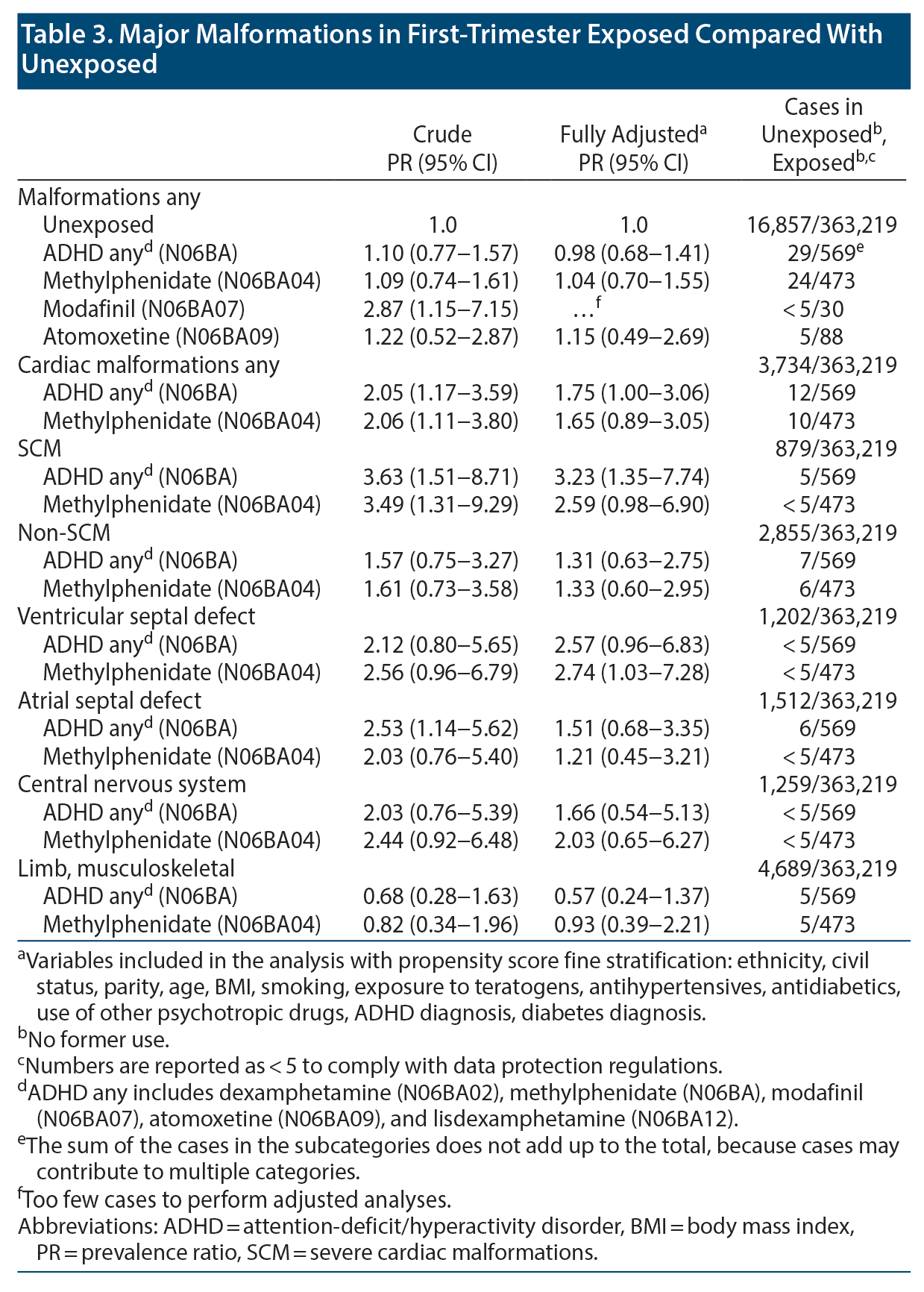
Most PR estimates became attenuated by improved control of confounding (Table 3). For methylphenidate, the fully adjusted PRs were 1.04 (95% CI, 0.70-1.55) for malformations overall, 1.65 (95% CI, 0.89-3.05) for cardiac malformations, and 2.59 (95% CI, 0.98-6.90) for SCM. In the pregnancies with cardiac malformations, 10 out of 12 exposed cases were septum defects, atrial septal defect (ASD) or ventricular septal defect (VSD), yielding fully adjusted PRs in methylphenidate-exposed pregnancies of 1.21 (95% CI, 0.45-3.21) for ASD and 2.74 (95% CI, 1.03-7.28) for VSD. The fully adjusted PR for CNS malformations was 2.03 (95% CI, 0.65 to 6.27). The estimates for SCM and CNS malformations were based on few exposed cases.
Sensitivity analyses, with exposure defined by 2 or more redeemed prescriptions during the first trimester, resulted in a decrease of cases of cardiac malformations among the exposed from 12 to 9. For methylphenidate, the fully adjusted PR for cardiac malformation was 1.75 (95% CI, 0.84-3.65) (data not shown). In analyses restricted to pregnancies ending in live births, the results were unchanged for any malformations and cardiac malformations, but crude estimates strengthened in cardiac malformation subgroups and CNS malformations, all based on few exposed cases (data not shown).
DISCUSSION
In this population-based study, methylphenidate was associated with a 1.65-fold increased risk of cardiac malformations, 2.74-fold increased risk of VSD, and 2.59-fold increased risk of SCM. Septal defects were present in 10 of 12 cases of exposed cardiac malformations.
We defined SCM as termination, miscarriage, death, or surgery. Previous studies did not consider severity; however, we find this outcome to be of major importance. Clinically, it is important because both health care providers and patients ask about the severity of outcomes. Methodologically, it is important because the classification can generate power by assembling rare outcomes and reduce the risk of surveillance bias in the severe group through the robust outcomes of death and surgery. This might contribute to the stronger association between methylphenidate and SCM than between methylphenidate and any cardiac malformations.
The results are in accordance with earlier studies, including a recent systematic review that reported a 1.3-fold association between first-trimester methylphenidate exposure and prevalence of overall cardiac malformation.3 However, our outcomes, according to the EUROCAT definition of major cardiac malformations, did not include patent ductus arteriosus and patent foramen ovale. The large study of Huybrechts et al4 included in the primary analysis women from the United States and replicated analyses adding pregnancy registry data from 5 Nordic countries (Denmark, Finland, Iceland, Norway, and Sweden). In this pooled analysis, the study found a 28% increase in cardiac malformations after methylphenidate exposure; furthermore, after patent ductus arteriosus and patent foramen ovale were excluded, the associations strengthened for methylphenidate: OR = 1.50 (95% CI, 1.05 to 2.14), in accordance with our findings.4 Moreover, similarly to earlier studies, we did not find an association between ADHD medication and overall malformations.1,4,18-20 Similarity of results is expected given the overlap of our study population with the studies by Hærvig et al1 (overlap birth years 2008, 2009) and Pottegård et al20 (overlap birth years 2008-2012) and the Danish population in the study by Huybrechts et al4 (overlap birth years 2008-2011), even with our use of prenatal data, as only 1 exposed case was lost prenatally. In this study, inclusion of prenatal data added 233 cases of cardiac malformations that were not in live births (terminated, miscarriage, or stillborn). Only 1 case was exposed to ADHD medication, resulting in the estimates of cardiac malformations being unchanged.
Similar to previous findings,1,19 we found that pregnancies exposed to ADHD medication were 4 times more likely to end in a termination of pregnancy than unexposed pregnancies. In addition, a Danish study highlighted the potential for cofounding by indication, as the authors found that exposure to ADHD but not to ADHD medications was associated with both spontaneous abortion and preterm birth.19
The estimates of exposure prevalence were similar to those in previous studies in the first conception years (eg, 0.05% in 2008),1,4 and the 5-fold increase is in continuation of the previously described increased use of ADHD medication.1 The prevalences of malformations per 1,000 singleton pregnancies (46 vs previously 35 per 1,000)4 and of cardiac malformations (10 vs previously 13 per 1,000),4 after excluding chromosomal anomalies, were in accordance with previous findings. The degree of selection bias was minimal in our study owing to the completeness and nationwide coverage of the data sources, allowing inclusion of nearly all pregnancies and all diagnosed malformations both prenatally and postpartum within 1 year.
A limitation of the study is the possibility of confounding by indication, even though several approaches were used to adjust for or assess the extent of confounding. Atomoxetine, which in contrast to methylphenidate is a nonstimulant ADHD drug, may have been an ideal negative control exposure as a result of the similar indication for use and pharmacologic effects, previously used by Cohen et al21; however, the number of atomoxetine-exposed pregnancies was too small. Furthermore, there might potentially be selection bias from non-random pregnancy loss before 11 weeks. However, from week 12 and onward, inclusion of nearly all eligible pregnancies and all diagnosed malformations both prenatally and up to 1 year of age, as well as all pregnancy losses regardless of cause, reduced important selection bias. We cannot rule out surveillance bias as pregnant women with ADHD might be more likely to undergo more prenatal or postnatal examination. However, the conclusions concerning SCM are very robust, as these are unlikely to be undiagnosed. There is a possibility of misclassification of exposure, by requiring only 1 or more redeemed prescriptions during the first trimester. Even though sensitivity analyses with 2 or more redeemed prescriptions showed similar findings, and ADHD is expected to be a more chronic than periodic condition, we do not know if the women actually ingested the redeemed medication. These misclassifications will take the estimate toward the null hypothesis and cannot explain the associations between use of methylphenidate and VSD.
We found no association with malformations overall. Exposure to methylphenidate was associated with an increased risk of cardiac malformations (PR = 1.65, NNH = 92), in particular VSDs. Septum defects were represented in 10 of 12 exposed cases with cardiac malformations. The result on SCM was based on few cases, and the 2-fold increase needs to be corroborated in future studies. We believe that the results add important information regarding safety of ADHD medication given the rapidly increasing use of ADHD medications during pregnancy and among women of reproductive age who may become pregnant inadvertently.
Submitted: May 11, 2020; accepted August 31, 2020.
Published online: January 5, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Drs Kolding, Ehrenstein, L. Pedersen, Sandager, Petersen, Uldbjerg, and L. H. Pedersen have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This study was funded by the University of Aarhus Denmark, Director Jacob Madsen and wife Olga Madsen’s Foundation, Ove William Buhl Olesen and Mrs Edith Buhl Olesen Memorial Foundation, Carl and Ellen Hertz’s scholarship, Professor Ann Tabor’s Foundation, the Solar Foundation of 1978, and Health Research Fund of Central Denmark Region. Dr Petersen holds a professorship funded by Novo Nordisk Foundation grant NNFSA170030576.
Role of the sponsor: The funders had no role in the design, analysis, interpretation, or publication of this study.
Acknowledgments: The authors thank the Danish Fetal Medicine Study Group. The Danish Fetal Medicine Study Group reports data to the database and has approved the study.
Additional information: Ethical approval: this study received the required approvals from the Danish Data Protection Agency (Aarhus University J.nr. 2016-051-000001/KEA-2017-24), from the Research Committee of the Danish Fetal Medicine Database, and from the Board of the Danish Health Service Prescription Database.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1.Haervig KB, Mortensen LH, Hansen AV, et al. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014;23(5):526-533. PubMed CrossRef
2.Ashton H, Gallagher P, Moore B. The adult psychiatrist’s dilemma: psychostimulant use in attention deficit/hyperactivity disorder. J Psychopharmacol. 2006;20(5):602-610. PubMed CrossRef
3.Jiang HY, Zhang X, Jiang CM, et al. Maternal and neonatal outcomes after exposure to ADHD medication during pregnancy: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2019;28(3):288-295. PubMed CrossRef
4.Huybrechts KF, Bröms G, Christensen LB, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the International Pregnancy Safety Study Consortium. JAMA Psychiatry. 2018;75(2):167-175. PubMed CrossRef
5.Svensson E, Ehrenstein V, Nørgaard M, et al. Estimating the proportion of all observed birth defects occurring in pregnancies terminated by a second-trimester abortion. Epidemiology. 2014;25(6):866-871. PubMed CrossRef
6.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563-591. PubMed CrossRef
7.Ekelund CK, Kopp TI, Tabor A, et al. The Danish Fetal Medicine Database. Clin Epidemiol. 2016;8:479-483. PubMed CrossRef
8.Pedersen LH, Petersen OB, Nørgaard M, et al. Linkage between the Danish National Health Service Prescription Database, the Danish Fetal Medicine Database, and other Danish registries as a tool for the study of drug safety in pregnancy. Clin Epidemiol. 2016;8:91-95. PubMed CrossRef
9.Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. PubMed CrossRef
10.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7 suppl):54-57. PubMed CrossRef
11.Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320-323. PubMed
12.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, et al. Existing data sources for clinical epidemiology: the Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303-313. PubMed CrossRef
13.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. PubMed CrossRef
14.Jimenez-Solem E, Andersen JT, Petersen M, et al. Exposure to selective serotonin reuptake inhibitors and the risk of congenital malformations: a nationwide cohort study. BMJ Open. 2012;2(3):e001148. PubMed CrossRef
15.European Surveillance of Congenital Anomalies. https://eu-rd-platform.jrc.ec.europa.eu/eurocat/data-collection/guidelines-for-data-registration_en. Accessed February 25, 2019.
16.Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9(2):175-196. CrossRef
17.Desai RJ, Rothman KJ, Bateman BT, et al. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249-257. PubMed CrossRef
18.Diav-Citrin O, Shechtman S, Arnon J, et al. Methylphenidate in pregnancy: a multicenter, prospective, comparative, observational study. J Clin Psychiatry. 2016;77(9):1176-1181. PubMed CrossRef
19.Bro SP, Kjaersgaard MI, Parner ET, et al. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015;7:139-147. PubMed CrossRef
20.Pottegård A, Hallas J, Andersen JT, et al. First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry. 2014;75(1):e88-e93. PubMed CrossRef
21.Cohen JM, Hernández-Díaz S, Bateman BT, et al. Placental complications associated with psychostimulant use in pregnancy. Obstet Gynecol. 2017;130(6):1192-1201. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top