Objective: To evaluate efficacy, safety, and tolerability of brexpiprazole adjunctive to antidepressant treatments (ADTs) in patients with major depressive disorder (as defined by DSM-IV-TR criteria) with inadequate response to ADTs.
Method: Patients still depressed despite 1-3 prior ADTs followed by 8 weeks of prospective physician-determined, open-label ADT were randomized (1:1:1) to double-blind brexpiprazole 3 mg/d, brexpiprazole 1 mg/d, or placebo for 6 weeks. The primary efficacy end point was change in Montgomery-Asberg Depression Rating Scale (MADRS) total score from baseline to week 6. The key secondary efficacy end point was change in Sheehan Disability Scale mean score. The Hochberg procedure corrected for multiplicity. The efficacy population comprised all patients who had ≥ 1 dose of study drug with baseline and ≥ 1 postrandomization MADRS scores; the efficacy population per final protocol consisted of efficacy population patients meeting amended criteria for inadequate response throughout the 8-week prospective ADT. The study was conducted between June 2011 and September 2013.
Results: In the efficacy population per final protocol, brexpiprazole 3 mg (n = 213) showed a greater improvement in MADRS total score versus placebo (n = 203; −8.29 vs −6.33; P = .0079), whereas brexpiprazole 1 mg did not (n = 211; −7.64 vs −6.33; P = .0737). The brexpiprazole groups showed comparable improvement in SDS mean score versus placebo (least squares [LS] mean difference: [1 mg] −0.49, P = .0158; [3 mg] −0.48, P = .0191). The most frequent adverse events were akathisia (4.4%, 13.5%, 2.3%), headache (9.3%, 6.1%, 7.7%), and weight increase (6.6%, 5.7%, 0.9%) in brexpiprazole 1-mg, 3-mg, and placebo groups, respectively. Mean changes from baseline in Abnormal Involuntary Movement Scale (LS mean difference = 0.08, P = .0141) and Barnes Akathisia Rating Scale (LS mean difference = 0.17, P = .0001) total scores were significantly greater with brexpiprazole 3 mg versus placebo.
Conclusions: Brexpiprazole 3 mg demonstrated efficacy versus placebo in the efficacy population per final protocol. Both doses of brexpiprazole were well tolerated.
Trial Registration: ClinicalTrials.gov identifier: NCT01360632
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
See article by Thase et al
ABSTRACT
Objective: To evaluate efficacy, safety, and tolerability of brexpiprazole adjunctive to antidepressant treatments (ADTs) in patients with major depressive disorder (as defined by DSM-IV-TR criteria) with inadequate response to ADTs.
Method: Patients still depressed despite 1-3 prior ADTs followed by 8 weeks of prospective physician-determined, open-label ADT were randomized (1:1:1) to double-blind brexpiprazole 3 mg/d, brexpiprazole 1 mg/d, or placebo for 6 weeks. The primary efficacy end point was change in Montgomery-Asberg Depression Rating Scale (MADRS) total score from baseline to week 6. The key secondary efficacy end point was change in Sheehan Disability Scale mean score. The Hochberg procedure corrected for multiplicity. The efficacy population comprised all patients who had ≥ 1 dose of study drug with baseline and ≥ 1 postrandomization MADRS scores; the efficacy population per final protocol consisted of efficacy population patients meeting amended criteria for inadequate response throughout the 8-week prospective ADT. The study was conducted between June 2011 and September 2013.
Results: In the efficacy population per final protocol, brexpiprazole 3 mg (n = 213) showed a greater improvement in MADRS total score versus placebo (n = 203; −8.29 vs −6.33; P = .0079), whereas brexpiprazole 1 mg did not (n = 211; −7.64 vs −6.33; P = .0737). The brexpiprazole groups showed comparable improvement in SDS mean score versus placebo (least squares [LS] mean difference: [1 mg] −0.49, P = .0158; [3 mg] −0.48, P = .0191). The most frequent adverse events were akathisia (4.4%, 13.5%, 2.3%), headache (9.3%, 6.1%, 7.7%), and weight increase (6.6%, 5.7%, 0.9%) in brexpiprazole 1-mg, 3-mg, and placebo groups, respectively. Mean changes from baseline in Abnormal Involuntary Movement Scale (LS mean difference = 0.08, P = .0141) and Barnes Akathisia Rating Scale (LS mean difference = 0.17, P = .0001) total scores were significantly greater with brexpiprazole 3 mg versus placebo.
Conclusions: Brexpiprazole 3 mg demonstrated efficacy versus placebo in the efficacy population per final protocol. Both doses of brexpiprazole were well tolerated.
Trial Registration: ClinicalTrials.gov identifier: NCT01360632
J Clin Psychiatry 2015;76(9):1232-1240
dx.doi.org/10.4088/JCP.14m09689
© Copyright 2015 Physicians Postgraduate Press, Inc.
aPerelman School of Medicine of the University of Pennsylvania, Philadelphia, Pennsylvania
bOtsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey
cH. Lundbeck A/S, Valby, Copenhagen, Denmark
*Corresponding author: Michael E. Thase, MD, Department of Psychiatry, Perelman School of Medicine of the University of Pennsylvania, 3535 Market St, Philadelphia, PA 19104 ([email protected]).
Notice of correction: As of September 17, 2015, the CGI-I Responder row in Table 2 has been corrected.
Major depressive disorder (MDD) is one of the world’s great public health problems. It has an estimated prevalence of 4.7%,1 and, in 2010, MDD was the second highest cause of years lived with disability.2 Furthermore, MDD has considerable economic impact,3 especially among patients not responding to treatment,4,5 causing loss of productivity and increased mortality.3
Although numerous antidepressant treatments (ADTs) are available, a significant minority of people suffering from MDD do not respond to first-line therapies.6,7 Treatment options following inadequate ADT response include changing to another ADT—either within the same class or in another ADT class—or augmenting ADT with another medication, such as a second-generation antipsychotic.6,8 In the United States, adjunctive aripiprazole and quetiapine are currently approved in MDD, while olanzapine combined with fluoxetine is approved for patients with treatment-resistant depression. Although efficacy has been established in many randomized controlled trials, tolerability profiles of these agents limit their clinical use.9,10 Side effects vary between medications but most commonly include akathisia for aripiprazole, increased appetite/weight gain for olanzapine-fluoxetine combination, and excessive sedation for quetiapine.11 Weight gain can be a particularly ominous side effect because it increases the lifetime risk for other metabolic abnormalities; extrapyramidal symptoms (EPS) are similarly associated with some eventual risk of tardive dyskinesia. Therefore, effective adjunctive treatments with an improved tolerability profile for patients who do not respond adequately to ADT monotherapy are needed.
Brexpiprazole is a rationally designed serotonin-dopamine activity modulator, with partial agonism at serotonin 5-HT1A and dopamine D2 receptors at similar potency and potent antagonism at 5-HT2A and norepinephrine α1B and α2C receptors. Brexpiprazole shows partial agonism at the D2 receptor with lower intrinsic activity than aripiprazole, suggesting a comparably lower potential to induce D2 agonist-mediated adverse effects, eg, akathisia. Furthermore, brexpiprazole has moderate affinity, relative to D2/5HT1A receptor affinity, for histamine H1 receptors, which may result in lower levels of sedation than other antipsychotic agents.12 Preclinical data suggest that brexpiprazole has therapeutic potential as an antipsychotic and as adjunctive treatment for MDD.13,14
Efficacy of adjunctive brexpiprazole (2 mg/d) was demonstrated in a phase 3 study (Pyxis; ClinicalTrials.gov identifier: NCT01360645) in patients with MDD and inadequate ADT response.15 The objective of this second phase 3 study (Polaris; ClinicalTrials.gov identifier: NCT01360632) was to evaluate efficacy, safety, and tolerability of brexpiprazole 1 mg/d and 3 mg/d in patients with MDD and inadequate response to ADTs.
METHOD
Patients
Adult outpatients aged 18-65 years were enrolled at 92 centers in the United States (71.7% of patients), Germany (9.2%), Ukraine (5.9%), Russia (5.7%), Hungary (3.5%), Canada (2.7%), and Romania (1.3%). Patients were diagnosed with a single or recurrent nonpsychotic episode of MDD according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria16 of at least 8 weeks’ duration. During the current episode, patients must have had inadequate response, defined as < 50% reduction in Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (ATRQ) score17 to an adequate trial of 1-3 ADTs. Eligible patients had 17-item Hamilton Depression Rating Scale (HDRS-17)18,19 total scores ≥ 18 at screening and at start of the prospective treatment phase. Key exclusion criteria and concomitant medication regulations are in eAppendix 1 (available at Psychiatrist.com).
The study was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice Consolidated Guideline. The protocol was approved by independent ethics committees, and all patients provided written informed consent.
Study Design
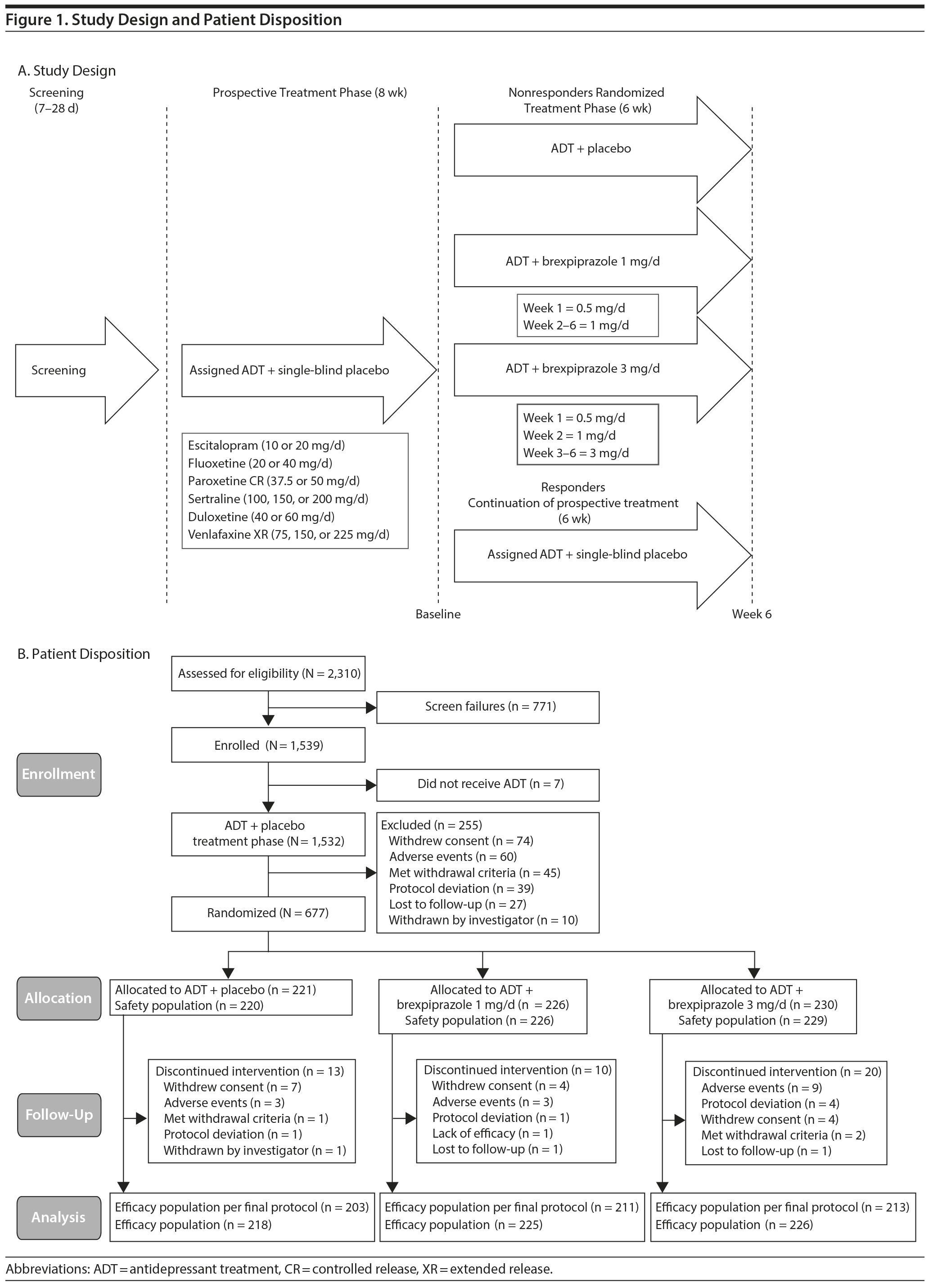
This randomized, double-blind, placebo-controlled, multicenter phase 3 study was conducted between June 2011 and September 2013 and comprised a 7- to 28-day screening phase; an 8-week single-blind, prospective treatment phase; and a 6-week double-blind, randomized treatment phase (Figure 1A).

- Availability of more effective and better tolerated antidepressant treatments remains a significant unmet need for patients with major depressive disorder (MDD); clinical use of adjunctive second-generation antipsychotics can be limited by their tolerability profiles.
- Adjunctive brexpiprazole 3 mg improved depressive symptoms compared with antidepressant monotherapy in patients with MDD and inadequate response to antidepressant treatment.
- Brexpiprazole was well tolerated in this population.
During the 8-week prospective treatment phase, patients received single-blind placebo adjunctive to a physician-determined open-label ADT from the following list: escitalopram, fluoxetine, paroxetine controlled release, sertraline, duloxetine, and venlafaxine extended release (XR). A minimum 24-hour washout period was applied if necessary. Antidepressant treatment was titrated to the maximum-tolerated dose to optimize the potential for response.
Following the prospective treatment phase, patients were eligible for entry into the double-blind randomized treatment phase if they had inadequate prospective ADT response, defined as < 50% reduction in HDRS-17 total score between baseline and end of the prospective phase, with an HDRS-17 total score of ≥ 14 and a Clinical Global Impressions-Improvement scale (CGI-I)20 score of ≥ 3 at the end of the prospective phase. While this study was ongoing, additional analyses were performed on data from a completed phase 2 study of similar design (reference 21 and data on file, Otsuka, Princeton, New Jersey). It was found that a small number of patients in that study had seemingly adequate improvement in Montgomery-Asberg Depression Rating Scale (MADRS)22,23 and CGI-I scores at various times during the prospective treatment period, but subsequent worse scores at time of randomization. These patients did not show a consistent lack of response and would have been considered adequate responders if evaluated at another time point during the prospective phase. A number of these patients showed significant improvement again during the randomized phase, even if continuing on ADT alone. In order to exclude patients with seemingly variable response to ADT, this study’s protocol was amended in March 2012 during the enrollment phase and prior to database lock to specify that patients had to meet more refined inadequate response criteria throughout prospective treatment (HDRS-17 score ≥ 14, < 50% reduction from baseline in HDRS-17 as well as < 50% reduction in MADRS total score between start of prospective treatment and each scheduled visit, and CGI-I score ≥ 3 at each scheduled visit) to be eligible for randomization. The investigator was also blinded to the revised criteria. Both the protocol amendment and the resulting primary analysis were discussed and agreed with the relevant regulatory authorities (US Food and Drug Administration). Eligible patients were randomized (1:1:1) to receive double-blind brexpiprazole 1 mg, brexpiprazole 3 mg, or placebo for 6 weeks adjunctive to continued stable-dose ADT. An interactive voice or web response system was used for assigning treatments using a fixed-block, computer-generated randomized schedule stratified by study center. Study visits took place weekly during double-blind treatment.
Outcome Measures
Efficacy assessments were made at baseline and during the double-blind treatment phase. The primary efficacy variable was MADRS total score and was measured at each weekly study visit. The key secondary efficacy variable was Sheehan Disability Scale (SDS)24,25 mean score and individual score items: family life, social life, and work/school, measured at the study visits at weeks 3 and 6. Other secondary variables were Clinical Global Impressions-Severity of Illness scale (CGI-S),20 Inventory of Depressive Symptomatology-Self-Report (IDS-SR),26 and CGI-I, which were measured at each weekly study visit. The HDRS-17 and Hamilton Anxiety Rating Scale (HARS)19,20,27 were measured at week 6 only.
Treatment-emergent adverse events (TEAEs), incidence, and severity were recorded by the investigator at each study visit. All adverse events were coded by preferred term using Medical Dictionary for Regulatory Activities (MedDRA) Version 15.0 (http://www.meddra.org/). EPS-related adverse events were defined as generalized rigidity, hyperkinesia, bradykinesia, akinesia, dystonia, akathisia, tremor, flexed posture, involuntary muscle contractions, athetosis, and chorea. Extrapyramidal symptoms were also evaluated by the Simpson-Angus Scale (SAS),26,28 Abnormal Involuntary Movement Scale (AIMS),20 and Barnes Akathisia Rating Scale (BARS)29 administered at baseline and at all study visits in the double-blind phase. Vital signs, including body weight, were measured at each study visit. Clinical laboratory tests and a 12-lead electrocardiogram (ECG) were taken at baseline and weeks 2, 4, and 6 of the double-blind phase. Suicidal behavior and ideation was evaluated by responses to the Columbia-Suicide Severity Rating Scale (C-SSRS)30,31 administered at each study visit. Patients completed the Massachusetts General Hospital Sexual Functioning Questionnaire (MSFQ)32 at baseline at and week 6.
Data Analysis
Full details of the data analysis are provided in eAppendix 1 (available at Psychiatrist.com).
The sample-size calculation was based on an expected “clinically significant” effect on the primary efficacy variable in the 2 active drug arms compared with placebo. On the basis of a previous phase 2 trial (reference 21 and data on file, Otsuka, Princeton, New Jersey), 603 evaluable patients (201 patients/arm) were required to detect with 90% power a between-group difference of 3.0 (SD = 8.5) in mean change from baseline to week 6 MADRS total score, at a 2-tailed significance level of .025. To allow for 5%-10% of patients in the double-blind phase being nonevaluable (eg, by having missing data), a total of 660 patients (220 patients/arm) were planned for randomization.
The safety population comprised all randomized patients who received ≥ 1 dose of double-blind investigational drug. The efficacy population comprised all patients in the safety population who had an evaluation for MADRS total score at baseline (end of the prospective phase) and ≥ 1 evaluation after randomization. The efficacy population per final protocol included all patients from the efficacy population who met the revised randomization criteria for inadequate response. Data reported here are for the efficacy population per final protocol. Data for the efficacy population are given in Supplementary eTable 1. Analyses of patients meeting the amended criteria for inadequate response were prespecified in the statistical analysis plan.
Baseline was defined as the last available measurement prior to randomization. The primary efficacy end point was change in MADRS total score from baseline to week 6. The primary analysis was conducted by fitting a mixed model repeated-measures (MMRM) analysis with an unstructured variance covariance structure using change from baseline to week 6 in MADRS total score as the dependent variable based on the observed cases dataset. The model included fixed class effect terms for treatment, trial site, visit week, and an interaction term of treatment-by-visit week. Also included was an interaction term of baseline MADRS total score values by visit week as covariates. Comparisons between brexpiprazole 1 mg versus placebo and brexpiprazole 3 mg versus placebo were tested using Hochberg procedure to adjust for multiplicity and maintain type I error at .05 (2-tailed).
The key secondary efficacy end point was change in SDS mean score from baseline to week 6, analyzed by using the same MMRM model as in the primary efficacy analysis. A hierarchical testing procedure was used for the key secondary efficacy end point and the SDS individual item scores to control for multiplicity and maintain overall type I error at .05.
The other secondary end points were analyzed at a nominal .05 level.
RESULTS
Patients
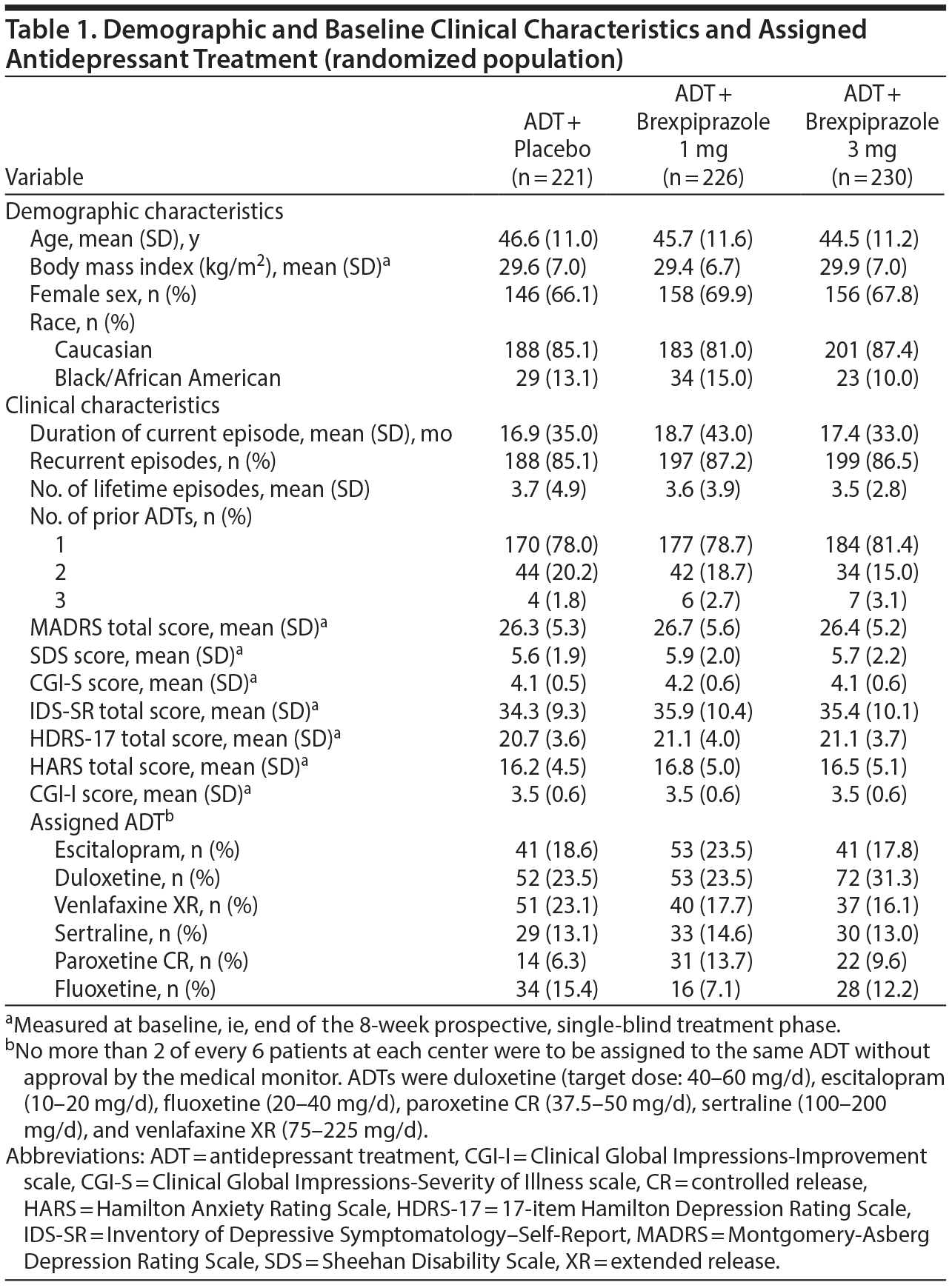
Six hundred seventy-seven patients were randomized to brexpiprazole 1 mg (n = 226), brexpiprazole 3 mg (n = 230), or placebo (n = 221) (Figure 1B). One patient from the brexpiprazole 3-mg group and 1 patient from the placebo group were randomized but did not receive study medication and were not included in the safety population. Six patients did not have valid assessments of MADRS score after randomization and were excluded from the efficacy population (brexpiprazole 1 mg, n = 1; brexpiprazole 3 mg, n = 3; placebo, n = 2). The efficacy population therefore consisted of 669 patients (brexpiprazole 1 mg, n = 225; brexpiprazole 3 mg, n = 226; placebo, n = 218). A further 42 patients did not meet revised criteria for persistent inadequate response according to the protocol amendment. Therefore, the efficacy population per final protocol consisted of 627 patients (brexpiprazole 1 mg, n = 211; brexpiprazole 3 mg, n = 213; placebo, n = 203). Of the randomized patients, 216/226 (95.6%) brexpiprazole 1-mg, 210/230 (91.3%) brexpiprazole 3-mg, and 208/221 (94.1%) placebo group patients completed the randomized treatment phase.
Baseline demographic and disease characteristics of the randomized population were similar between groups (Table 1). At the end of the prospective phase, mean MADRS total score was 26.5, indicating moderate depression.
Efficacy
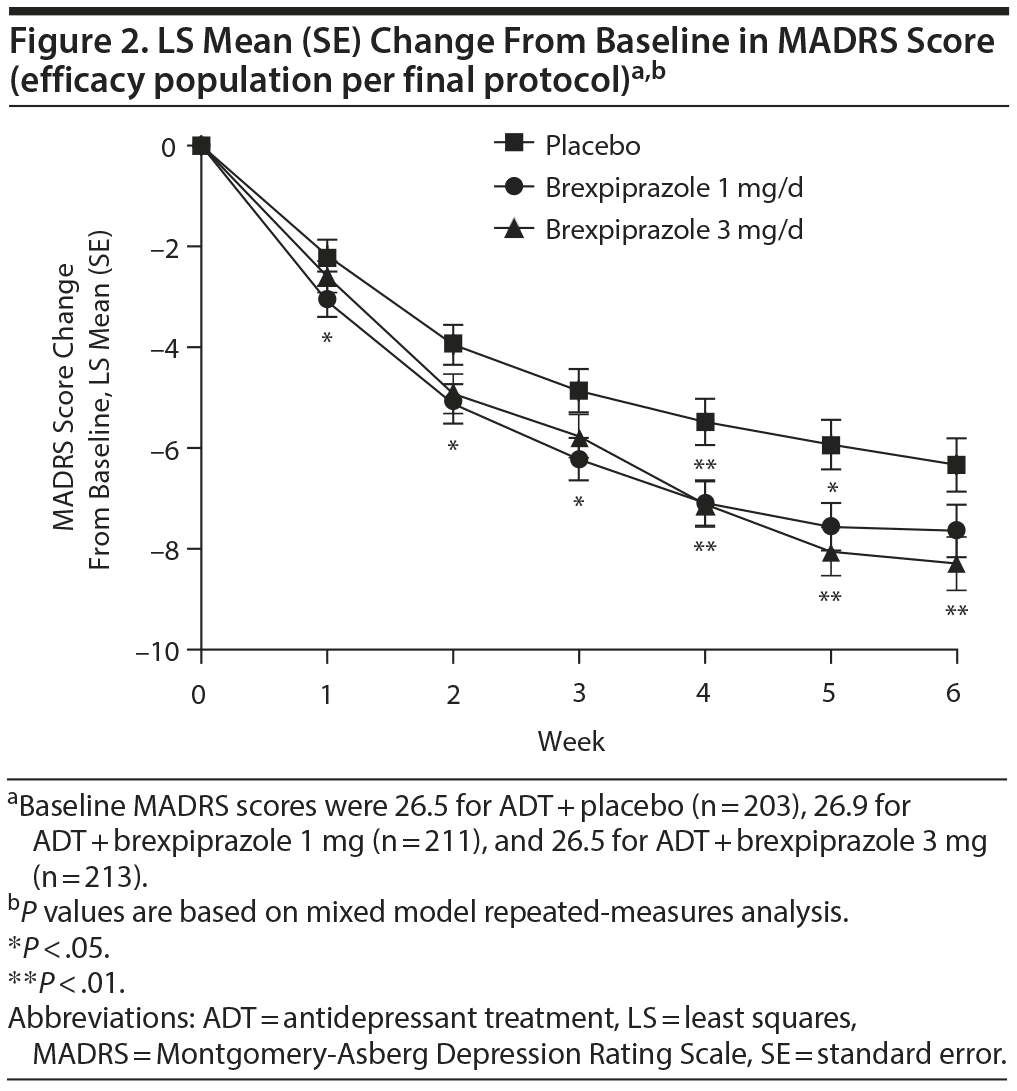
MADRS score (primary end point). In the efficacy population per final protocol, mean reduction from baseline to week 6 in MADRS total score for brexpiprazole 3 mg showed greater improvement (−8.29) compared with placebo (−6.33; least squares [LS] mean difference = −1.95; 95% CI, −3.39 to −0.51; P = .0079) (Figure 2). Mean change in MADRS total score for brexpiprazole 1 mg was −7.64 versus −6.33 for placebo (LS mean difference = −1.30; 95% CI, −2.73 to 0.13; P = .0737) (Figure 2).
Mean change in MADRS total score for the efficacy population also showed improvement for brexpiprazole 3 mg versus placebo (−1.52; 95% CI, −2.92 to −0.13; P = .0327) but did not reach the level of statistical significance required for multiple comparisons according to the prespecified statistical analysis. The mean improvement for brexpiprazole 1 mg versus placebo was less than that for 3 mg (−1.19; 95% CI, −2.58 to 0.20; P = .0925) (Supplementary eFigure 1).
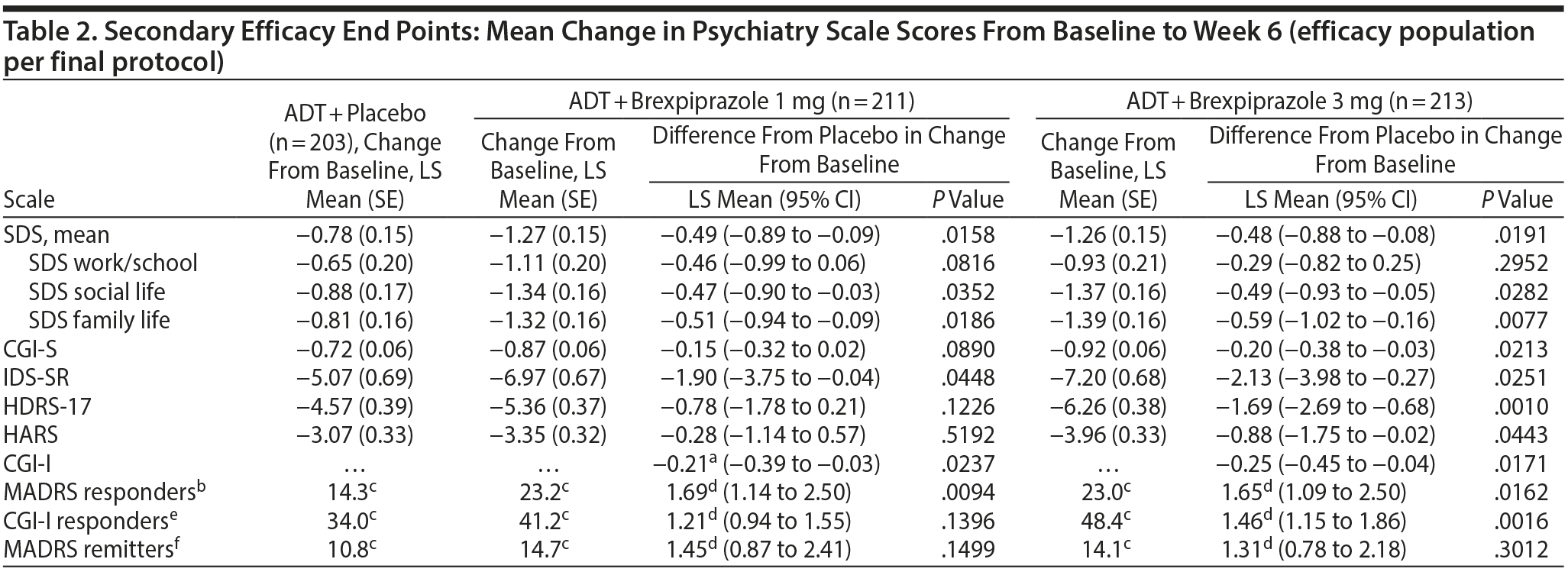
Secondary end points. In the efficacy population per final protocol, brexpiprazole 1 mg and 3 mg showed greater improvement than placebo for the key secondary efficacy parameter, SDS mean score (Table 2). Mean reductions from baseline to week 6 were greater for family life and social life for both doses of brexpiprazole versus placebo (Table 2). Brexpiprazole 1 mg showed greater efficacy than placebo (P < .05) on MADRS-defined response rate and CGI-I at week 6 (Table 2). Brexpiprazole 3 mg showed greater efficacy than placebo (P < .05) on MADRS-defined response rate, CGI-I-defined response rate, and CGI-I at week 6 and in mean change from baseline at week 6 in CGI-S, HDRS-17, HARS, and IDS-SR (Table 2).
Safety and Tolerability
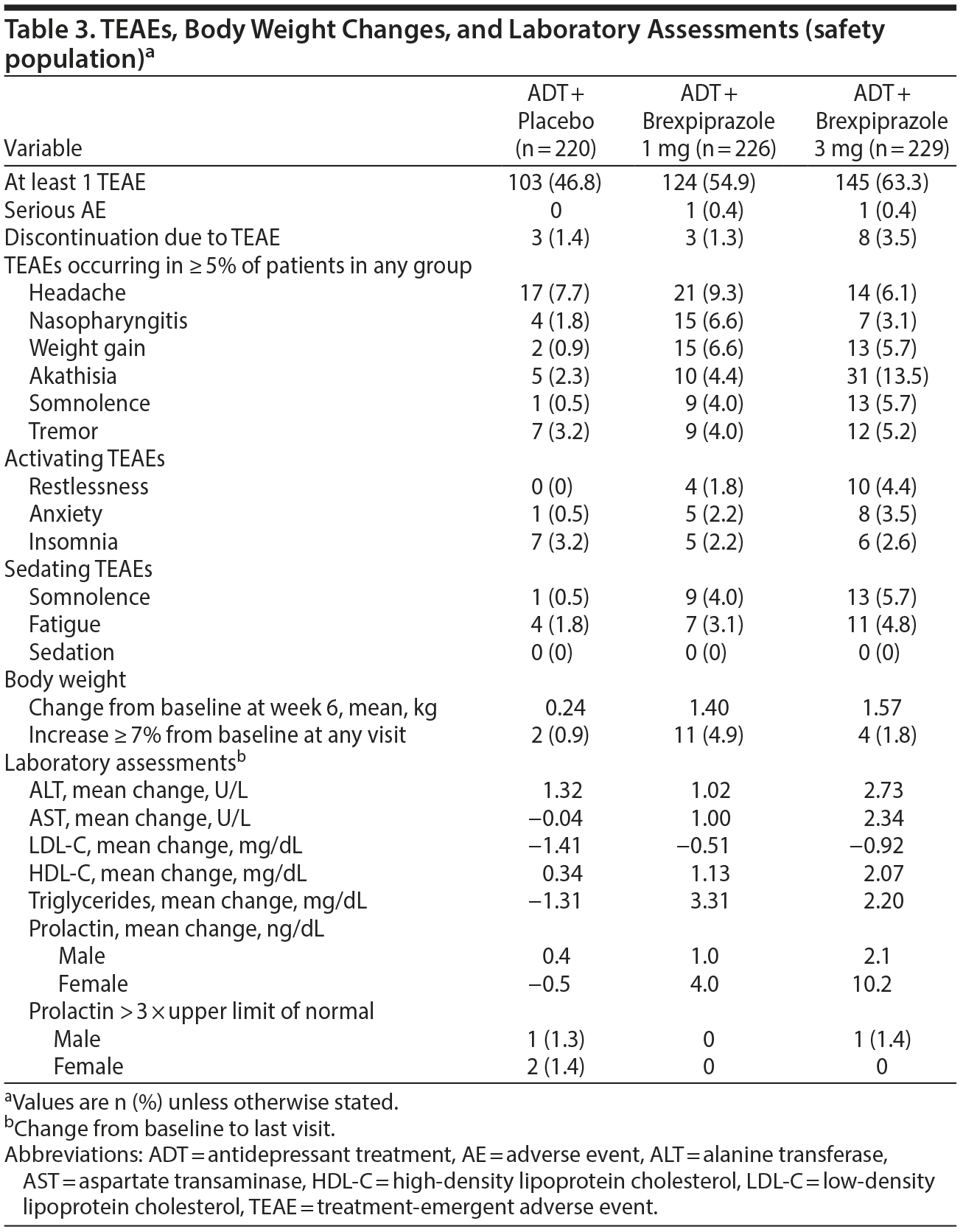
The most frequently (> 5%) reported TEAEs were headache, nasopharyngitis, and weight gain in the brexpiprazole 1-mg group and akathisia, headache, somnolence, weight gain, and tremor in the brexpiprazole 3-mg group (Table 3). Most TEAEs were considered mild-to-moderate severity by investigators. Activating TEAEs were infrequently reported: restlessness, 1.8% vs 4.4% vs 0%; anxiety, 2.2% vs 3.5% vs 0.5%; and insomnia, 2.2% vs 2.6% vs 3.2% in the brexpiprazole 1-mg, 3-mg, and placebo groups, respectively. Sedating TEAEs such as somnolence (4.0% vs 5.7% vs 0.5%), fatigue (3.1% vs 4.8% vs 1.8%), and sedation (0% vs 0% vs 0%) were also infrequent (Table 3).
Fourteen patients discontinued due to TEAEs; 5 patients in the 3-mg brexpiprazole group discontinued due to akathisia (Table 3). Serious TEAEs were reported by 1 patient taking brexpiprazole 1 mg (pneumonia) and 1 patient taking brexpiprazole 3 mg (epilepsy). There were no deaths and no reports of suicide or attempted suicide during the study.
Mean (SD) body weight increased from 83.1 (20.8) kg at baseline to 84.6 (21.0) kg in the brexpiprazole 1-mg group at week 6 (observed cases) and from 85.3 (21.6) kg to 85.8 (22.0) kg in the placebo group (LS mean gain: 1.40 kg vs 0.24 kg; LS mean difference: 1.17 kg, P < .0001). It increased from 84.6 kg to 87.0 kg in the 3-mg group (LS mean gain: 1.57 kg vs 0.24 kg for placebo; LS mean difference: 1.33 kg, P < .0001). Increased body weight ≥ 7% was seen at any visit in 11/225 (4.9%) brexpiprazole 1 mg, 4/228 (1.8%) brexpiprazole 3 mg, and 2/217 (0.9%) placebo patients.
With respect to laboratory tests, mean low-density lipoprotein cholesterol values decreased from baseline in all 3 groups, and there were no clinically relevant changes in levels of high-density lipoprotein cholesterol and triglycerides between treatment groups. There were small mean increases in prolactin level with brexpiprazole compared with placebo; no patients taking brexpiprazole 1 mg, 0.4% of patients taking brexpiprazole 3 mg, and 1.4% of placebo patients had prolactin levels > 3 times upper limit of normal. No clinically meaningful effects were observed for liver parameters (alanine transaminase and aspartate transaminase). No meaningful differences between brexpiprazole groups and placebo were seen in ECGs and vital signs.
Extrapyramidal symptom rating scales showed small increases in the brexpiprazole 3-mg group during the double-blind phase. Statistically significant mean changes from baseline to last visit were recorded for brexpiprazole 3 mg versus placebo for AIMS total score (0.08 versus 0.00; LS mean difference = 0.08; P = .0141) and for BARS total score (0.18 versus 0.01; LS mean difference = 0.17; P = .0001). Mean change from baseline to last visit for brexpiprazole 3 mg versus placebo in SAS total score was 0.12 versus 0.00 (LS mean difference = 0.13, P = .0529). The incidence of EPS-related TEAEs was higher in the brexpiprazole groups versus placebo: most frequently akathisia (1 mg, 4.4%; 3 mg, 13.5%; placebo, 2.3%) and tremor (1 mg, 4.0%; 3 mg, 5.2%; placebo, 3.2%).
No suicidal behavior was reported in the C-SSRS during the double-blind treatment phase. Incidence of emergent suicidal ideation was similar in all treatment groups (1 mg, 4.0%; 3 mg, 2.6%; placebo, 6.4%). One patient taking brexpiprazole 1 mg reported a TEAE of suicide ideation during the treatment phase.
Mean MSFQ scores suggested that sexual function was near normal at baseline and improved slightly in all treatment groups during the double-blind phase. Mean change in MSFQ overall sexual satisfaction scores for brexpiprazole 1 mg, 3 mg, and placebo were −0.28, −0.46, and −0.27, respectively (brexpiprazole 3 mg vs placebo indicated an improvement: −0.19, P = .0561).
DISCUSSION
In this study, adjunctive brexpiprazole 3 mg improved depressive symptoms, as measured by MADRS, compared with ADT monotherapy in patients with MDD and inadequate response to standard ADTs. Primary end point results were supported by nominal improvements in several secondary efficacy end points (SDS mean score, CGI-S, IDS-SR, HDRS-17, HARS, CGI-I, MADRS response rate, and CGI-I response rate). The efficacy outcomes for the group that received brexpiprazole 1 mg tended to be intermediate, and the outcome was not statistically significant. SDS total mean score was improved with brexpiprazole 3 mg and 1 mg versus placebo. The individual SDS items of social life and family were also improved with brexpiprazole; however, work/school was not.
The final study protocol included a more robust definition of inadequate response. During the enrollment phase and prior to database lock, the protocol was amended to introduce an additional criterion (< 50% reduction in MADRS total score) to determine inadequate response to prospective ADT. Furthermore, patients were required to meet the definition of inadequate response at each visit, rather than at only the final visit of the prospective treatment phase. These amendments resulted in randomization of a population that is more relevant to clinical practice, where clinicians rely on broad experience with a particular patient and the recent disease course to judge response to treatment rather than severity scale scores during a single consultation. This is also consistent with the American Psychiatric Association Guidelines for MDD recommendation of evaluating 4-8 weeks of treatment.33 Blinding the response data aimed to reduce potential bias to further improve generalizability of the data. The efficacy population per final protocol had 42 fewer patients than the efficacy population but more significant P values in many end points, implying a more distinct effect could be observed with this better-defined population.
Some have suggested that a clinically relevant change requires a difference of at least 2 points over placebo in MADRS total score; the data in this study are consistent with that hypothesis (1.95-point reduction).34,35 In the current study, mean reductions in MADRS total score for brexpiprazole 3 mg were comparable with that in the Pyxis study of brexpiprazole 2 mg.15 However, given the long duration of current depressive episode (17.6 months), this change may be more meaningful, since the population appeared to have a prolonged period of inadequate response to ADT; certainly other adjunctive studies have excluded patients with > 12 months in the current episode.36,37
Previous studies have evaluated the efficacy of adjunctive aripiprazole38-40 and quetiapine XR36,37 for the treatment of patients with MDD and inadequate response to ADT. However, direct comparisons between studies of different agents must be made cautiously due to methodological differences. For example, fixed doses of brexpiprazole and quetiapine XR were evaluated, while aripiprazole was dosed flexibly. There are also limitations inherent in comparing MDD studies conducted at different points in time, particularly since the placebo response rate has been observed to increase over time.41,42 Unlike the aripiprazole studies, the brexpiprazole and quetiapine XR studies had 3 treatment arms, which may have influenced the placebo response rate. Nevertheless, the absolute reductions in MADRS total score observed with adjunctive brexpiprazole (8.3; placebo, 6.4) and aripiprazole (8.5 to 10.1; placebo 5.7 to 6.4)38-40 are broadly similar. In the studies of quetiapine XR, reductions in MADRS total score versus placebo at the 300-mg dose were 15.0 versus 12.236 and 14.7 versus 11.737; however, it should be noted that these studies did not select patients on the basis of inadequate response to a prospective ADT phase.
In this trial, both doses of brexpiprazole were reasonably well tolerated, and there were few discontinuations due to TEAEs. The TEAE profile was consistent with that observed in Pyxis.15 It has been hypothesized that the unique receptor binding profile for brexpiprazole at 5-HT1A, D2 5-HT2A, α1B, and α2C receptors makes brexpiprazole a more suitable choice for adjunctive treatment of MDD than currently approved products. Sedation and somnolence rates are low compared with those reported for quetiapine, and magnitude of weight gain is low compared with that reported for olanzapine-fluoxetine combination.11 Mean changes from baseline in EPS rating scale scores were small in all treatment groups. Although akathisia was reported at 13.5% for brexpiprazole 3 mg, the incidence was dose-related and is still substantially lower than that reported in similar studies of adjunctive aripiprazole.11,43 Neuroleptic malignant syndrome (NMS) and tardive dyskinesia (TD) are rare but serious events that may occur with administration of second-generation antipsychotics.9 There were no cases of NMS or TD observed in this study. Thus, the tolerability profile of brexpiprazole observed in the current study and Pyxis15 confirms the tolerability anticipated from its pharmacologic profile.
Limitations of the study included the relatively short double-blind treatment phase duration and lack of active comparator. We also note that the response rate for the ADT monotherapy group was higher than that in Pyxis; higher placebo responses are a recognized factor in limiting signal detection in controlled studies of antidepressant efficacy.42 Once short-term efficacy is established, it will be important to evaluate long-term efficacy and safety of brexpiprazole, particularly to try to identify the optimal duration of adjunctive therapy.
In conclusion, adjunctive brexpiprazole 3 mg demonstrated efficacy in this randomized, placebo-controlled phase 3 study, based on the efficacy population per final protocol, which included only patients who had inadequate response throughout the 8 weeks of prospective ADT. Both doses of adjunctive brexpiprazole are well tolerated in patients with MDD and inadequate response to antidepressant therapy.
Drug names: aripiprazole (Abilify and others), duloxetine (Cymbalta and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), olanzapine (Zyprexa and others), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel and others), sertraline (Zoloft and others).
Submitted: November 26, 2014; accepted May 14, 2015.
Online first: August 4, 2015.
Potential conflicts of interest: Dr Thase has received grants from Agency for Healthcare Research and Quality, Alkermes, Forest, National Institute of Mental Health, Otsuka, PharmaNeuroboost and Roche; has acted as an advisor or consultant for Alkermes, AstraZeneca, Bristol-Myers Squibb, Cerecor, Eli Lilly, Forest, Gerson Lehman Group, GlaxoSmithKline, Guidepoint Global, Lundbeck, MedAvante, Merck, Neuronetics, Novartis, Ortho-McNeil, Otsuka, Pamlab, Pfizer, Shire, Sunovion, and Takeda; has received royalties from American Psychiatric Association, Guilford Publications, Herald House, and W. W. Norton & Company; and holds equity in MedAvante Inc. Dr Thase’s spouse is an employee of Peloton Advantage. Drs Youakim, Skuban, Hobart, Zhang, McQuade, Nyilas, Carson, and Sanchez are employees of Otsuka Pharmaceutical Development & Commercialization. Dr Eriksson is an employee of H. Lundbeck A/S.
Funding/support: Funding for this study was provided by Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark).
Role of sponsors: The sponsors were responsible for the study design and conduct and the collection, management, analysis, and interpretation of the data. The authors, some of whom are employed by the sponsors, were also responsible for writing and reviewing this article. All authors approved the final version.
Acknowledgment: Sally Cotterill, PhD (QXV Communications, Macclesfield, United Kingdom) provided writing support, which was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark).
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. Ferrari AJ, Somerville AJ, Baxter AJ, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43(3):471-481. PubMed doi:10.1017/S0033291712001511
2. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease study 2010. PLoS Med. 2013;10(11):e1001547. PubMed doi:10.1371/journal.pmed.1001547
3. Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1-14. PubMed doi:10.1016/j.psc.2011.11.005
4. Luppa M, Heinrich S, Angermeyer MC, et al. Cost-of-illness studies of depression: a systematic review. J Affect Disord. 2007;98(1-2):29-43. PubMed doi:10.1016/j.jad.2006.07.017
5. Olchanski N, McInnis Myers M, Halseth M, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512-522. PubMed doi:10.1016/j.clinthera.2012.09.001
6. Connolly KR, Thase ME. If at first you don’ t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71(1):43-64. PubMed doi:10.2165/11587620-000000000-00000
7. Möller HJ. Outcomes in major depressive disorder: the evolving concept of remission and its implications for treatment. World J Biol Psychiatry. 2008;9(2):102-114. PubMed doi:10.1080/15622970801981606
8. Fleurence R, Williamson R, Jing Y, et al. A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacol Bull. 2009;42(3):57-90. PubMed
9. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991. PubMed doi:10.1176/appi.ajp.2009.09030312
10. Wright BM, Eiland EH 3rd, Lorenz R. Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy. 2013;33(3):344-359. PubMed doi:10.1002/phar.1204
11. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed doi:10.1371/journal.pmed.1001403
12. Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. PubMed doi:10.1124/jpet.114.213793
13. Amada N, Maeda K, Akazawa H, et al. Brexpiprazole, a novel serotonin-dopamine activity modulator: in vivo evaluation of its antipsychotic-like profile. Society of Biological Psychiatry 69th Annual Meeting; May 8-10, 2014; New York, NY. Abstract P410.
14. Hirose T, Maeda K, Stensbøl TB, et al. Synergistic effects of brexpiprazole with SSRI/SNRI/diazepam on forced swim test and marble burying behaviour in mice. Biol Psychiatry. 2014;75(suppl 1):132S.
15. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 2015;76(00):00-00.
16. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
17. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. PubMed doi:10.1111/j.1755-5949.2009.00102.x
18. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
19. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278-296. PubMed doi:10.1111/j.2044-8260.1967.tb00530.x
20. Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976:218-222.
21. Thase ME, Fava M, Hobart M, et al. Efficacy of adjunctive OPC-34712 across multiple outcome measures in major depressive disorder: a phase II, randomized, placebo-controlled study. Neuropsychopharmacology. 2011;36:S302-S304.
22. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
23. Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52-58. PubMed doi:10.1192/bjp.bp.106.032532
24. Sheehan DV. The Anxiety Disease. New York, NY: Charles Scribner & Sons; 1983.
25. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed doi:10.1097/00004850-199606003-00015
26. Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. PubMed doi:10.1017/S0033291700035558
27. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
28. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;45(S212):11-19. PubMed doi:10.1111/j.1600-0447.1970.tb02066.x
29. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed doi:10.1192/bjp.154.5.672
30. Chappell P, Feltner DE, Makumi C, et al. Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale. Am J Psychiatry. 2012;169(6):662-663, author reply 663. PubMed doi:10.1176/appi.ajp.2012.12010123
31. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed doi:10.1176/appi.ajp.2011.10111704
32. Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70(4):221-225. PubMed doi:10.1159/000056257
33. Thase ME, Gelenberg AJ, Freeman MP, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, 3rd ed. Arlington, VA: American Psychiatric Association; 2010.
34. Duru G, Fantino B. The clinical relevance of changes in the Montgomery-Asberg Depression Rating Scale using the minimum clinically important difference approach. Curr Med Res Opin. 2008;24(5):1329-1335. PubMed doi:10.1185/030079908X291958
35. Montgomery SA, Möller HJ. Is the significant superiority of escitalopram compared with other antidepressants clinically relevant? Int Clin Psychopharmacol. 2009;24(3):111-118. PubMed doi:10.1097/YIC.0b013e32832a8eb2
36. Bauer M, Pretorius HW, Constant EL, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70(4):540-549. PubMed doi:10.4088/JCP.08m04629
37. El-Khalili N, Joyce M, Atkinson S, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2010;13(7):917-932. PubMed doi:10.1017/S1461145710000015
38. Berman RM, Marcus RN, Swanink R, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843-853. PubMed doi:10.4088/JCP.v68n0604
39. Berman RM, Fava M, Thase ME, et al. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14(4):197-206. PubMed
40. Marcus RN, McQuade RD, Carson WH, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156-165. PubMed doi:10.1097/JCP.0b013e31816774f9
41. Dunlop BW, Thase ME, Wun CC, et al. A meta-analysis of factors impacting detection of antidepressant efficacy in clinical trials: the importance of academic sites. Neuropsychopharmacology. 2012;37(13):2830-2836. PubMed doi:10.1038/npp.2012.153
42. Walsh BT, Seidman SN, Sysko R, et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840-1847. PubMed doi:10.1001/jama.287.14.1840
43. Thase ME, Trivedi MH, Nelson JC, et al. Examining the efficacy of adjunctive aripiprazole in major depressive disorder: a pooled analysis of 2 studies. Prim Care Companion J Clin Psychiatry. 2008;10(6):440-447. PubMed doi:10.4088/PCC.v10n0603
This PDF is free for all visitors!
Save
Cite