Abstract
Importance: Schizophrenia is a complex syndrome with taxing symptoms and for which treatment challenges remain. Current dopamine D2 receptor–blocking antipsychotics have well-known limitations, including ineffectively treating across all symptom domains and generating common side effects such as motor disturbances, weight gain, and metabolic dysfunction. New approaches are sorely needed to address the continued unmet treatment needs for individuals living with schizophrenia.
Observations: Although current antipsychotic drugs indicated for the treatment of schizophrenia interact with various neurotransmitter receptors, they all commonly act as dopamine D2 receptor antagonists or partial agonists. While antipsychotics primarily relieve positive symptoms, residual positive symptoms are still common, and management of negative symptoms and cognitive impairment remains an unmet need. Problematic side effects are common with current agents and can contribute to nonadherence. In addition to alterations in dopaminergic pathways, increasing evidence indicates that the pathophysiology of schizophrenia also includes dysfunction in other neurotransmitter systems including glutamate, acetylcholine, serotonin, and γ-aminobutyric acid. While the pathophysiology of schizophrenia is complex, treatments with novel pharmacologic actions that target these systems are of interest as adjunctive treatment for individuals with schizophrenia.
Conclusion and Relevance: An unmet need exists for effective treatment of all the core symptoms of schizophrenia. Novel antipsychotics with a nondopaminergic mechanism of action may be useful candidates for antipsychotic adjunctive treatment in people with schizophrenia who are showing inadequate responses, treatment resistance, or low tolerance to dopamine D2 receptor–blocking antipsychotics.
J Clin Psychiatry 2024;85(3):23nr15240
Author affiliations are listed at the end of this article.
Schizophrenia is a complex and chronic syndrome that affects approximately 24 million people worldwide1 and remains associated with considerable disability,1 premature mortality,2 and significant economic burden.3 The primary symptom domains of schizophrenia include positive symptoms such as hallucinations and delusions, negative symptoms such as social withdrawal, and cognitive dysfunction. Approved treatments for schizophrenia are limited in their efficacy across symptom domains and are associated with side effects including motor symptoms, somnolence/sedation, weight gain, hormonal abnormalities, and metabolic dysregulation.4 When an individual living with schizophrenia does not have an adequate response to treatment, alternative strategies are often employed, including dose increases, switching to another antipsychotic drug, or combination with a second psychotropic medication, such as antipsychotics, antidepressants, anxiolytics, stimulants, and mood stabilizers.5–7 There are currently no approved pharmacologic combinations for the treatment of schizophrenia.
While the classical dopamine hypothesis of psychosis has been an enduring theory in schizophrenia, this hypothesis has been refined, with additional neurotransmitter systems implicated in the pathophysiology of schizophrenia.8 In addition to dopaminergic dysfunction, alterations in glutamate, γ-aminobutyric acid (GABA), serotonin, and acetylcholine neurotransmitter systems have been reported.9–13 All currently approved antipsychotics are dopamine D2 receptor antagonists or partial agonists, and many bind with varying affinities to other receptors, contributing to both their efficacy and side effect profiles.9,14 To date, strong evidence is lacking to support the combination of an antipsychotic drug with a second psychotropic medication for improved symptom reduction.7 Novel pharmacologic approaches are profoundly needed.
This article reviews existing pharmacologic treatments for schizophrenia and investigational antipsychotic and psychotropic agents with novel, non-D2 receptor mechanisms of action, with a focus on adjunctive or cotreatments for schizophrenia. In this article, the term “adjunctive treatment” is used to describe a therapy given to enhance the main or current treatment to maximize its effectiveness. This term is used interchangeably with similar terms such as “augmentation,” “add-on,” “combination treatment,” or “cotreatment” to reflect the term used in the cited literature. It is acknowledged that these terms are not necessarily synonymous in meaning and may, in some instances, denote 2 cotreatments given together to enhance the effectiveness of either given alone.
LIMITATIONS OF CURRENT ANTIPSYCHOTIC MEDICATIONS
Current antipsychotic medications address positive symptoms of schizophrenia without clear evidence of efficacy in controlling primary negative or cognitive symptoms, except for cariprazine and amisulpride in low doses.15 No or partial responses to antipsychotics are common. An analysis of 16 randomized, controlled trials showed that 67% of individuals with acute exacerbation of schizophrenia had ≤50% reduction in overall symptoms, and 20% had no reduction at all.16 Approximately 24%–30% of people with schizophrenia have treatment-resistant schizophrenia,17–19 defined as failure to respond to ≥2 trials of adequate dose and duration of antipsychotic medications.20 Further, up to 60% of people with treatment-resistant schizophrenia do not respond to clozapine,21 the only antipsychotic agent specifically indicated for this condition.22 Even when psychosis responds to the antipsychotic used and positive symptoms are reduced extensively, the overall therapeutic effect is usually not a cure or complete remission. Manifestations of the illness are persistent, especially those related to negative symptoms and cognitive impairments. Residual symptoms are also associated with higher relapse rates.23,24
Antipsychotics are associated with increased risks of extrapyramidal symptoms (EPS) including antipsychotic drug–induced parkinsonism, akathisia, and rescue use of antiparkinson medications, as well as weight gain, metabolic side effects, and elevated serum prolactin levels.25–29 Among these side effects, EPS and increased prolactin are attributable to D2 receptor blockade.30 In general, antipsychotics with relatively lower D2 antagonism and higher 5-hydroxytryptamine 2A (5-HT2A) receptor antagonism tend to carry a lower risk of EPS and prolactin elevation, but a greater risk of weight gain.31 Studies in individuals taking typical and atypical antipsychotics reported prevalence rates ranging from approximately 20%–35% for antipsychotic-induced parkinsonism and tardive dyskinesia.32 A systematic review and meta-analysis of 15 studies that included users of typical and/or atypical antipsychotics found overall pooled prevalence of approximately 20%, 11%, and 7% for antipsychotic drug-induced parkinsonism, akathisia, and tardive dyskinesia, respectively.33 Unfavorable side effects of antipsychotics also contribute to treatment discontinuation, poor compliance, and subsequent relapse.34,35 A systematic review of 39 studies found an estimated nonadherence rate of 40%–50% in individuals with schizophrenia.36 Approximately 75% of people with schizophrenia stopped the first antipsychotic prescribed during the first 18 months of treatment.37
Both negative and cognitive symptoms are common in most individuals with schizophrenia and are associated with functional impairment.38–40 Primary negative symptoms are intrinsic to the underlying pathophysiology and present throughout the course of the disease, while secondary negative symptoms are thought to be related to other factors such as positive symptoms, side effects of medications including certain antipsychotics, other comorbidities, social deprivation, and substance abuse; the 2 types can be clinically indistinguishable.41 While antipsychotics can reduce negative symptoms in individuals with schizophrenia and exacerbations of psychosis, it is unclear whether their effects are on primary or secondary negative symptoms.41 A meta-analysis revealed that amisulpride was the only antipsychotic drug to improve primary negative symptoms with evidence from placebo controlled trials, while drugs like olanzapine and zotepine did not outperform placebo, and cariprazine was the only antipsychotic that was clearly better than another antipsychotic (risperidone) in this regard.15 Commonly prescribed antipsychotic medications for schizophrenia have limited cognitive benefits.42 Meta analyses found only mild neurocognitive improvements for certain atypical antipsychotics, mainly compared to haloperidol, and the effect sizes were generally small.43–46 Moreover, neurocognitive improvements may be the result of improvements in secondary factors such as EPS.47,48
ADJUNCTIVE AND COMBINATION TREATMENT STRATEGIES
Combining multiple antipsychotic medications or adding a nonantipsychotic agent to an antipsychotic agent is a common practice for addressing inadequate responses to antipsychotic monotherapy. The goal is to improve general or selective symptoms, functional outcome, and/or tolerability.6,49
Inherent challenges exist to demonstrate the superiority of combinations vs monotherapy. A comprehensive, systematic overview of 29 meta analyses for 42 combinations (mostly antipsychotic/nonantipsychotic combinations) showed significantly more benefit with some strategies versus their antipsychotic monotherapy controls in positive or negative symptoms.7 Fourteen agents, including certain antidepressants, antioxidants, and mood stabilizers, combined with antipsychotics were found superior to controls for total symptom reductions, while all clozapine augmentation strategies did not outperform controls.7 However, the effect sizes of the pooled interventions were inversely correlated with the quality of the studies included in the meta-analyses, lowering the confidence in recommending any of the combination strategies.7
Results from a meta-analysis showed that augmentation of a current antipsychotic with a different antipsychotic appeared to be more effective than monotherapy in overall symptom reduction in open label or low-quality studies, but not in double-blind, high quality studies.50 In a meta-analysis of clozapine augmentation studies, only aripiprazole among the 10 add-on antipsychotics analyzed exhibited a greater effect vs placebo in reducing total psychosis,51 consistent with the real-world data that clozapine augmented with aripiprazole reduces rehospitalization risks compared with clozapine alone.52 However, the effect of the clozapine/aripiprazole combination on total psychosis was not statistically different when poor-quality studies were excluded from the meta-analysis.51 It was speculated that the treatment response resulting from D2 receptor blockade may already be maximized with clozapine, and therefore, adding a second dopamine blocker would provide limited benefit,53 although clozapine at clinical doses was shown to only transiently occupy 47%–72% of D2 receptors without saturating the receptors.54–56 Preliminary data from a small pilot study suggest that clozapine augmentation with cariprazine, which has partial D2/D3 receptor agonism, may improve overall, negative, and cognition symptoms.57 The positive effects of cariprazine were hypothesized to be related to its unique pharmacodynamic profile of partial agonism at D3/D2 receptors and 5-HT1A receptors along with antagonism of 5-HT2A and 5-HT2B receptors.57,58 Further evaluations in large, randomized controlled studies are needed to confirm these effects. A meta-analysis of 2 randomized controlled trials with head-to-head comparisons of augmentation with another antipsychotic versus switching to a new antipsychotic found no significant differences between the 2 strategies in controlling total, positive, or negative symptoms.59 Overall, current evidence remains inconclusive to show the superior efficacy of antipsychotic combinations compared with monotherapies. This is especially notable considering that most studies included in these meta analyses had small sample sizes, which can cause potential biases in meta-analysis as smaller studies tend to overestimate treatment effects.60
Antidepressants, anxiolytics, mood stabilizers, and antioxidants have been studied as adjunctive therapies with antipsychotics.5,61 Results from meta-analyses suggest the beneficial effects of adjunctive antidepressants (eg, selective serotonin reuptake inhibitors) on reducing negative or depressive symptoms are small,62,63 particularly for people with chronic schizophrenia.64 A comparative, real-world effectiveness study showed that adjunctive antidepressants were associated with lower risks of psychiatric hospitalization and emergency department visits compared with alternative antipsychotic therapy, while poorer outcomes were observed for adjunctive benzodiazepines and mood stabilizers.65 However, data from 2 earlier meta-analyses did not provide support for the effectiveness of adjunctive antidepressants.66,67 Despite mixed findings, these combination strategies are common in clinical practice. Real-world data from Europe showed that in outpatient treatment, 8%–23%, 7%–19%, and 22%–37% of individuals with schizophrenia who took antipsychotics received adjunctive antidepressants, mood stabilizers, and anxiolytics, respectively.68 A nationwide register based study among individuals with schizophrenia in inpatient or specialized outpatient care in Finland and Sweden found a rate of 25%–30% for adjunctive antidepressants, 17%–18% for adjunctive mood stabilizers, and 22–33% for adjunctive benzodiazepines and related drugs.69
Positive effects with adjunctive anti-inflammatory agents or azapirones have been reported.70,71 A systematic review and meta-analysis of 7 randomized, controlled studies showed that adjunctive N-acetylcysteine versus placebo reduced total and negative schizophrenia symptoms after 24 weeks of treatment and improved the cognitive domain of working memory.72 However, the absolute changes may be too small to be clinically meaningful.73 In a more recent, randomized, placebo controlled study in people with treatment-resistant schizophrenia who were taking clozapine, no significant benefits were observed in negative symptoms or cognition function at any time point over 1 year of treatment with adjunctive N-acetylcysteine.74
Augmenting cognitive remediation therapy with novel medications has also been studied in individuals with schizophrenia. Amphetamine75,76 and memantine77 were found to enhance auditory discrimination and learning in cognitive training for schizophrenia. When combined with auditory cognitive remediation, D-serine improved plasticity compared with placebo.78 However, no significant effects on cognitive performance were observed for D-serine,79 PF-03463275,80 and iclepertin,81 when these agents were combined with computerized cognitive training compared with training alone.
Concerns exist regarding the potential exacerbation of D2 receptor blocking–related adverse effects with concurrent use of multiple antipsychotics. Combinations of multiple antipsychotics have been reported to cause more side effects than monotherapies, particularly antipsychotic drug-induced parkinsonism, rescue anticholinergic use, and hyperprolactinemia.82 An overall greater total dose of antipsychotics can contribute to the increased risk of side effects.82,83 Antipsychotic combinations may also have detrimental effects on cognition,82 although a causal relationship has not been definitively established.84 However, less insomnia was seen when combining 2 D2 receptor antagonists compared with monotherapy.50 Adjunctive aripiprazole, a D2 receptor partial agonist, was also found to lower prolactin levels and reduce body weight.50,85
While augmentation of clozapine with other agents has been recommended for clozapine-refractory schizophrenia by the Treatment Response and Resistance in Psychosis Working Group,86 current treatment guidelines lack direction for other augmentation strategies. The National Institute for Health and Care Excellence guideline recommends against the regular combined use of antipsychotic medications except for short periods of time such as when changing medication.87 The American Psychiatric Association practice guidelines suggest augmentation approaches for individuals showing no or partial response to antipsychotics, although a trial of clozapine should not be delayed.88 Combining multiple antipsychotics for refractory schizophrenia is possible by the Royal Australian and New Zealand College of Psychiatrists guidelines, but careful monitoring is required due to potentially increased side effects, hospitalization, and mortality.89
RATIONALE FOR TARGETING MULTIPLE NEUROTRANSMITTER SYSTEMS
Increasing evidence suggests that the pharmacologic actions of current antipsychotics, all of which share the ability to block the D2 receptor, are insufficient to adequately control all symptoms of schizophrenia for all individuals. Postmortem and neuroimaging studies revealed differences in dopamine neurotransmission between treatment-responsive and treatment-resistant schizophrenia.90–93 Based on these findings, 2 subtypes of schizophrenia were hypothesized, one with increased dopamine synthesis and release capacity in the striatum (hyperdopaminergic) and the other with unaltered dopaminergic function (normodopaminergic), with the latter type likely involving nondopaminergic mechanisms.94 Given that all current antipsychotics are D2 receptor blockers, a sizable subgroup of people with schizophrenia with little dopaminergic pathophysiology would not be expected to respond well to current antipsychotic treatments.94,95 Moreover, positive symptoms of schizophrenia are associated with dopamine hyperactivity in the striatum, and negative and cognitive symptoms have been hypothesized to be associated with reduced cortical dopamine signaling.10,96,97 Current antipsychotic drugs acting as D2 receptor functional antagonists could therefore actually impair cognition and secondarily increase negative symptoms. This is supported by evidence that administration of antipsychotics can induce negative symptoms and cognitive dysfunction in healthy volunteers98,99 and increase or cause secondary negative symptoms in people with schizophrenia.30
Therapies combining complementary mechanisms have been shown to provide more clinical benefits than monotherapy when treating diseases with diverse mechanisms, such as diabetes, hypertension, and cancer.100–102 Similarly, targeting multiple mechanisms in schizophrenia may improve treatment effects. Perturbations in acetylcholine, glutamate, GABA, and serotonin neurotransmitter systems have been implicated in schizophrenia and may contribute to negative and cognitive symptoms.38,97 Treatments targeting these nondopaminergic pathways may produce beneficial effects on these symptom domains. The rationale for adjunctive therapy with multiple antipsychotics targeting different mechanisms also stems from the observation that high-efficacy antipsychotic drugs, particularly clozapine, have complex actions with pleiotropic effects on multiple neurotransmitter systems. Compared with other antipsychotics, clozapine is more effective in controlling overall and secondary negative symptoms,27,103 although no clear difference was found between olanzapine and clozapine.104,105 Clozapine is also associated with lower risks of EPS, hospitalization, and all-cause discontinuation.106 While not the first-line drug of choice due to its safety profile, clozapine is the only antipsychotic drug approved by the US Food and Drug Administration (FDA) for treatment-resistant schizophrenia.22 The superior efficacy of clozapine has been postulated to be attributable to its low D2 receptor occupancy, rapid dissociation from D2 receptors, a higher affinity ratio for 5-HT2A versus D2 receptors, and high D4 receptor affinity.107 The ability of clozapine to interact with other receptors, including the muscarinic (M1, M2, M3, and M5), histamine, and α1-adrenergic receptors may also contribute to its superior efficacy.107 Additionally, clozapine’s effects on glycine transport inhibition108 and glutamatergic modulation,109,110 as well as the M1 and M4 agonism attributable to norclozapine,109 may be involved in its unique efficacy.
Advantages of combining traditional antipsychotic agents with a nondopaminergic agent have been reported. For example, adjunctive sodium benzoate, a D-amino acid oxidase (DAAO) inhibitor that indirectly enhances N-methyl-D-aspartate (NMDA) receptor functions, was shown to improve a variety of symptom domains and cognitive function when added to an ongoing antipsychotic medication.111,112 Improvements in several domains of cognition were also observed for combinations of antipsychotics with acetylcholinesterase inhibitors.113 Taken together, these data suggest that individuals suffering from schizophrenia very likely have complex and heterogeneous pathophysiology. There is hope that they may demonstrate more favorable outcomes when treated with pharmacologic approaches that go beyond D2 receptor blockade.
NOVEL PSYCHOTROPIC AGENTS THAT DO NOT BLOCK DOPAMINE RECEPTORS
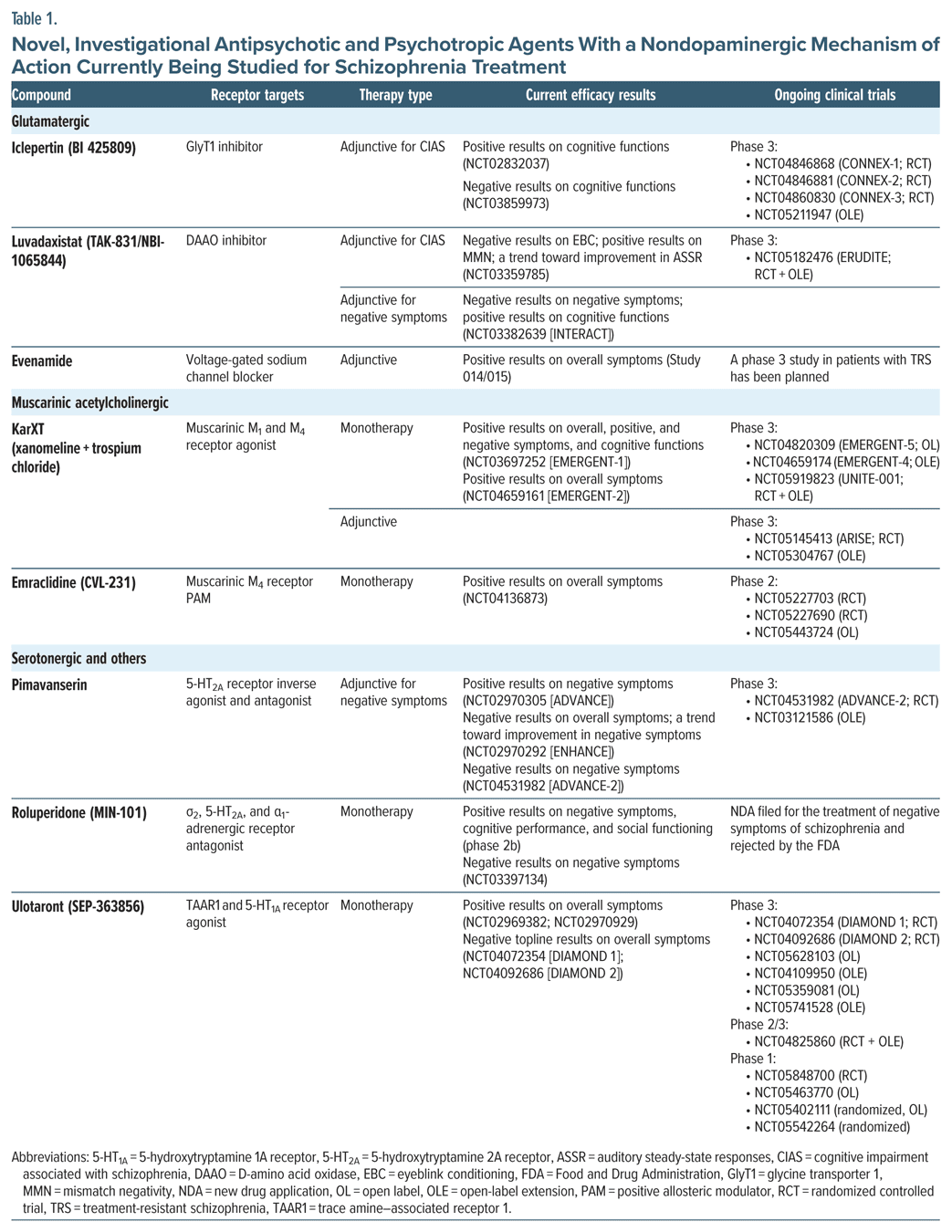
Considerable efforts have been dedicated to the research and development of non-D2 agents for schizophrenia over the past 20 years,114 although none of these new agents has received FDA approval, with some being unsuccessful in phase 3 programs. For instance, encenicline, a selective partial α7 nicotinic receptor agonist, demonstrated only limited cognitive effects when added to stable antipsychotics in 2 large phase 3 trials.115 Three phase 3 studies evaluating adjunctive bitopertin, a glycine transporter 1 (GlyT1) inhibitor, missed their primary efficacy end point of significant improvement in negative symptoms.116 Pomaglumetad methionil, a mGlu2/3 receptor agonist, failed to show greater improvement in psychotic symptoms compared with placebo in its pivotal, phase 2 study,117 nor did it demonstrate superiority over aripiprazole in a phase 3 study.118 However, there is continued interest in the development of novel, nondopaminergic psychotropics for people with schizophrenia who do not respond to approved antipsychotics properly. Several new agents are currently at their later stage of clinical development (Table 1).119
Targeting the Glutamate Receptors
Glutamatergic dysfunction is implicated in the pathophysiology of schizophrenia,10,11 so there is great interest in investigating the therapeutic potential of glutamatergic compounds. However, most of these compounds (eg, mGlu2/3 receptor agonists, riluzole, and memantine) have failed to demonstrate definitive efficacy on positive, negative, or cognitive symptoms.38,117 Results from meta-analyses suggest that NMDA receptor–enhancing agents have little to negligible effect on improving overall cognition when added to antipsychotics.120,121 However, adjunctive NMDA receptor modulators as a group exhibited a small effect on negative symptoms,122 and adjunctive NMDA receptor coagonists, such as glycine, D-serine, and D-alanine, have been reported to improve both positive and negative symptoms in some but not in all studies.123–127 In addition, a review of current findings suggests that indirect modulators of the NMDA receptor glycine modulatory site, such as GlyT1 inhibitors or DAAO inhibitors, may offer more benefits, at least on cognition, than direct modulators.128
Iclepertin (BI 425809) is a GlyT1 inhibitor that indirectly potentiates glutamate acting on the NMDA receptor, resulting in normalization of the NMDA receptor–mediated cortical excitatory-inhibitory imbalance.129 A phase 2, randomized controlled study showed significantly greater improvement in cognition with oral, adjunctive iclepertin vs placebo.130 The treatment was well tolerated, with similar incidence of adverse events (AEs) across treatment groups and low frequencies of serious AEs and AE-related study discontinuations.130 Several phase 3 trials are ongoing to further investigate the efficacy and safety of adjunctive iclepertin in treating impaired cognition and daily functioning associated with schizophrenia (Table 1).129
Luvadaxistat (TAK-831 or NBI-1065844) is a DAAO inhibitor that increases the synaptic levels of the glutamate coagonist D-serine, thereby indirectly modulating the NMDA receptor activity.131 A small, phase 2a, randomized controlled, crossover study did not demonstrate any effect of adjunctive luvadaxistat at an oral, daily dose of 50-mg or 500-mg on learning as assessed with eyeblink conditioning (primary end point). However, the 50-mg dose improved or showed a trend toward improving mismatch negativity (MMN) and auditory steady-state responses, which are the neurocircuitry biomarkers implicated in NMDA receptor function and schizophrenia.131 Luvadaxistat was well tolerated in the study with mild, nontreatment-related AEs.131 The phase 2, randomized controlled, INTERACT study showed that 12 weeks of treatment with luvadaxistat added to current antipsychotics did not improve negative symptoms of schizophrenia (primary end point) in adults with schizophrenia.132 However, INTERACT met its secondary end points of improving cognitive function,132 and luvadaxistat is being further investigated for the treatment of cognitive impairment in schizophrenia (Table 1).
Evenamide is a highly selective, voltage-gated sodium channel blocker that normalizes excessive glutamate release due to NMDA receptor hypofunction.133 In a pilot, phase 2, open-label trial, evenamide added to an ongoing antipsychotic was shown to improve the overall symptoms of schizophrenia from baseline to 6 weeks, 6 months, and 1 year, with favorable tolerability, in people with treatment-resistant schizophrenia.133 Evenamide also demonstrated progressively increasing efficacy as assessed with the Clinical Global Impression (CGI)- Severity and CGI-Change scales.133 No clinically important weight gains, metabolic syndrome, or EPS were noted in the study.133 A phase 3, randomized controlled study has been planned to further evaluate evenamide in treatment resistant schizophrenia (Table 1).134
Targeting the Muscarinic Acetylcholine Receptors
KarXT (xanomeline and trospium chloride) is an oral, investigational treatment that combines xanomeline, a dual M1 and M4 preferring muscarinic acetylcholine receptor agonist, with trospium chloride, a peripherally restricted pan-muscarinic acetylcholine receptor antagonist.135 Xanomeline was initially developed to treat the cognitive symptoms of Alzheimer disease but unexpectedly demonstrated efficacy in reducing and preventing psychosis symptoms (eg, delusions and hallucinations) in people with Alzheimer disease.136 A later, proof-of-concept, phase 2 trial demonstrated antipsychotic-like effects of xanomeline in people with schizophrenia or schizoaffective disorder.137 However, further clinical development of xanomeline was hampered by its significant peripheral cholinergic side effects.138 The addition of trospium chloride to xanomeline reduces the incidence of these cholinergic AEs in healthy volunteers, making the development of the combination, KarXT, for schizophrenia possible.139 In the phase 2, randomized controlled, EMERGENT-1 trial, KarXT significantly improved overall, positive, and negative symptoms vs placebo in people with acute exacerbations of schizophrenia.135 Post hoc analyses from EMERGENT 1 also revealed cognitive improvement with KarXT that was independent of the improvement in positive symptoms in participants with baseline clinical cognitive impairment.140 Procholinergic (nausea or vomiting) or anticholinergic (dry mouth or constipation) AEs occurred early in treatment and were transient in nature.141 Incidence of weight gain and EPS was similar with KarXT and placebo, and no clinically meaningful changes in metabolic parameters were observed.135,141 The randomized, placebo-controlled, phase 3, EMERGENT-2 trial further demonstrated the efficacy of KarXT, which significantly reduced the Positive and Negative Syndrome Scale (PANSS) total score versus placebo in acutely psychotic hospitalized adults with schizophrenia after 5 weeks of treatment (primary end point).142 Frequencies of treatment-emergent EPS, akathisia, weight gain, and somnolence were similar across treatment groups.142 KarXT is being further investigated as a monotherapy and as an adjunctive therapy for schizophrenia in several other ongoing phase 3 studies (Table 1).
Emraclidine (CVL-231) is an oral, brain-penetrant, highly selective positive allosteric modulator of the muscarinic M4 receptor.143 A two-part, phase 1b trial evaluated the safety, tolerability, and pharmacology of multiple ascending doses of emraclidine monotherapy in people with schizophrenia.143 The study demonstrated a favorable safety profile and potential antipsychotic activities of emraclidine without need for dose titration.143 Both the 30-mg (daily) and 20-mg (twice daily) doses significantly improved overall symptoms, and the 30-mg dose also significantly improved the CGI-Severity score.143 No treatment effects on EPS were noted, gastrointestinal AEs were infrequent, and emraclidine-associated increases in heart rate and blood pressure were modest and transient.143 Emraclidine is currently in phase 2 of clinical development as monotherapy (Table 1).
Different from KarXT, emraclidine selectively stimulates the M4 receptor and therefore may reduce peripheral side effects, such as increased gastrointestinal motility and increased secretions associated with peripheral M1 receptor stimulation.144 Dosing schedules are different for the 2 agents. Flexible dosing (twice daily) is used for KarXT and could complicate efficacy evaluation and affect patient adherence compared with the constant dosing (daily) used for emraclidine.
Targeting the Serotonin and Other Neurotransmitter Receptors
Pimavanserin is an oral, selective inverse agonist and antagonist of 5-HT2A145 approved for the treatment of hallucinations and delusions associated with Parkinson disease.146 The activity of pimavanserin for the treatment of schizophrenia was tested in 2 randomized controlled trials.147–149 In the phase 2, ADVANCE study, adjunctive pimavanserin significantly improved negative symptoms compared with placebo in people with predominant negative symptoms of schizophrenia who were receiving a background antipsychotic medication; the treatment was well tolerated, with no clinically significant differences in vital signs, body weight, and EPS between the 2 treatment groups.148 Higher pimavanserin exposure was associated with improved response, without increasing the incidence of key AEs, such as anxiety, headache, insomnia, and somnolence.147 In the phase 3, ENHANCE study in adult outpatients with schizophrenia and an inadequate response to their ongoing antipsychotics, adjunctive pimavanserin failed to demonstrate an improvement in the PANSS total score (primary end point) but did show a trend toward improvement in negative symptoms.149 Pimavanserin was well tolerated in the study, with a low rate of serious AEs and AE-related discontinuations.149 However, adjunctive pimavanserin did not demonstrate significant improvement in negative symptoms of schizophrenia (primary end point) compared with adjunctive placebo in the phase 3, randomized controlled ADVANCE-2 study150 (Table 1), and further development of pimavanserin has been stopped.
Roluperidone (MIN-101) is an oral antagonist of σ2 and 5-HT2A receptors; it also binds to α1-adrenergic receptors but has no or low binding affinity for dopaminergic, muscarinic, cholinergic, and histaminergic receptors.151 Improvements in negative symptoms, cognitive performance, and social functioning with roluperidone monotherapy were observed in individuals with stable positive and concurrent negative symptoms of schizophrenia in a phase 2b, randomized controlled trial.151–154 No changes in vital signs, laboratory values, and EPS ratings were observed in the study and changes in body weight were small.151 A larger, phase 3 trial failed to demonstrate statistical significance on the primary outcome of negative symptom improvement of the low (32-mg/day) and high (64-mg/day) roluperidone doses as planned, although the higher dose did demonstrate nominally statistically significant improvement vs placebo in the modified intent-to-treat population.155 Similarly as in the phase 2b trial, roluperidone was not associated with notable changes in weight, plasma prolactin, and EPS ratings.155 Open-label extension studies of the 2 trials demonstrated sustained effects of roluperidone up to 40 weeks on negative symptoms and social functioning.156 The new drug application for roluperidone for the treatment of negative symptoms in schizophrenia was filed last year but was recently rejected by the FDA.157
Ulotaront (SEP-363856) is an oral agonist of trace amine–associated receptor 1 (TAAR1) and 5-HT1A receptors that has no binding affinity for D2 or 5-HT2A receptor.158 Ulotaront monotherapy was shown to improve total symptoms of schizophrenia compared with placebo in people with an acute exacerbation of schizophrenia in a 4-week, phase 2, randomized controlled trial,159 and the improvement persisted during the subsequent 26-week, open-label extension study.160 Ulotaront was generally well tolerated; the incidence rates of AEs related to EPS, metabolic syndrome, and serum prolactin levels were similar across treatment groups during the placebo-controlled phase159 and remained minimal during the extension phase.160 A relatively high completion rate (67%) was observed in the extension study.160 Moreover, preclinical evidence suggests that ulotaront can reduce weight gain associated with common antipsychotics, such as olanzapine.161 Further investigations on the efficacy and safety of ulotaront for the treatment of schizophrenia are ongoing (Table 1). However, recent results from the phase 3, randomized controlled, DIAMOND 1 and DIAMOND 2 trials showed no significant improvement in overall symptoms with ulotaront versus placebo in acutely psychotic adults with schizophrenia, although the high response to placebo in the 2 trials may possibly obscure a significant drug effect.162
PERSONALIZED TREATMENT
Schizophrenia is a highly heterogeneous disease, and significant interindividual differences likely exist in clinical characteristics and underlying pathophysiology.90,93,163–165 Response to antipsychotic medications varies considerably across individuals with schizophrenia.17,21,166 By tailoring the treatment to the clinical, or ideally biological, characteristics of each individual, a personalized approach could optimize the treatment response. As part of the approach, identifying biomarkers that are predictive of antipsychotic drug outcomes could markedly facilitate informed selection of treatment for individuals with schizophrenia.167,168
Pharmacogenetic studies have identified a number of genetic variants that are associated with antipsychotic efficacy and side effects, such as antipsychotic-induced weight gain, metabolic syndrome, risk of tardive dyskinesia, antipsychotic related prolactin levels, and clozapine-induced agranulocytosis.167,169,170 In particular, dose reductions for aripiprazole and clozapine are recommended by the FDA for the CYP2D6 poor metabolizers who carry nonfunctioning variants of the cytochrome P450 2D6 enzyme to prevent potential drug-associated AEs caused by increasing drug exposure.22,171 However, the clinical relevance of these identified genetic markers may be limited due to their small effect sizes and the need for them to be validated in large, well-designed studies.167,169
Electroencephalography (EEG) features have been linked to antipsychotic treatment responses172 and can serve as a useful research tool for studying the treatment effect of an antipsychotic agent. One of the EEG-based biomarkers is MMN. In the recent phase 2a study of luvadaxistat, MMN responses to treatment aligned with the cognitive improvement produced by luvadaxistat at the same dose in the parallel, INTERACT study, suggesting the potential of MMN as a predictive biomarker for antipsychotic effects.131 With the potential establishment of procholinergic antipsychotics for schizophrenia treatment, markers that can predict response to procholinergic vs dopaminergic agents will be useful when choosing effective treatment. Possible muscarinic deficit markers include resistance to antidopaminergic agents, visual hallucinations, severe cognitive deficits, reduced MMN, presence of antimuscarinic antibodies, and reduced M1 receptor availability on radionucleotide imaging.173 Additional neuroimaging, proteomic, and metabolic biomarkers have also been identified for schizophrenia.168,174
It is believed that biomarker testing may help stratify individuals with heterogeneous characteristics of schizophrenia into relatively more homogeneous subgroups and aid in the selection of optimal antipsychotic treatment.175 This may also ultimately provide a framework for a rational approach to combination treatment strategies.
CONCLUSIONS
Current antipsychotic medications are all D2 receptor antagonists or partial agonists and are associated with suboptimal efficacy and intolerable side effects in many individuals with schizophrenia. While combinations of currently prescribed antipsychotics are commonly used to improve inadequate treatment response, evidence supporting their superior efficacy over antipsychotic monotherapies is inconclusive. It is highly likely that the etiology and pathophysiology of schizophrenia is quite heterogeneous, and thus, subsets of people with schizophrenia are likely unable to achieve optimal treatment response to the dopamine receptor–blocking agents, highlighting the need for targeting other, non-D2 receptors. Clinical findings on investigational antipsychotic agents and innovative psychotropics that act via novel, nondopaminergic mechanisms are encouraging. If the results with these novel agents can be corroborated, they may serve as useful adjunctive or cotreatments in people with schizophrenia who do not derive sufficient benefits from current treatment strategies.
Article Information
Published Online: August 19, 2024. https://doi.org/10.4088/JCP.23nr15240
© 2024 Physicians Postgraduate Press, Inc.
Submitted: December 29, 2023; accepted May 23, 2024.
To Cite: Kinon BJ, Leucht S, Tamminga C, et al. Rationale for adjunctive treatment targeting multiple mechanisms in schizophrenia. J Clin Psychiatry. 2024;85(3):23nr15240.
Author Affiliations: Karuna Therapeutics, Boston, Massachusetts (Kinon and Paul (former employees), Bristol Myers Squibb, Princeton, New Jersey (Marcus); Department of Psychiatry and Psychotherapy, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany (Leucht); Department of Psychiatry, University of Texas Southwestern Medical School, Dallas, Texas (Tamminga); Department of Psychiatry, Indiana University School of Medicine, Indianapolis, Indiana (Breier).
Corresponding Author: Bruce J. Kinon, MD ([email protected]), c/o Alicia Meli, MS, Bristol Myers Squibb Corporate Headquarters, Route 206 & Province Line Road, Princeton, NJ 08543
Relevant Financial Relationships: Drs Kinon and Paul are former employees of Karuna Therapeutics. Dr Marcus is an employee of Bristol Myers Squibb. Dr Leucht has received honoraria as an advisor/consultant and/or for lectures and/or for educational material from Alkermes, Angelini, Apsen, Eisai, Gedeon Richter, Janssen, Karuna, Kynexis, Lundbeck, Medichem, Medscape, Merck Sharp and Dome, Mitsubishi, Neurotorium, Novo Nordisk, Otsuka, Recordati, Roche, Rovi, Sanofi Aventis, and Teva. Dr Tamminga has received honoraria as a consultant from Karuna and Kynexis and is a founding partner of Kynexis. Dr Breier is a consultant for Karuna, BioXcel, and Neumarker.
Funding/Support: Karuna Therapeutics, a Bristol Myers Squibb company, provided support for the medical writing assistance.
Role of the Sponsor: The Sponsor had the opportunity to review during manuscript development, but the authors had final control of the content included in the manuscript.
Acknowledgment: We thank Hui Zhang, PhD (Precise Publications); Alicia Meli, MS (Bristol Myers Squibb); and Nichole Neugebauer, PhD (Bristol Myers Squibb) for their medical writing and editorial assistance. Hui Zhang declares no conflicts of interest. Alicia Meli and Nichole Neugebauer are employees of Bristol Myers Squibb.
Clinical Points
- Currently available antipsychotics act as D2 receptor antagonists/partial agonists, which may lack effectiveness for some individuals with schizophrenia.
- Treatments combining multiple mechanisms of action hold promise in addressing some of the unmet treatment needs in schizophrenia.
- Novel agents without direct D2 receptor blocking may be useful to augment current antipsychotics’ effects in individuals with inadequate response/intolerance to current schizophrenia treatment.
References (175)

- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137–150. PubMed CrossRef
- Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. PubMed CrossRef
- Kadakia A, Catillon M, Fan Q, et al. The economic burden of schizophrenia in the United States. J Clin Psychiatry. 2022;83(6):22m14458. PubMed CrossRef
- Paul SM, Yohn SE, Popiolek M, et al. Muscarinic acetylcholine receptor agonists as novel treatments for schizophrenia. Am J Psychiatry. 2022;179(9):611–627. PubMed CrossRef
- Baandrup L. Polypharmacy in schizophrenia. Basic Clin Pharmacol Toxicol. 2020;126(3):183–192. PubMed CrossRef
- Lähteenvuo M, Tiihonen J. Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs. 2021;81(11):1273–1284. PubMed
- Correll CU, Rubio JM, Inczedy-Farkas G, et al. Efficacy of 42 pharmacologic cotreatment strategies added to antipsychotic monotherapy in schizophrenia: systematic overview and quality appraisal of the meta-analytic evidence. JAMA Psychiatry. 2017;74(7):675–684. PubMed CrossRef
- Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187–191. PubMed CrossRef
- Stepnicki P, Kondej M, Kaczor AA. Current concepts and treatments of schizophrenia. Molecules. 2018;23(8):2087. PubMed
- McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19(1):15–33. PubMed CrossRef
- Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29(2):97–115. PubMed CrossRef
- Dean B, Bakker G, Ueda HR, et al. A growing understanding of the role of muscarinic receptors in the molecular pathology and treatment of schizophrenia. Front Cell Neurosci. 2023;17:1124333. PubMed CrossRef
- Kidambi N, Elsayed OH, El-Mallakh RS. Xanomeline-trospium and muscarinic involvement in schizophrenia. Neuropsychiatr Dis Treat. 2023;19:1145–1151. PubMed
- Lobo MC, Whitehurst TS, Kaar SJ, et al. New and emerging treatments for schizophrenia: a narrative review of their pharmacology, efficacy and side effect profile relative to established antipsychotics. Neurosci Biobehav Rev. 2022;132:324–361. PubMed CrossRef
- Krause M, Zhu Y, Huhn M, et al. Antipsychotic drugs for patients with schizophrenia and predominant or prominent negative symptoms: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2018;268(7):625–639. PubMed CrossRef
- Samara MT, Nikolakopoulou A, Salanti G, et al. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr Bull. 2019;45(3):639–646. PubMed CrossRef
- Kane JM, Agid O, Baldwin ML, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80(2):18com12123. PubMed CrossRef
- Correll CU, Brevig T, Brain C. Patient characteristics, burden and pharmacotherapy of treatment-resistant schizophrenia: results from a survey of 204 US psychiatrists. BMC Psychiatry. 2019;19(1):362. PubMed CrossRef
- Siskind D, Orr S, Sinha S, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. 2022;220(3):115–120. PubMed CrossRef
- Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. PubMed CrossRef
- Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772–777. PubMed CrossRef
- CLOZARIL® (clozapine) tablets, for oral use [prescribing information]. Novartis Pharmaceuticals Corporation; 2020.
- Schennach R, Obermeier M, Meyer S, et al. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2012;63(1):87–90. PubMed CrossRef
- Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107–116. PubMed CrossRef
- Wu H, Siafis S, Hamza T, et al. Antipsychotic-induced weight gain: dose-response meta-analysis of randomized controlled trials. Schizophr Bull. 2022;48(3):643–654. PubMed CrossRef
- Wu H, Siafis S, Wang D, et al. Antipsychotic-induced akathisia in adults with acute schizophrenia: a systematic review and dose-response meta-analysis. Eur Neuropsychopharmacol. 2023;72:40–49. PubMed
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):P939–P951. PubMed CrossRef
- Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. PubMed CrossRef
- Rognoni C, Bertolani A, Jommi C. Second-generation antipsychotic drugs for patients with schizophrenia: systematic literature review and meta-analysis of metabolic and cardiovascular side effects. Clin Drug Investig. 2021;41(4):303–319. PubMed CrossRef
- Kaar SJ, Natesan S, McCutcheon R, et al. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. PubMed CrossRef
- Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638–645. PubMed
- Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs. Tardive dyskinesia-key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2):233–248. PubMed CrossRef
- Ali T, Sisay M, Tariku M, et al. Antipsychotic-induced extrapyramidal side effects: a systematic review and meta-analysis of observational studies. PLoS One. 2021;16(9):e0257129. PubMed CrossRef
- Doane MJ, Raymond K, Saucier C, et al. Unmet needs with antipsychotic treatment in schizophrenia and bipolar I disorder: patient perspectives from qualitative focus groups. BMC Psychiatry. 2023;23(1):245. PubMed
- Doane MJ, Sajatovic M, Weiden PJ, et al. Antipsychotic treatment experiences of people with schizophrenia: patient perspectives from an online survey. Patient Prefer Adherence. 2020;14:2043–2054. PubMed CrossRef
- Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. PubMed CrossRef
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. PubMed CrossRef
- McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023;28:1902–1918. PubMed CrossRef
- Mosolov SN, Yaltonskaya PA. Primary and secondary negative symptoms in schizophrenia. Front Psychiatry. 2021;12:766692. PubMed CrossRef
- Harvey PD. Disability in schizophrenia: contributing factors and validated assessments. J Clin Psychiatry. 2014;75(suppl 1):15–20. PubMed CrossRef
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. PubMed CrossRef
- Sharma T. Impact on cognition of the use of antipsychotics. Curr Med Res Opin. 2002;18(suppl 3):s13–s17. PubMed CrossRef
- Woodward ND, Purdon SE, Meltzer HY, et al. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8(3):457–472. PubMed CrossRef
- Nielsen RE, Levander S, Kjaersdam Telleus G, et al. Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131(3):185–196. PubMed CrossRef
- Clissold M, Crowe SF. Comparing the effect of the subcategories of atypical antipsychotic medications on cognition in schizophrenia using a meta-analytic approach. J Clin Exp Neuropsychol. 2019;41(1):26–42. PubMed CrossRef
- Baldez DP, Biazus TB, Rabelo-da-Ponte FD, et al. The effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: network meta-analyses of randomized controlled trials. Neurosci Biobehav Rev. 2021;126:265–275. PubMed CrossRef
- Monteleone P, Cascino G, Monteleone AM, et al. Prevalence of antipsychotic-induced extrapyramidal symptoms and their association with neurocognition and social cognition in outpatients with schizophrenia in the [L8D2Q2M0]real-life[R8D2Q2M1]. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110250. PubMed CrossRef
- Lindgren M, Therman S, Avellan A, et al. Extrapyramidal symptoms predict cognitive performance after first-episode psychosis. Schizophr (Heidelb). 2022;8(1):64.
- Ballon J, Stroup TS. Polypharmacy for schizophrenia. Curr Opin Psychiatry. 2013;26(2):208–213. PubMed CrossRef
- Galling B, Roldán A, Hagi K, et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry. 2017;16(1):77–89. PubMed CrossRef
- Siskind DJ, Lee M, Ravindran A, et al. Augmentation strategies for clozapine refractory schizophrenia: a systematic review and meta-analysis. Aust N Z J Psychiatry. 2018;52(8):751–767. PubMed CrossRef
- Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499–507. PubMed CrossRef
- Gunduz-Bruce H, Oliver S, Gueorguieva R, et al. Efficacy of pimozide augmentation for clozapine partial responders with schizophrenia. Schizophr Res. 2013;143(2–3):344–347. PubMed CrossRef
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286–293. PubMed CrossRef
- Seeman P. Clozapine, a fast-off-D2 antipsychotic. ACS Chem Neurosci. 2014;5(1):24–29. PubMed CrossRef
- Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161(9):1620–1625. PubMed CrossRef
- Pappa S, Kalniunas A, Sharma H, et al. Efficacy and safety of cariprazine augmentation in patients treated with clozapine: a pilot study. Ther Adv Psychopharmacol. 2022;12:20451253221132087. PubMed CrossRef
- Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9(2):193–206. PubMed CrossRef
- Rubio JM, Guinart D, Kane JM, et al. Early non-response to antipsychotic treatment in schizophrenia: a systematic review and meta-analysis of evidence-based management options. CNS Drugs. 2023;37(6):499–512. PubMed CrossRef
- Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. 2013;17(1):R2. PubMed CrossRef
- Hong J, Bang M. Anti-inflammatory strategies for schizophrenia: a review of evidence for therapeutic applications and drug repurposing. Clin Psychopharmacol Neurosci. 2020;18(1):10–24. PubMed
- Helfer B, Samara MT, Huhn M, et al. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry. 2016;173(9):876–886. PubMed CrossRef
- Galling B, Vernon JA, Pagsberg AK, et al. Efficacy and safety of antidepressant augmentation of continued antipsychotic treatment in patients with schizophrenia. Acta Psychiatr Scand. 2018;137(3):187–205. PubMed CrossRef
- Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174–179. PubMed CrossRef
- Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508–515. PubMed
- Rummel C, Kissling W, Leucht S. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev. 2006;2006(3):CD005581. PubMed CrossRef
- Sepehry AA, Potvin S, Elie R, et al. Selective serotonin reuptake inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68(4):604–610. PubMed CrossRef
- Haro JM, Salvador-Carulla L. The SOHO (Schizophrenia Outpatient Health Outcome) study: implications for the treatment of schizophrenia. CNS Drugs. 2006;20(4):293–301. PubMed CrossRef
- Taipale H, Puranen A, Mittendorfer-Rutz E, et al. Antipsychotic use among persons with schizophrenia in Sweden and Finland, trends and differences. Nord J Psychiatry. 2021;75(5):315–322. PubMed CrossRef
- Yamada R, Wada A, Stickley A, et al. Effect of 5-HT1A receptor partial agonists of the azapirone class as an add-on therapy on psychopathology and cognition in schizophrenia: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2023;26(4):249–258. PubMed CrossRef
- Cho M, Lee TY, Kwak YB, et al. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry. 2019;53(8):742–759. PubMed CrossRef
- Yolland CO, Hanratty D, Neill E, et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry. 2020;54(5):453–466. PubMed CrossRef
- Andrade C. Antipsychotic augmentation with N-acetylcysteine for patients with schizophrenia. J Clin Psychiatry. 2022;83(5):22f14664. PubMed CrossRef
- Neill E, Rossell SL, Yolland C, et al. N-acetylcysteine (NAC) in schizophrenia resistant to clozapine: a double-blind, randomized, placebo-controlled trial targeting negative symptoms. Schizophr Bull. 2022;48(6):1263–1272. PubMed CrossRef
- Swerdlow NR, Tarasenko M, Bhakta SG, et al. Amphetamine enhances gains in auditory discrimination training in adult schizophrenia patients. Schizophr Bull. 2017;43(4):872–880. PubMed CrossRef
- Swerdlow NR, Bhakta SG, Talledo J, et al. Auditory discrimination and frequency modulation learning in schizophrenia patients: amphetamine within-subject dose response and time course. Psychol Med. 2023;53(1):140–148. PubMed
- Swerdlow NR, Bhakta SG, Talledo J, et al. Memantine effects on auditory discrimination and training in schizophrenia patients. Neuropsychopharmacology. 2020;45(13):2180–2188. PubMed CrossRef
- Sehatpour P, Iosifescu DV, De Baun HM, et al. Dose-dependent augmentation of neuroplasticity-based auditory learning in schizophrenia: a double-blind, placebo-controlled, randomized, target engagement clinical trial of the NMDA glutamate receptor agonist d-serine. Biol Psychiatry. 2023;94(2):164–173. PubMed CrossRef
- D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492–503. PubMed
- Surti TS, Ranganathan M, Johannesen JK, et al. Randomized controlled trial of the glycine transporter 1 inhibitor PF-03463275 to enhance cognitive training and neuroplasticity in schizophrenia. Schizophr Res. 2023;256:36–43. PubMed CrossRef
- ClinicalTrials. This study tests whether BI 425809 together with brain training using a computer improves mental functioning in patients with schizophrenia (NCT03859973); 2023. Accessed March 2, 2024. https://clinicaltrials.gov/study/NCT03859973
- Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf. 2012;11(4):527–542. PubMed CrossRef
- Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol. 2014;17(7):1083–1093. PubMed CrossRef
- Kontis D, Theochari E, Kleisas S, et al. Doubtful association of antipsychotic polypharmacy and high dosage with cognition in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1333–1341. PubMed CrossRef
- Srisurapanont M, Suttajit S, Maneeton N, et al. Efficacy and safety of aripiprazole augmentation of clozapine in schizophrenia: a systematic review and meta-analysis of randomized-controlled trials. J Psychiatr Res. 2015;62:38–47. PubMed CrossRef
- Wagner E, Kane JM, Correll CU, et al. Clozapine combination and augmentation strategies in patients with schizophrenia -recommendations from an international expert survey among the Treatment Response and Resistance in Psychosis (TRRIP) Working Group. Schizophr Bull. 2020;46(6):1459–1470. PubMed CrossRef
- National Institute for Health and Care Excellence: Guidelines. Psychosis and schizophrenia in adults: prevention and management (CG178). NICE; 2014.
- The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. 3rd ed. American Psychiatric Association; 2021.
- Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410–472. PubMed CrossRef
- Demjaha A, Murray RM, McGuire PK, et al. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169(11):1203–1210. PubMed CrossRef
- Roberts RC, Roche JK, Conley RR, et al. Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse. 2009;63(6):520–530. PubMed CrossRef
- Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97(14):8104–8109. PubMed CrossRef
- Demjaha A, Egerton A, Murray RM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–e13. PubMed CrossRef
- Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic). Br J Psychiatry. 2014;205(1):1–3. PubMed CrossRef
- Gillespie AL, Samanaite R, Mill J, et al. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? A systematic review. BMC Psychiatry. 2017;17(1):12. PubMed CrossRef
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. PubMed CrossRef
- Galderisi S, Merlotti E, Mucci A. Neurobiological background of negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2015;265(7):543–558. PubMed CrossRef
- Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488–493. PubMed CrossRef
- Kim E, Howes OD, Turkheimer FE, et al. The relationship between antipsychotic D2 occupancy and change in frontal metabolism and working memory: a dual [(11)C]raclopride and [(18) F]FDG imaging study with aripiprazole. Psychopharmacology (Berl). 2013;227(2):221–229. PubMed CrossRef
- Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. PubMed CrossRef
- Guerrero-García C, Rubio-Guerra AF. Combination therapy in the treatment of hypertension. Drugs Context. 2018;7:212531. PubMed
- Xie X, Wu C, Hao Y, et al. Benefits and risks of drug combination therapy for diabetes mellitus and its complications: a comprehensive review. Front Endocrinol (Lausanne). 2023;14:1301093. PubMed CrossRef
- Qubad M, Bittner RA. Second to none: rationale, timing, and clinical management of clozapine use in schizophrenia. Ther Adv Psychopharmacol. 2023;13:20451253231158152. PubMed CrossRef
- Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia: a network meta-analysis. JAMA Psychiatry. 2016;73(3):199–210. PubMed
- Dong S, Schneider-Thoma J, Bighelli I, et al. A network meta-analysis of efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2024;274(4):917–928. PubMed CrossRef
- Masuda T, Misawa F, Takase M, et al. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. 2019;76(10):1052–1062. PubMed CrossRef
- Nucifora FC Jr., Mihaljevic M, Lee BJ, et al. Clozapine as a model for antipsychotic development. Neurotherapeutics. 2017;14(3):750–761. PubMed CrossRef
- Javitt DC, Duncan L, Balla A, et al. Inhibition of system A-mediated glycine transport in cortical synaptosomes by therapeutic concentrations of clozapine: implications for mechanisms of action. Mol Psychiatry. 2005;10(3):275–287. PubMed CrossRef
- de Bartolomeis A, Vellucci L, Barone A, et al. Clozapine[R8S2Q1M7]s multiple cellular mechanisms: what do we know after more than fifty years? A systematic review and critical assessment of translational mechanisms relevant for innovative strategies in treatment-resistant schizophrenia. Pharmacol Ther. 2022;236:108236. PubMed CrossRef
- McQueen G, Sendt KV, Gillespie A, et al. Changes in brain glutamate on switching to clozapine in treatment-resistant schizophrenia. Schizophr Bull. 2021;47(3):662–671. PubMed CrossRef
- Lin CH, Lin CH, Chang YC, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2018;84(6):422–432. PubMed
- Lane HY, Lin CH, Green MF, et al. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry. 2013;70(12):1267–1275. PubMed CrossRef
- Singh J, Kour K, Jayaram MB. Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst Rev. 2012;1(1):CD007967. PubMed CrossRef
- Hopkins SC, Lew R, Zeni C, et al. Challenges in the clinical development of non-D2 compounds for schizophrenia. Curr Med Res Opin. 2023;39(3):467–471. PubMed
- Brannan S. 32.2 Two Global Phase III trials of encenicline for cognitive impairment in chronic schizophrenia patients: red flags and lessions learned. Schizophr Bull. 2019;45(suppl 2):S141–S142.
- Bugarski-Kirola D, Blaettler T, Arango C, et al. Bitopertin in negative symptoms of schizophrenia-results from the Phase III FlashLyte and DayLyte studies. Biol Psychiatry. 2017;82(1):8–16. PubMed CrossRef
- Downing AM, Kinon BJ, Millen BA, et al. A double-blind, placebo-controlled comparator study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry. 2014;14:351. PubMed CrossRef
- Adams DH, Zhang L, Millen BA, et al. Pomaglumetad methionil (LY2140023 monohydrate) and aripiprazole in patients with schizophrenia: a phase 3, multicenter, double-blind comparison. Schizophr Res Treat. 2014;2014:758212.
- de Bartolomeis A, Ciccarelli M, Vellucci L, et al. Update on novel antipsychotics and pharmacological strategies for treatment-resistant schizophrenia. Expert Opin Pharmacother. 2022;23(18):2035–2052. PubMed CrossRef
- Chang CH, Lane HY, Tseng PT, et al. Effect of N-methyl-D-aspartate-receptor-enhancing agents on cognition in patients with schizophrenia: a systematic review and meta-analysis of double-blind randomised controlled trials. J Psychopharmacol. 2019;33(4):436–448. PubMed CrossRef
- Iwata Y, Nakajima S, Suzuki T, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20(10):1151–1160. PubMed CrossRef
- Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859–885. PubMed CrossRef
- Heresco-Levy U, Ermilov M, Lichtenberg P, et al. High-dose glycine added to olanzapine and risperidone for the treatment of schizophrenia. Biol Psychiatry. 2004;55(2):165–171. PubMed CrossRef
- Heresco-Levy U, Javitt DC, Ebstein R, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57(6):577–585. PubMed CrossRef
- Tsai GE, Yang P, Chang YC, et al. D-alanine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2006;59(3):230–234. PubMed CrossRef
- Tsai G, Yang P, Chung LC, et al. D-serine added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 1998;44(11):1081–1089. PubMed CrossRef
- Goh KK, Wu TH, Chen CH, et al. Efficacy of N-methyl- D-aspartate receptor modulator augmentation in schizophrenia: A meta-analysis of randomised, placebo-controlled trials. J Psychopharm. 2021;35(3):236–252.
- Pei JC, Luo DZ, Gau SS, et al. Directly and indirectly targeting the glycine modulatory site to modulate NMDA receptor function to address unmet medical needs of patients with schizophrenia. Front Psychiatry. 2021;12:742058. PubMed CrossRef
- Rosenbrock H, Desch M, Wunderlich G. Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023;273:1557–1566. PubMed CrossRef
- Fleischhacker WW, Podhorna J, Gröschl M, et al. Efficacy and safety of the novel glycine transporter inhibitor BI 425809 once daily in patients with schizophrenia: a double-blind, randomised, placebo-controlled phase 2 study. Lancet Psychiatry. 2021;8(3):191–201. PubMed
- O’Donnell P, Dong C, Murthy V, et al. The D-amino acid oxidase inhibitor luvadaxistat improves mismatch negativity in patients with schizophrenia in a randomized trial. Neuropsychopharmacology. 2023;48(7):1052–1059. PubMed
- Neurocrine Biosciences. Neurocrine Biosciences announces top-Line results from phase II INTERACT study evaluating luvadaxistat (NBI-1065844) for the treatment of negative symptoms and cognitive impairment associated with schizophrenia (CIAS). Neurocrine Biosciences, Inc; 2021. Accessed July 29, 2023. https://neurocrine.gcs-web.com/news-releases/news-release-details/neurocrine-biosciences-announces-top-line-results-phase-ii
- Anand R, Turolla A, Chinellato G, et al. Phase 2 results indicate evenamide, a selective modulator of glutamate release, is associated with clinically important long-term efficacy when added to an antipsychotic in patients with treatment-resistant schizophrenia. Int J Neuropsychopharmacol. 2023;26:523–528. PubMed CrossRef
- Newron reports compelling topline results from all patients in Study 014, its phase II clinical trial evaluating evenamide as add-on therapy for treatment resistant schizophrenia. Newron Pharmaceuticals SpA; 2023. Accessed June 19, 2023. https://www.newron.com/news-and-media/regulatory-news/newron-reports-compelling-topline-results-all-patients-study-014-its
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726. PubMed
- Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54(4):465–473. PubMed CrossRef
- Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033–1039. PubMed CrossRef
- Vaidya S, Guerin AA, Walker LC, et al. Clinical effectiveness of muscarinic receptor-targeted interventions in neuropsychiatric disorders: a systematic review. CNS Drugs. 2022;36(11):1171–1206. PubMed CrossRef
- Breier A, Brannan SK, Paul SM, et al. Evidence of trospium’s ability to mitigate cholinergic adverse events related to xanomeline: Phase 1 study results. sychopharmacology (Berl). 2023;240(5):1191–1198.
- Sauder C, Allen LA, Baker E, et al. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled Phase 2 study. Transl Psychiatry. 2022;12(1):491. PubMed CrossRef
- Correll CU, Angelov AS, Miller AC, et al. Safety and tolerability of KarXT (xanomeline-trospium) in a phase 2, randomized, double-blind, placebo-controlled study in patients with schizophrenia. Schizophrenia (Heidelb). 2022;8(1):109. PubMed CrossRef
- Kaul I, Sawchak S, Correll CU, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose Phase 3 trial. Lancet. 2024;403(10422):160–170. PubMed
- Krystal JH, Kane JM, Correll CU, et al. Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: a two-part, randomised, double-blind, placebo-controlled, Phase 1b trial. Lancet. 2022;400(10369):2210–2220. PubMed CrossRef
- Jones SE, Harvey PD. Cross-diagnostic determinants of cognitive functioning: the muscarinic cholinergic receptor as a model system. Transl Psychiatry. 2023;13(1):100. PubMed
- Vanover KE, Weiner DM, Makhay M, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N[R8S2Q1M7]-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006;317(2):910–918. PubMed CrossRef
- NUPLAZID® (pimavanserin) capsules/tablets, for oral use [prescribing information]. Acadia Pharmaceuticals Inc; 2020.
- Darwish M, Bugarski-Kirola D, Passarell J, et al. Pimavanserin exposure-response analyses in patients with schizophrenia: results from the Phase 2 ADVANCE study. J Clin Psychopharmacol. 2022;42(6):544–551. PubMed CrossRef
- Bugarski-Kirola D, Arango C, Fava M, et al. Pimavanserin for negative symptoms of schizophrenia: results from the ADVANCE Phase 2 randomised, placebo-controlled trial in North America and Europe. Lancet Psychiatry. 2022;9(1):46–58. PubMed
- Bugarski-Kirola D, Bitter I, Liu IY, et al. ENHANCE: Phase 3, randomized, double-blind, placebo-controlled study of adjunctive pimavanserin for schizophrenia in patients with an inadequate response to antipsychotic treatment. Schizophr Bull Open. 2022;3(1):sgac006. PubMed CrossRef
- Acadia Pharmaceuticals announces top-line results from phase 3 ADVANCE 2 trial of pimavanserin in negative symptoms of schizophrenia. Acadia Pharmaceuticals; Accessed March 11, 2024. https://acadia.com/media/newsreleases/acadia-pharmaceuticals-announces-top-line-results-from-phase-3-advance-2-trial-of-pimavanserin-in-negative-symptoms-of-schizophrenia/
- Davidson M, Saoud J, Staner C, et al. Efficacy and safety of MIN-101: a 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am J Psychiatry. 2017;174(12):1195–1202. PubMed CrossRef
- Harvey PD, Saoud JB, Luthringer R, et al. Effects of roluperidone (MIN-101) on two dimensions of the negative symptoms factor score: reduced emotional experience and reduced emotional expression. Schizophr Res. 2020;215:352–356. PubMed CrossRef
- Rabinowitz J, Badescu S, Palamarchuk P, et al. Personal and social adjustment effects of roluperidone in patients with schizophrenia and negative symptoms: results from an exploratory outcome of a randomized placebo-controlled trial. Schizophr Res. 2019;211:103–104. PubMed CrossRef
- Keefe RSE, Harvey PD, Khan A, et al. Cognitive effects of MIN-101 in patients with schizophrenia and negative symptoms: results from a randomized controlled trial. J Clin Psychiatry. 2018;79(3):17m11753. PubMed CrossRef
- Davidson M, Saoud J, Staner C, et al. Efficacy and safety of roluperidone for the treatment of negative symptoms of schizophrenia. Schizophr Bull. 2022;48(3):609–619. PubMed CrossRef
- Rabinowitz J, Staner C, Saoud J, et al. Long-term effects of roluperidone on negative symptoms of schizophrenia. Schizophr Res. 2023;255:9–13. PubMed
- Minerva Neurosciences receives complete response letter from FDA for new drug application for roluperidone for the treatment of negative symptoms in patients with schizophrenia. Minerva Neurosciences, Inc; 2024. Accessed February 27, 2024. https://ir.minervaneurosciences.com/news-releases/newsrelease-details/minerva-neurosciences-receives-complete-response-letter-fdanew
- Dedic N, Jones PG, Hopkins SC, et al. SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. J Pharmacol Exp Ther. 2019;371(1):1–14. PubMed CrossRef
- Koblan KS, Kent J, Hopkins SC, et al. A non-D2-receptor-binding drug for the treatment of schizophrenia. N Engl J Med. 2020;382(16):1497–1506. PubMed
- Correll CU, Koblan KS, Hopkins SC, et al. Safety and effectiveness of ulotaront (SEP-363856) in schizophrenia: results of a 6-month, open-label extension study. NPJ Schizophr. 2021;7(1):63. PubMed CrossRef
- Liang L, Ren X, Xu J, et al. Effect of co-treatment of olanzapine with SEP-363856 in mice models of schizophrenia. Molecules. 2022;27(8):2550. PubMed
- umitomo Pharma and Otsuka announce topline results from Phase 3 DIAMOND 1 and DIAMOND 2 clinical studies evaluating ulotaront in schizophrenia. Sumitomo Pharma Co, Ltd and Otsuka Pharmaceutical Co, Ltd; 2023. Accessed August 14, 2023. https://www.otsuka-us.com/news/sumitomopharma-and-otsuka-announce-topline-results-phase-3-diamond-1-and-diamond2-clinical
- Sun X, Liu J, Ma Q, et al. Disrupted intersubject variability architecture in functional connectomes in schizophrenia. Schizophr Bull. 2021;47(3):837–848. PubMed CrossRef
- Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74(11):1104–1111. PubMed CrossRef
- Dickinson D, Pratt DN, Giangrande EJ, et al. Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophr Bull. 2018;44(1):101–113. PubMed
- Correll CU, Shaikh L, Gallego JA, et al. Antipsychotic polypharmacy: a survey study of prescriber attitudes, knowledge and behavior. Schizophr Res. 2011;131(1–3):58–62. PubMed CrossRef
- Teng Y, Sandhu A, Liemburg EJ, et al. The progress and pitfalls of pharmacogenetics-based precision medicine in schizophrenia spectrum disorders: a systematic review and meta-analysis. J Pers Med. 2023;13(3):471. PubMed
- Rodrigues-Amorim D, Rivera-Baltanás T, López M, et al. Schizophrenia: a review of potential biomarkers. J Psychiatr Res. 2017;93:37–49. PubMed CrossRef
- Islam F, Hain D, Lewis D, et al. Pharmacogenomics of clozapine-induced agranulocytosis: a systematic review and meta-analysis. Pharmacogenomics J. 2022;22(4):230–240. PubMed CrossRef
- Zhang JP, Lencz T, Zhang RX, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis. Schizophr Bull. 2016;42(6):1418–1437. PubMed CrossRef
- ABILIFY® (aripiprazole) tablets, for oral use. ABILIFY DISCMELT® (aripiprazole) orally disintegrating tablets, ABILIFY® (aripiprazole) oral solution, ABILIFY® (aripiprazole) injection for intramuscular use only [prescribing information]. Otsuka America Pharmaceutical, Inc; 2022.
- De Pieri M, Rochas V, Sabe M, et al. Pharmaco-EEG of antipsychotic treatment response: a systematic review. Schizophrenia (Heidelb). 2023;9(1):85. PubMed CrossRef
- Stuke H. Markers of muscarinic deficit for individualized treatment in schizophrenia. Front Psychiatry. 2022;13:1100030. PubMed CrossRef
- Patel S, Sharma D, Uniyal A, et al. Recent advancements in biomarker research in schizophrenia: mapping the road from bench to bedside. Metab Brain Dis. 2022;37(7):2197–2211. PubMed CrossRef
- Kantrowitz JT, Correll CU, Jain R, et al. New developments in the treatment of schizophrenia: an expert roundtable. Int J Neuropsychopharmacol. 2023;26(5):322–330. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite