The use of attention-deficit/hyperactivity disorder (ADHD) medications during pregnancy has increased in recent years. An earlier article in this column examined whether psychostimulant medications, used to treated ADHD and related disorders, increase the risk of major congenital malformations in pregnancies with first trimester exposure to these drugs. This article examines whether amphetamines, methylphenidate, and atomoxetine exposure during early and late pregnancy are associated with other adverse gestational outcomes. One large and 4 small studies provided data relevant to the inquiry. In unadjusted analyses, amphetamines and methylphenidate were associated with an increased risk of most of the adverse outcomes under study. However, in analyses adjusted for potential confounds, amphetamine exposure during early pregnancy was associated only with an increased risk of preeclampsia; otherwise, amphetamine and methylphenidate exposure was not associated with the risk of preeclampsia, placental abruption, small for gestational age, or preterm birth. Late gestational exposure to psychostimulants was associated with an increased risk of preterm birth but not with the other adverse outcomes. In sensitivity analyses, such as one that examined exposure during weeks 8-18 of gestation, amphetamines were associated with an increased risk of preeclampsia, placental abruption, and preterm birth, and methylphenidate, with an increased risk of preeclampsia. For reasons explained in the text, it may be prudent to err on the side of caution, but in the worst case scenario, the number needed to harm is about 63 for amphetamines exposure and preeclampsia and larger (eg, > 500, indicating less risk) for other adverse outcomes. Atomoxetine was not associated with any adverse gestational outcome, but it is not clear whether this is a true finding or a result of underpowered analyses. In conclusion, women need to weigh the benefits of the ADHD medication that they are using against potential gestational risks when deciding whether or not to continue treatment during pregnancy.


ABSTRACT
The use of attention-deficit/
hyperactivity disorder (ADHD) medications during pregnancy has increased in recent years. An earlier article in this column examined whether psychostimulant medications, used to treated ADHD and related disorders, increase the risk of major congenital malformations in pregnancies with first trimester exposure to these drugs. This article examines whether amphetamines, methylphenidate, and atomoxetine exposure during early and late pregnancy are associated with other adverse gestational outcomes. One large and 4 small studies provided data relevant to the inquiry. In unadjusted analyses, amphetamines and methylphenidate were associated with an increased risk of most of the adverse outcomes under study. However, in analyses adjusted for potential confounds, amphetamine exposure during early pregnancy was associated only with an increased risk of preeclampsia; otherwise, amphetamine and methylphenidate exposure was not associated with the risk of preeclampsia, placental abruption, small for gestational age, or preterm birth. Late gestational exposure to psychostimulants was associated with an increased risk of preterm birth but not with the other adverse outcomes. In sensitivity analyses, such as one that examined exposure during weeks 8-18 of gestation, amphetamines were associated with an increased risk of preeclampsia, placental abruption, and preterm birth, and methylphenidate, with an increased risk of preeclampsia. For reasons explained in the text, it may be prudent to err on the side of caution, but in the worst case scenario, the number needed to harm is about 63 for amphetamines exposure and preeclampsia and larger (eg, > 500, indicating less risk) for other adverse outcomes. Atomoxetine was not associated with any adverse gestational outcome, but it is not clear whether this is a true finding or a result of underpowered analyses. In conclusion, women need to weigh the benefits of the ADHD medication that they are using against potential gestational risks when deciding whether or not to continue treatment during pregnancy.
J Clin Psychiatry 2018;79(1):18f12136
To cite: Andrade C. Adverse gestational outcomes associated with attention-deficit/hyperactivity disorder medication exposure during pregnancy. J Clin Psychiatry. 2018;79(1):18f12136.
To share: https://doi.org/10.4088/JCP.18f12136
© Copyright 2018 Physicians Postgraduate Press, Inc.
The use of attention-deficit/hyperactivity disorder (ADHD) medications during pregnancy is increasing. For example, Danish health registry data showed that, between 2003 and 2010, the use of these medications by pregnant women rose from 5 per 100,000 person-years to 533 per 100,000 person-years.1 North American data suggest similar trends; one study reported that the use of ADHD medications in pregnancy rose from 0.2% during 1997-1998 to 1.3% during 2013.2
An earlier article in this column3 examined the risk of major congenital malformations following first trimester gestational exposure to psychostimulant medications; in summary, there did not seem to be sufficient evidence, at present, to suggest that methylphenidate and amphetamines are teratogenic. The present article examines whether ADHD medications are associated with other adverse gestational outcomes; data from 1 large4 and 4 smaller1,5-7 studies are specifically reviewed.
Placental Complications Associated With Early and Late Pregnancy Exposure to ADHD Medications
Cohen et al4 observed that first-line ADHD medications have vasoconstrictive properties and may therefore impair placental perfusion; this effect could increase the risk of gestational complications such as preeclampsia, placental abruption, fetal growth restriction, and preterm birth. They therefore examined these outcomes in a population-based cohort study using 2000-2010 data from the Medicaid program in the United States.
The sample comprised 5,299 women who were exposed to ADHD medications in monotherapy during early pregnancy and 1,461,493 unexposed controls. In this context, exposure was defined as at least 1 filled prescription for a single ADHD drug during the first 140 days of pregnancy, starting from the first day of the last menstrual period (LMP), with no prescription for any other ADHD drug from 90 days before to 140 days after the LMP. Nonexposure was defined as the absence of a prescription for any ADHD medication from 90 days before to 140 days after the LMP.
The exposed sample included 3,331 women who had received an amphetamine, 1,515 who had received methylphenidate, and 453 who had received atomoxetine. Of these, 1,353 women continued their medication beyond week 20 (140 days) and 3,946 did not; this allowed a comparison between early and late pregnancy exposure and reduced (but did not eliminate) confounding by indication.
Relative to unexposed controls, women exposed to ADHD medications were younger; more likely to be white; more likely to use alcohol, tobacco, and other substances; more likely to have psychiatric comorbidity; and more likely to use medical and psychotropic drugs. Exposed women were also more likely to have other diagnoses for which ADHD medications may be prescribed, including bipolar disorder, chronic fatigue syndrome, and narcolepsy. Relative to women who discontinued ADHD medication in early pregnancy, those who continued the medication were more likely to be older, multiparous, and diagnosed with ADHD; they were also more likely to be users of other medications. Whereas indices of illness severity were far greater in exposed relative to unexposed women, these indices were similar in those who continued vs those who discontinued ADHD medications.
Analyses were adjusted using propensity score fine stratification for a wide range of potential confounding variables, including sociodemographic variables; obstetric variables; use of alcohol, tobacco, and other substances; medical and psychiatric illness variables; medication variables; and measures of illness severity and health care utilization.
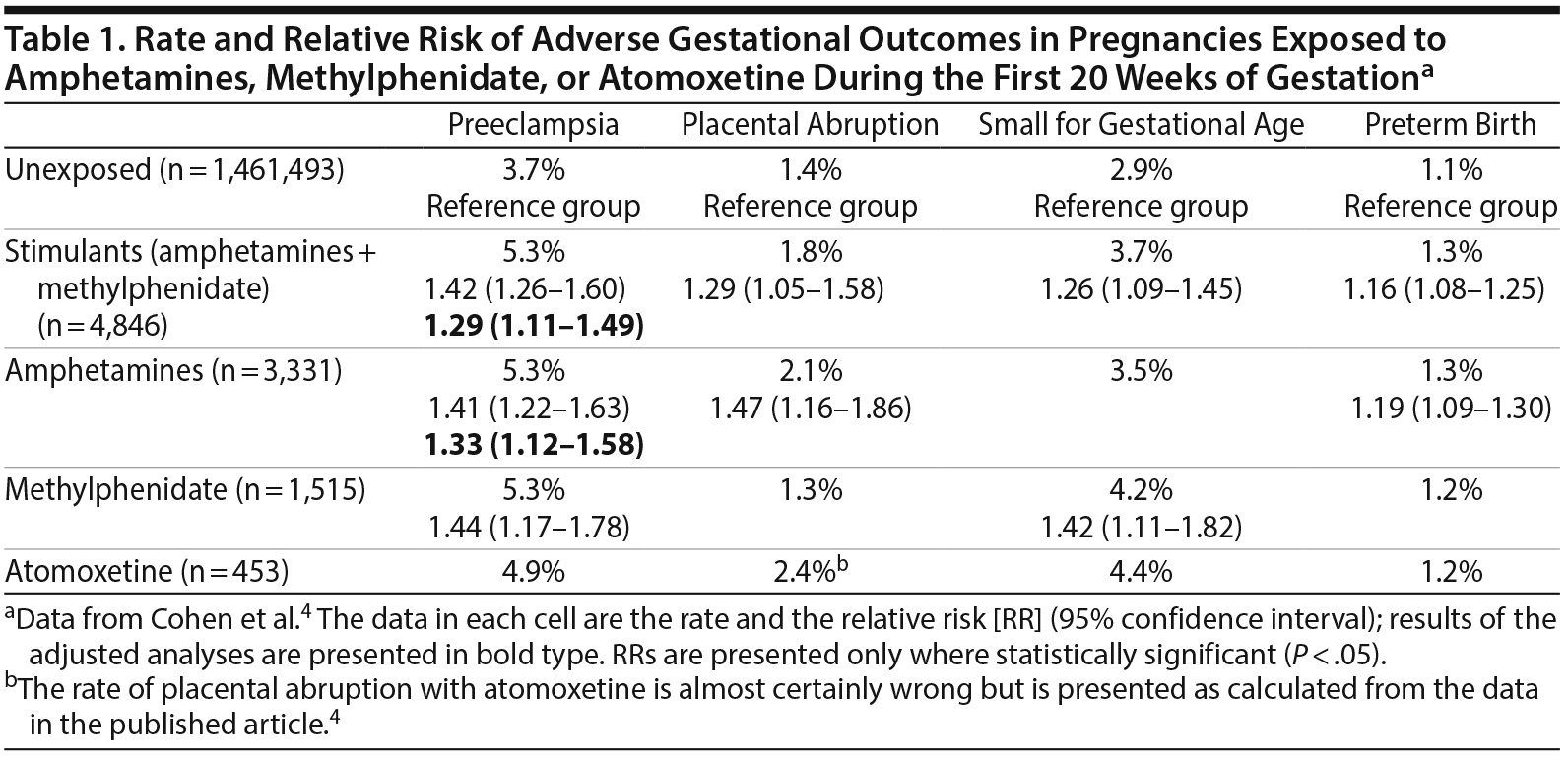
Findings. Important findings for early gestational exposure are presented in Table 1. In summary:
- In unadjusted analyses, amphetamines were associated with an increased risk of preeclampsia, placental abruption, and preterm birth, and methylphenidate was associated with an increased risk of preeclampsia and small for gestational age.
- In adjusted analyses, amphetamines were associated only with an increased risk of preeclampsia, and methylphenidate was not associated with an increased risk of any adverse outcome.
- Atomoxetine was not associated with an increased risk of any adverse outcome in either unadjusted or adjusted analyses.
When late and early gestational exposure were compared in women who continued (n = 1,319) vs discontinued (n = 3,527) amphetamines or methylphenidate with a cutoff at week 20, important findings were as follows:
- Late gestational exposure to psychostimulants was not associated with increased risk of preeclampsia or placental abruption in either unadjusted or adjusted analyses.
- In unadjusted analyses, late gestational exposure to psychostimulants was associated with an increased risk of small for gestational age (relative risk [RR] = 1.36) and preterm birth (RR = 1.42).
- In adjusted analyses, late exposure to psychostimulants was associated with only preterm birth (RR = 1.30; 95% confidence interval [CI], 1.10-1.55).
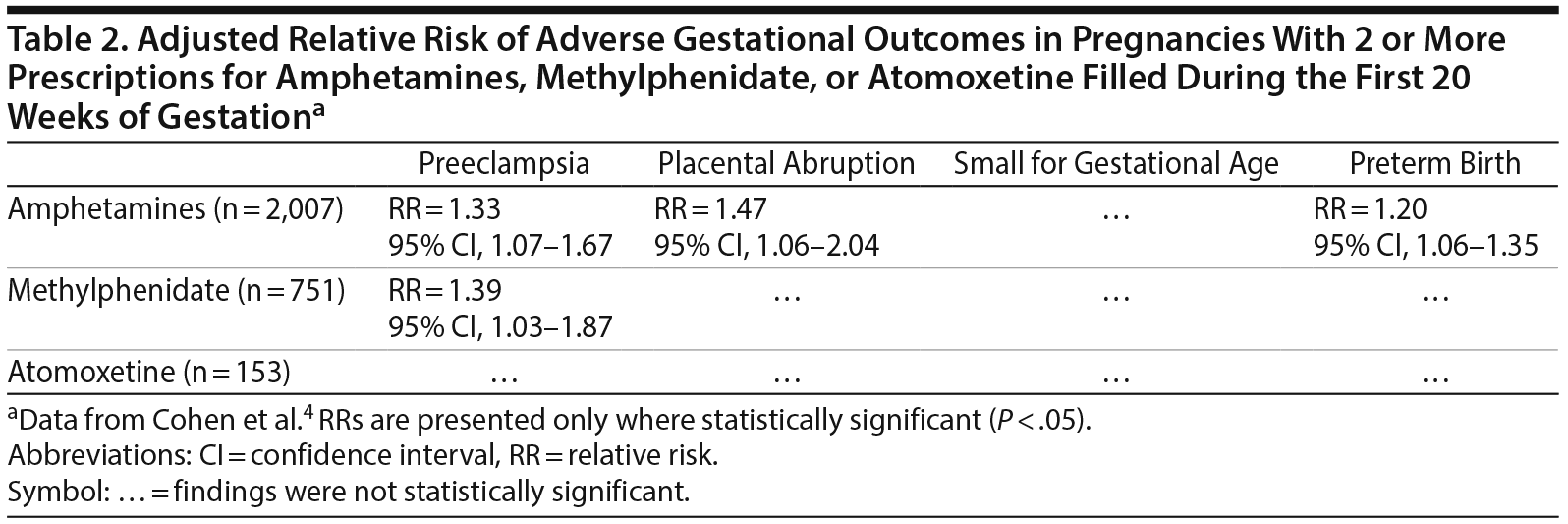
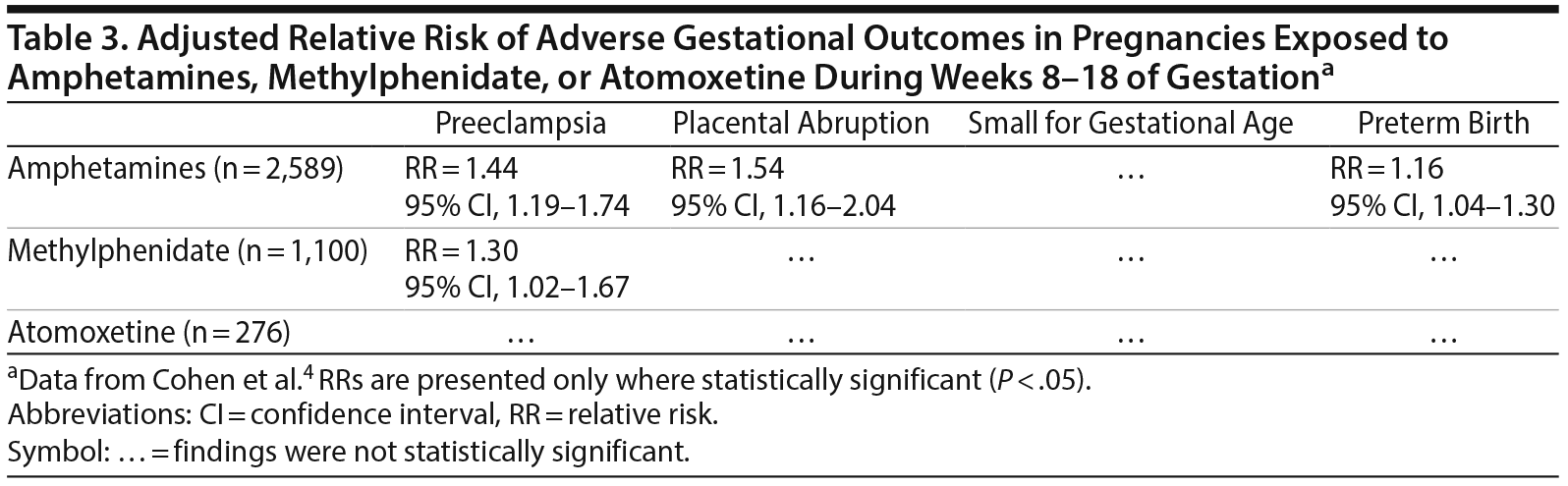
Sensitivity analyses examined the risk of adverse gestational outcomes associated with the filling of at least 2 prescriptions for ADHD medication (in monotherapy) during the first 20 weeks of gestation, and the filling of at least 1 prescription during weeks 8-18 of gestation, which period is critical for placentation. The results of these sensitivity analyses are presented in Tables 2 and 3. In summary, in both analyses, after adjustment for confounding variables, amphetamines were associated with an increased risk of preeclampsia, placental abruption, and preterm birth (but not small for gestational age), and methylphenidate was associated with (only) preeclampsia. Atomoxetine was not associated with any adverse outcome.
Finally, when dose-dependent medication supply effects were examined, a dose-dependent risk in steps between 30 days’ and > 90 days’ supply appeared evident only for the risk of preeclampsia associated with amphetamines; for other adverse outcomes, only > 90 days’ supply of amphetamines was associated with an increased risk, and this only for preterm birth. For methylphenidate, only > 60 days’ supply was associated with increased risk, and this only for preeclampsia. For atomoxetine, there was no significant increase in risk at any level of supply.
Other Recent Studies
In a study that extracted 1999-2010 data from Danish registries, Haervig et al1 identified 1,054,494 pregnancies, of which 480 had been exposed to methylphenidate, atomoxetine, or modafinil. Exposure was defined as redeeming a prescription for one of these drugs anytime between 4 weeks before the first day of the LMP to the end of pregnancy. Exposure to these medications was associated with an increased risk of induced abortion on maternal request (odds ratio [OR] = 4.70; 95% CI, 3.77-5.85), induced abortion due to special indication (OR = 2.99; 95% CI, 1.34-6.67), and miscarriage (OR = 2.07; 95% CI, 1.51-2.84).
In a study that extracted 1997-2008 data from Danish registries, Bro et al5 identified 989,932 pregnancies, of which 186 had been exposed to methylphenidate or atomoxetine. Exposure was defined as 1 or more redeemed prescriptions from 30 days before the estimated date of conception to the end of pregnancy. There were 275 other pregnancies in which the mother had ADHD but did not use ADHD medication during the pregnancy. There were 989,471 pregnancies that were not exposed to either ADHD or ADHD medications. In adjusted analyses, the authors found that exposure to untreated ADHD (RR = 1.56; 95% CI, 1.11-2.20) and exposure to ADHD medications (RR = 1.55; 95% CI, 1.03-2.36) were associated with an almost identically increased risk of spontaneous abortion; that exposure to untreated ADHD was associated with preterm birth (RR = 1.82; 95% CI, 1.01-3.29); that exposure to ADHD medication (RR = 2.06; 95% CI, 1.11-3.82) but not to untreated ADHD was associated with an Apgar score < 10; and that exposure to ADHD medication or untreated ADHD was otherwise not significantly associated with differences in birth weight, gestational age, small for gestational age, and low birth weight.
In a prospective study of data from teratology information services in 4 countries, Diav-Citrin et al6 identified 382 pregnancies characterized by methylphenidate use (indication: ADHD in 90% of women) anytime during pregnancy. The median dose of methylphenidate was 20 mg/d. A quarter of the women were taking other psychotropic medications, as well. Half the women discontinued methylphenidate treatment by week 6 of gestation. Methylphenidate-exposed pregnancies were matched by maternal age, gestational age, and year of initial contact with an equal number of pregnancies in which counseling had been provided for nonteratogenic exposure. Methylphenidate, relative to nonteratogenic exposure, was associated with an increased risk of elective termination of pregnancy (8.1% vs 2.6%), miscarriage (14.1% vs 7.1%), and perinatal complications (23.6% vs 13.5%). The commonest perinatal complication was neonatal jaundice. Perinatal complications did not differ between women who stopped vs those who continued methylphenidate during pregnancy, suggesting that the complications were not treatment related. The methylphenidate exposed and unexposed cohorts otherwise did not differ in median gestational age at delivery, rates of preterm delivery, or median birth weight.
In a prospective study of 686 women, Newport et al7 found that psychostimulant exposure during pregnancy was higher in women with hypertensive disorders of pregnancy (n = 86) than in controls (OR = 6.11; 95% CI, 1.79-20.90). However, this analysis was based on just 12 pregnancies exposed to psychostimulants after week 20.
Critical Comments
The study by Cohen et al4 dominates the literature in the field because the sample, comprising nearly 1.5 million pregnancies, included almost 5,300 women who had been exposed to ADHD medications; the data were extensively analyzed for individual drugs from different perspectives and for different outcomes, and the analyses were adjusted for potential confounds. In contrast, the number of exposed pregnancies in each of the other studies was small, mostly well below 500. On the one hand, analyses in these smaller studies would not have been able to adequately adjust for confounding variables, predisposing to the identification of false positive associations; on the other hand, analyses in these studies would have been underpowered, predisposing to the failure to detect potentially significant associations.
The smaller studies, nevertheless, did provide some useful information. For example, Bro et al5 were the only authors to examine outcomes in women with a diagnosis of ADHD who did not use ADHD medication during pregnancy; these authors found that exposure to ADHD but not to ADHD medications was associated with both spontaneous abortion and preterm birth. The findings suggest that confounding by indication could explain associations of (some) adverse gestational outcomes with ADHD medications.
Although Cohen et al4 made commendable efforts toward adjusting for confounds, no amount of statistical manipulation can control for unknown and unmeasured, known but unmeasured, and known but inadequately well-measured confounds. In effect, observational studies can identify significant associations but can never ascribe causality to those associations.8,9 As an example, if there was systematic bias in prescribing psychostimulants to more severely ill patients and atomoxetine to less severely ill patients, and if the ADHD behaviors that predisposed to adverse gestational outcomes were not adequately measured and adjusted for in the analyses, then no amount of statistical manipulation would satisfactorily adjust for confounding by indication. This could well be a reason why atomoxetine was not associated with adverse gestational outcomes, although psychostimulants were.
There is also the possibility that the atomoxetine analyses were underpowered because there were too few pregnancies exposed to this drug; the crude rates of adverse outcomes with atomoxetine (Table 1) suggest that significant associations with atomoxetine may have emerged had the atomoxetine exposure sample size been larger. However, even had the atomoxetine associations been found statistically significant, for reasons already stated, no causality in the relationships could be inferred.
Of note, Cohen et al4 tested a very large number of hypotheses without adjusting for the inflated type I error risk, and so some of the findings may have been false positives. It is worth noting here that some analyses actually found amphetamines to be protective or near significantly protective against adverse outcomes in the small for gestational age analyses.
Up to this point, the discussion has cast doubts on the possible etiologic influence of ADHD medications on adverse gestational outcomes. However, the results of the sensitivity analyses must give us pause. Usually, when the sample size decreases, confidence intervals widen, and it becomes more difficult to establish statistical significance. Nevertheless, despite the smaller numbers of exposed pregnancies in the sensitivity analyses, these seem to have more robustly associated the psychostimulants (but not atomoxetine) with adverse outcomes (Tables 2 and 3). This is indeed food for thought because the sensitivity analyses examined situations that were theoretically associated with higher risk and because the results of the analyses confirmed the theoretical expectations. This is especially food for thought because in one of the analyses (the 8-18 week analysis; Table 3), confounding because of illness severity or other reasons does not seem likely as an explanation.
If one chooses to err on the side of caution and accept that the sensitivity analyses indicate that amphetamines are associated with an increased risk of preeclampsia, placental abruption, and preterm birth, and that methylphenidate is associated with an increased risk of preeclampsia, then what is the worst case scenario? With an RR of 1.44 for amphetamine and preeclampsia (Table 3) and a base rate of 3.7% for this outcome (reference group, Table 1), pregnancies exposed to amphetamines could have a 5.3% rate of preeclampsia; that is, an absolute increase by 1.6%. This translates to a number needed to harm (NNH) value of 63. The NNH values for other outcomes would be higher, indicating lower risks. As an example, if amphetamine increases the risk of preterm birth by 16% (Table 3) and if the base rate for this adverse outcome is 1.1% (reference group, Table 1), then amphetamine would be associated with a 1.28% risk of preterm birth; that is, an absolute increase of 0.18%. The corresponding NNH is 556.
Finally, if a risk factor is admitted, what explains the risk? Cohen et al4 suggested that psychostimulants cause vasoconstriction, and the resultant impairment of placental perfusion can result in complications such as those that they studied. In this context, Newport et al7 found that, besides psychostimulant exposure, selective norepinephrine reuptake inhibitor exposure after week 20 of gestation was also significantly associated with hypertensive disorders of pregnancy. However, if this speculation is correct, the absence of risk with atomoxetine is curious, given that atomoxetine is also a noradrenergic drug. Perhaps, as already stated, the atomoxetine analyses by Cohen et al4 were underpowered.
Conclusions
It cannot be asserted that psychostimulants are responsible for adverse gestational outcomes because observational studies cannot determine causality in statistically significant relationships. Nevertheless, the available data do suggest the possibility that psychostimulants, especially amphetamines, may increase the risk of preeclampsia and possibly certain other adverse gestational outcomes; the absolute risk, however, is low, with NNH values ranging from about 60 to over 500. No adverse associations have been identified with atomoxetine, but this could be because of underpowered analyses. Women with ADHD should therefore balance the benefits of their medications against the potential risks associated with these drugs during pregnancy when they consider their options during pregnancy.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Haervig KB, Mortensen LH, Hansen AV, et al. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014;23(5):526-533. PubMed CrossRef
2. Louik C, Kerr S, Kelley KE, et al. Increasing use of ADHD medications in pregnancy. Pharmacoepidemiol Drug Saf. 2015;24(2):218-220. PubMed CrossRef
3. Andrade C. Risk of major congenital malformations associated with the use of methylphenidate or amphetamines in pregnancy. J Clin Psychiatry. 2018;79(1):18f12108.
4. Cohen JM, Hernandez-Diaz S, Bateman BT, et al. Placental complications associated with psychostimulant use in pregnancy. Obstet Gynecol. 2017;130(6):1192-1201. PubMed CrossRef
5. Bro SP, Kjaersgaard MI, Parner ET, et al. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015;7:139-147. PubMed CrossRef
6. Diav-Citrin O, Shechtman S, Arnon J, et al. Methylphenidate in pregnancy: a multicenter, prospective, comparative, observational study. J Clin Psychiatry. 2016;77(9):1176-1181. PubMed CrossRef
7. Newport DJ, Hostetter AL, Juul SH, et al. Prenatal psychostimulant and antidepressant exposure and risk of hypertensive disorders of pregnancy. J Clin Psychiatry. 2016;77(11):1538-1545. PubMed CrossRef
8. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052-e1056. PubMed CrossRef
9. Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017;78(2):e162-e165. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top