Major congenital malformation risks in association with gestational exposure to antiepileptic drugs (AEDs) have been extensively studied. Less information is available on other adverse outcomes associated with the use of these drugs during pregnancy. This article critically examines the risk of fetal loss, intrauterine growth retardation (IUGR), and preterm birth following gestational exposure to 14 AEDs, based on information obtained from a recent network meta-analysis of mostly nonrandomized, observational studies. The AEDs studied were carbamazepine, clobazam, clonazepam, ethosuximide, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbitone, phenytoin, primidone, topiramate, valproate, and vigabatrin. The results show that very few AEDs are significantly associated with each adverse outcome and that the implicated AEDs are different for different outcomes. Furthermore, when one discounts findings obtained in small sample analyses, almost no significant associations remain. Next, even these associations become questionable when one considers that they could have been due to confounding by indication. Finally, the few significant associations may have been false-positive findings because they were identified after performing a very large number of statistical tests. These caveats notwithstanding, guidance should err on the side of caution. Therefore, a conservative conclusion is that whereas analyses based on exposures in 2,000-4,000 pregnancies suggest that lamotrigine and carbamazepine are not associated with an increased risk of fetal loss, IUGR, and preterm birth, there is insufficient evidence to either firmly indict or firmly exonerate the other AEDs with regard to these outcomes.


ABSTRACT
Major congenital malformation risks in association with gestational exposure to antiepileptic drugs (AEDs) have been extensively studied. Less information is available on other adverse outcomes associated with the use of these drugs during pregnancy. This article critically examines the risk of fetal loss, intrauterine growth retardation (IUGR), and preterm birth following gestational exposure to 14 AEDs, based on information obtained from a recent network meta-analysis of mostly nonrandomized, observational studies. The AEDs studied were carbamazepine, clobazam, clonazepam, ethosuximide, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbitone, phenytoin, primidone, topiramate, valproate, and vigabatrin. The results show that very few AEDs are significantly associated with each adverse outcome and that the implicated AEDs are different for different outcomes. Furthermore, when one discounts findings obtained in small sample analyses, almost no significant associations remain. Next, even these associations become questionable when one considers that they could have been due to confounding by indication. Finally, the few significant associations may have been false-positive findings because they were identified after performing a very large number of statistical tests. These caveats notwithstanding, guidance should err on the side of caution. Therefore, a conservative conclusion is that whereas analyses based on exposures in 2,000-4,000 pregnancies suggest that lamotrigine and carbamazepine are not associated with an increased risk of fetal loss, IUGR, and preterm birth, there is insufficient evidence to either firmly indict or firmly exonerate the other AEDs with regard to these outcomes.
J Clin Psychiatry 2018;79(4):18f12467
To cite: Andrade C. Adverse pregnancy outcomes associated with gestational exposure to antiepileptic drugs. J Clin Psychiatry. 2018;79(4):18f12467.
To share: https://doi.org/10.4088/JCP.18f12467
© Copyright 2018 Physicians Postgraduate Press, Inc.
Previous articles in this series examined recent research and regulatory responses related to the use of valproate in women of reproductive age1 and major congenital malformations (MCMs) associated with exposure to antiepileptic drugs (AEDs) during pregnancy.2 However, there are many other gestational outcomes that need to be studied in the context of medication exposure during pregnancy, including exposure during late pregnancy. Examples of outcomes that have been studied in the context of antidepressant drugs, especially the selective serotonin reuptake inhibitors, include spontaneous abortions, intrauterine growth retardation (IUGR), preterm delivery, stillbirths, complications of delivery such as cesarean section and postpartum hemorrhage, the poor neonatal adaptation syndrome, persistent pulmonary hypertension of the newborn, and others; many of these outcomes had previously been examined in this column.3-11
There is far less information available about gestational outcomes after AED exposure during pregnancy. This article therefore summarizes and critically examines important findings relevant to prenatal outcomes; MCM data are not considered because these had already been examined earlier.1,2
Veroniki et al12 described a systematic review and Bayesian random effects network meta-analysis of prenatal outcomes following gestational exposure to AEDs. They searched electronic databases, reference lists, and other sources and identified 75 cohort studies, 2 case-control studies, and 1 randomized controlled trial (RCT) for 14 AEDs. Older AEDs included carbamazepine, clobazam, clonazepam, ethosuximide, phenobarbital, phenytoin, primidone, and valproate, and the newer AEDs included gabapentin, lamotrigine, levetiracetam, oxcarbazepine, topiramate, and vigabatrin. Comparisons were drawn between AED-exposed pregnancies and pregnancies in untreated women with epilepsy.
Fetal Loss
In this meta-analysis,12 fetal loss was studied as a single variable, not decomposed into early pregnancy abortions and late pregnancy stillbirths. There were 29 cohort studies, 1 case-control study, and 1 RCT that contributed data to this outcome; the pooled sample included 13,487 pregnancies.
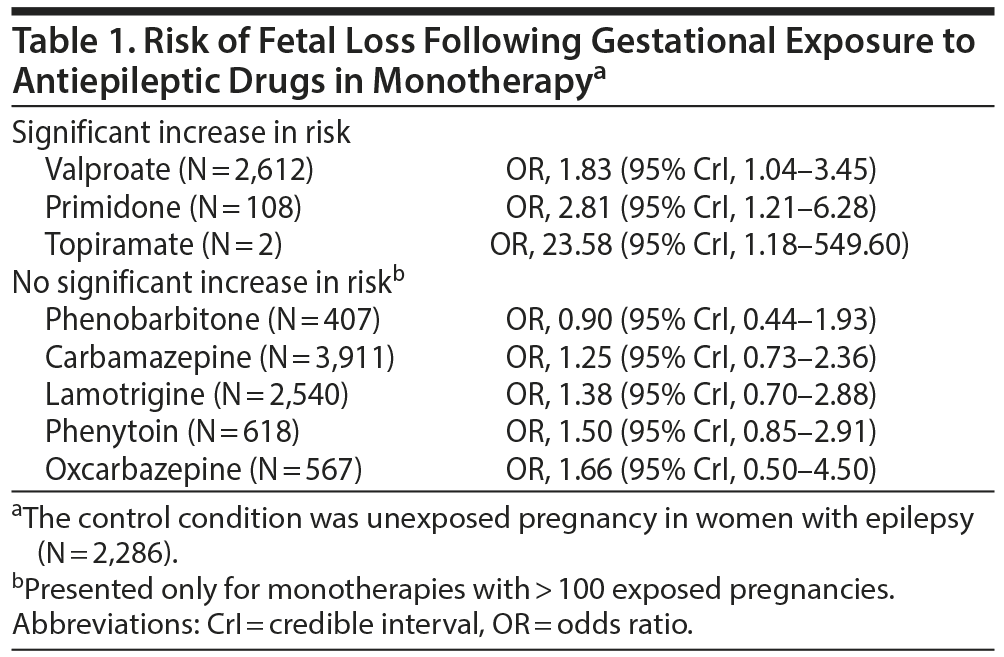
Table 1 presents important findings for the risk of fetal loss following AED exposure. In summary, only valproate, primidone, and topiramate were associated with a significantly increased risk in monotherapy; valproate was also associated with a significant increase in risk when combined with carbamazepine or phenytoin. Only the valproate finding should be taken seriously because the other analyses were based on very small numbers of exposed patients (Table 1).
AEDs administered in monotherapy that were not associated with a significantly increased risk of fetal loss included phenobarbitone, carbamazepine, lamotrigine, phenytoin, and oxcarbazepine. Other monotherapies, levetiracetam and clobazam, were also not associated with a significant increase in risk, but there were very few pregnancies exposed to these drugs, and so the conclusions are weak.
Of interest, a sensitivity analysis restricted to studies with > 300 pregnancies and a low risk of bias found that no AED was associated with an increased risk of fetal loss. However, although this sensitivity analysis included 4 cohort studies with 10,224 women exposed to 10 treatments, the analyses for individual treatments could have been underpowered. Other sensitivity analyses were also presented, and whereas the trends were similar to those reported in the main analysis, they were also mostly underpowered.
Intrauterine Growth Retardation
The meta-analysis12 included 16 cohort studies with 18,117 pregnancies that contributed data for an analysis of IUGR outcomes after gestational exposure to AEDs in monotherapy and polytherapy. IUGR was not defined and was presumably based on how authors in the original articles operationalized outcomes such as small-for-dates and low birth weight.
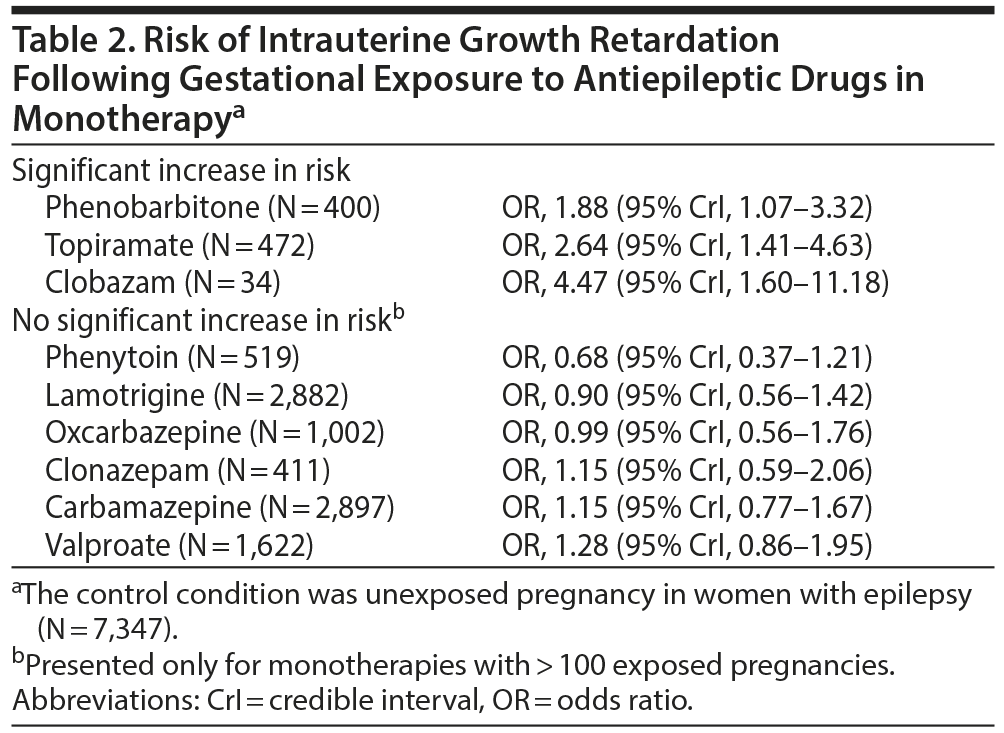
Table 2 presents important findings for the risk of IUGR following AED exposure. In summary, only phenobarbitone, topiramate, and clobazam, all in monotherapy, were associated with significantly increased risk, and the finding for clobazam should be interpreted with caution because it was based on a very small number of exposed patients (Table 2).
AEDs administered in monotherapy that were not associated with a significantly increased risk of IUGR included phenytoin, lamotrigine, oxcarbazepine, clonazepam, carbamazepine, and valproate. Other monotherapies, such as with levetiracetam, gabapentin, and primidone, were also not associated with a significant increase in risk, but there were very few pregnancies exposed to these drugs, and so the conclusions are weak. AED polytherapies were also not associated with increased risk, but there were too few exposed pregnancies for each polytherapy for firm conclusions to be possible. Isolated significant findings were obtained in sensitivity analyses, but these are not summarized here because the analyses were mostly underpowered based on small numbers and hence potentially unreliable.
Preterm Birth
The meta-analysis12 included 17 cohort studies with 17,133 pregnancies that contributed data for an analysis of preterm birth after gestational exposure to AEDs in monotherapy and polytherapy. Preterm birth was not defined and was presumably based on how authors in the original articles defined outcomes.
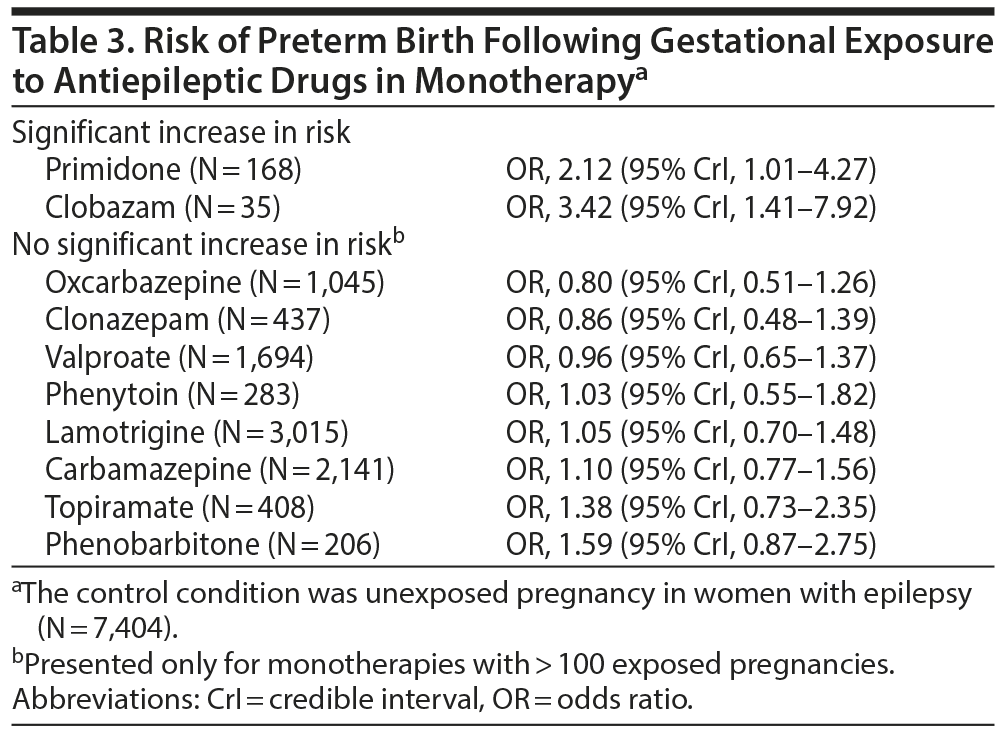
Primidone and clobazam were the only AEDs that, in monotherapy, were significantly associated with an increased risk of preterm birth (Table 3); these findings must be cautiously interpreted because of the small number of exposed pregnancies. There was no significant increase in the risk of preterm birth with oxcarbazepine, clonazepam, valproate, phenytoin, lamotrigine, carbamazepine, topiramate, and phenobarbitone (Table 3). The numbers were too small for interpretation of the results for vigabatrin, levetiracetam, ethosuximide, and gabapentin. The numbers were also too small for interpretation of the results for polytherapies.
Summary and Critical Comments
Summarizing the findings, gestational exposure to valproate, primidone, and topiramate was associated with an increased risk of fetal loss; exposure to 3 other AEDs, phenobarbitone, topiramate, and clobazam, was associated with an increased risk of IUGR; and primidone and clobazam were associated with an increased risk of preterm birth (Tables 1, 2, and 3).
Many of these significant findings emerged from analyses with few exposed pregnancies and even fewer adverse events of interest. These analyses could therefore be problematic to interpret because 1 fewer or 1 more positive outcome could result in a numerically large change in the OR. It could therefore be prudent to examine outcomes only in analyses where the number of exposed pregnancies exceeds an arbitrary threshold. If this threshold is set at 500 pregnancies, then the only important result is an increased risk of fetal loss associated with exposure to valproate. If the threshold is lowered to 400 to accommodate several analyses that had exposures in the window of 400-500 pregnancies, then phenobarbitone and topiramate would also be included for their significant associations with IUGR.
Importantly, with this threshold of 400 exposures, phenobarbitone, carbamazepine, lamotrigine, phenytoin, and oxcarbazepine showed no significant association with fetal loss; phenytoin, lamotrigine, oxcarbazepine, clonazepam, carbamazepine, and valproate showed no association with IUGR; and oxcarbazepine, clonazepam, valproate, lamotrigine, carbamazepine, and topiramate showed no association with preterm birth. Furthermore, lamotrigine and carbamazepine, which had exposures in 2,000-4,000 pregnancies in various analyses, were not associated with any of the adverse outcomes studied. Lamotrigine and carbamazepine, therefore, have the best evidence of safety with regard to these outcomes.
When one considers that a large number of analyses were performed without correction for a type I error, then even the very few significant associations identified may be questioned. Finally, when one considers that patients with more severe forms of epilepsy would have been more likely to continue their AEDs during pregnancy, then confounding by indication becomes an additional complication in the interpretation of the results. That is, variables related to severe epilepsy, the indication for which the AEDs were prescribed and continued during pregnancy, and not the AEDs themselves, may have predisposed to the adverse gestational outcomes.
The bottom line appears to be that gestational exposure to lamotrigine and carbamazepine does not appear to be associated with fetal loss, IUGR, and preterm birth and that there is insufficient evidence to indict or exonerate the other AEDs with regard to these pregnancy outcomes. It goes without saying that full information must nevertheless be communicated to patients and that decision-making should be shared.
Limitations
Conclusions drawn from a study are only as strong or weak as the study itself. This meta-analysis12 had many limitations. An important limitation is that exposure was not segmented by pregnancy trimester. In partial justification, if patients require AEDs for severe epilepsy or bipolar illness, then the risks associated with the illness probably outweigh the risks associated with continuous treatment with AEDs, making issues such as trimester of exposure merely academic. However, valproate exposure in early pregnancy is an obvious exception.1
Another limitation is that outcomes were clubbed, so we do not know the deconstructed effects of AED exposure on spontaneous abortion and stillbirth. In this regard, the association of valproate with fetal loss may well have been due to voluntary termination of pregnancy by women who did not wish to risk MCMs after valproate exposure.
The meta-analysis12 could not examine dose-dependent effects, something that is important, for example, in the context of MCMs.1,2 The meta-analysis could not adjust for confounds, and there is no assurance that the source studies conscientiously adjusted for confounds. It is possible that the limitations listed in this section could prejudice the direction of risk in either direction.
Finally, as stated early in this article, there are many more pregnancy outcomes that remain to be studied in the context of AED exposure during pregnancy.
Published online: July 31, 2018.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C. Valproate in pregnancy: recent research and regulatory responses. J Clin Psychiatry. 2018;79(3):18f12351. PubMed CrossRef
2. Andrade C. Major congenital malformations associated with exposure to antiepileptic drugs during pregnancy. J Clin Psychiatry. 2018;79(4):18f12449.
3. Andrade C. Continuing medical education: SSRIs and pregnancy. Indian J Psychiatry. 2010;52(1):83-86. PubMed CrossRef
4. Andrade C, Sandarsh S, Chethan KB, et al. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565-1575. PubMed CrossRef
5. Andrade C. Selective serotonin reuptake inhibitors and persistent pulmonary hypertension of the newborn. J Clin Psychiatry. 2012;73(5):e601-e605. PubMed CrossRef
6. Andrade C. Antenatal exposure to selective serotonin reuptake inhibitors and duration of gestation. J Clin Psychiatry. 2013;74(7):e633-e635. PubMed CrossRef
7. Andrade C. Adverse outcomes following serotonin reuptake inhibitor exposure during pregnancy. J Clin Psychiatry. 2016;77(2):e199-e200. PubMed CrossRef
8. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: meta-review of meta-analyses. J Clin Psychiatry. 2017;78(8):e1047-e1051. PubMed CrossRef
9. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052-e1056. PubMed CrossRef
10. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The effect of prenatal antidepressant exposure on neonatal adaptation: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e309-e320. PubMed CrossRef
11. Jiang HY, Xu LL, Li YC, et al. Antidepressant use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta-analysis. J Psychiatr Res. 2016;83:160-167. PubMed CrossRef
12. Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. PubMed CrossRef