Objective: This systematic review and meta-analysis examined the association between maternal antenatal anxiety (AA) and a range of perinatal outcomes.
Data Sources: Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, PsycINFO, CINAHL, Embase, and the Cochrane Library were searched to May 31, 2016, using controlled vocabulary and keywords (eg, prenatal, anxiety, preterm).
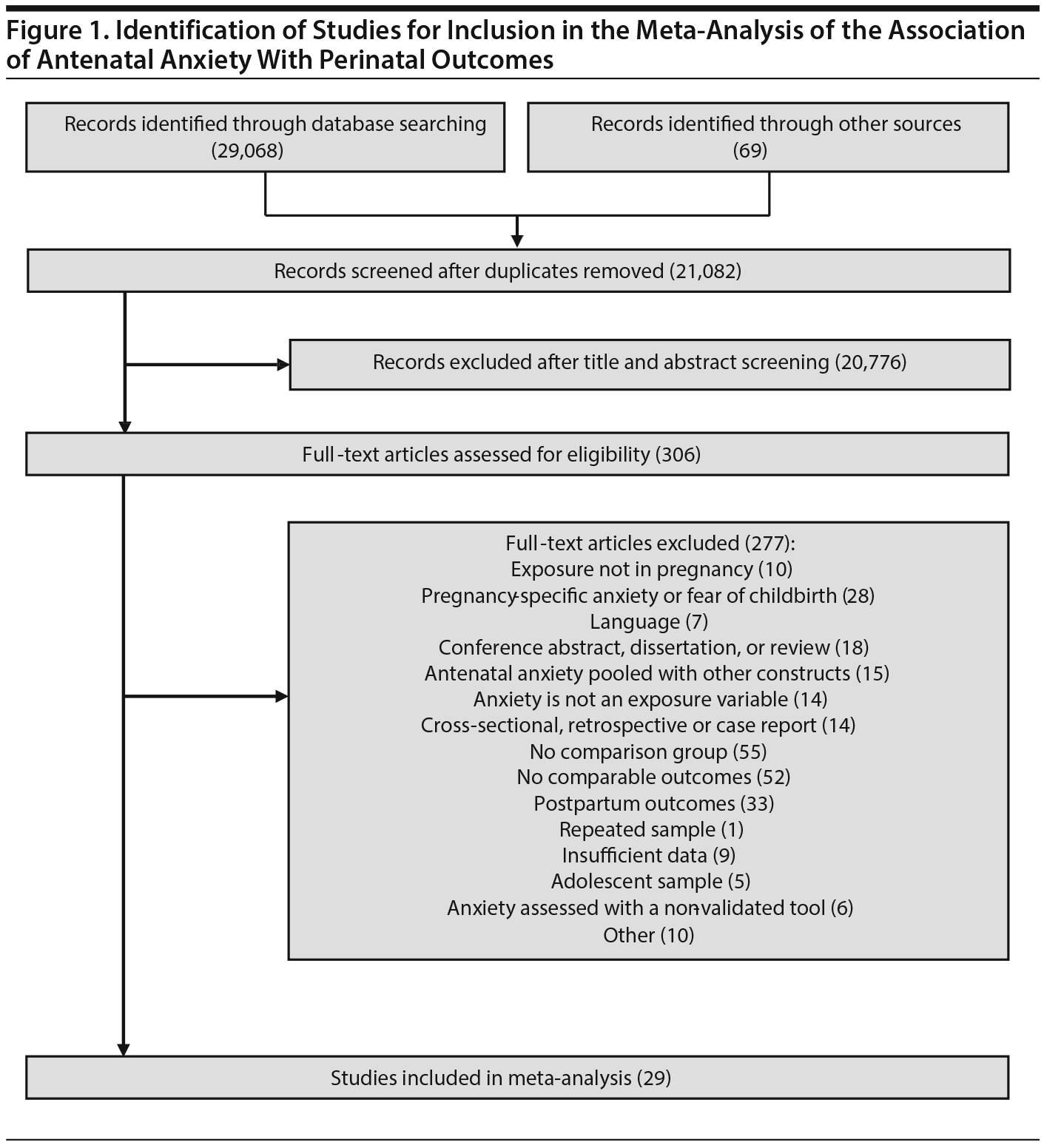
Study Selection: Perinatal outcomes of women with and without AA (diagnosed or self-reported using validated scale) derived from English language, prospectively collected data were included. 1,458 abstracts were reviewed, 306 articles were retrieved, and 29 articles were included.
Data Extraction: Two independent reviewers extracted data and assessed quality. Random-effects models were utilized for outcomes (≥ 3 studies). Subanalyses examined potential effect moderators including study quality and diagnostic versus self-reported anxiety among others.
Results: Antenatal anxiety was associated with increased odds for preterm birth (pooled odds ratio [OR] = 1.54; 95% confidence interval [CI], 1.39 to 1.70, 16 studies) and spontaneous preterm birth (OR = 1.41; 95% CI, 1.13 to 1.75), lower mean birth weight (mean difference = −55.96 g; 95% CI, −93.62 to −18.31 g), increased odds for low birth weight (OR = 1.80; 95% CI, 1.48 to 2.18), earlier gestational age (mean difference = −0.13 wk; 95% CI, −0.22 to −0.04 wk), increased odds for being small for gestational age (OR = 1.48; 95% CI, 1.26 to 1.74), and smaller head circumference (mean difference = −0.25 cm; 95% CI, −0.45 to −0.06 cm). Heterogeneity between studies was not significant for most outcomes. Subanalyses for birth weight found women with diagnosed anxiety had infants with significantly lower birth weight (P < .03) compared to those identified with rating scales (although both subanalyses were significant [P < .01]). Associations between anxiety and preeclampsia, cesarean delivery, and Apgar scores were nonsignificant.
Conclusions: Antenatal anxiety is associated with multiple adverse perinatal outcomes and is not benign. The impact of treating anxiety on these associations is unknown.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Maternal Anxiety During Pregnancy and the Association With Adverse Perinatal Outcomes:
Systematic Review and Meta-Analysis

ABSTRACT
Objective: This systematic review and meta-analysis examined the association between maternal antenatal anxiety (AA) and a range of perinatal outcomes.
Data Sources: Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, PsycINFO, CINAHL, Embase, and the Cochrane Library were searched to May 31, 2016, using controlled vocabulary and keywords (eg, prenatal, anxiety, preterm).
Study Selection: Perinatal outcomes of women with and without AA (diagnosed or self-reported using validated scale) derived from English language, prospectively collected data were included. 1,458 abstracts were reviewed, 306 articles were retrieved, and 29 articles were included.
Data Extraction: Two independent reviewers extracted data and assessed quality. Random-effects models were utilized for outcomes (≥ 3 studies). Subanalyses examined potential effect moderators including study quality and diagnostic versus self-reported anxiety among others.
Results: Antenatal anxiety was associated with increased odds for preterm birth (pooled odds ratio [OR] = 1.54; 95% confidence interval [CI], 1.39 to 1.70, 16 studies) and spontaneous preterm birth (OR = 1.41; 95% CI, 1.13 to 1.75), lower mean birth weight (mean difference = −55.96 g; 95% CI, −93.62 to −18.31 g), increased odds for low birth weight (OR = 1.80; 95% CI, 1.48 to 2.18), earlier gestational age (mean difference = −0.13 wk; 95% CI, −0.22 to −0.04 wk), increased odds for being small for gestational age (OR = 1.48; 95% CI, 1.26 to 1.74), and smaller head circumference (mean difference = −0.25 cm; 95% CI, −0.45 to −0.06 cm). Heterogeneity between studies was not significant for most outcomes. Subanalyses for birth weight found women with diagnosed anxiety had infants with significantly lower birth weight (P < .03) compared to those identified with rating scales (although both subanalyses were significant [P < .01]). Associations between anxiety and preeclampsia, cesarean delivery, and Apgar scores were nonsignificant.
Conclusions: Antenatal anxiety is associated with multiple adverse perinatal outcomes and is not benign. The impact of treating anxiety on these associations is unknown.
J Clin Psychiatry 2018;79(5):17r12011
To cite: Grigoriadis S, Graves L, Peer M, et al. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J Clin Psychiatry. 2018;79(5):17r12011.
To share: https://doi.org/10.4088/JCP.17r12011
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Sunnybrook Health Sciences Centre, and the University of Toronto, Toronto, Ontario, Canada
bFamily and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, Michigan
cEvaluative Clinical Sciences, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
dBiostatistics and Department of Medicine, Toronto General Hospital, and the University of Toronto, Toronto, Ontario, Canada
eDepartment of Psychiatry, Women’s College Hospital, and the University of Toronto, Toronto, Ontario, Canada
fLawrence S. Bloomberg Faculty of Nursing, University of Toronto, Toronto, Ontario, Canada
gDepartment of Psychiatry and Behavioural Neurosciences, St Joseph’s Healthcare Hamilton and McMaster University, Hamilton, Ontario, Canada
hDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
iHealth Nexus, Toronto, Ontario, Canada
jDivision of Neurology, St Michael’s Hospital, Toronto, Ontario, Canada
*Corresponding author: Sophie Grigoriadis, MD, Women’s Mood and Anxiety Clinic: Reproductive Transitions, Department of Psychiatry, FG 29, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, Ontario, M4N 3M5, Canada ([email protected]).
Anxiety disorders are widespread psychiatric disorders appearing across the lifespan, including during pregnancy (the pooled prevalence during pregnancy is 15.2% for any anxiety disorder and 22.9% for anxiety symptoms).1 Some studies suggest elevated rates of certain anxiety disorders such as generalized anxiety disorder (8.5%-9.5%)2,3 compared to rates in the general population (3.1%)4 over 1 year. As the disorders are common, understanding their effects on maternal and infant outcomes is important, yet studies to date5,6 have not provided uniform conclusions, resulting in a lack of information critical to evidence-based treatment decisions for anxiety during pregnancy.
Attention has historically focused on perinatal depression. For example, one systematic review7 noted 14 studies examining the effect of maternal depression on preterm birth (PTB) and only 4 studies examining maternal anxiety. Although research is beginning to focus on antenatal anxiety, syntheses of its impact on perinatal outcomes have not kept pace. To date, meta-analyses of the effects of anxiety during pregnancy have been narrowly focused on preterm birth8,9 and low birth weight,9 and while 2 systematic reviews10,11 have addressed a broader range of outcomes, results were inconsistent and, with newer published work, need to be readdressed. Given the high prevalence of antenatal anxiety and the preliminary recognition of its negative effect on maternal and infant outcomes, it is important to systematically summarize the literature across diverse outcomes.
Our work addresses the significant gap in the literature by extending previous meta-analytic work to include a broad range of clinically relevant outcomes. The purpose of this systematic review and meta-analysis was to synthesize available data on the association between exposure to antenatal anxiety and the risk of various perinatal outcomes. We pooled results from studies comparing outcomes in pregnancies exposed and unexposed to maternal anxiety. Moreover, we examined the potential effect of variables that may account for heterogeneity as modifying variables on outcomes such as a diagnosis of anxiety versus anxiety measured by rating scales, among others.
METHODS
Search Strategy and Selection Criteria
We conducted a systematic review and meta-analyses following the guidelines of the Meta-Analysis Of Observational Studies in Epidemiology group,12 using methods previously described.13 Briefly, the review was guided by an advisory committee of key stakeholders. Independent literature searches were conducted by 2 professional librarians with expertise in psychiatry and psychopharmacology. As part of a broader search for studies of the impact of antenatal anxiety and medication use, 1 librarian searched Ovid MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, PsycINFO, CINAHL, Embase, and the Cochrane Library from their start date to May 31, 2016. Keywords included, but were not limited to, anxiety/phobia, posttraumatic stress disorder, panic disorder, obsessive compulsive disorder, pregnancy, prenatal or antenatal, congenital malformations, cardiac malformation, preterm birth or premature delivery, low birth weight, birth weight, gestational age, Apgar, NICU, neonatal/infant outcomes, delivery outcomes, and preeclampsia. The second experienced medical information specialist’s search strategy was developed and tested through an iterative process in consultation with the review team, and the same databases were searched. This core strategy was reviewed prior to execution by another senior information specialist using the Evidence Based Checklist for the Peer Review of Electronic Search Strategies.14 Strategies utilized a combination of controlled vocabulary (eg, pregnancy, anxiety disorders) and keywords (eg, prenatal, anxiety, preterm) (full search strategy available from the authors upon request). Vocabulary and syntax were adjusted across databases. Results were limited to the English language, removing animal-only records and opinion pieces. Reference lists of reviews and meta-analyses were also searched. We followed the PRISMA statement for reporting systematic reviews and meta-analyses.15
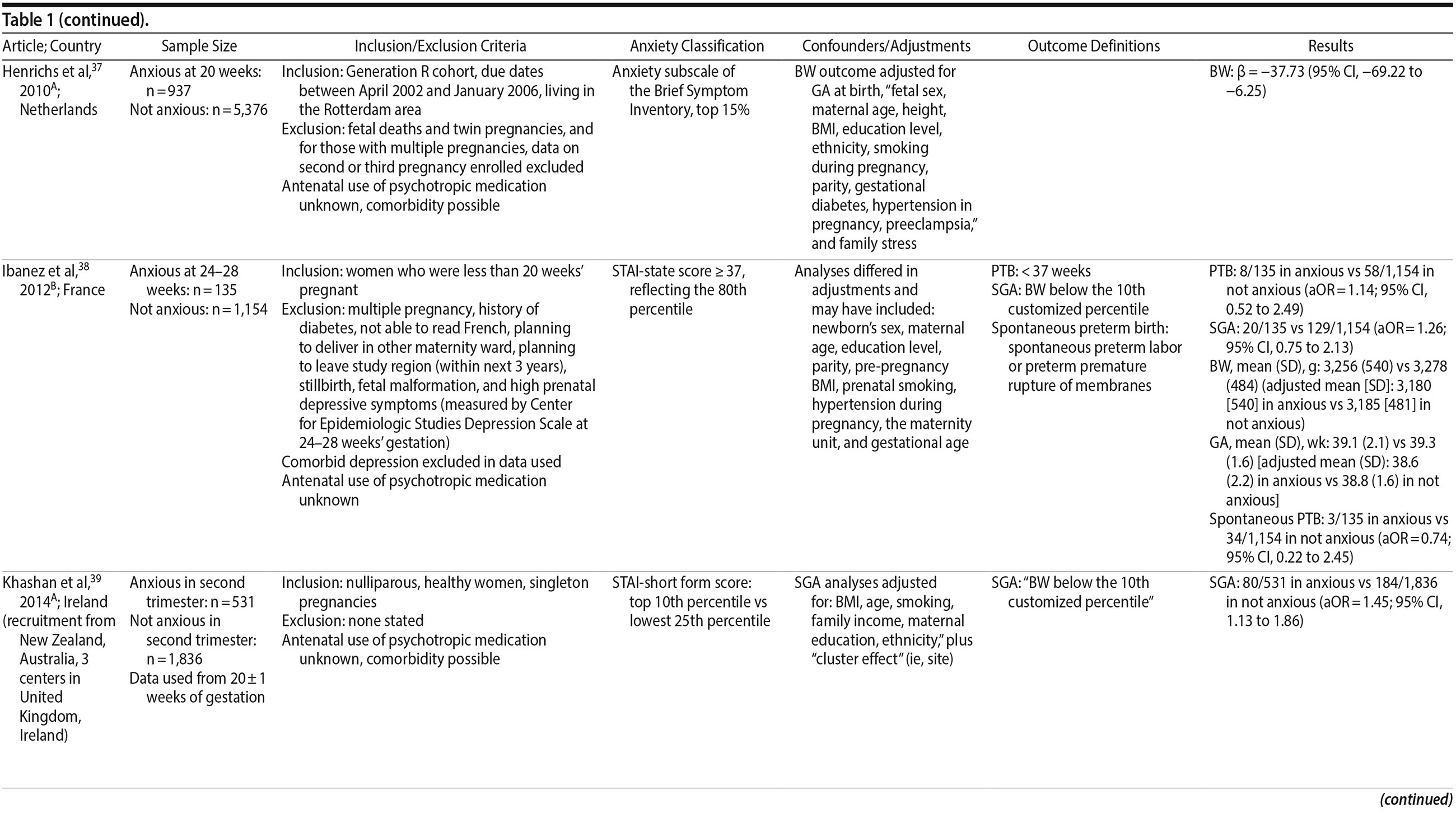
Original prospectively collected data with an assessment of maternal anxiety any time during pregnancy and an unexposed comparison group of pregnant women without anxiety were considered. Maternal anxiety required either a clinical diagnosis of any anxiety disorder or use of a validated self-report anxiety measure with a cutoff score for high anxiety. Pregnancy-specific or pregnancy-related anxiety was not considered, as it is believed to represent a distinct concept not well measured by current scales.16 Outcomes in the infant or mother in the gestational and delivery periods were acceptable. When a sample was repeated in more than one publication, the article providing the most valid data relevant to our research question was chosen. One article presented 2 time points; as both were from the second trimester, the second time point with the larger sample size was used. For cases in which antenatal anxiety was assessed using multiple self-report measures, data representing the largest sample size were included. We excluded studies that pooled antenatal and postpartum anxiety scores, studies with adolescent samples, abstracts, conference proceedings, and unpublished data. Two reviewers independently screened all titles and abstracts and retrieved all eligible studies. Requests for raw data, clarification of methodology, or data analysis were sent to 9 authors; 4 responses were received.

- Treatment decisions for women with anxiety during pregnancy must consider the risks of anxiety itself on mothers and their infants, yet current understanding of these risks is incomplete.
- Anxiety during pregnancy is associated with several negative birth outcomes, and pregnant women should therefore be screened for anxiety and be informed about these risks.
Data Analysis
The quality assessment and data extraction methods were based on previously published methodology developed by our team.13 Two reviewers conducted quality assessments and extracted data. Disagreements were resolved on a case-by-case basis by discussion with the study leads (S.G., L.G.), until consensus was reached. Study quality was assessed with the Systematic Assessment of Quality in Observational Research (SAQOR),13 adapted from the Downs and Black17 checklist and the Newcastle-Ottawa Scale.18 Articles were assessed under the following categories: (1) sample, (2) control group, (3) quality of exposure/outcome measure, (4) follow-up, and (5) distorting influences/control for confounders. Based on the SAQOR criteria and a modification of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system,19 each article was assigned a quality rating of high, moderate, low, or very low quality, further dichotomized into “above quality threshold” (high, moderate, and low) and “below quality threshold” (very low).
Data extraction procedures were based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria.20 Extracted data included authors, year of publication, source country, details of study design, participants (sample, control, demographics, and clinical characteristics), inclusion/exclusion criteria, method and type of anxiety assessment, timing of anxiety assessment, outcomes and their assessment methods and definitions, statistical adjustment for confounders, and loss to follow-up. Adjusted estimates with their variances were extracted when available; when adjusted estimates were not provided in the published data, we calculated crude odds ratios or mean differences and sample variances. Before calculating the odds ratio for studies that included cells with a 0 count, we added 0.5 to these cells. Data were extracted by one reviewer and checked by another and the primary author (S.G.); disagreements were resolved by the primary author. Outcomes were as defined by the authors of the original publication.
DerSimonian and Laird21 random effects models were used to pool estimates of the odds ratio for binary outcomes and the weighted mean difference for continuous outcomes. In the few instances in which relative risks were given, they were treated as odds ratios, given that events were typically rare. We visually inspected funnel plots portraying individual study estimates (on the log scale for odds ratios) against their standard error to assess for the possibility of publication bias. The Egger weighted regression method was used to formally test for publication bias.22 Cochrane Q and visual inspection of forest plots were used to assess between-study heterogeneity, which was then quantified by I2, the percentage of the observed between-study variance in effects that is in excess of what is expected due to chance. Larger values of I2 suggest that the effect varies quantitatively from study to study. The amount of heterogeneity may be characterized as low, moderate, or high (I2 = 25%, 50%, or 75%, respectively).23 However I2 does not indicate qualitative variation, whether the direction of the effect varies—high heterogeneity in the size of an effect can still exist in the context of a large degree of certainty about the direction of the effect.
Regardless of the statistical significance of the Q statistic for an outcome, we explored sources of heterogeneity through subgroup analyses as they were planned a priori. These subgroup analyses examined within-group effects and between-group differences in pooled effects based on the following study characteristics: (1) study quality above versus below threshold; (2) anxiety identification using clinical diagnosis versus validated self-report measure with cutoff score; (3) adjusted versus unadjusted estimates; (4) antenatal assessment time; (5) registry versus non-registry data; and (6) source country. Additional subgroup analyses were completed for the following outcomes when data were available: (1) controlling for depression (preterm birth); (2) effect of specific anxiety diagnoses (preterm birth); (3) adjustment for gestational age (birth weight); and (4) percentile threshold (small for gestational age). Fixed-effects models were used in analyses with < 3 articles. Statistical analyses were completed with the metafor package in R (3.3.2) and similar to our other work.24 Forest plots were generated with Review Manager (RevMan) 5.3 for Windows (Review Manager version 5.3, Cochrane Collaboration, http://tech.cochrane.org/revman).
RESULTS
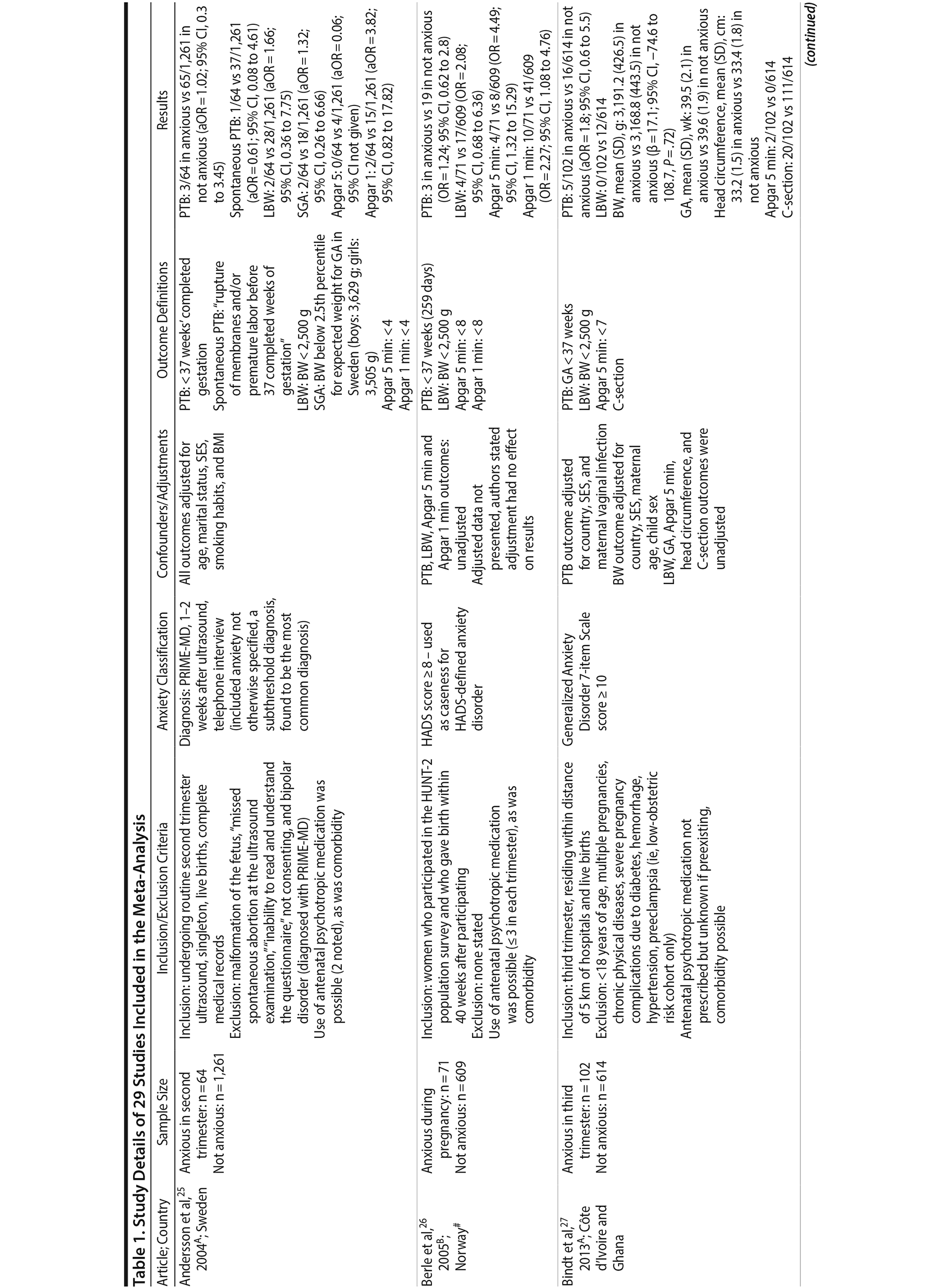
Of the 1,458 articles for which abstracts were reviewed, 1,152 were excluded based on title and abstract. Of the remaining 306 retrieved and assessed articles, 29 studies met the inclusion criteria (Figure 1) and were included in meta-analyses5,6,25-51; 16 were above quality threshold and 13 were below. Detailed study characteristics are provided in Table 1. Suitable data were available for a total of 11 outcomes.
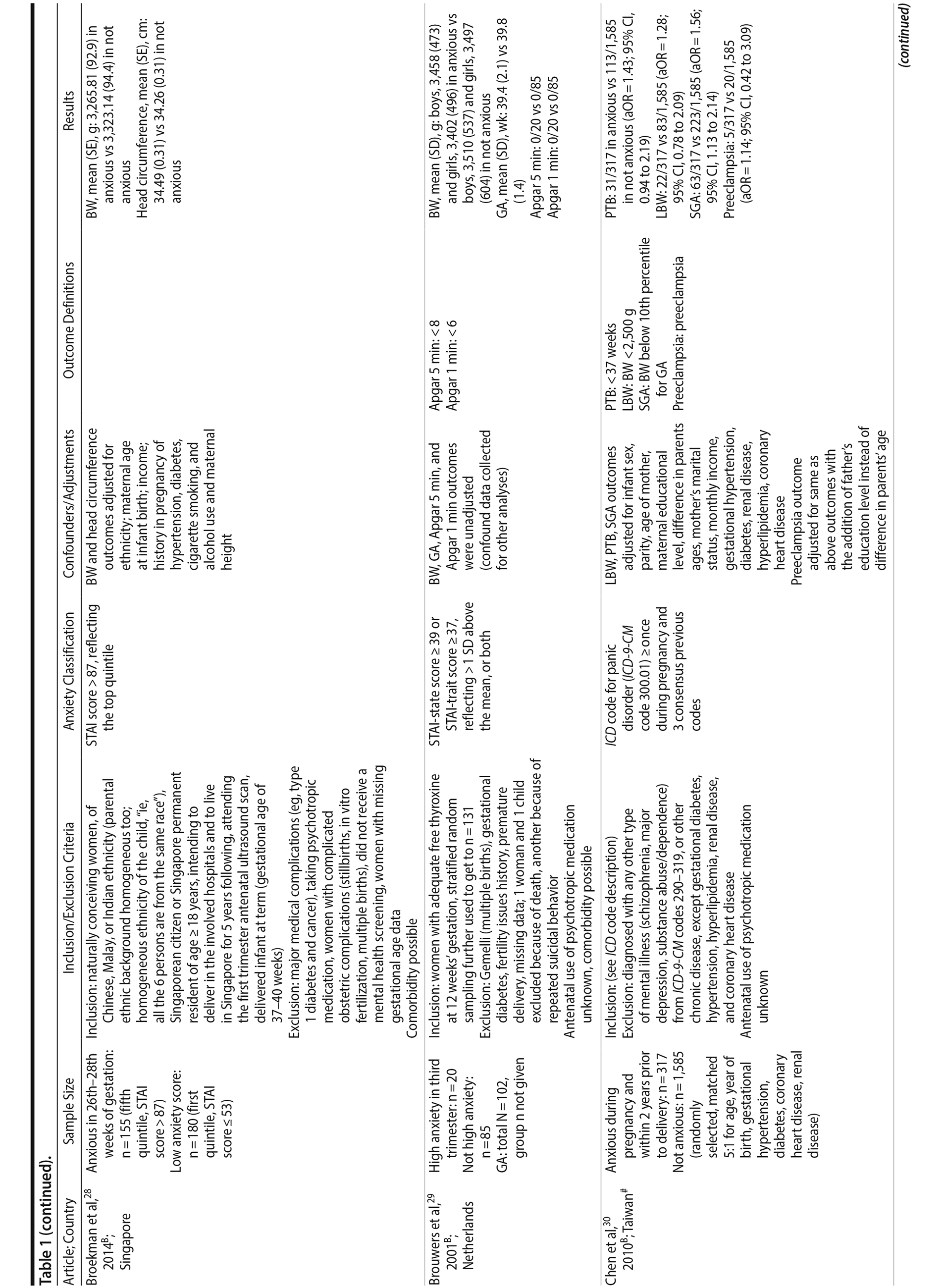
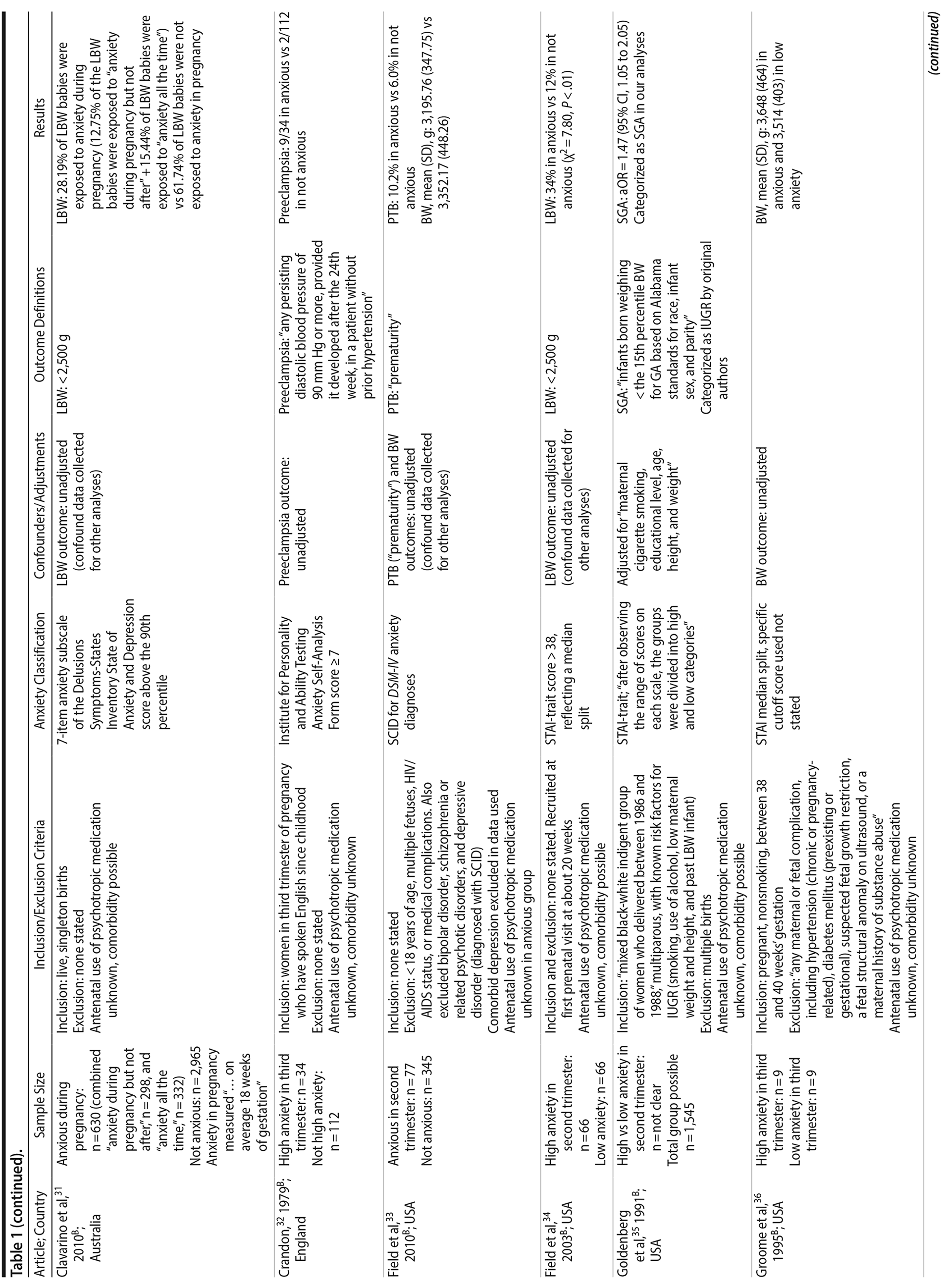
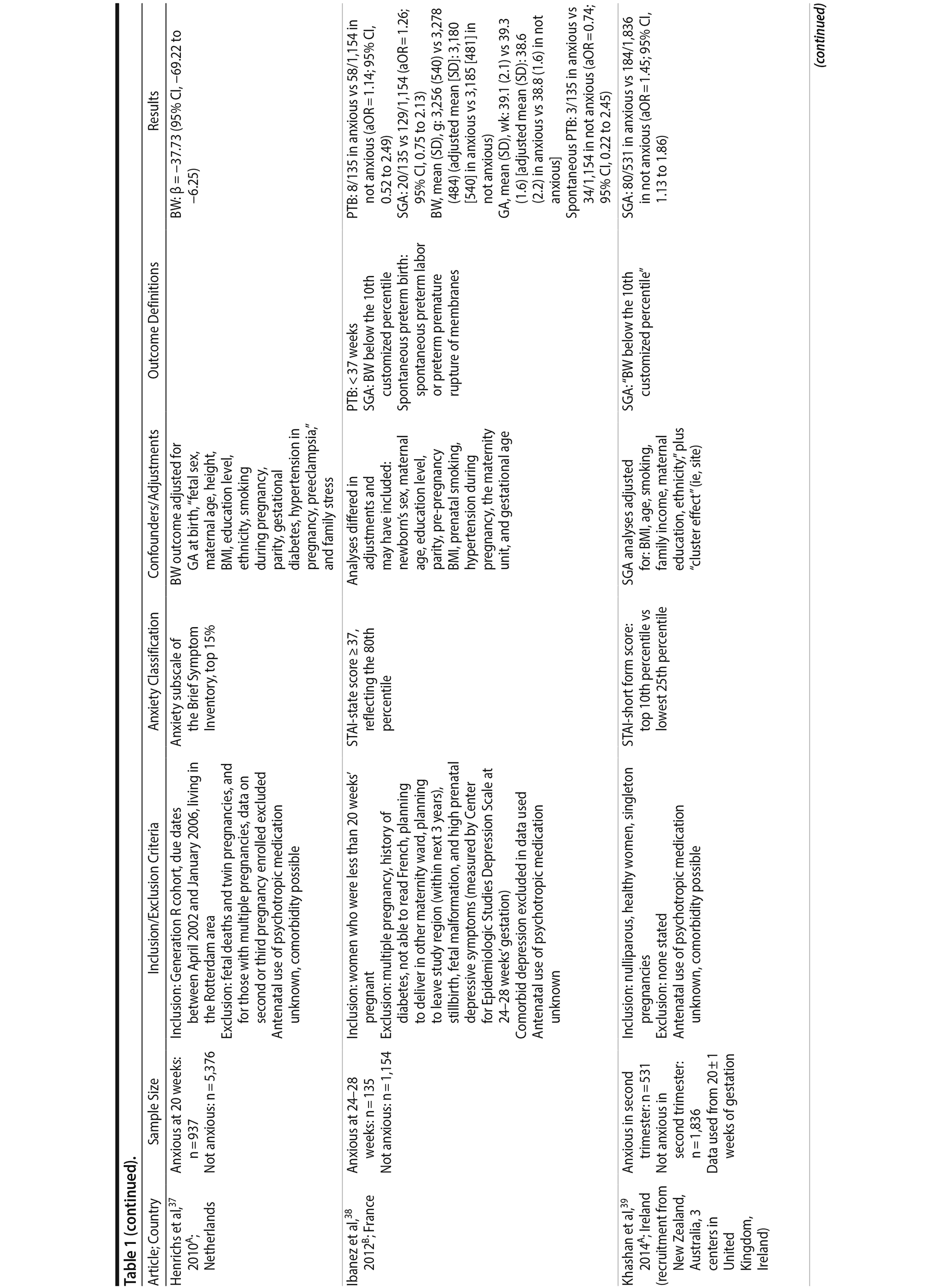
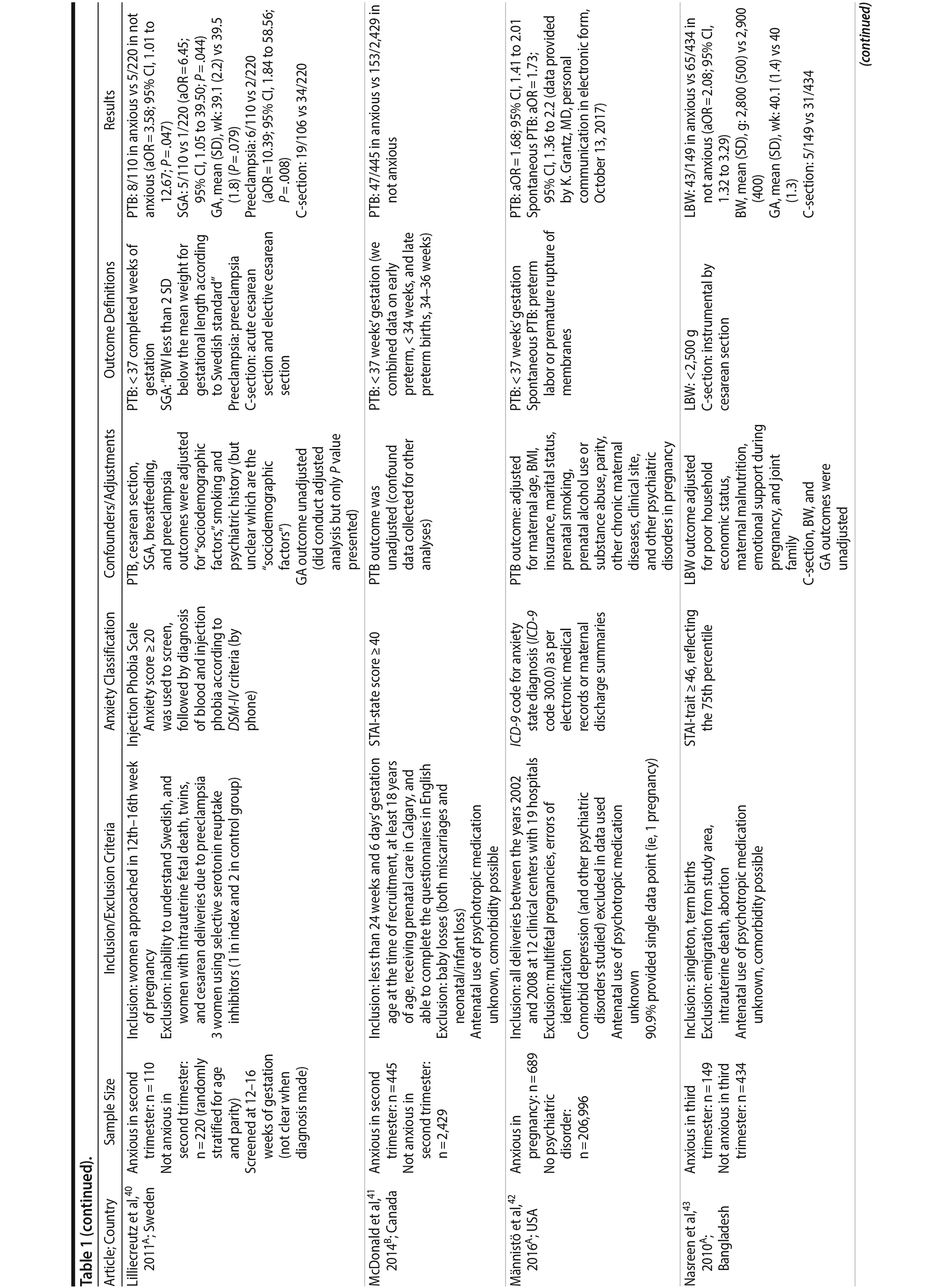
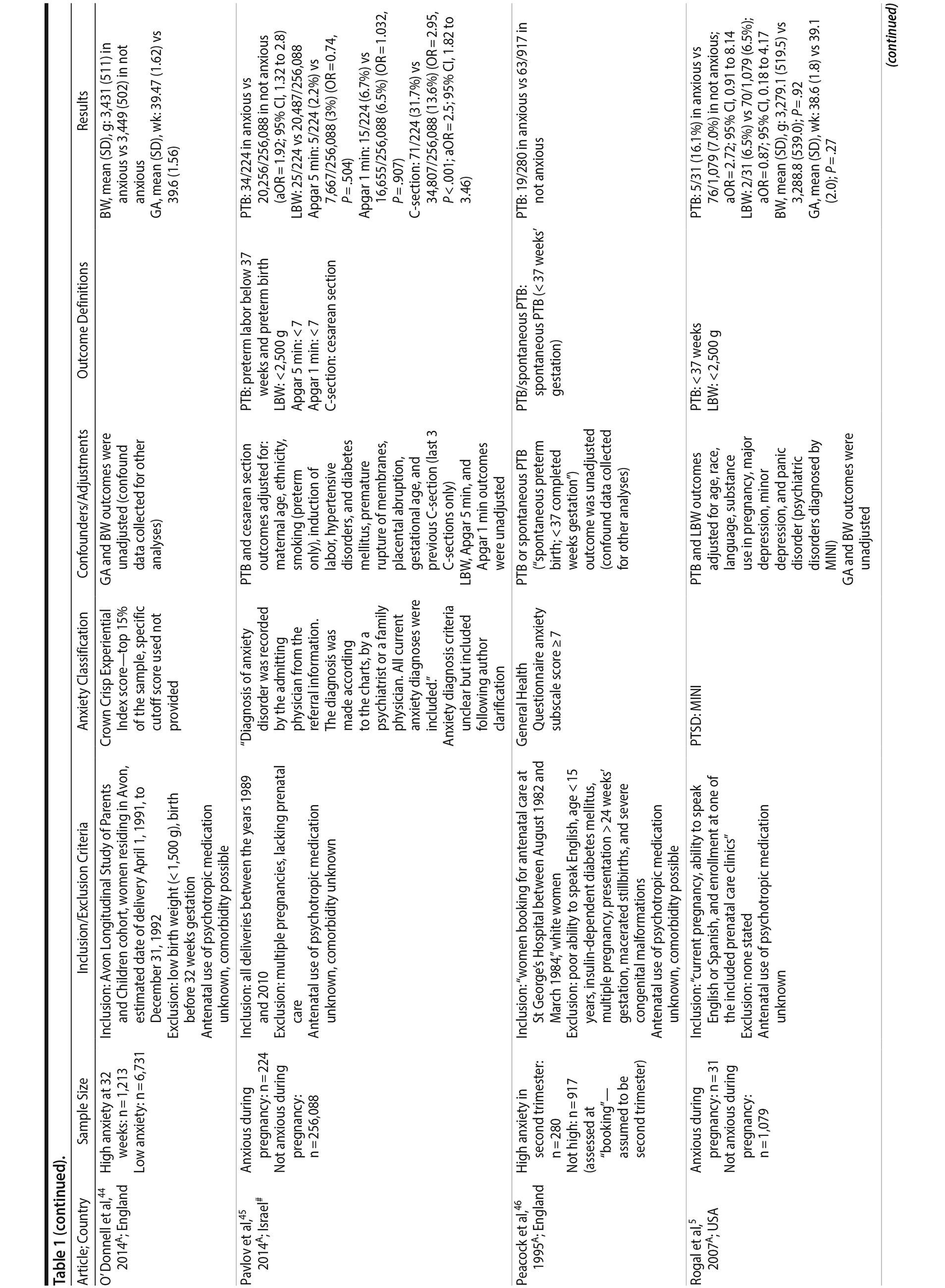
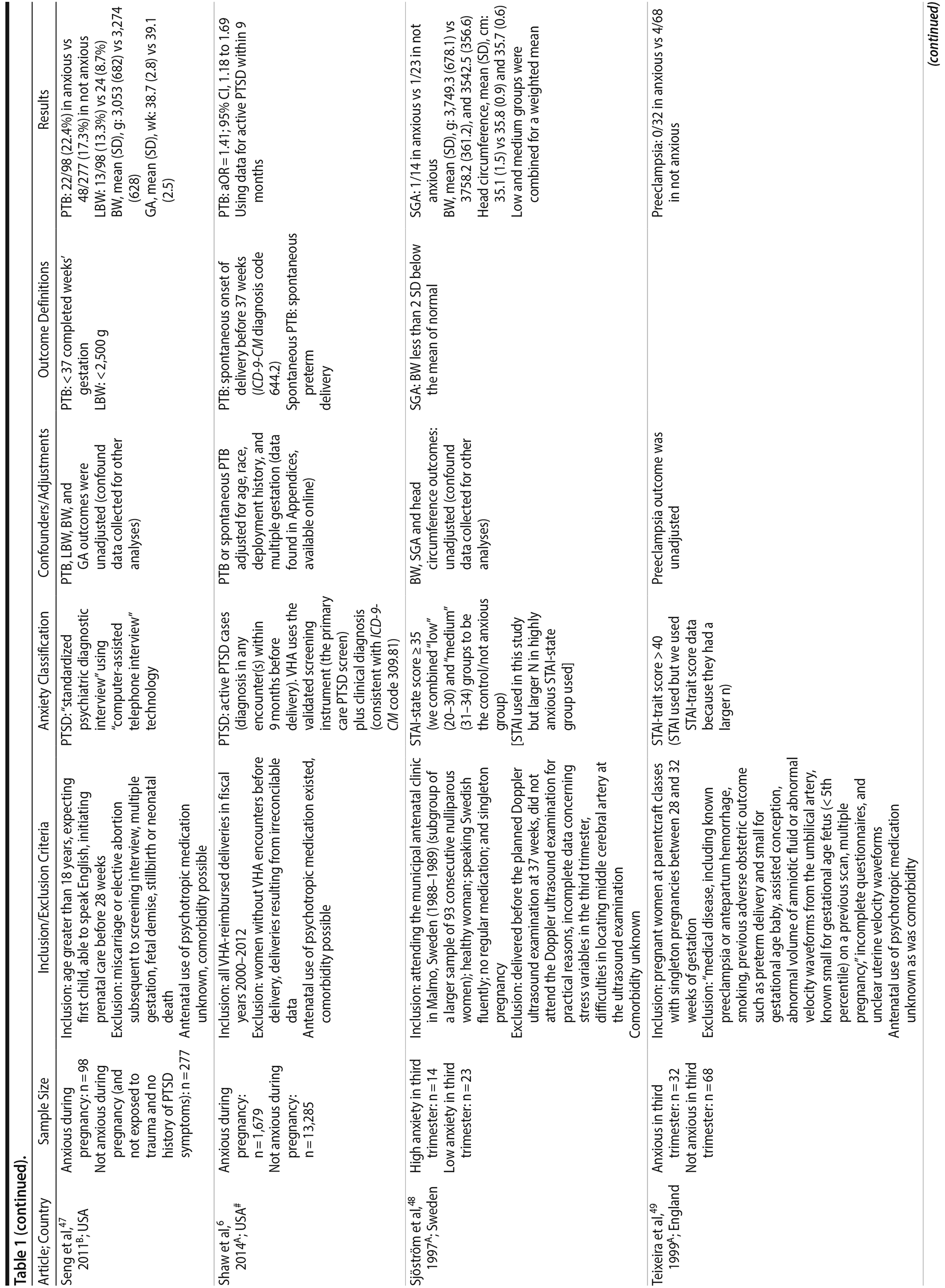
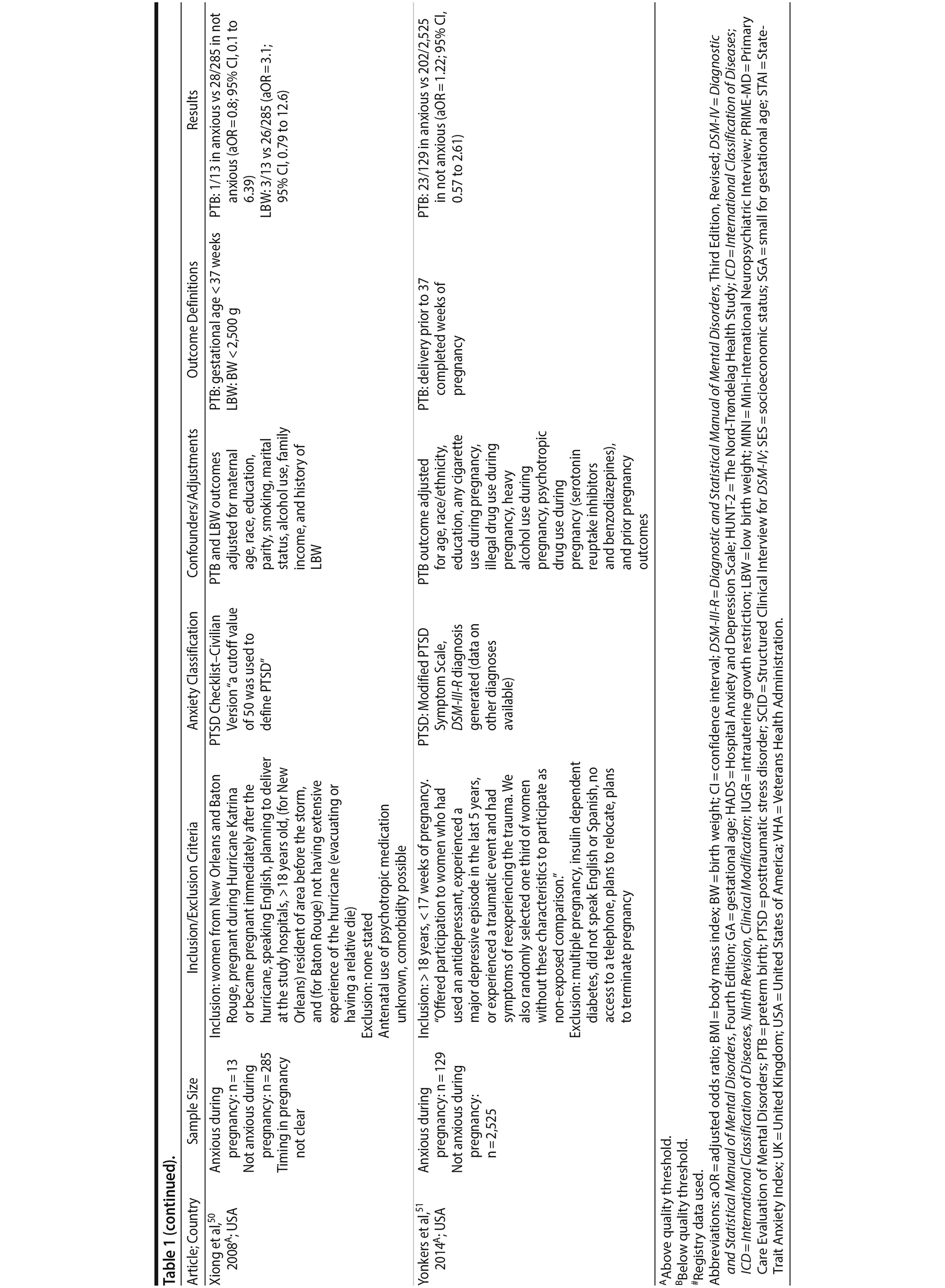
In 16 pooled studies, maternal antenatal anxiety was significantly associated with preterm birth (odds ratio [OR] = 1.54; 95% CI, 1.39 to 1.70; P < .0001; Figure 2A). Heterogeneity across studies was not significant (Q15 = 11.71, P = .70; I2 = 0%) as were none of the moderator variables (Table 2). Five studies with women experiencing antenatal posttraumatic stress disorder/symptoms specifically pooled to an OR similar to the main analyses and no heterogeneity (OR = 1.41; 95% CI, 1.20 to 1.67, P < .01). Regarding spontaneous preterm birth, 5 studies pooled to a significant effect (OR = 1.41; 95% CI, 1.13 to 1.75; P < .01; Figure 2B), similar in magnitude to the main analysis, without heterogeneity. Interestingly, the moderators registry/non-registry data and source country crossed the significance threshold (both Q1 = 3.80, P = .05), but the subgroups contained only 2 or 3 studies, while identification of anxiety by diagnostic versus non-diagnostic (self-report) measures approached borderline significance (Q1 = 3.23, P = .07); the remainder of the moderator analyses were not significant (Table 2).
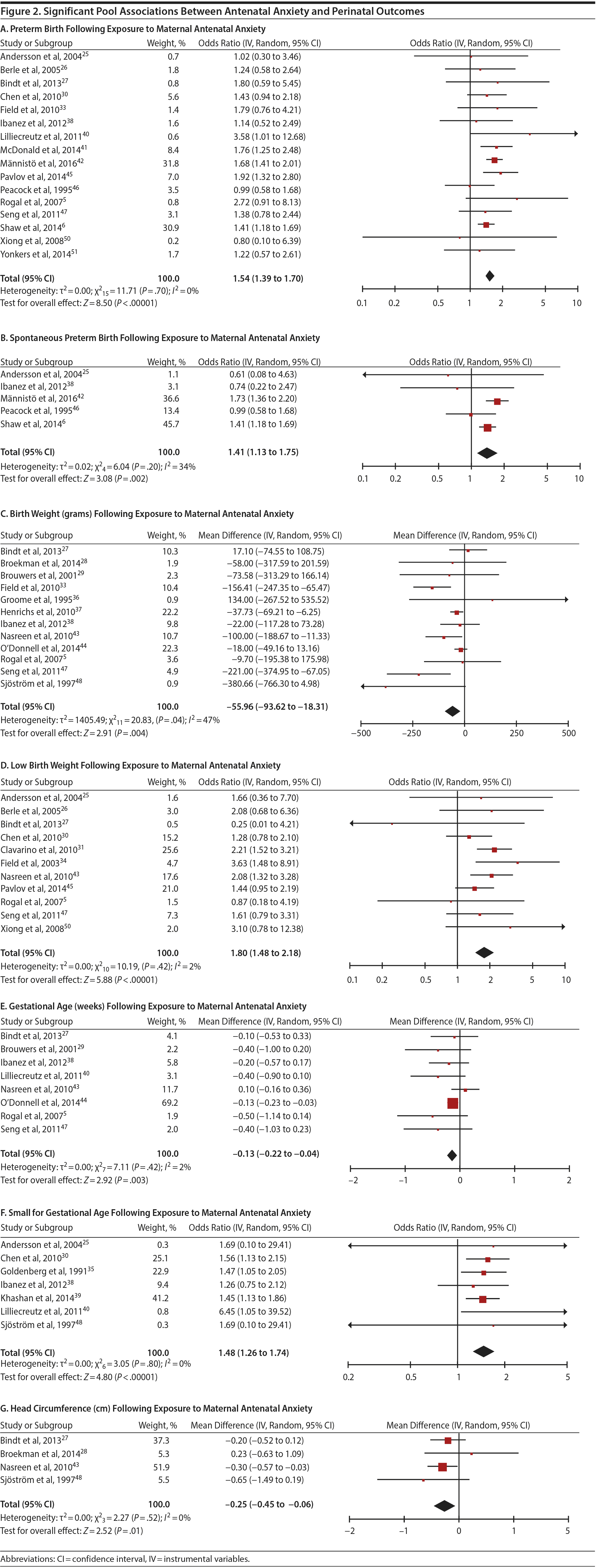
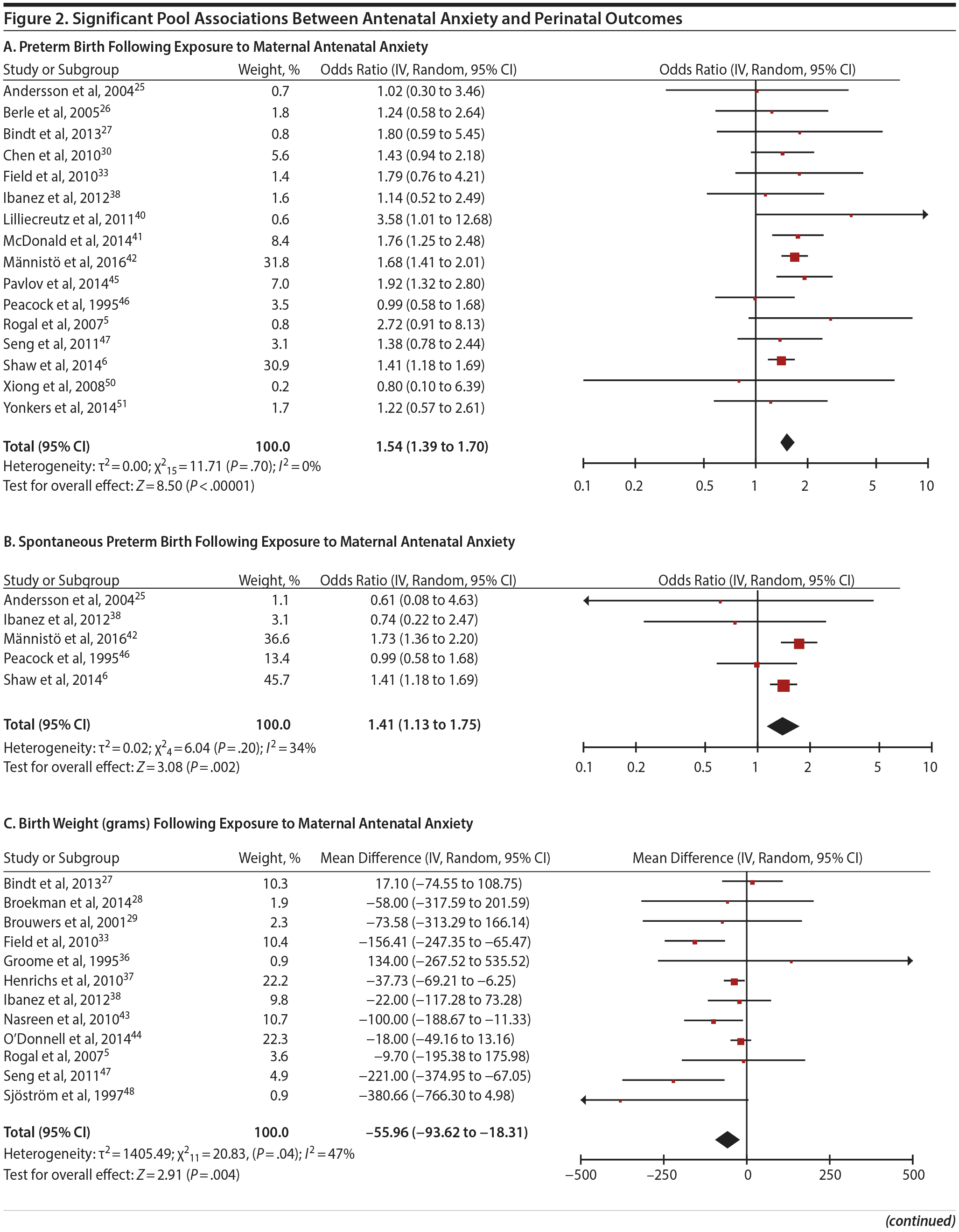
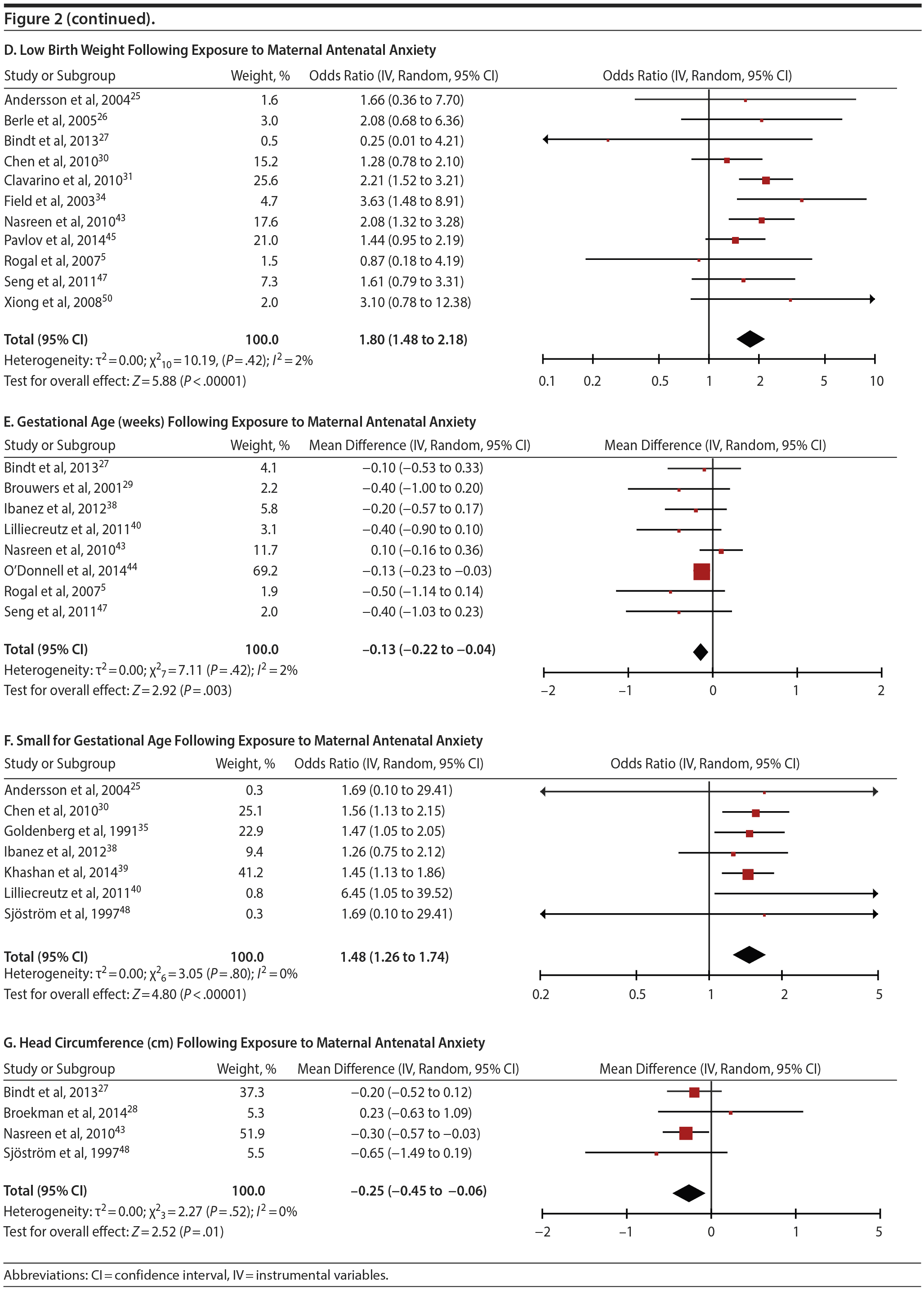
Antenatal anxiety was associated with lower infant birth weight (continuous variable, 12 studies, mean difference = −55.96 g; 95% CI, −93.62 to −18.31 g; P = .004; Figure 2C). Significant heterogeneity across studies was present, accounting for 47% of the variance (Q11 = 20.83, P = .04; I2 = 47%). Infants of mothers experiencing diagnosed clinical anxiety were lower in birth weight than those whose mothers’ anxiety was assessed using a self-reported measure (mean difference = −143.47 g [95% CI, −240.27 to −46.67 g] versus −30.42 g [−51.87 to −8.97 g]; Q1 = 4.99, P = .03; Table 2). An additional subanalysis examining adjustment for gestational age as a potential moderator was not significant.
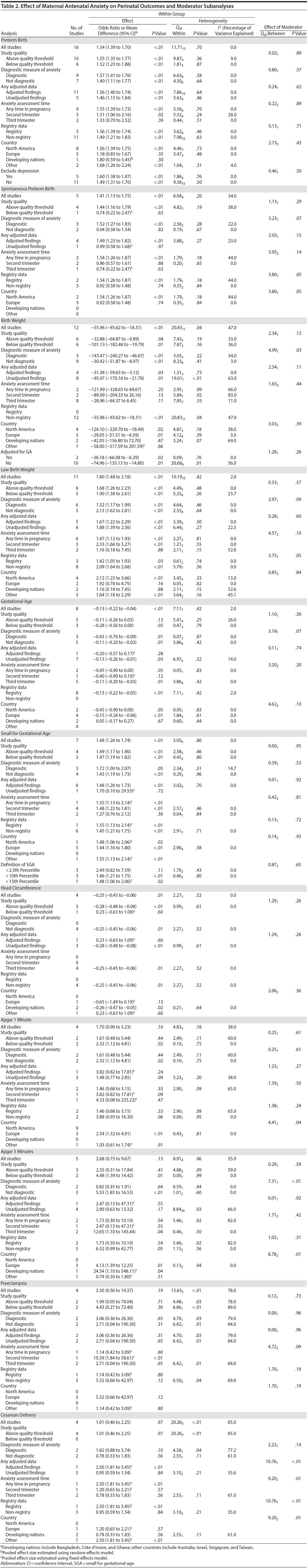
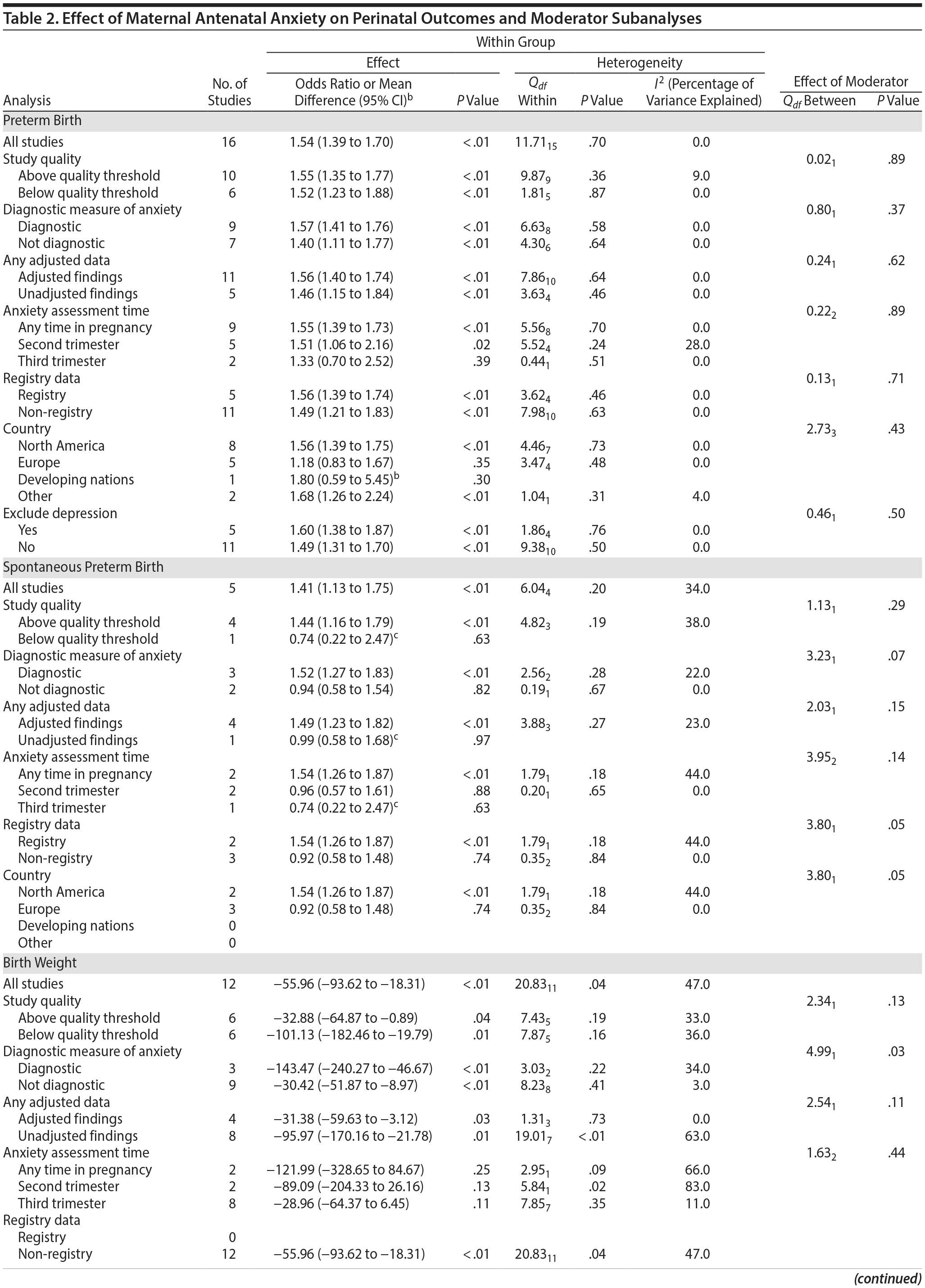
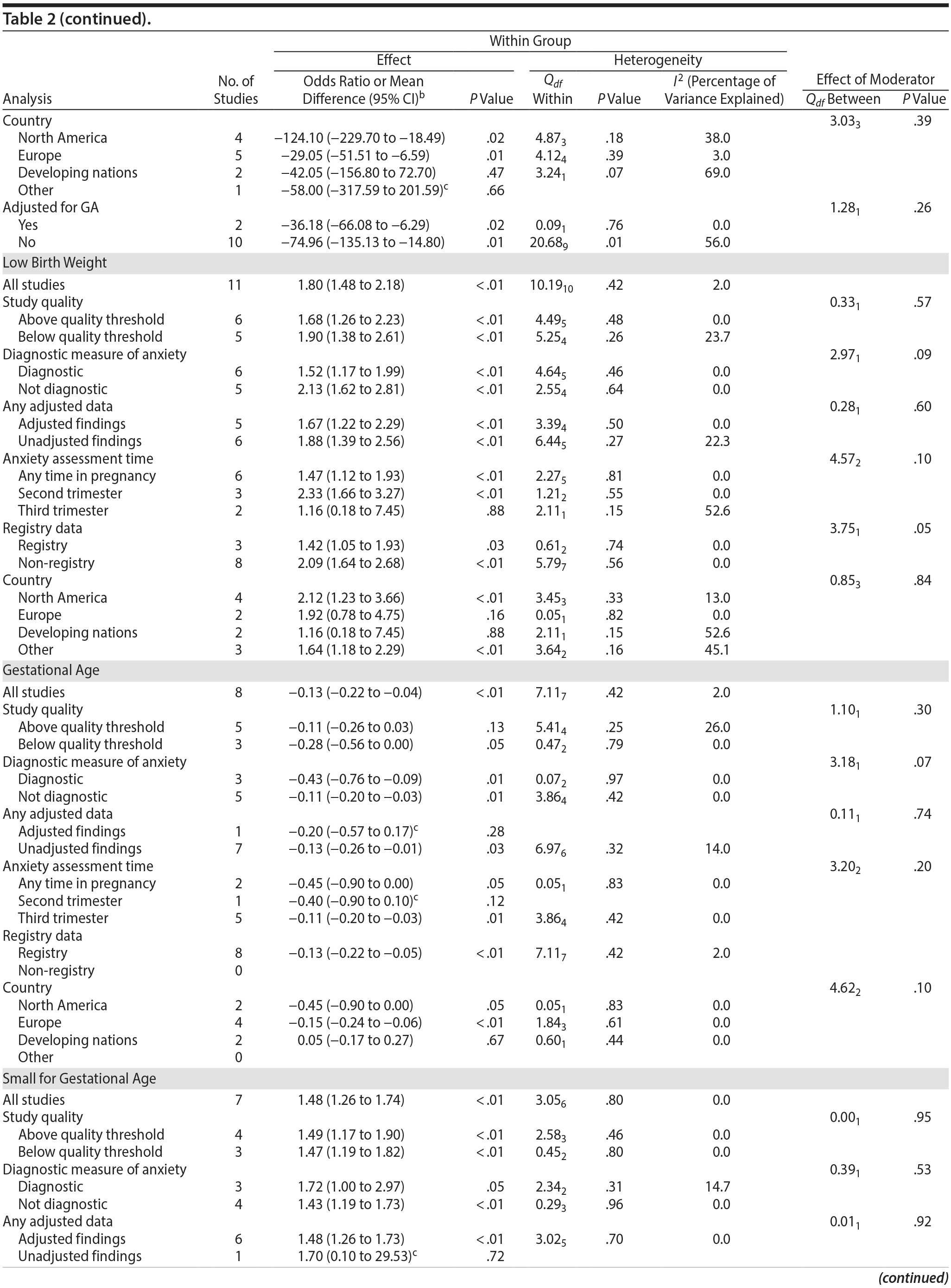
The outcome “low birth weight“ (typically defined as < 2,500 g, 11 studies, OR = 1.80; 95% CI, 1.48 to 2.18; P < .00001; Figure 2D) was significant, but heterogeneity across studies was not (Q10 = 10.19, P = .42; I2 = 2.0%). Although data from non-registry studies pooled to a considerably higher odds ratio (2.09) compared to registry studies (1.42) with a significant moderator (Q1 = 3.75, P = .05), both subanalyses were significant (Table 2).
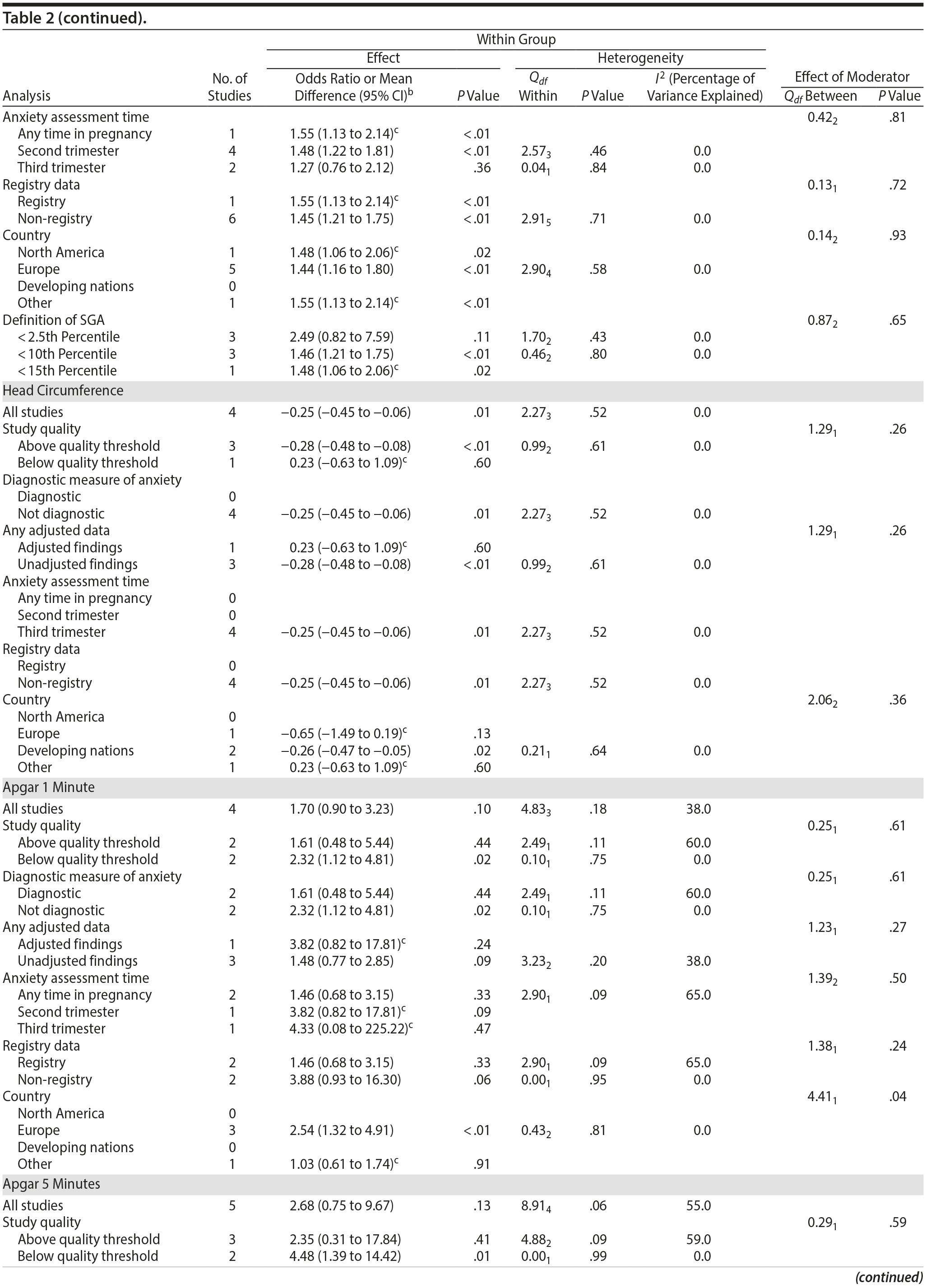
Among 8 studies, gestational age (GA) at birth pooled to a significant effect (mean difference = −0.13 weeks; 95% CI, −0.22 to −0.04, P = .004; Figure 2E). Heterogeneity was not significant (Q7 = 7.11, P = .42; I2 = 2%), nor were any of the subgroup analyses (Table 2). The variable “small for gestational age” (SGA) was significantly associated with maternal anxiety (7 studies, OR = 1.48; 95% CI, 1.26 to 1.74; P < .0001; Figure 2F). Heterogeneity was not significant (Q6 = 3.05, P = .80; I2 = 0%), nor were any of the moderators (Table 2) including the definition of SGA (birth weight below 15th, 10th, or 2.5th percentile for GA). Smaller head circumference was also a significant outcome (4 studies, mean difference = −0.25 cm; 95% CI, −0.45 to −0.06 cm; P = .012. Figure 2G), and heterogeneity across studies was not significant (Q3 = 2.27, P = .52; I2 = 0%), nor were there any significant moderators (Table 2).
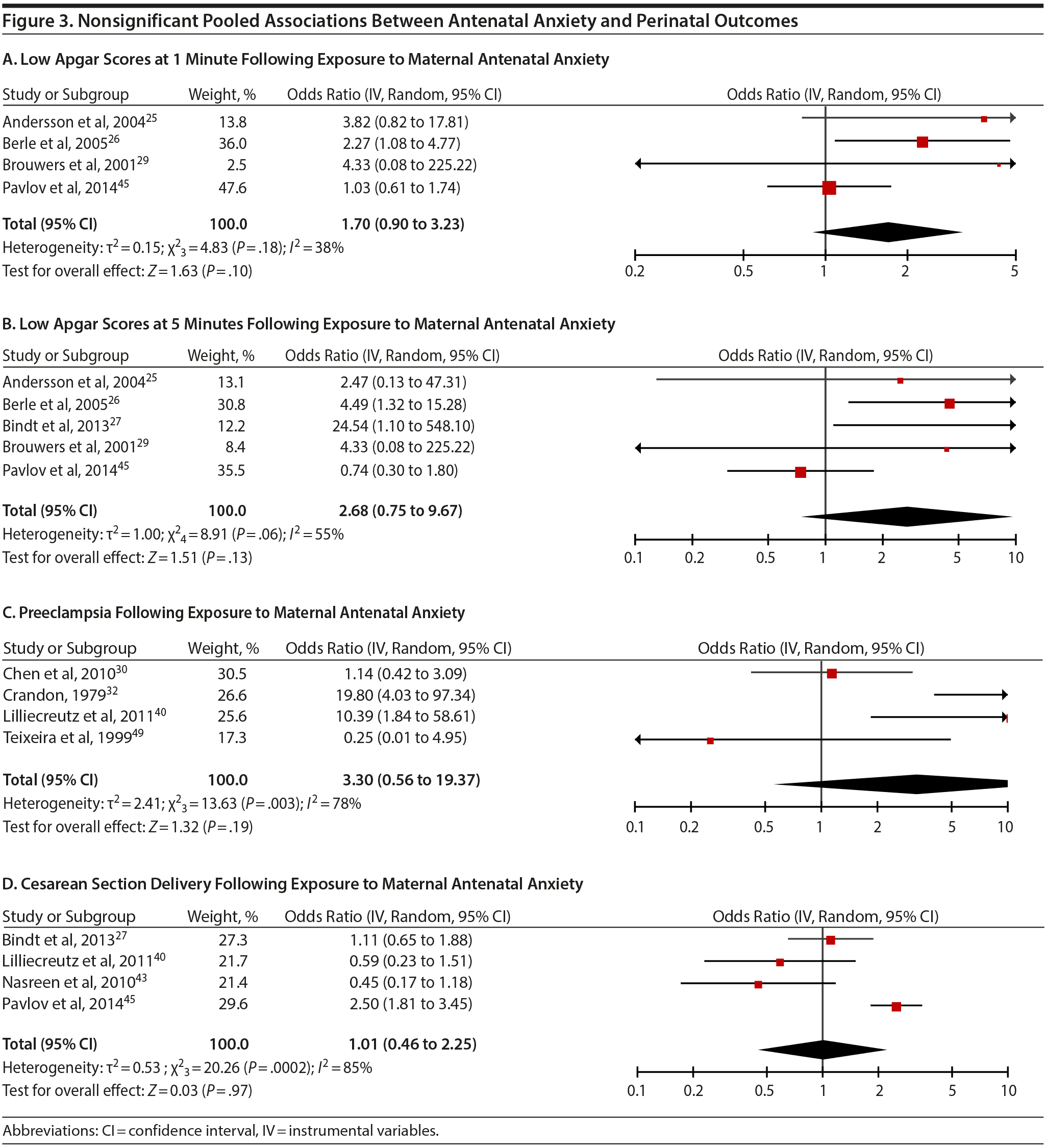
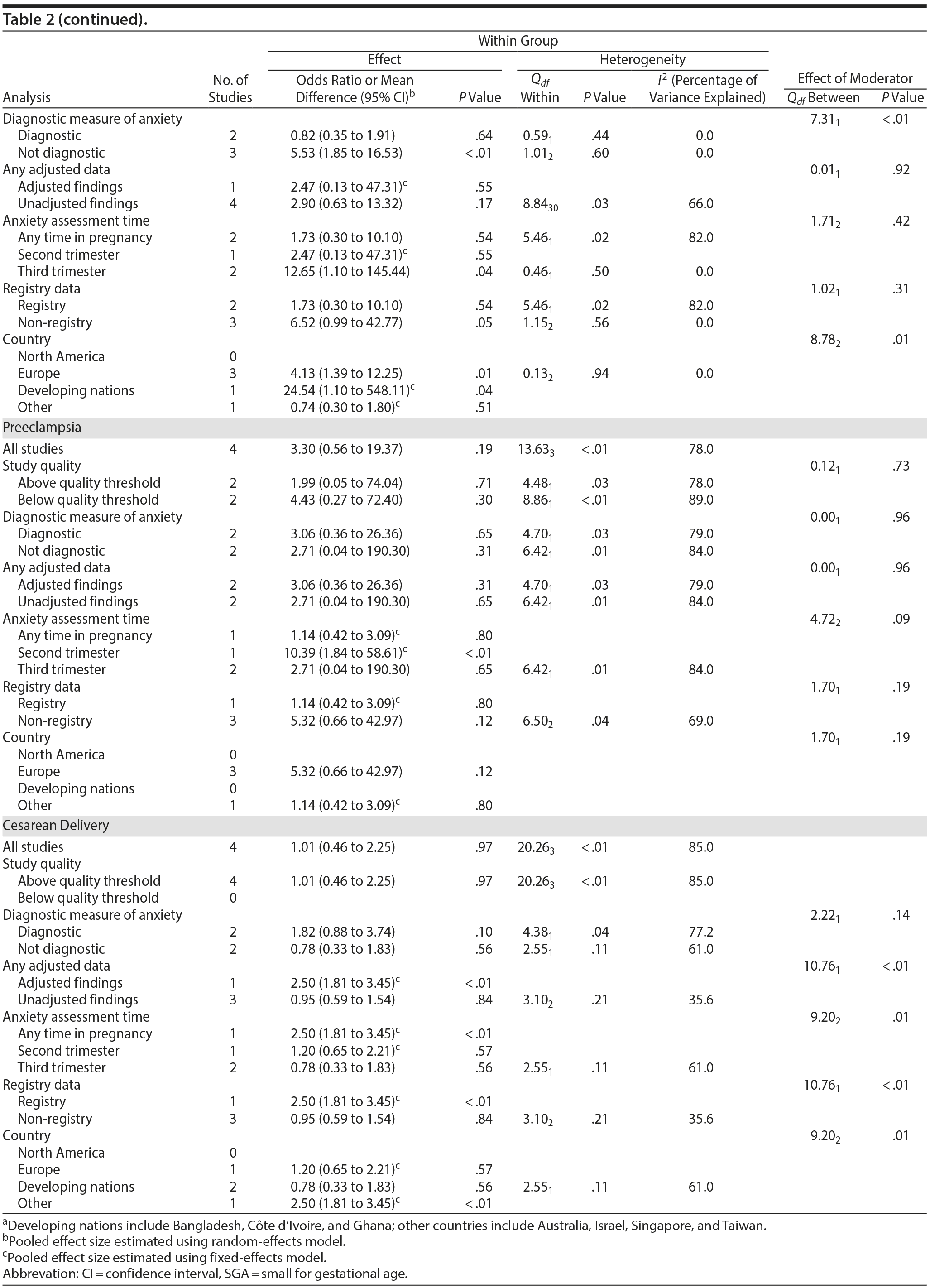
Apgar scores at 1 and 5 minutes did not pool to significant estimates. Four studies (OR = 1.70, 95% CI, 0.90 to 3.23; P = .10; Figure 3A) with minimal heterogeneity were used for the 1-minute score (Q3 = 4.83, P = .18; I2 = 38%) and 5 studies for the 5-minute outcome (OR = 2.68; 95% CI, 0.75 to 9.67; P = .13; Figure 3B); heterogeneity was slightly greater for the latter analysis (Q4 = 8.91, P = .06; I2 = 55%). Exposure to prenatal maternal anxiety was not significantly associated with preeclampsia (4 studies, OR = 3.30; 95% CI, 0.56 to 19.37; P = .19; Figure 3C); however, there was significant heterogeneity across studies, accounting for 78% of the variance (Q3 = 13.63, P < .01; I2 = 78%). None of the moderator subanalyses were significant (Table 2).
Based on 4 studies, exposure to antenatal anxiety was not associated with delivery by cesarean section (OR = 1.01, 95% CI, 0.46 to 2.25; P = .97; Figure 3D), although there was significant heterogeneity accounting for 82.1% of the variance (Q3 = 20.26, P < .01; I2 = 85.0%). A number of moderator variables emerged as significant, including adjustment for confounders, anxiety assessment time, use of registry versus non-registry data, and country (Table 2), but the majority of subanalyses contained too few studies. When there was a sufficient number of studies in the subanalysis for meaningful interpretation (ie, > 3 studies), the ORs were not significant.
The assessment of publication bias was possible only for preterm birth, low birth weight, and birth weight, as data were not sufficient to produce a funnel plot for other outcomes. There was no evidence of asymmetry based on visual inspection, and the Egger test was not statistically significant for the aforementioned outcomes.
DISCUSSION
To our knowledge, this is the first meta-analysis to examine a wide range of perinatal outcomes in relation to maternal anxiety during pregnancy. We found significant associations between antenatal anxiety and 7 of the 11 perinatal outcomes including preterm and spontaneous preterm birth, low birth weight and birth weight, gestational age, small for gestational age, and head circumference at birth. These observed associations were consistently significant, with little heterogeneity and few moderators significantly affecting the relationship. Only in studies that examined birth weight did we find significant between-studies heterogeneity among those that included a clinical diagnosis of anxiety versus those that included a self-report measure. This was also seen (albeit at a trend level) in the analysis for spontaneous preterm birth and is a noteworthy finding as it provides preliminary evidence for an effect of anxiety severity on low infant birth weight and spontaneous preterm birth. While we found no significant associations between antenatal anxiety and preeclampsia, cesarean delivery, or Apgar scores at 1 and at 5 minutes, these outcomes were examined based on 5 or fewer studies and the first 2 outcomes had significant heterogeneity across the included studies. Overall, our findings suggest a strong and robust association between maternal antenatal anxiety and adverse perinatal outcomes, leading to a broader understanding of the potential impact of antenatal anxiety that will inform screening and treatment decisions.
We assessed for the effects of moderator variables because of the known methodological limitations from observational studies and applied a rigorous quality assessment to separate studies based on quality, although we found study quality was not a significant source of heterogeneity. Our results were consistent with prior work, arguing for the strength of the relationship between antenatal anxiety and adverse outcomes previously summarized. Specifically, we replicated the effects on risk of preterm birth (OR = 1.54), spontaneous preterm birth (OR = 1.41), and low birth weight (OR = 1.80), despite differing definitions of anxiety and the number of included studies. Previously, a pooled OR of 1.46 (based on 7 studies)8 and a pooled RR of 1.50 (based on 12 studies)9 were reported for preterm birth in association with antenatal anxiety. These prior meta-analyses8,9 included measurement of anxiety as a continuous variable and pregnancy-specific anxiety, and one9 included a retrospective study, which we excluded. We expand upon prior work by including more studies and a larger pooled sample size. Similarly, our low birth weight outcome yielded an effect comparable to that of a previous meta-analysis9 (OR = 1.76), but again we included a greater number of studies (11 vs 6) with a larger pooled sample size.
To our knowledge, the association between antenatal anxiety and the variables of small for gestational age, gestational age, birth weight, and head circumference has not been previously summarized. It is not surprising that we found significant pooled effects, as those outcomes may be related to preterm birth and low birth weight, further reinforcing the strength of the relationship. In a previous meta-analysis,24 we examined the effects of depression on diverse perinatal outcomes and did not find such consistent and numerous adverse delivery outcomes, speaking to the potentially more grave effects of antenatal anxiety. It has already been suggested that antenatal anxiety may be more prevalent than depression52-55; it appears the consequences of this disorder may also be more adverse as well.
The primary limitations of this study stem from methodological issues of the included original articles. The definition of anxiety by self-report varied across studies with respect to the scales and cutoff scores used. Even for the State-Trait Anxiety Inventory (STAI), the most commonly used measure of antenatal anxiety, the cutoff scores used were not uniform and only half of the studies employed the STAI-state suggested cutoff (39-40).56 The category of diagnosed anxiety disorder was likewise a heterogeneous grouping. We included all types of anxiety disorders as there were not enough studies examining perinatal outcomes in association with specific anxiety disorders, other than for posttraumatic stress disorder (PTSD) and preterm birth. Additionally, we included 1 study with unclear diagnostic criteria,44 as correspondence with the primary author confirmed an active clinical diagnosis of anxiety (E. Sheiner, MD, PhD, personal communication in electronic form, April 18, 2016). A study just published57 that examined generalized anxiety disorder (GAD), panic disorder (PD), PTSD, and comorbidity of these disorders with major depression found significance for preterm birth only in the adjusted models for comorbid PTSD and depression but not for any of the individual disorders (although several unadjusted were significant) nor for any other delivery outcome. This is the same cohort as that in Yonkers et al,51 included in our analyses, which also did not find significance for the individual disorders and preterm birth (but similarly was found for comorbid PTSD and major depression in adjusted models). Our main analyses included the nonsignificant Yonkers et al51 data on PTSD only as we deemed this the most rigorous diagnostically (PD had a small sample size and the GAD diagnosis was based on only 1 month of symptoms instead of 6). There were enough data for us to investigate pooled PTSD data from several articles on preterm birth, and this analysis was similar to the main one and was significant. The timing of the anxiety assessments was also not consistent across the studies, and we grouped them by trimester. Our inclusion criteria specified that the initial assessment occur in pregnancy and we do not believe any occurred just prior to delivery, although some articles were vague in their descriptions (ie, Nasreen et al43 assessed during the third trimester, and Shaw et al6 included a diagnosis that was documented within 9 months before delivery). Most studies did not report on active treatments for anxiety, and thus we cannot confirm whether the associations between prenatal anxiety and outcomes truly reflect untreated anxiety. A majority of studies (22/29) did not report whether psychotropic medication was used, and only 1 study51 controlled for psychotropic use and found evidence for an effect of antidepressants (same cohort published in 2017,57 which found an effect for medications but not in adjusted models with individual anxiety disorders); psychotherapy use was equally not identified. Comorbidity was also not considered, and this may confound the data as anxiety is often seen with other disorders (eg, depression) and this may increase the chance of negative perinatal/neonatal outcomes, although to date data are not consistent.33,38,42 For example, Mפnnistö et al42 reported an adjusted odds ratio for PTB in pregnant women with comorbid depression and anxiety that was higher than the adjusted odds ratio in association with any singular maternal psychiatric disorder (OR = 2.31, 1.93-2.78); a similar result was observed for spontaneous PTB (OR = 2.24, 1.73-2.91) (K. Grantz, MD, personal communication in electronic form, October 13, 2017). On the contrary, Ibanez et al38 found no significant risk of PTB in pregnant women with comorbid depression and anxiety, but a significantly increased risk of spontaneous PTB. When statistical adjustments were made, different variables were used across studies, and given that certain factors (eg, smoking, SES) are known to be associated with both anxiety and negative perinatal outcomes, this inconsistency may have also confounded associations. Finally, we limited our search to English-language publications as we did not originally budget for translation costs, which may have biased the results. However, only 7 studies were excluded based on this criterion, and given the strength of the significant associations (P < .0001), any such bias is likely to minimally impact the results.
Additional research must address whether treatment of antenatal anxiety decreases the risk of adverse perinatal outcomes. Unfortunately there is a dearth of studies examining interventions to treat antenatal anxiety—eg, a recent systematic review and meta-analysis58 found 28 trials for the treatment of prenatal depressive disorders and only 1 for the treatment of anxiety disorders. Nevertheless, some treatments are common to both depressive and anxiety disorders and may suggest areas for initial research focus. For example, use of selective serotonin reuptake inhibitors (SSRIs) in pregnant women with psychiatric diagnoses has been associated with a lower risk for some negative perinatal outcomes; however, effects were outcome-specific (eg, PTB and cesarean section only) and require replication.59 Research in this area is further complicated by the fact that medications to treat anxiety (SSRIs and benzodiazepines) have been associated with potential adverse effects60,61; evaluating the effect of non-pharmacologic treatments such as psychotherapy should also be a focus of future studies. Moreover, whether there exists a period of heightened susceptibility, or a differential impact of longer exposure to anxiety, should be the focus of future research. Although we did not find a significant effect for timing of anxiety as a moderator, few studies identified the trimester of anxiety assessment. Lastly, the potential underlying mechanisms linking antenatal anxiety with poor perinatal outcomes, while recently summarized elsewhere,62 remain an important topic for further study as they may ultimately inform treatment optimization.
Our findings are clinically important given the high prevalence of anxiety in pregnancy, as previously noted, coupled with low treatment uptake. For example, a large US study63 found that past-year pregnant women sought treatment for an anxiety disorder at nearly half the rate of non-pregnant women (6.1% versus 11.6%). The multiple adverse perinatal outcomes associated with antenatal anxiety highlight the need to both identify and manage pregnant women with high levels of anxiety, particularly those with the most severe forms of anxiety (eg, diagnosed anxiety disorders). Here, we provide data that will be useful to clinicians and patients in their decision making. Women with antenatal anxiety disorders have higher rates of psychotropic medication nonadherence compared to other prenatal psychiatric disorders,64 and those declining medications have significantly more anxiety symptoms across pregnancy than those adherent to their medication regimen.65 While psychological therapies are both first-line treatment for mild anxiety and the preferred modality for most women during pregnancy,66 these treatments are nevertheless also linked with variable rates of adherence/attrition.58 Along with our data, the preceding provides a strong argument for not only identifying antenatal anxiety but additionally addressing treatment preferences, barriers, and adherence in women with antenatal anxiety to mitigate potential adverse perinatal outcomes.
Submitted: November 8, 2017; accepted January 17, 2018.
Published online: September 4, 2018.
Potential conflicts of interest: Dr Grigoriadis has received other fees from Allergan, personal fees from Pfizer, other fees from Sage, and personal fees from Bristol-Myers Squibb, outside the submitted work; she has no other relationships or activities that could appear to have influenced the submitted work. Drs Graves, Peer, Tomlinson, Vigod, Dennis, Steiner, Brown, Cheung, Rector, and Richter and Mmes Mamisashvili, Dawson, and Guenette have no conflicts to disclose.
Funding/support: This study was funded by The Canadian Institutes of Health Research (CIHR; Ottawa Ontario, Canada), FRN 141002.
Role of the sponsor: The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclaimer: The funder is independent of the research team.
Previous presentation: Presented at the 66th Annual Conference of the Canadian Psychiatric Association; September 23, 2016; Toronto, Ontario, Canada, ▪ the 25th Anniversary Congress on Women’s Health; April 28-30, 2017; Washington, DC ▪ the 2017 Annual Meeting of the American Psychiatric Association; May 23, 2017; San Diego, California.
REFERENCES
1. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315-323. PubMed CrossRef
2. Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, et al. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. Eur Psychiatry. 2004;19(8):459-463. PubMed CrossRef
3. Buist A, Gotman N, Yonkers KA. Generalized anxiety disorder: course and risk factors in pregnancy. J Affect Disord. 2011;131(1-3):277-283. PubMed CrossRef
4. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627. PubMed CrossRef
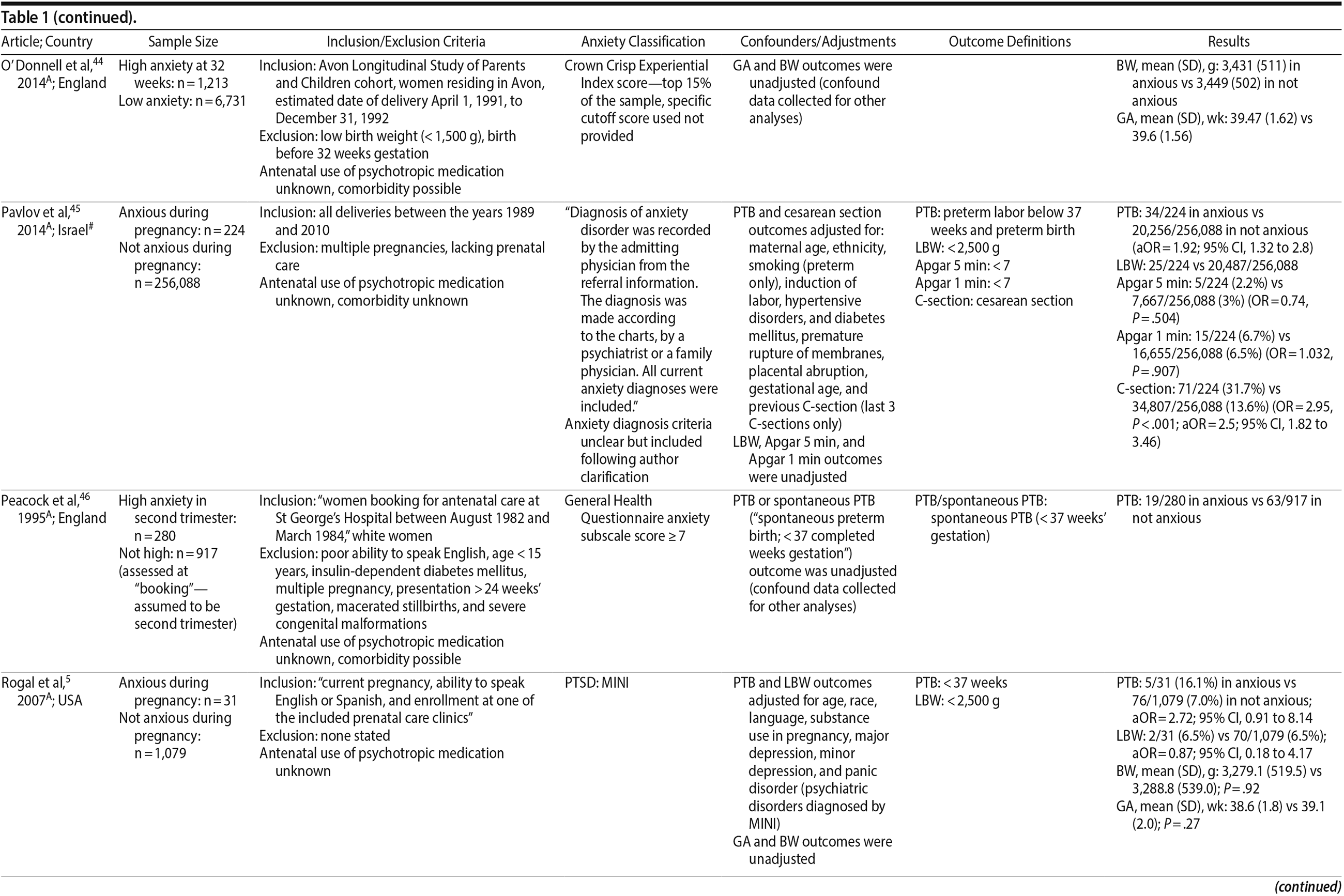
5. Rogal SS, Poschman K, Belanger K, et al. Effects of posttraumatic stress disorder on pregnancy outcomes. J Affect Disord. 2007;102(1-3):137-143. PubMed CrossRef
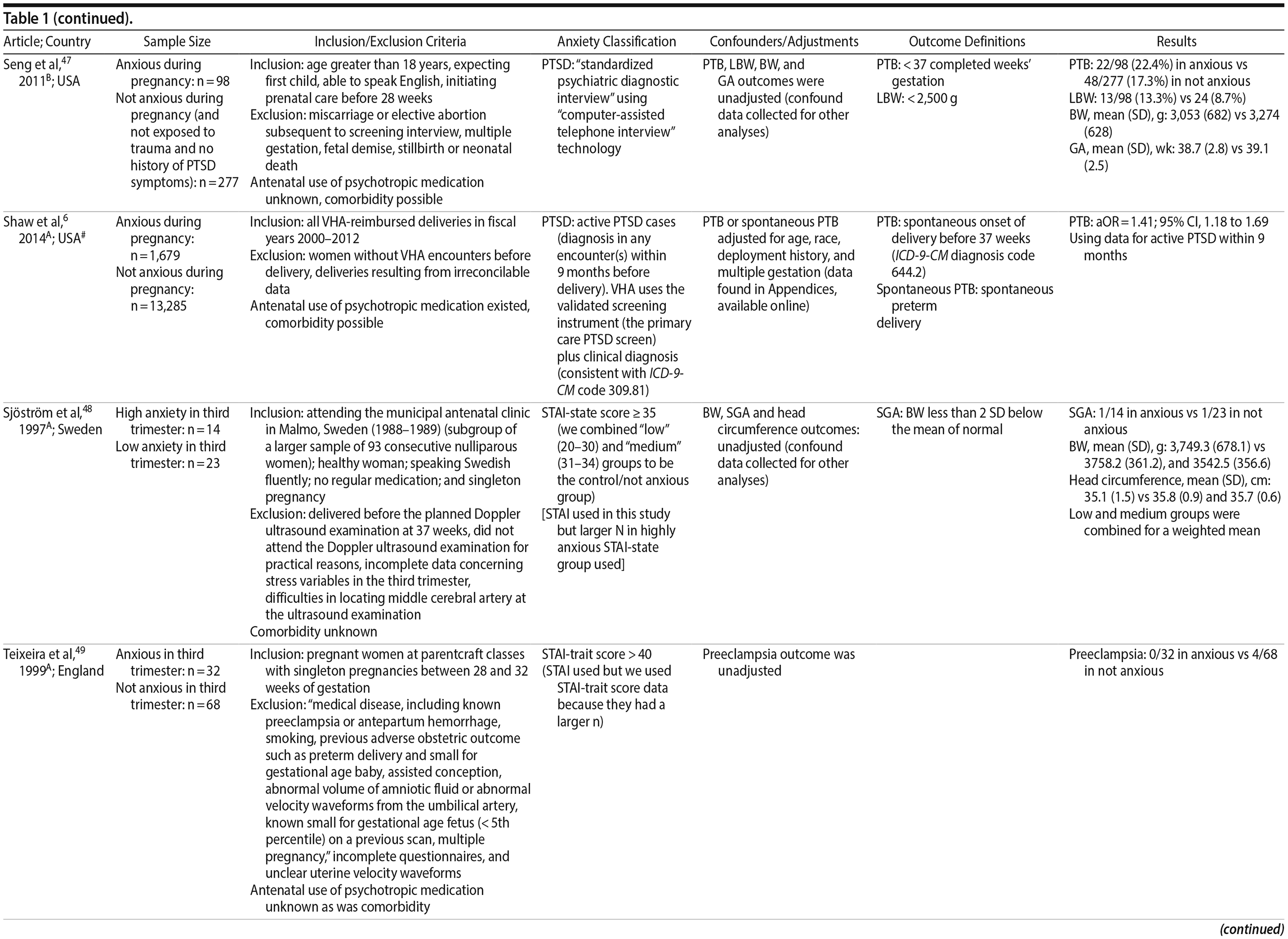
6. Shaw JG, Asch SM, Kimerling R, et al. Posttraumatic stress disorder and risk of spontaneous preterm birth. Obstet Gynecol. 2014;124(6):1111-1119. PubMed CrossRef
7. Staneva A, Bogossian F, Pritchard M, et al. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015;28(3):179-193. PubMed CrossRef
8. Rose MS, Pana G, Premji S. Prenatal maternal anxiety as a risk factor for preterm birth and the effects of heterogeneity on this relationship: a systematic review and meta-analysis. BioMed Res Int. 2016;2016:8312158. PubMed CrossRef
9. Ding XX, Wu YL, Xu SJ, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103-110. PubMed CrossRef
10. Littleton HL, Breitkopf CR, Berenson AB. Correlates of anxiety symptoms during pregnancy and association with perinatal outcomes: a meta-analysis. Am J Obstet Gynecol. 2007;196(5):424-432. PubMed CrossRef
11. Goodman JH, Chenausky KL, Freeman MP. Anxiety disorders during pregnancy: a systematic review. J Clin Psychiatry. 2014;75(10):e1153-e1184. PubMed CrossRef
12. Stroup DF, Berlin JA, Morton SC, et al; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting:. JAMA. 2000;283(15):2008-2012. PubMed CrossRef
13. Ross LE, Grigoriadis S, Mamisashvili L, et al. Quality assessment of observational studies in psychiatry: an example from perinatal psychiatric research. Int J Methods Psychiatr Res. 2011;20(4):224-234. PubMed CrossRef
14. McGowan JSM, Lefebvre C. An Evidence Based Checklist for the Peer Review of Electronic Search Strategies (PRESS EBC). Evid Based Libr Inf Pract. 2010;5(1):149-154. CrossRef
15. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. PubMed CrossRef
16. Bayrampour H, Ali E, McNeil DA, et al. Pregnancy-related anxiety: a concept analysis. Int J Nurs Stud. 2016;55:115-130. PubMed CrossRef
17. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed CrossRef
18. Wells GASB, O’ Connell D, Peterson J, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Jan 2, 2016.
19. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. PubMed CrossRef
20. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. PubMed CrossRef
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. PubMed CrossRef
22. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. PubMed CrossRef
23. Huedo-Medina TB, Sánchez-Meca J, Mar×n-Mart×nez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193-206. PubMed CrossRef
24. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321-e341. PubMed CrossRef
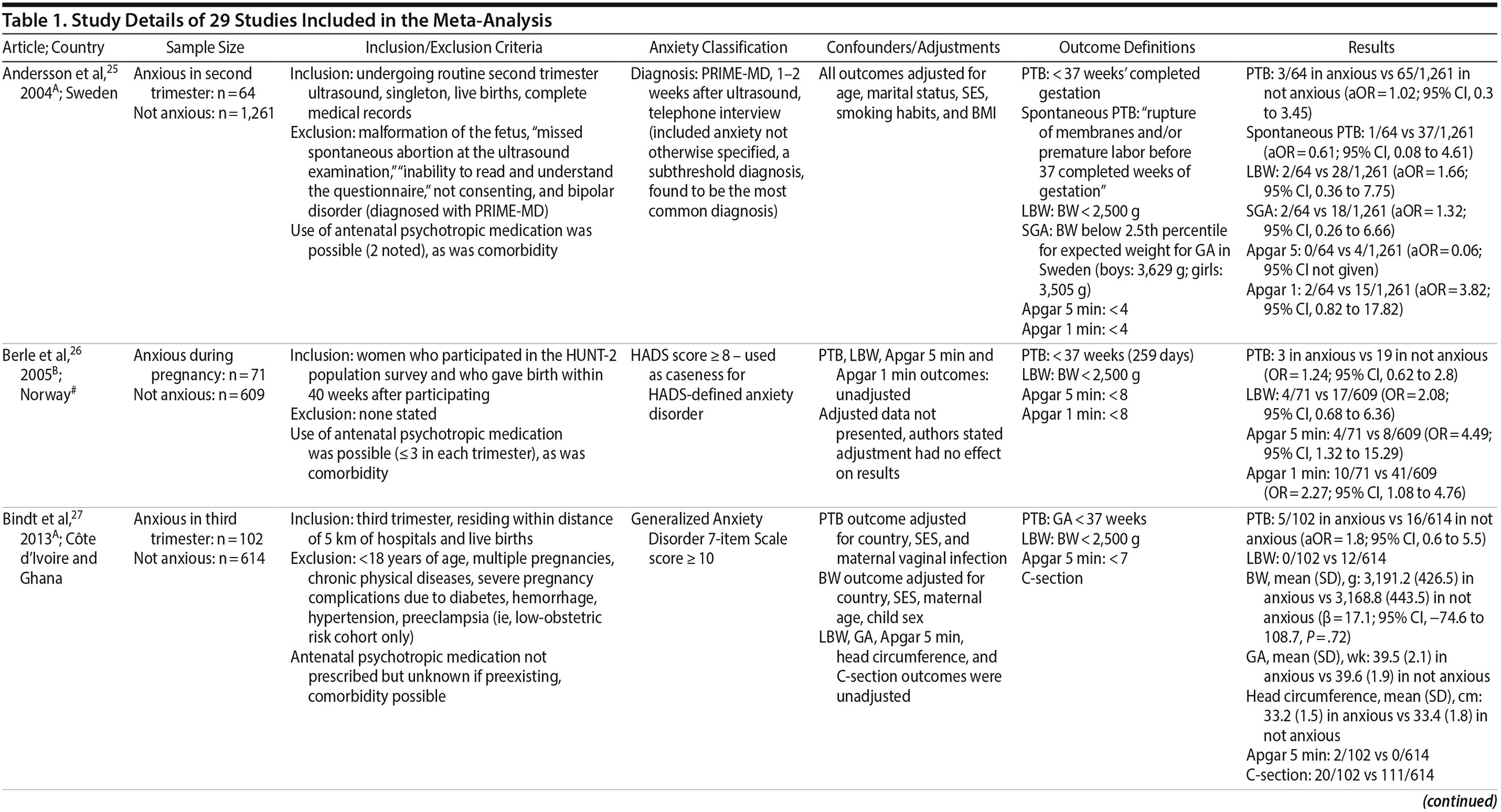
25. Andersson L, Sundström-Poromaa I, Wulff M, et al. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am J Epidemiol. 2004;159(9):872-881. PubMed CrossRef
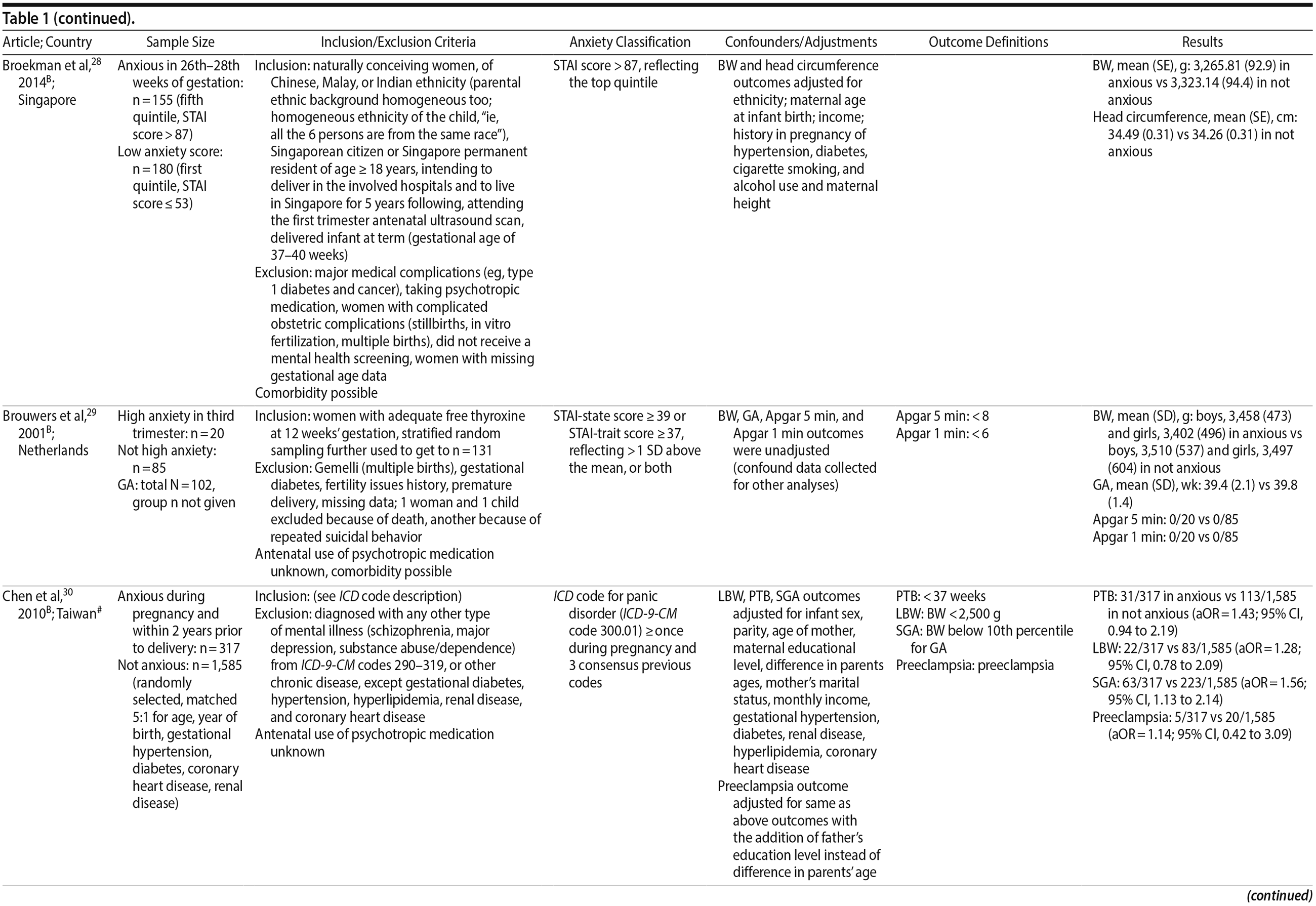
26. Berle JO, Mykletun A, Daltveit AK, et al. Neonatal outcomes in offspring of women with anxiety and depression during pregnancy: a linkage study from The Nord-Trøndelag Health Study (HUNT) and Medical Birth Registry of Norway. Arch Women Ment Health. 2005;8(3):181-189. PubMed CrossRef
27. Bindt C, Guo N, Bonle MT, et al; International CDS Study Group. No association between antenatal common mental disorders in low-obstetric risk women and adverse birth outcomes in their offspring: results from the CDS study in Ghana and C×´te D’ Ivoire. PLoS One. 2013;8(11):e80711. PubMed CrossRef
28. Broekman BF, Chan YH, Chong YS, et al; GUSTO Research Group. The influence of anxiety and depressive symptoms during pregnancy on birth size. Paediatr Perinat Epidemiol. 2014;28(2):116-126. PubMed CrossRef
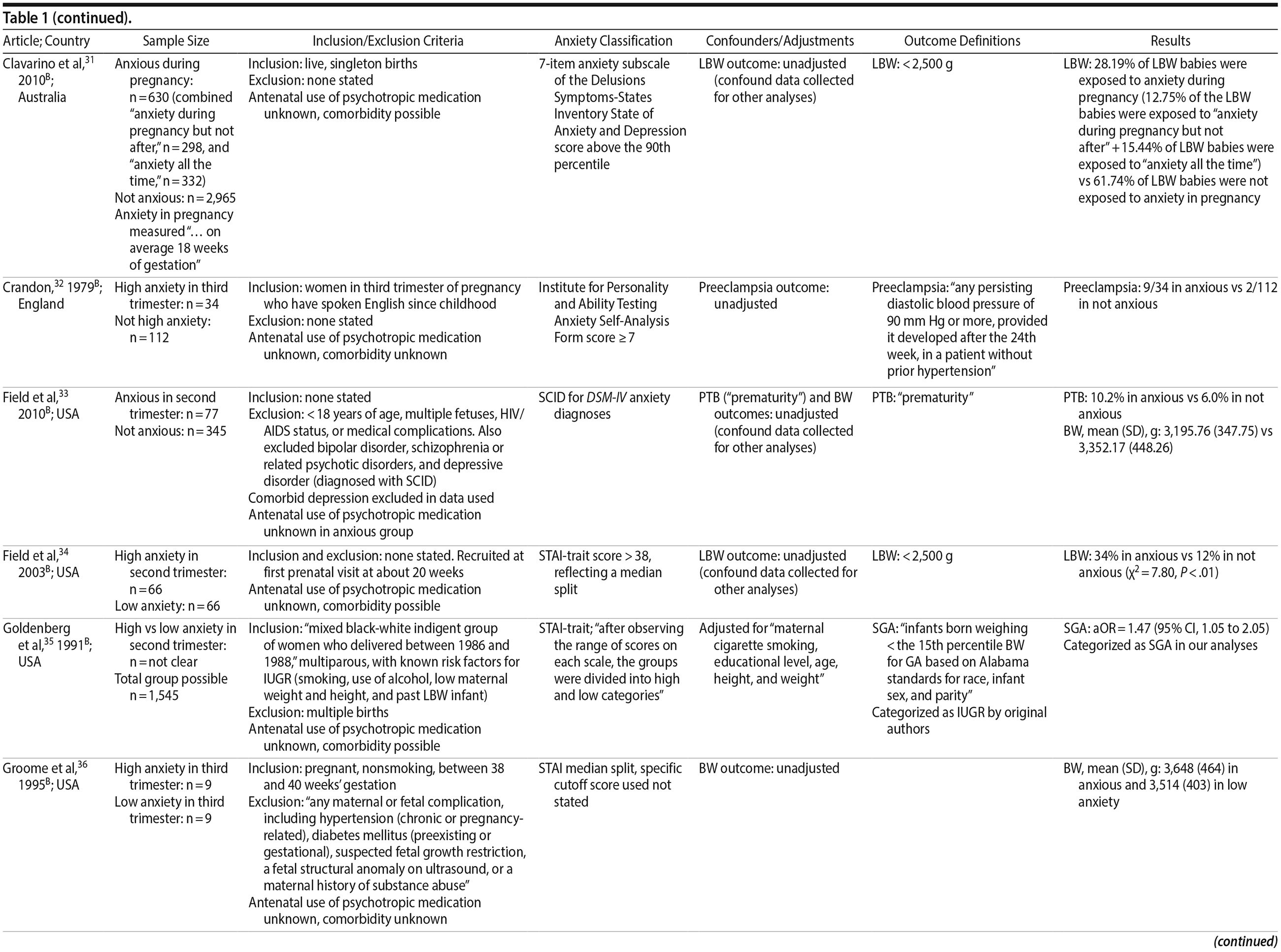
29. Brouwers EPM. van Baar AL, Pop VJ. Maternal anxiety during pregnancy and subsequent infant development. Infant Behav Dev. 2001;24(1):95-106. CrossRef
30. Chen YH, Lin HC, Lee HC. Pregnancy outcomes among women with panic disorder—do panic attacks during pregnancy matter? J Affect Disord. 2010;120(1-3):258-262. PubMed CrossRef
31. Clavarino AM, Mamun AA, O’ Callaghan M, et al. Maternal anxiety and attention problems in children at 5 and 14 years. J Atten Disord. 2010;13(6):658-667. PubMed CrossRef
32. Crandon AJ. Maternal anxiety and obstetric complications. J Psychosom Res. 1979;23(2):109-111. PubMed CrossRef
33. Field T, Diego M, Hernandez-Reif M, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behav Dev. 2010;33(1):23-29. PubMed CrossRef
34. Field T, Diego M, Hernandez-Reif M, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17(3):140-151. PubMed CrossRef
35. Goldenberg RL CS, Cutter GR, Hoffman HJ, et al. Maternal psychological characteristics and intrauterine growth retardation. Pre- and Peri-natal Psychology Journal. 1991;6(2):129-134.
36. Groome LJ, Swiber MJ, Bentz LS, et al. Maternal anxiety during pregnancy: effect on fetal behavior at 38 to 40 weeks of gestation. J Dev Behav Pediatr. 1995;16(6):391-396. PubMed CrossRef
37. Henrichs J, Schenk JJ, Roza SJ, et al. Maternal psychological distress and fetal growth trajectories: the Generation R Study. Psychol Med. 2010;40(4):633-643. PubMed CrossRef
38. Ibanez G, Charles MA, Forhan A, et al; EDEN Mother-Child Cohort Study Group. Depression and anxiety in women during pregnancy and neonatal outcome: data from the EDEN mother-child cohort. Early Hum Dev. 2012;88(8):643-649. PubMed CrossRef
39. Khashan AS, Everard C, McCowan LM, et al. Second-trimester maternal distress increases the risk of small for gestational age. Psychol Med. 2014;44(13):2799-2810. PubMed CrossRef
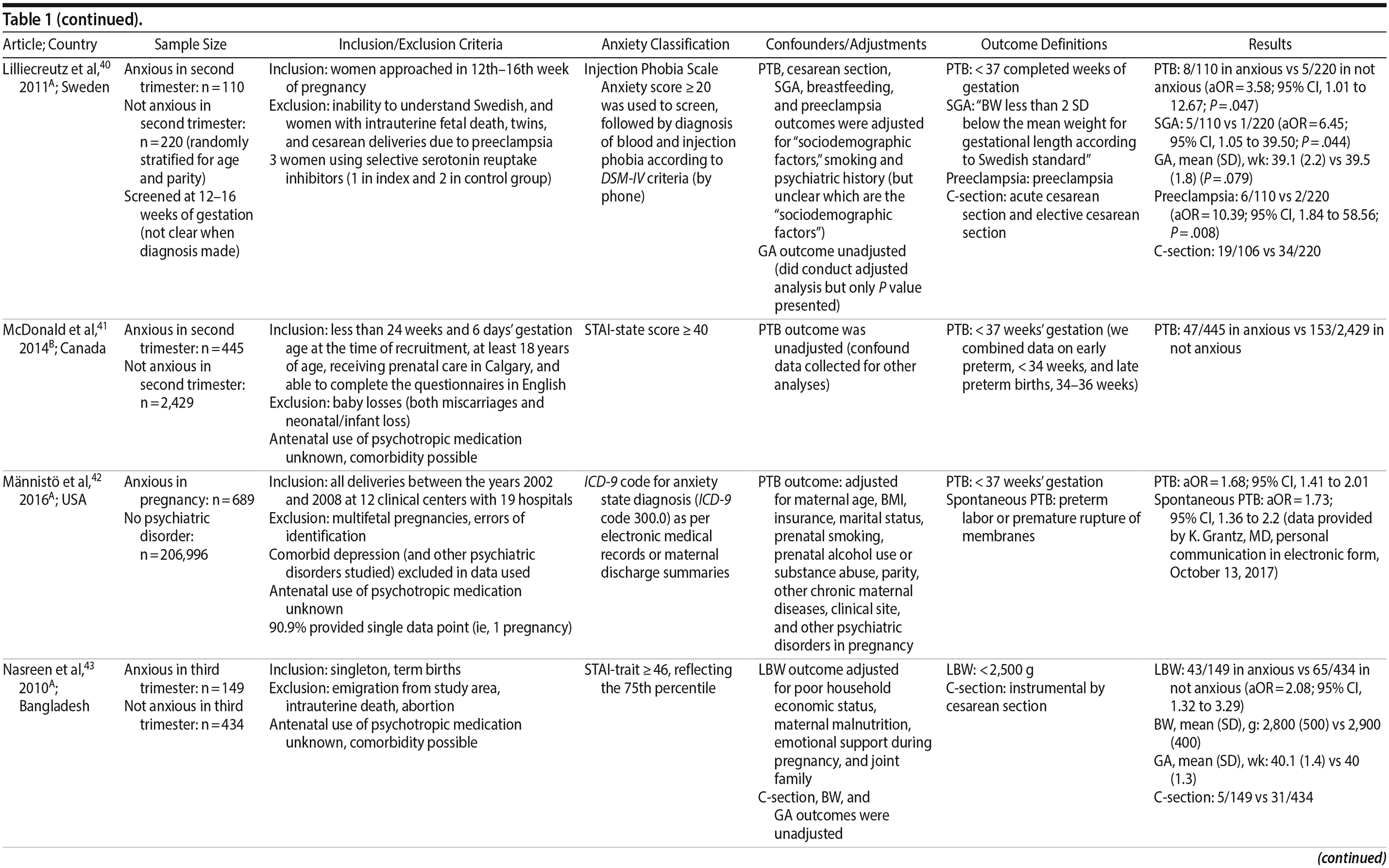
40. Lilliecreutz C, Sydsjö G, Josefsson A. Obstetric and perinatal outcomes among women with blood- and injection phobia during pregnancy. J Affect Disord. 2011;129(1-3):289-295. PubMed CrossRef
41. McDonald SW, Kingston D, Bayrampour H, et al. Cumulative psychosocial stress, coping resources, and preterm birth. Arch Women Ment Health. 2014;17(6):559-568. PubMed CrossRef
42. Mפnnistö T, Mendola P, Kiely M, et al. Maternal psychiatric disorders and risk of preterm birth. Ann Epidemiol. 2016;26(1):14-20. PubMed CrossRef
43. Nasreen HE, Kabir ZN, Forsell Y, et al. Low birth weight in offspring of women with depressive and anxiety symptoms during pregnancy: results from a population based study in Bangladesh. BMC Public Health. 2010;10(1):515. PubMed CrossRef
44. O’ Donnell KJ, Glover V, Barker ED, et al. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol. 2014;26(2):393-403. PubMed CrossRef
45. Pavlov M, Steiner N, Kessous R, et al. Obstetric and neonatal outcome in patients with anxiety disorders. J Matern Fetal Neonatal Med. 2014;27(13):1339-1342. PubMed CrossRef
46. Peacock JL, Bland JM, Anderson HR. Preterm delivery: effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ. 1995;311(7004):531-535. PubMed CrossRef
47. Seng JS, Low LK, Sperlich M, et al. Post-traumatic stress disorder, child abuse history, birthweight and gestational age: a prospective cohort study. BJOG. 2011;118(11):1329-1339. PubMed CrossRef
48. Sjöström K, Valentin L, Thelin T, et al. Maternal anxiety in late pregnancy and fetal hemodynamics. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):149-155. PubMed CrossRef
49. Teixeira JM, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318(7177):153-157. PubMed CrossRef
50. Xiong X, Harville EW, Mattison DR, et al. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008;336(2):111-115. PubMed CrossRef
51. Yonkers KA, Smith MV, Forray A, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry. 2014;71(8):897-904. PubMed CrossRef
52. Fairbrother N, Janssen P, Antony MM, et al. Perinatal anxiety disorder prevalence and incidence. J Affect Disord. 2016;200:148-155. PubMed CrossRef
53. Martini J, Petzoldt J, Einsle F, et al. Risk factors and course patterns of anxiety and depressive disorders during pregnancy and after delivery: a prospective-longitudinal study. J Affect Disord. 2015;175:385-395. PubMed CrossRef
54. Grant KA, McMahon C, Austin MP. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord. 2008;108(1-2):101-111. PubMed CrossRef
55. Lee AM, Lam SK, Sze Mun Lau SM, et al. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstet Gynecol. 2007;110(5):1102-1112. PubMed CrossRef
56. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S467-S472. PubMed CrossRef
57. Yonkers KA, Gilstad-Hayden K, Forray A, et al. Association of panic disorder, generalized anxiety disorder, and benzodiazepine treatment during pregnancy with risk of adverse birth outcomes. JAMA Psychiatry. 2017;74(11):1145-1152. PubMed CrossRef
58. van Ravesteyn LM, Lambregtse-van den Berg MP, Hoogendijk WJ, et al. Interventions to treat mental disorders during pregnancy: a systematic review and multiple treatment meta-analysis. PLoS One. 2017;12(3):e0173397. PubMed CrossRef
59. Malm H, Sourander A, Gissler M, et al. Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. Am J Psychiatry. 2015;172(12):1224-1232. PubMed CrossRef
60. Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70(4):436-443. PubMed CrossRef
61. Kפllén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel). 2013;6(10):1221-1286. PubMed CrossRef
62. Newman LJF, Komiti A. Developmental implications of maternal antenatal anxiety mechanisms and approaches to intervention. Transl Dev Psychiatry. 2017;5(1):1309879. CrossRef
63. Vesga-López O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815. PubMed CrossRef
64. Lupattelli A, Spigset O, Björnsdóttir I, et al. Patterns and factors associated with low adherence to psychotropic medications during pregnancy—a cross-sectional, multinational web-based study. Depress Anxiety. 2015;32(6):426-436. PubMed CrossRef
65. Misri S, Eng AB, Abizadeh J, et al. Factors impacting decisions to decline or adhere to antidepressant medication in perinatal women with mood and anxiety disorders. Depress Anxiety. 2013;30(11):1129-1136. PubMed CrossRef
66. Arch JJ. Cognitive behavioral therapy and pharmacotherapy for anxiety: treatment preferences and credibility among pregnant and non-pregnant women. Behav Res Ther. 2014;52:53-60. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at [email protected].
This PDF is free for all visitors!
Save
Cite