Objective: Older individuals with schizophrenia are at risk of being treated with anticholinergic medications due to the prevalence of medical comorbidities and polypharmacy. High anticholinergic burden impairs cognition and is a risk factor for Alzheimer’s dementia. Thus, we assessed the impact of anticholinergic burden on Alzheimer’s dementia-related and schizophrenia-related cognitive functions in older patients with schizophrenia.
Methods: Anticholinergic burden was measured using the Anticholinergic Cognitive Burden scale (ACB) in 60 community-dwelling patients aged ≥ 50 years who met DSM-IV criteria for schizophrenia between May 2007 and November 2011. Cognitive domains affected early in the course of Alzheimer’s dementia were assessed using the Cambridge Neuropsychological Test Automated Battery (CANTAB) Alzheimer’s Dementia Battery and the Repeatable Battery for the Assessment of Neuropsychological Status. Two CANTAB tests of executive function were used to assess deficits common in schizophrenia. Regression analyses were used to assess the relationships between anticholinergic burden and cognition. A receiver operating characteristic curve was constructed to determine an ACB cutoff score to identify those at risk of cognitive impairment.
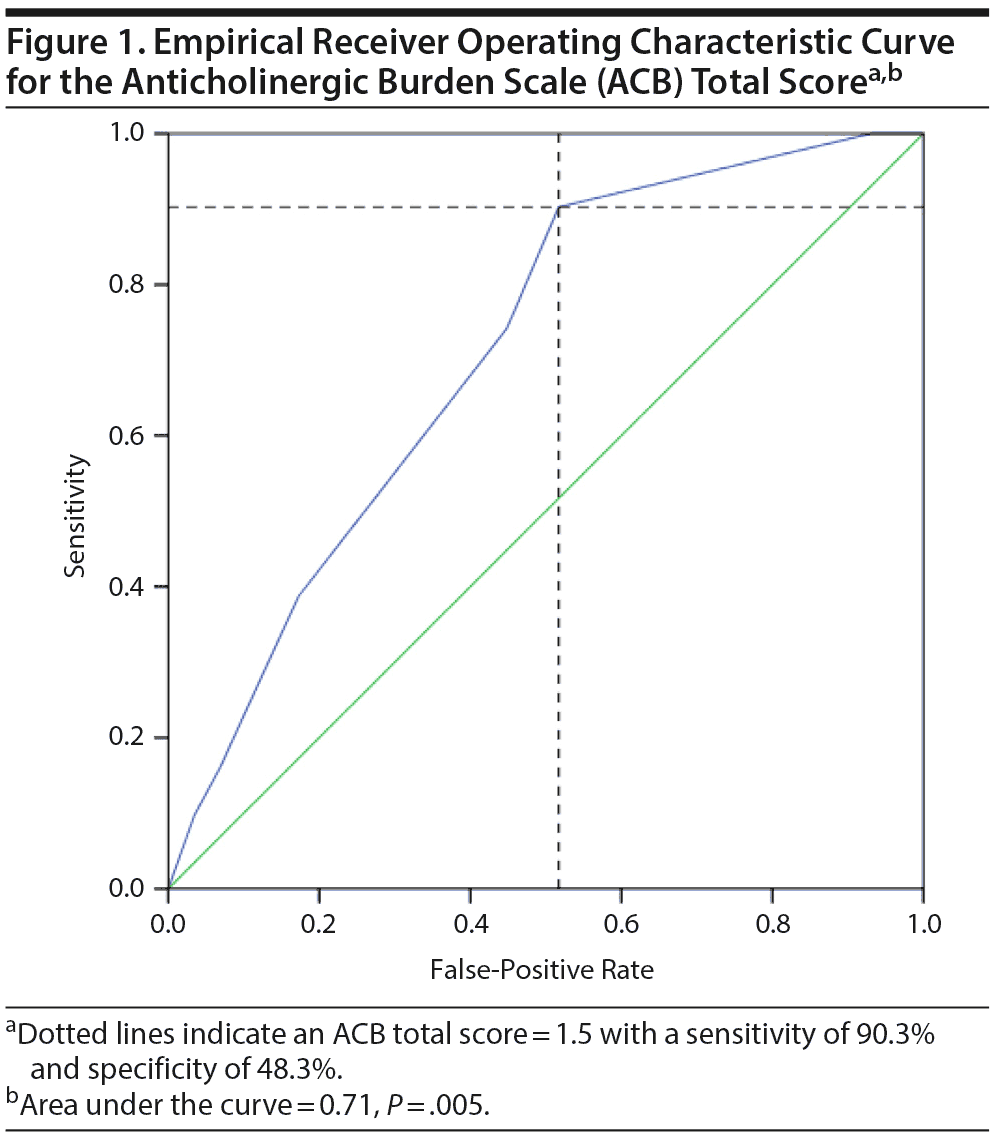
Results: ACB scores were associated with spatial working (P = .04) and immediate (P = .004) memory and visuospatial ability (P = .02) and showed a trend toward association with impaired learning (P = .06), but were not associated with attention, executive function, language, or reaction time. An ACB cutoff score of ≤ 1.5 can detect cognitive impairment with a sensitivity of 90.3% and specificity of 48.3%.
Conclusions: High anticholinergic burden contributes to specific cognitive deficits in older individuals with schizophrenia that resemble those commonly observed early in the course of Alzheimer’s dementia. The ACB is a potentially useful screening tool that can help identify patients at risk of developing anticholinergic-related cognitive impairment.

Anticholinergic Burden and Cognition in Older Patients With Schizophrenia

ABSTRACT
Objective: Older individuals with schizophrenia are at risk of being treated with anticholinergic medications due to the prevalence of medical comorbidities and polypharmacy. High anticholinergic burden impairs cognition and is a risk factor for Alzheimer’s dementia. Thus, we assessed the impact of anticholinergic burden on Alzheimer’s dementia-related and schizophrenia-related cognitive functions in older patients with schizophrenia.
Methods: Anticholinergic burden was measured using the Anticholinergic Cognitive Burden scale (ACB) in 60 community-dwelling patients aged ≥ 50 years who met DSM-IV criteria for schizophrenia between May 2007 and November 2011. Cognitive domains affected early in the course of Alzheimer’s dementia were assessed using the Cambridge Neuropsychological Test Automated Battery (CANTAB) Alzheimer’s Dementia Battery and the Repeatable Battery for the Assessment of Neuropsychological Status. Two CANTAB tests of executive function were used to assess deficits common in schizophrenia. Regression analyses were used to assess the relationships between anticholinergic burden and cognition. A receiver operating characteristic curve was constructed to determine an ACB cutoff score to identify those at risk of cognitive impairment.
Results: ACB scores were associated with spatial working (P = .04) and immediate (P = .004) memory and visuospatial ability (P = .02) and showed a trend toward association with impaired learning (P = .06), but were not associated with attention, executive function, language, or reaction time. An ACB cutoff score of ≤ 1.5 can detect cognitive impairment with a sensitivity of 90.3% and specificity of 48.3%.
Conclusions: High anticholinergic burden contributes to specific cognitive deficits in older individuals with schizophrenia that resemble those commonly observed early in the course of Alzheimer’s dementia. The ACB is a potentially useful screening tool that can help identify patients at risk of developing anticholinergic-related cognitive impairment.
J Clin Psychiatry 2017;78(9):e1284-e1290
https://doi.org/10.4088/JCP.17m11523
© Copyright 2017 Physicians Postgraduate Press, Inc.
aDivision of Geriatric Psychiatry, Centre for Addiction and Mental Health, Toronto, Canada
bDepartment of Psychiatry, University of Toronto, Toronto, Canada
cDepartment of Psychiatry, University of British Columbia, Vancouver, Canada
*Corresponding author: Tarek K. Rajji, MD, Centre for Addiction and Mental Health, 80 Workman Way, 6th floor, Room 6312, Toronto, ON M6J1H4 ([email protected]).
With the growing number of older individuals with schizophrenia, 20% of them will be over the age of 65 years by 2025.1 Thus, the prevalence of dementia is expected to increase markedly in this population.2 Several studies have shown that schizophrenia is associated with an increased risk of dementia.3-5 Compared to the general population, individuals with schizophrenia have a 2-fold higher risk of developing dementia before the age of 80 years.5 The etiology underlying the development of dementia in schizophrenia remains unknown. Aside from age,2 other risk factors common among patients with schizophrenia increase their vulnerability to dementia; they include low educational attainment,6,7 premorbid cognitive dysfunction,8,9 cardiovascular disease,10,11 polypharmacy,12,13 history of alcohol and/or substance abuse,14,15 and apolipoprotein E ε4 genotype.16,17 There is also increasing evidence that anticholinergic medications increase the risk of Alzheimer’s dementia.18-20 Chronic high anticholinergic burden has been associated with deleterious effects on cognition in individuals with21-24 and without schizophrenia.25-29 These deficits are typically of smaller magnitude in contrast to those observed following acute administration of potent anticholinergic agents (eg, scopolamine).27 However, they are often long-lasting and may continue to persist following a reduction of anticholinergic medications.18,28,30 Further, chronic anticholinergic burden predicts longitudinal cognitive decline and eventual diagnosis of mild cognitive impairment.26,31 Thus, sustained cognitive dysfunction secondary to anticholinergic burden may result in neurodegenerative changes in cholinergic pathways implicated in cognition.29
Anticholinergic burden accounts for the anticholinergic load of multiple medications and their metabolites rather than that of a single compound.32 Commonly prescribed medications such as analgesics, antihistamines, antiemetics, antiarrhythmics, medications for urinary incontinence, and bronchodilators have anticholinergic properties.33,34 Additionally, several medications used in the treatment of schizophrenia such as antipsychotics, antidepressants, mood stabilizers, and antiparkinsonian agents are known have central anticholinergic activity.23,35,36 Thus, given the increased prevalence of medical comorbidities37-39 and polypharmacy12 in patients with schizophrenia, many of them have a high anticholinergic burden. Furthermore, the adverse effects associated with a high anticholinergic burden may be more severe in patients with schizophrenia because of their preexisting brain changes and cognitive impairment.40-45 This may be especially true for older individuals with schizophrenia who have increased sensitivity to anticholinergic side effects due to age-related changes in pharmacokinetics and pharmacodynamics, reduced muscarinic receptor density, and increased blood-brain barrier permeability.46,47 Thus, given the growing number of older community-dwelling individuals with schizophrenia,1,48 characterizing the extent to which anticholinergic burden impairs their cognitive performance is of great clinical importance.
To date, no studies have directly investigated the effects of anticholinergic burden on cognition in older patients with schizophrenia. The cognitive deficits commonly observed in individuals with schizophrenia remain relatively stable over time49 and are consistent with that of neurodevelopmental process. To the contrary, anticholinergic burden may contribute to progressive cognitive impairment seen in neurodegenerative processes such as Alzheimer’s dementia. Alzheimer’s dementia, especially early on in the course of the disease, is known to be associated with characteristic cognitive deficits in learning and delayed recall whereas schizophrenia is associated with more prominent executive dysfunction.50,51 Thus, using 2 independent cognitive batteries that mainly assess cognitive domains affected in Alzheimer’s dementia (hereafter referred to Alzheimer’s dementia-related cognitive functions), we predicted that there will be an association between anticholinergic burden and these functions in community-dwelling individuals with schizophrenia aged 50 years or above. We also aimed at contrasting this association with that between anticholinergic burden and cognitive tests that mainly assess executive dysfunction given that the latter is more linked to schizophrenia than Alzheimer’s dementia.

- Older individuals with schizophrenia are at risk of experiencing high anticholinergic burden, which is known to cause cognitive impairment.
- Anticholinergic burden may contribute to a pattern of cognitive deficits similar to those observed early in the course of Alzheimer’s dementia.
- An Anticholinergic Cognitive Burden scale total score cutoff of ≤ 1.5 may be used to screen patients at risk of developing Alzheimer’s dementia-like cognitive impairment secondary to anticholinergic burden.
METHODS
Participants
Sixty patients with schizophrenia or schizoaffective disorder were recruited at the Centre for Addiction and Mental Health (CAMH) in Toronto, Canada, using advertisements and physician referrals between May 2007 and November 2011. The study was approved by the CAMH Research Ethics Board, and all participants provided written informed consent.
Eligibility criteria were (1) age of 50 years or older; (2) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)52 criteria for a current diagnosis of schizophrenia or schizoaffective disorder; (3) clinically stable as operationalized by (a) not having been hospitalized within 3 months and (b) having had no change in antipsychotic medication dosage within 4 weeks prior to assessment; (4) not meeting criteria for a cognitive disorder secondary to a neurologic disease or brain injury; (5) Mini-Mental State Examination (MMSE)53 score of 18 or more because individuals with very low MMSE scores are unlikely to be able to complete a neuropsychological battery; (6) not having a current DSM-IV major depressive or manic episode; (7) no DSM-IV alcohol or other substance abuse within the past 6 months; (8) no electroconvulsive therapy within 6 months prior to assessment; (9) ability and willingness to speak English; (10) adequate corrected hearing and visual acuity; and (11) ability and willingness to provide written informed consent.
Measures
Diagnosis and clinical symptoms. Diagnosis was confirmed through the Structured Clinical Interview for DSM-IV Disorders.54 Symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS).55
Cognition. To assess Alzheimer’s dementia-related cognitive functions, we used the Cambridge Neuropsychological Test Automated Battery (CANTAB) Alzheimer’s Dementia Battery (CANTAB-AD) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The CANTAB-AD assesses episodic memory, working memory, and reaction time. This battery has been clinically validated to characterize cognitive deficits commonly observed in Alzheimer’s dementia.51,56 The corresponding tests and outcome measures were as follows: Paired Associates Learning (PAL) total errors adjusted, Spatial Working Memory (SWM) between errors, and Reaction Time (RTI) 5-choice reaction time. Outcome measures were transformed to z-scores (mean = 0, SD = 1) such that higher scores represented poorer performance. An Alzheimer’s dementia composite z-score was generated by averaging the z-scores for all 3 tests combined.
Similarly, the RBANS57 is commonly used to detect cognitive deficits associated with Alzheimer’s dementia. It has been shown to reliably differentiate healthy individuals from those with mild cognitive impairment (MCI) and Alzheimer’s dementia.58,59 It assesses 5 cognitive domains and comprises 12 subtests. The domains and corresponding subtests were as follows: Attention (Digit Span, Symbol Coding), Language (Picture Naming, Semantic Fluency), Visuospatial-Construction (Figure Copy, Line Orientation), Immediate Memory (List Learning, Story Memory), and Delayed Memory (List Recall, List Recognition, Story Recall, and Figure Recall). Age-adjusted index scores and total scale scores (mean = 100, SD = 50) were generated using normative data available from the RBANS manual.
The impact of anticholinergic burden on the CANTAB-AD Battery and RBANS performance was contrasted with its impact on other cognitive functions core to schizophrenia using 2 additional CANTAB tests of executive function: Stockings of Cambridge (SOC) problems solved; and Intra-Extra Dimensional Set Shift (IED) total errors adjusted. Individuals with schizophrenia have impaired, yet stable, performance on the SOC and IED throughout the course of their illness.60
Anticholinergic burden. The Anticholinergic Cognitive Burden scale (ACB)33 is a validated expert-based list of medications with possible or definite anticholinergic effects.61 We used the 2012 update of the ACB62 to quantify the overall anticholinergic burden of medications used by our participants. Medication data were collected from electronic health records, chart review, and self-report. Both prescription and over-the-counter medications were included in the calculation of the ACB total score. Each medication was rated on a 4-point Likert-type scale (0: no anticholinergic activity; 1: possible anticholinergic activity; 2 or 3: definite anticholinergic activity), and the total score is calculated by summing the ratings of all medications. An ACB total score ≥ 3 suggests definite anticholinergic activity.
Data Analysis
Our primary analyses assessed the relationship between the CANTAB-AD composite score and demographic and clinical variables, including ACB total score. We conducted a regression analysis in which CANTAB-AD composite score was the dependent variable and age, gender, education, PANSS Positive and Negative Scores, and ACB total score were entered simultaneously as the independent variables. We then assessed whether the relationship between ACB total score and CANTAB-AD composite score was driven by 1 of the 3 CANTAB tests (PAL, SWM, and RTI), replacing the CANTAB-AD composite by the z-score of each of the 3 tests separately.
To validate the results generated with the CANTAB-AD, we also assessed the relationship between performance on RBANS and the same demographic and clinical variables. For these regression analyses, the dependent variables were the RBANS total score or the scores for each domain. The covariates were gender, education, PANSS positive and negative scores, and ACB total score. We did not include age as a covariate in the analyses because the RBANS scores are adjusted for age.
To contrast these analyses with an analysis focusing on schizophrenia-related cognitive functions rather than Alzheimer’s dementia-related cognitive functions, we additionally performed 2 similar multiple regressions with either the SOC or IED score as the dependent variable.
Lastly, we constructed a receiver operating characteristic (ROC) curve to determine an ACB cutoff score using the CANTAB-AD composite score to define cognitive status. We dichotomized our sample into “high” versus “low” performance using the median of the CANTAB-AD composite score to identify a threshold for risk of cognitive impairment.
All analyses listed above were corrected for multiple comparisons using Bonferroni correction when appropriate. SPSS, version 21 (SPSS Inc, Chicago, Illinois) was used for all analyses.
RESULTS
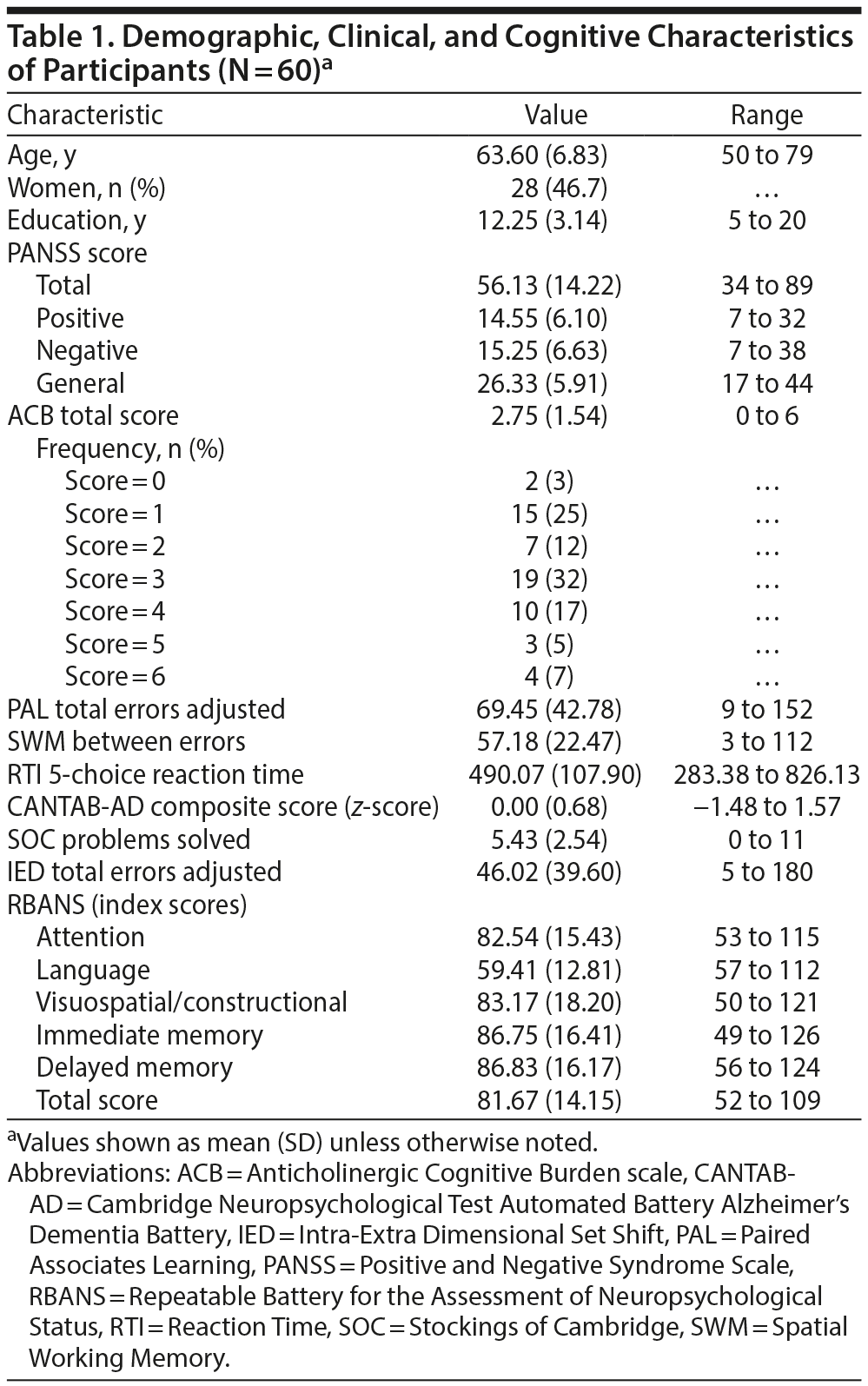
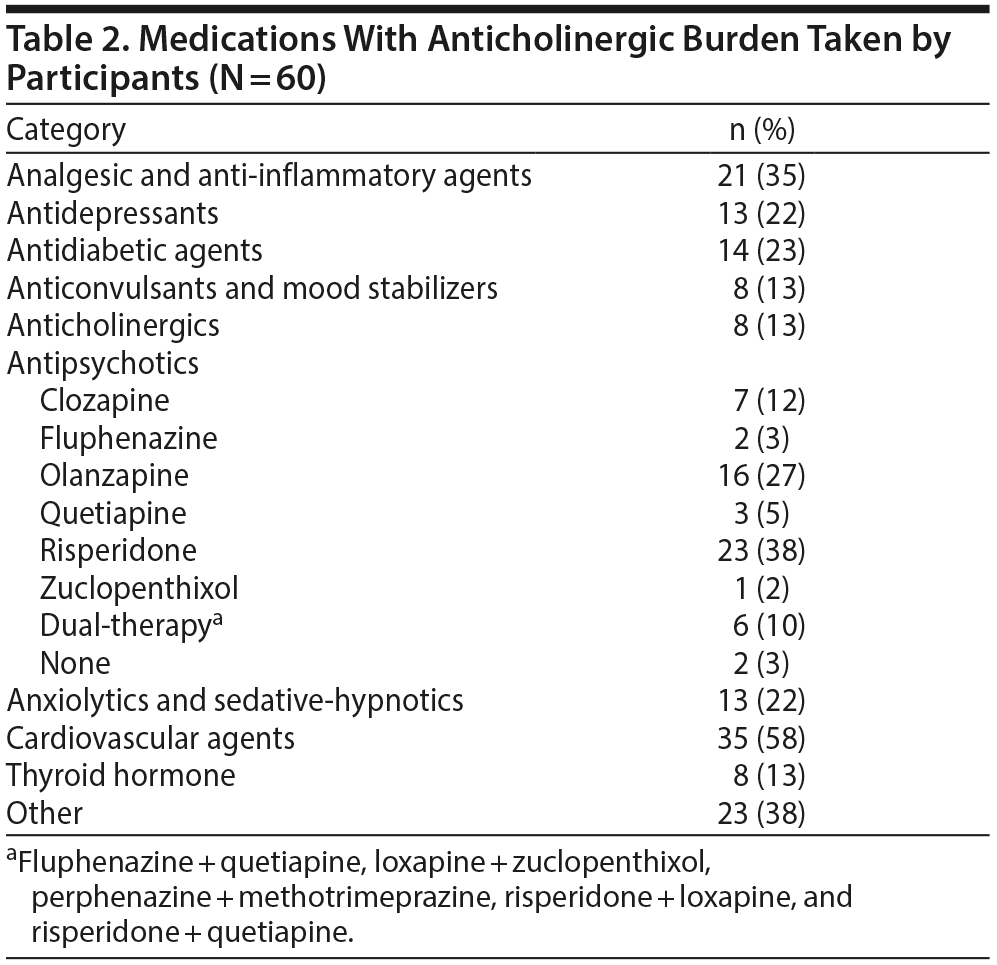
Table 1 summarizes the demographic, clinical, and cognitive characteristics of the 60 participants who completed the study. Table 2 summarizes the participants’ medications at the time of assessment.
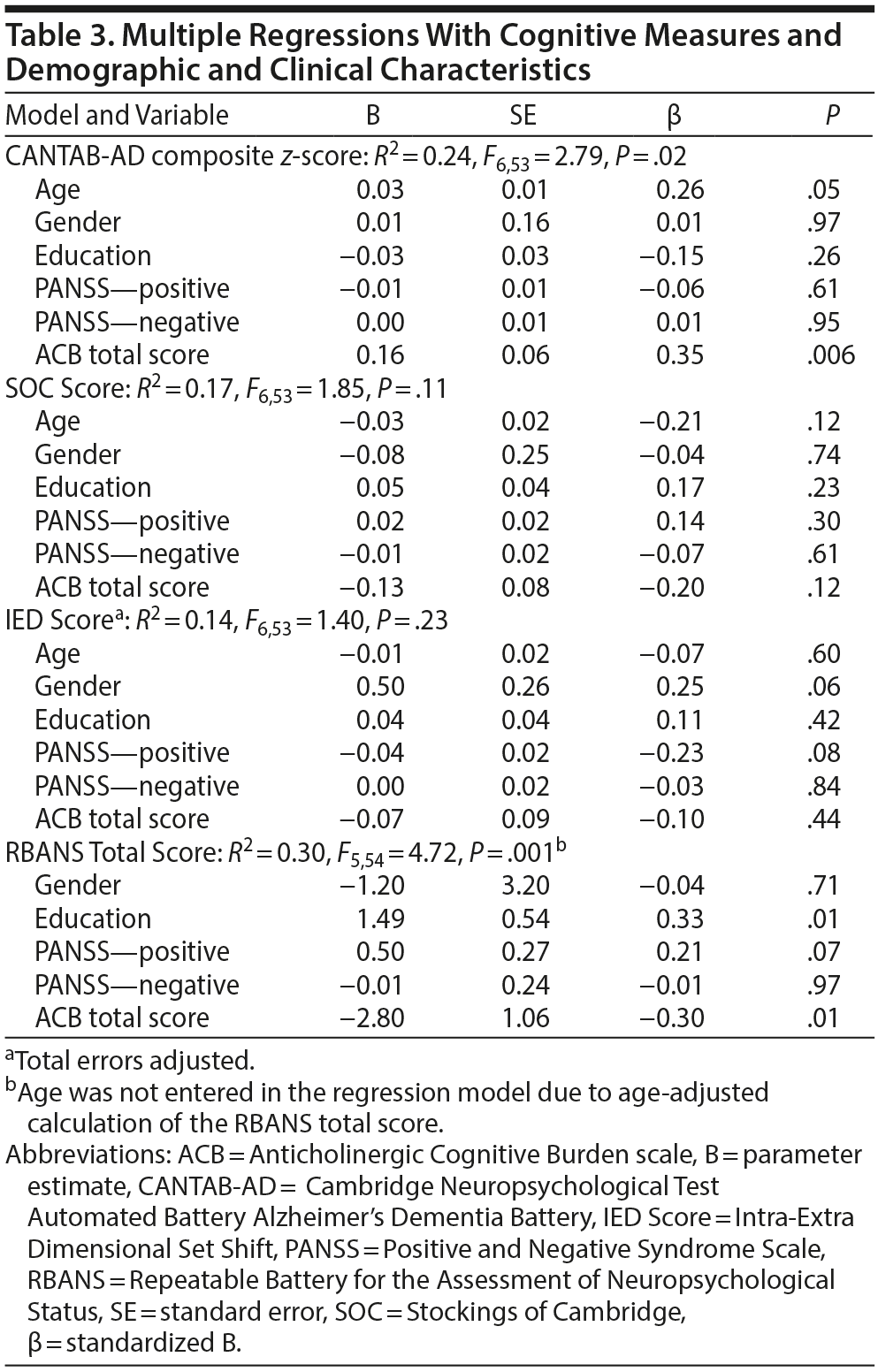
As predicted, the ACB total score was positively associated with the CANTAB-AD composite score and negatively associated with the RBANS total score (Table 3). No associations were observed between the ACB total score and the SOC or IED scores (Table 3).
In the multiple regressions with each of the 3 CANTAB tests constituting the Alzheimer’s Dementia composite score, the ACB total score was associated with spatial working memory (SWM: B = 0.18, SE = 0.09, β = .27, P = .04), trended with paired associates learning (as assessed by the PAL: B = 0.16, SE = 0.08, β = .24, P = .06), and was not associated with reaction time (RTI: B = 0.13, SE = 0.09, B = 0.21, P = .13).
In the multiple regressions with each of the 5 RBANS domains, the ACB total score was associated with Immediate Memory (B = −3.45, SE = 1.16, β = −0.32, P = .004) and Visuospatial-Construction (B = −3.29, SE = 1.39, β = −0.28, P = .02) and Delayed Memory (B = −4.00, SE = 1.20, β = −0.38, P = .002), but not Language (B = −1.62, SE = 0.94, β = −0.19, P = .09) or Attention (B = 0.24, SE = 1.33, β = .02, P = .86).
The ROC curve was found to slope off at an ACB total score cutoff of 1.5 (Figure 1). At this cutoff, the ACB total score sensitivity was 90.3% with a specificity of 48.3%. ACB total score cutoffs of 2.5 and 3.5 had sensitivities of 74.2% and 38.7% with specificities of 55.2% and 82.8%, respectively. The area under the curve for the ROC curve was 0.71 (P = .005).
DISCUSSION
We found a robust association between anticholinergic burden and Alzheimer’s dementia-related cognitive functions among older community-dwelling patients with schizophrenia. Higher anticholinergic burden was specifically associated with poorer performance on measures of delayed memory, episodic memory, immediate memory, spatial working memory, and visuospatial-construction, but not attention, executive function, language, or reaction time. Further, an ACB total score cutoff of ≤ 1.5 may be used to identify individuals at risk of experiencing adverse cognitive consequences.
Compared to the general population, individuals with schizophrenia have a 2-fold higher risk of developing dementia before the age of 80 years.5 The etiology underlying the development of dementia in schizophrenia is currently unknown and cannot be explained by traditional dementia risk factors.5 Our results suggest that high anticholinergic burden is common among patients with schizophrenia, and it may account for some of their increased risk for dementia. However, additional studies are warranted to fully elucidate this potential relationship and characterize the extent of the contribution made by anticholinergic burden.
Our results are congruent with those of previous studies assessing the relationship between anticholinergic burden and cognition in adults with schizophrenia. Both the literature and our findings show that high anticholinergic burden contributes to impairment in learning, memory, and visuospatial ability.21-24 Negative associations between anticholinergic burden and complex attention have also been described.22 Our study did not directly assess complex attention, which relies on higher-order cognitive abilities.22,63,64 Deficits in this domain may have been indirectly captured by our measure of spatial working memory given that intact visuospatial ability, response inhibition, and maintenance and retrieval of stored information are required to perform well on this task.65
Our findings are also consistent with the effects of anticholinergic burden on cognition in healthy elderly. Similar to our results, high anticholinergic burden has been associated with poorer performance on measures of learning, memory, and visuospatial ability in healthy elderly individuals.25-27,29 However, deficits in attention, executive function, and processing speed have also been observed in older healthy individuals.26,27,31 Thus, it appears that anticholinergic burden contributes to global cognitive dysfunction in healthy older individuals and selective impairments in older individuals with schizophrenia. Schizophrenia-related cognitive deficits in the domains of attention, executive function, and processing speed could be masking any anticholinergic deleterious impact (“floor effect”). This finding further supports a possible specific contribution of anticholinergic burden in older individuals with schizophrenia to cognitive deficits that are associated with Alzheimer’s dementia and are of a neurodegenerative and progressive nature rather than neurodevelopmental and stable.
There have been several studies linking high anticholinergic burden with increased risk of MCI and Alzheimer’s dementia in nondemented elderly individuals.18-20,26 In one longitudinal cohort study26 of individuals aged 60 years or older, 80% of participants with high anticholinergic burden at baseline were classified as having MCI at 1-year follow-up. Another longitudinal cohort study in individuals aged 65 years or older found that cumulative anticholinergic exposure over 10 years increased the risk of incident dementia and Alzheimer’s dementia.18 Further, this risk was found to persist even when other Alzheimer’s dementia risk factors had been accounted for (eg, age, education, medical comorbidity, and apolipoprotein E ε4 status).18,26 Taken together, these studies suggest that high anticholinergic burden may be a modifiable risk factor for MCI and Alzheimer’s dementia. However, it is still unclear as to whether reducing anticholinergic burden will improve cognitive outcomes.30,66
The pathologic changes that occur early in the course of Alzheimer’s dementia manifest as impairments in episodic memory.61,67-69 In the present study, high anticholinergic burden was predictive of impaired episodic memory. Poor episodic memory is routinely observed in schizophrenia, but typically occurs in the context of global cognitive dysfunction.70 This finding is consistent with the general observation that anticholinergic burden impairs selected cognitive domains in individuals with schizophrenia.21,22 Our results further support this relationship by demonstrating that anticholinergic burden was associated with the RBANS indices of immediate memory, delayed memory, and visuospatial-construction, but not attention or language. The RBANS indices of immediate and delayed memory have high sensitivity for Alzheimer’s dementia and reliably differentiate between individuals with and without dementia.58 Further, in addition to episodic memory, visuospatial ability is also compromised in early Alzheimer’s dementia,71 whereas deficits in attention and language are typically later manifestations.63 Taken together, these results suggest that high anticholinergic burden may give rise to cognitive deficits in schizophrenia that are phenotypic of preclinical Alzheimer’s dementia. However, this is not to minimize the impact and severity of schizophrenia-related cognitive deficits, but rather to emphasize the importance of screening for potentially reversible risk factors that may cause impairment in certain cognitive domains.
Moreover, our patients with schizophrenia had an anticholinergic burden that was about 50% higher than what is reported in the general population.33,72 The majority of this burden was accounted for by the anticholinergic side effects of their antipsychotics and other psychotropic medications. Anticholinergic activity and cognitive impairment have been observed with several of these medications even at therapeutic doses.23,36 Long-term use of these agents may have exacerbated preexisting cognitive deficits in our patients. There is some evidence suggesting that permanent cognitive deficits may be a consequence of chronic anticholinergic exposure28,29 and may result from neuroanatomical changes analogous to that observed in Alzheimer’s dementia (eg, increased amyloid and neurofibrillary pathology).29,73
Our study has several limitations. First, we excluded patients with very low cognitive function. Some of these patients may have had Alzheimer’s dementia or other dementia, and they may have been more sensitive to the detrimental effects of anticholinergic medications. However, severe cognitive deficits could also override any impact from anticholinergic burden, thus weakening its association with cognitive performance. Second, we assessed anticholinergic burden based on prescribed medications, and we did not collect data on medication adherence. This may have contributed to an overestimate of anticholinergic burden. Third, much like other scales that measure anticholinergic burden, the ACB yields an imprecise estimation of anticholinergic activity72 because it does not take into account drug dosages/levels, interactions, or metabolism. Our group has previously published on the use of a serum anticholinergic activity assay as a direct method of assessing anticholinergic burden.32 However, a pitfall of that method was that it was limited in its ability to differentiate between procholinergic and anticholingeric activity. For example, some medications, like clozapine, that have high anticholinergic burden produce metabolites that have procholinergic and procognitive effects.74 Therefore, we believe that using this assay would have not provided an advantage over the ACB given the extent of polypharmacy among participants in our sample. Moreover, given its availability and ease of administration, the ACB would be better suited for use in clinical settings compared to this assay. Lastly, because of our cross-sectional design and limited sample size, we are unable to infer true causality between anticholinergic burden and cognitive impairment. We further realize that our use of a convenience sample may additionally limit the generalizability of our findings to other older individuals with schizophrenia.
Notwithstanding these limitations, our results have implications for clinical practice. Clinicians should be aware that high anticholinergic burden in older individuals with schizophrenia may contribute to a pattern of cognitive deficits characteristic of early Alzheimer’s dementia. These deficits may be misattributed to progression of underlying disease (ie, schizophrenia) or to the onset of dementia rather than to a secondary treatable cause (ie, anticholinergic burden). The ACB total score with a cutoff of ≤ 1.5 may be utilized as a screening measure to identify patients at risk of developing cognitive impairment. Doing so may facilitate early interventions to mitigate further cognitive decline (ie, introduction of an acetylcholinesterase inhibitor). However, regular assessment of cognition is still necessary to fully capture impairment that extends beyond that which can be attributed to schizophrenia itself. Similarly, the high level of anticholinergic burden among these patients highlights the importance of regular medication reviews. When conducting these reviews, prescribers should be aware that anticholinergic burden may increase insidiously over time in patients with multiple comorbidities. Pharmacokinetic and pharmacodynamic changes associated with aging may also require a gradual decrease in the dosage of antipsychotic medications.75,76 Anticholinergic burden should influence the pharmacologic management of these older patients, and alternative agents should be considered. When no therapeutic alternatives exist, the lowest effective dose should be prescribed.
In summary, high anticholinergic burden selectively impairs learning, memory, and visuospatial ability in older patients with schizophrenia. Consequently, medications should be assessed in terms of relative contribution to anticholinergic burden before being prescribed to these patients. Longitudinal studies are required to determine whether anticholinergic burden confers an increased risk of dementia in this population and whether this increased risk can be mitigated by optimal pharmacologic management.
Submitted: February 12, 2017; accepted June 19, 2017.
Published online: November 28, 2017.
Potential conflicts of interest: Dr Mulsant receives research support from Brain Canada, the Canadian Institutes of Health Research (CIHR), and the US National Institutes of Health (NIH). During the past 5 years he has received medications (and matching placebo pills) for NIH-funded clinical trials from Bristol-Myers Squibb, Eli Lilly, and Pfizer. He directly owns stocks of General Electric (less than $5,000). Dr Rajji receives research support from Brain Canada, Brain and Behavior Research Foundation, Canadian Foundation for Innovation, CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, NIH, and the W. Garfield Weston Foundation. Mr Tsoutsoulas and Drs Kumar, Ghazala, Voineskos, Menon, and Pollock have no conflict of interest.
Funding/support: CIHR provided financial support for the present study from the following grants: CIHR 200017 to Dr Mulsant and CIHR 180087 to Dr Rajji.
Role of the sponsor: CIHR was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
REFERENCES
1. Goeree R, Farahati F, Burke N, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin. 2005;21(12):2017-2028. PubMed doi:10.1185/030079905X75087
2. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137-152. PubMed doi:10.1038/nrneurol.2011.2
3. Zilkens RR, Bruce DG, Duke J, et al. Severe psychiatric disorders in mid-life and risk of dementia in late- life (age 65-84 years): a population based case-control study. Curr Alzheimer Res. 2014;11(7):681-693. PubMed doi:10.2174/1567205011666140812115004
4. de Vries PJ, Honer WG, Kemp PM, et al. Dementia as a complication of schizophrenia. J Neurol Neurosurg Psychiatry. 2001;70(5):588-596. PubMed doi:10.1136/jnnp.70.5.588
5. Ribe AR, Laursen TM, Charles M, et al. Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiatry. 2015;72(11):1095-1101. PubMed doi:10.1001/jamapsychiatry.2015.1546
6. Farmer ME, Kittner SJ, Rae DS, et al. Education and change in cognitive function: the Epidemiologic Catchment Area Study. Ann Epidemiol. 1995;5(1):1-7. PubMed doi:10.1016/1047-2797(94)00047-W
7. Swanson CL Jr, Gur RC, Bilker W, et al. Premorbid educational attainment in schizophrenia: association with symptoms, functioning, and neurobehavioral measures. Biol Psychiatry. 1998;44(8):739-747. PubMed doi:10.1016/S0006-3223(98)00046-8
8. Corral M, Rodríguez M, Amenedo E, et al. Cognitive reserve, age, and neuropsychological performance in healthy participants. Dev Neuropsychol. 2006;29(3):479-491. PubMed doi:10.1207/s15326942dn2903_6
9. Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549-559. PubMed doi:10.1176/appi.ajp.157.4.549
10. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004;49(11):753-760. PubMed doi:10.1177/070674370404901106
11. Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135-145. PubMed
12. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18-28. PubMed doi:10.1016/j.schres.2012.03.018
13. Lai SW, Lin CH, Liao KF, et al. Association between polypharmacy and dementia in older people: a population-based case-control study in Taiwan. Geriatr Gerontol Int. 2012;12(3):491-498. PubMed doi:10.1111/j.1447-0594.2011.00800.x
14. Winklbaur B, Ebner N, Sachs G, et al. Substance abuse in patients with schizophrenia. Dialogues Clin Neurosci. 2006;8(1):37-43. PubMed
15. Hulse GK, Lautenschlager NT, Tait RJ, et al. Dementia associated with alcohol and other drug use. Int Psychogeriatr. 2005;17(suppl 1):S109-S127. PubMed doi:10.1017/S1041610205001985
16. Dean B, Laws SM, Hone E, et al. Increased levels of apolipoprotein E in the frontal cortex of subjects with schizophrenia. Biol Psychiatry. 2003;54(6):616-622. PubMed doi:10.1016/S0006-3223(03)00075-1
17. Hata T, Kunugi H, Nanko S, et al. Possible effect of the APOE epsilon 4 allele on the hippocampal volume and asymmetry in schizophrenia. Am J Med Genet. 2002;114(6):641-642. PubMed doi:10.1002/ajmg.10556
18. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401-407. PubMed doi:10.1001/jamainternmed.2014.7663
19. Carrière I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317-1324. PubMed doi:10.1001/archinternmed.2009.229
20. Jessen F, Kaduszkiewicz H, Daerr M, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S111-S115. PubMed doi:10.1007/s00406-010-0156-4
21. Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055-1062. PubMed doi:10.1176/appi.ajp.2009.09010017
22. Minzenberg MJ, Poole JH, Benton C, et al. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161(1):116-124. PubMed doi:10.1176/appi.ajp.161.1.116
23. Tune LE, Strauss ME, Lew MF, et al. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139(11):1460-1462. PubMed doi:10.1176/ajp.139.11.1460
24. Tracy JI, Monaco C, Giovannetti T, et al. Anticholinergicity and cognitive processing in chronic schizophrenia. Biol Psychol. 2001;56(1):1-22. PubMed doi:10.1016/S0301-0511(00)00083-1
25. Ray PG, Meador KJ, Loring DW, et al. Central anticholinergic hypersensitivity in aging. J Geriatr Psychiatry Neurol. 1992;5(2):72-77. PubMed
26. Ancelin ML, Artero S, Portet F, et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455-459. PubMed doi:10.1136/bmj.38740.439664.DE
27. Nebes RD, Pollock BG, Halligan EM, et al. Cognitive slowing associated with elevated serum anticholinergic activity in older individuals is decreased by caffeine use. Am J Geriatr Psychiatry. 2011;19(2):169-175. PubMed doi:10.1097/JGP.0b013e3181e4490d
28. Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain—how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. PubMed doi:10.1111/bcpt.12140
29. Risacher SL, McDonald BC, Tallman EF, et al; Alzheimer’s Disease Neuroimaging Initiative. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721-732. PubMed doi:10.1001/jamaneurol.2016.0580
30. Kersten H, Molden E, Tolo IK, et al. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(3):271-278. PubMed doi:10.1093/gerona/gls176
31. Bottiggi KA, Salazar JC, Yu L, et al. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14(11):980-984. PubMed doi:10.1097/01.JGP.0000224619.87681.71
32. Mulsant BH, Pollock BG, Kirshner M, et al. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60(2):198-203. PubMed doi:10.1001/archpsyc.60.2.198
33. Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509X.4.3.311
34. Chew ML, Mulsant BH, Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333-1341. PubMed doi:10.1111/j.1532-5415.2008.01737.x
35. Perlick D, Stastny P, Katz I, et al. Memory deficits and anticholinergic levels in chronic schizophrenia. Am J Psychiatry. 1986;143(2):230-232. PubMed doi:10.1176/ajp.143.2.230
36. Chew ML, Mulsant BH, Pollock BG, et al. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr Res. 2006;88(1-3):63-72. PubMed doi:10.1016/j.schres.2006.07.011
37. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21(11):1133-1137. PubMed doi:10.1111/j.1525-1497.2006.00563.x
38. Jeste DV, Gladsjo JA, Lindamer LA, et al. Medical comorbidity in schizophrenia. Schizophr Bull. 1996;22(3):413-430. PubMed doi:10.1093/schbul/22.3.413
39. Tsoutsoulas C, Mulsant BH, Kalache SM, et al. The influence of medical burden severity and cognition on functional competence in older community-dwelling individuals with schizophrenia. Schizophr Res. 2016;170(2-3):330-335. PubMed doi:10.1016/j.schres.2015.12.009
40. Voineskos AN, Lobaugh NJ, Bouix S, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133(pt 5):1494-1504. PubMed doi:10.1093/brain/awq040
41. Felsky D, Voineskos AN, Lerch JP, et al. Myelin-associated glycoprotein gene and brain morphometry in schizophrenia. Front Psychiatry. 2012;3:40. PubMed doi:10.3389/fpsyt.2012.00040
42. Rajji TK, Chow TW, Voineskos AN, et al. Cholinergic pathways and cognition in patients with schizophrenia: a pilot study. Schizophr Res. 2012;139(1-3):46-52. PubMed doi:10.1016/j.schres.2012.06.006
43. Voineskos AN, Felsky D, Kovacevic N, et al. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex. 2013;23(9):2044-2057. PubMed doi:10.1093/cercor/bhs188
44. Nazeri A, Chakravarty MM, Felsky D, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38(10):1954-1962. PubMed doi:10.1038/npp.2013.93
45. Wheeler AL, Chakravarty MM, Lerch JP, et al. Disrupted prefrontal interhemispheric structural coupling in schizophrenia related to working memory performance. Schizophr Bull. 2014;40(4):914-924. PubMed doi:10.1093/schbul/sbt100
46. Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225-233. PubMed
47. Dewey SL, Volkow ND, Logan J, et al. Age-related decreases in muscarinic cholinergic receptor binding in the human brain measured with positron emission tomography (PET). J Neurosci Res. 1990;27(4):569-575. PubMed doi:10.1002/jnr.490270418
48. Lafeuille MH, Dean J, Fastenau J, et al. Burden of schizophrenia on selected comorbidity costs. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):259-267. PubMed doi:10.1586/14737167.2014.894463
49. Rajji TK, Voineskos AN, Butters MA, et al. Cognitive performance of individuals with schizophrenia across seven decades: a study using the MATRICS consensus cognitive battery. Am J Geriatr Psychiatry. 2013;21(2):108-118. PubMed doi:10.1016/j.jagp.2012.10.011
50. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140. PubMed doi:10.1016/j.schres.2008.03.015
51. Egerházi A, Berecz R, Bartók E, et al. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):746-751. PubMed doi:10.1016/j.pnpbp.2007.01.011
52. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
53. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
54. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, New York: Biometrics Research, New York State Psychiatric Institute; November 2002.
55. Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23(1):99-110. PubMed doi:10.1016/0165-1781(88)90038-8
56. Swainson R, Hodges JR, Galton CJ, et al. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12(4):265-280. PubMed doi:10.1159/000051269
57. Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. PubMed doi:10.1076/jcen.20.3.310.823
58. Duff K, Humphreys Clark JD, O’ Bryant SE, et al. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603-612. PubMed doi:10.1016/j.acn.2008.06.004
59. Karantzoulis S, Novitski J, Gold M, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): utility in detection and characterization of mild cognitive impairment due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28(8):837-844. PubMed doi:10.1093/arclin/act057
60. Tyson PJ, Laws KR, Roberts KH, et al. Stability of set-shifting and planning abilities in patients with schizophrenia. Psychiatry Res. 2004;129(3):229-239. PubMed doi:10.1016/j.psychres.2004.09.007
61. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. PubMed doi:10.1186/s12877-015-0029-9
62. Aging Brain Care. Anticholinergic Cognitive Burden Scale—2012 Update. www. agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf.
63. Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006171. PubMed doi:10.1101/cshperspect.a006171
64. Santangelo V, Macaluso E. The contribution of working memory to divided attention. Hum Brain Mapp. 2013;34(1):158-175. PubMed doi:10.1002/hbm.21430
65. Badcock JC, Michiel PT, Rock D. Spatial working memory and planning ability: contrasts between schizophrenia and bipolar I disorder. Cortex. 2005;41(6):753-763. PubMed doi:10.1016/S0010-9452(08)70294-6
66. Yeh YC, Liu CL, Peng LN, et al. Potential benefits of reducing medication-related anticholinergic burden for demented older adults: a prospective cohort study. Geriatr Gerontol Int. 2013;13(3):694-700. PubMed doi:10.1111/ggi.12000
67. Small BJ, Fratiglioni L, Viitanen M, et al. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57(6):839-844. PubMed doi:10.1001/archneur.57.6.839
68. Bפckman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124(pt 1):96-102. PubMed doi:10.1093/brain/124.1.96
69. Howieson DB, Dame A, Camicioli R, et al. Cognitive markers preceding Alzheimer’s dementia in the healthy oldest old. J Am Geriatr Soc. 1997;45(5):584-589. PubMed doi:10.1111/j.1532-5415.1997.tb03091.x
70. Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat. 2006;2(4):531-536. PubMed doi:10.2147/nedt.2006.2.4.531
71. Johnson DK, Storandt M, Morris JC, et al. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66(10):1254-1259. PubMed doi:10.1001/archneurol.2009.158
72. Kashyap M, Belleville S, Mulsant BH, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. J Am Geriatr Soc. 2014;62(2):336-341. PubMed doi:10.1111/jgs.12632
73. Perry EK, Kilford L, Lees AJ, et al. Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol. 2003;54(2):235-238. PubMed doi:10.1002/ana.10639
74. Rajji TK, Mulsant BH, Davies S, et al. Prediction of working memory performance in schizophrenia by plasma ratio of clozapine to N-desmethylclozapine. Am J Psychiatry. 2015;172(6):579-585. PubMed doi:10.1176/appi.ajp.2015.14050673
75. Uchida H, Mamo DC, Mulsant BH, et al. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 2009;70(3):397-405. PubMed doi:10.4088/JCP.08r04171
76. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 receptor occupancy study. JAMA Psychiatry. 2015;72(9):927-934. PubMed doi:10.1001/jamapsychiatry.2015.0891
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
Please sign in or purchase this PDF for $40.00.
Save
Cite