Many observational studies published during the past 15 years have found an association between anticholinergic drug exposure and the risk of incident dementia. Animal data suggest plausible causal mechanisms for this finding. The results of a recent, large, and well-conducted study on the subject were widely disseminated by the lay and scientific media. This study addressed protopathic bias by examining anticholinergic drug exposure in time windows 1-11, 3-13, and 5-20 years before the identification of dementia. In brief, the study found that, pooling anticholinergic drug exposure across 11 drug categories, exposure in smallest to largest cumulative dosing groups was significantly associated with incident dementia risk, with an apparently dose-dependent relationship in unadjusted as well as adjusted analyses in all time windows prior to dementia identification. Whereas these findings appear compelling, there are at least 4 elephants in the room. First, only 3 of 11 anticholinergic drug categories were consistently associated with an increased risk of dementia; this suggests that anticholinergic activity may be an irrelevant common denominator. Second, for 2 of these 3 categories (antidepressant and antipsychotic drugs), confounding by indication seemed a distinct possibility. Third, in many analyses it seemed that exposure for as little as the equivalent of 1-90 days sufficed to increase the risk of dementia at a time interval of up to 20 years later; a causal mechanism here would need to have strong neurotoxic effects to result in the widespread brain changes that characterize dementia. Finally, the associations were almost uniformly stronger for vascular dementia than for Alzheimer’s disease, making the identification of a causal mechanism even more challenging. Deprescribing anticholinergics to reduce state-dependent cognitive impairment, or to reduce the risk of delirium in vulnerable demographic and medical populations, is reasonable. Deprescribing anticholinergics to reduce the risk of future dementia is presently unwarranted.

ABSTRACT
Many observational studies published during the past 15 years have found an association between anticholinergic drug exposure and the risk of incident dementia. Animal data suggest plausible causal mechanisms for this finding. The results of a recent, large, and well-conducted study on the subject were widely disseminated by the lay and scientific media. This study addressed protopathic bias by examining anticholinergic drug exposure in time windows 1-11, 3-13, and 5-20 years before the identification of dementia. In brief, the study found that, pooling anticholinergic drug exposure across 11 drug categories, exposure in smallest to largest cumulative dosing groups was significantly associated with incident dementia risk, with an apparently dose-dependent relationship in unadjusted as well as adjusted analyses in all time windows prior to dementia identification. Whereas these findings appear compelling, there are at least 4 elephants in the room. First, only 3 of 11 anticholinergic drug categories were consistently associated with an increased risk of dementia; this suggests that anticholinergic activity may be an irrelevant common denominator. Second, for 2 of these 3 categories (antidepressant and antipsychotic drugs), confounding by indication seemed a distinct possibility. Third, in many analyses it seemed that exposure for as little as the equivalent of 1-90 days sufficed to increase the risk of dementia at a time interval of up to 20 years later; a causal mechanism here would need to have strong neurotoxic effects to result in the widespread brain changes that characterize dementia. Finally, the associations were almost uniformly stronger for vascular dementia than for Alzheimer’s disease, making the identification of a causal mechanism even more challenging. Deprescribing anticholinergics to reduce state-dependent cognitive impairment, or to reduce the risk of delirium in vulnerable demographic and medical populations, is reasonable. Deprescribing anticholinergics to reduce the risk of future dementia is presently unwarranted.
J Clin Psychiatry 2019;80(4):19f13000
To cite: Andrade C. Anticholinergic drug exposure and the risk of dementia: there is modest evidence for an association but not for causality. J Clin Psychiatry. 2019;80(4):19f13000.
To share: 10.4088/JCP.19f13000
© Copyright 2019 Physicians Postgraduate Press, Inc.
A well-known proverb tells us that prevention is better than cure. This is especially true for disorders, such as dementia, that are mostly irreversible; after all, dead neurons cannot be brought back to life. In this context, many groups of drugs have been associated with an increased risk of dementia; these include benzodiazepines,1,2 other sedative-hypnotics,3 antidepressants,4,5 and even specific agents, such as tamsulosin.6,7 This article examines the evidence associating anticholinergic drugs with dementia. One study8 is examined in detail because the results of this very recent study were widely disseminated by the scientific and lay media.
Why Anticholinergic Drugs?
Acetylcholine is an important neurotransmitter in the brain. Muscarinic acetylcholine receptor antagonists induce state-dependent cognitive deficits and are therefore employed in animal models of learning and memory.9 Greater anticholinergic burden in a prescription is associated with greater cognitive impairment in older persons,10 including those with schizophrenia11 or depression.12 In contrast, cholinergic drugs are effective treatments for Alzheimer’s disease.13
Anticholinergic drugs have been suggested to disturb neural networks involved in learning and memory,14 decrease levels of brain phosphatidylcholine, and increase the formation of β-amyloid.15 Anticholinergics may increase tau-driven neurodegeneration.16 Anticholinergics may also drive neuroinflammation.17 These findings suggest that long-term anticholinergic drug use might predispose to the development of dementia, particularly dementia of the Alzheimer type.
In sharp contrast, however, in a study18 of 420 community-based autopsy samples with use of anticholinergic medication examined across a 10-year period, anticholinergic exposure was not associated with neuritic plaque scores or neurofibrillary degeneration, and, surprisingly, heavy use of anticholinergics was significantly associated with lower cerebral microinfarct burden. There was no association between anticholinergic exposure and macroscopic infarcts or atherosclerosis.
Anticholinergic Drugs and Dementia: Clinical Evidence
There are no clinical trials of anticholinergic drugs with the risk of dementia examined as an outcome. However, many observational studies have suggested that an association does exist. A few of these are briefly examined.
In a French cohort study19 of 372 subjects, aged > 60 years, none of whom had dementia at recruitment, 9.2% had continuously used anticholinergic drugs during the year before cognitive assessment. These subjects displayed poorer performance on many but not all neuropsychological measures. These subjects were also more likely to have mild cognitive impairment relative to nonusers (80% vs 35%, respectively). However, the risk of incident dementia did not differ significantly between users and nonusers at an 8-year follow-up.
In another, larger, French cohort study20 of 6,912 subjects (67% female) aged 65 years and older, the risk of incident dementia across a 4-year follow-up period was elevated in continuous users of anticholinergic drugs (hazard ratio [HR], 1.65; 95% confidence interval [CI], 1.00-2.73) but not in those who discontinued these drugs (HR, 1.28; 95% CI, 0.59-2.76).
In a German cohort study21 of 2,605 dementia-free subjects aged > 75 years at recruitment, the use of anticholinergic drugs was associated with a significantly increased risk of incident dementia at a 54-month follow-up (HR, 2.08; 95% CI not provided). The HRs were higher in association with greater potency of anticholinergic activity.
In a 1-year retrospective North American study22 of 3,690 subjects aged 65 years and older, some but not all analyses found an association between anticholinergic drug exposure and mild cognitive impairment. No analysis found an association between anticholinergic exposure and dementia.
In a North American cohort study,23 3,434 subjects aged 65 years and older were followed for a mean of 7.3 years; 23.2% of the subjects developed dementia. The risk of dementia was examined in persons who used versus did not use anticholinergic drugs such as (most commonly) tricyclic antidepressants, first-generation antihistamines, and bladder antimuscarinics; use of these drugs in the first year before the diagnosis of dementia was not considered. In an analysis that adjusted for confounding variables, exposure to anticholinergic drugs for > 1,095 total standardized daily doses (TSDDs) was associated with an increased risk of dementia (HR, 1.54; 95% CI, 1.21-1.96); however, lesser exposure was not associated with statistically significant increase in risk. These findings were supported in secondary, sensitivity, and post hoc analyses.
In a British case-control study,24 40,770 patients with dementia, aged 65 to 99 years, were compared with 283,933 controls. In analyses that adjusted for confounding variables, anticholinergic exposure in the years 4-20 before diagnosis of dementia was compared between cases and controls. Subjects with dementia had an increased risk of prior anticholinergic exposure (odds ratio [OR], 1.11; 95% CI, 1.08-1.14). This risk was elevated in an exposure-dependent manner for antidepressant, urologic, and antiparkinsonian drugs with high anticholinergic activity, but not clearly so for gastrointestinal drugs. Strikingly, for antidepressant and urological drug classes, the association between strong anticholinergic drug use and the risk of dementia was apparent in 4-10 and 10-15 as well as 15-20 year windows of exposure prior to the diagnosis of dementia, indicating that these drugs were significantly associated with dementia regardless of when the exposure occurred in the past.
In other studies, increasing use of strong anticholinergic drugs was associated with increased odds of transition from normal cognition to mild cognitive impairment.25 Anticholinergic drugs were also associated with an increased risk of dementia in diagnostic subgroups, such as patients with diabetes mellitus26 and Parkinson disease.27
Confounding by Indication, Protopathic Bias, and Reverse Causation
In observational studies that examine the relationship between an exposure and an adverse outcome, the outcome may occur not because of the exposure but because of the indication for the exposure. This is known as confounding by indication. As a real example, leaving a night-light on during sleeping hours was associated with an increased risk of infants developing myopia in later life. However, the increased risk of myopia is not necessarily because the night-light was left on; it is more likely to be due to the heritability of axial myopia, and the need of myopic parents to have a night-light on in the child’s room when they check on a sleeping child.28
In like manner, confounding by indication may explain a part or the whole of the risk of suicidality associated with antidepressant drugs, especially in children,29 and the risk of autism spectrum disorder (ASD) after gestational exposure to antidepressant medication.30,31 In the former situation, the indication for the antidepressant (depression) may explain the suicidal behavior, and in the latter situation, genetic determinants of severe depression, biological changes associated with severe depression, or even merely adverse behaviors associated with severe depression may separately or together predispose to ASD.
Confounding by indication includes a broad range of situations. Protopathic (literally, “before the disease”) bias is a special situation wherein although the exposure occurs before the outcome, it occurs because of the outcome. That is, there is reverse causation. As an example that is relevant to this article, persons with unrecognized, subclinical symptoms of Alzheimer’s disease may experience anxiety and depression and may consequently be treated with antidepressant medications. A longitudinal observational study that compares elderly subjects who did versus did not receive antidepressant drugs will then find more cases of incident (new-onset) dementia in the antidepressant cohort than in the control cohort. One conclusion could be that the disease occurred because of the antidepressant prescription. Another explanation is that the antidepressant prescription occurred because of the early, unrecognized symptoms of the disease; that is, this is an example of protopathic bias as a special case of confounding by indication. In the study by Richardson et al,24 summarized in the previous section, protopathic bias was addressed in the analysis by examining exposure in 4-10, 10-15, and 15-20 year time windows before the diagnosis of dementia. This was to exclude the possibility that the anticholinergic drugs were prescribed for symptoms that antedated the recognition of dementia.
Anticholinergic Drugs and Dementia: A New Study
Coupland et al8 described a nested case-control study of subjects drawn from a general practice database in England. Cases (n = 58,769) were subjects identified with dementia, of which Alzheimer’s disease and mixed dementia formed the largest group (60%), followed by vascular dementia (36%); those with specific subtypes of dementia, such as Huntington disease, were excluded. For each case, 5 age-, sex-, and date-matched controls were selected; there were 225,574 controls.
The mean age of the sample was 82 years. The sample was 63% female and 97% white. The mean body mass index (BMI) was 27. About 58% of subjects were nonsmokers. A third consumed no alcohol. Hypertension was the commonest comorbidity, present in a third of the sample. Prevalences for all comorbidities and medication use were slightly higher in the cases.
Exposure to 56 strong anticholinergic medications in 11 different classes by indication was compared between cases and controls. In these comparisons, exposure was reckoned in terms of TSDDs, which is a way of expressing the total number of days for which a subject would have received the medication in the minimum effective daily dose. The analyses were adjusted for a wide range of confounding variables, including ethnicity, BMI, smoking, alcohol use, deprivation score, comorbidities, and medication use, as assessed at the start of the window of exposure.
To preclude protopathic bias, different windows of exposure were examined: 1-11 years, 3-13 years, and 5-20 years before the diagnosis of dementia. Exposure was examined in different cumulative dosing categories: nil, 1-90, 91-365, 366-1,095, and > 1,095 TSDDs. In this context, it may be noted that 90, 365, and 1,095 represent notional exposure for 3 months, 1 year, and 3 years, respectively.
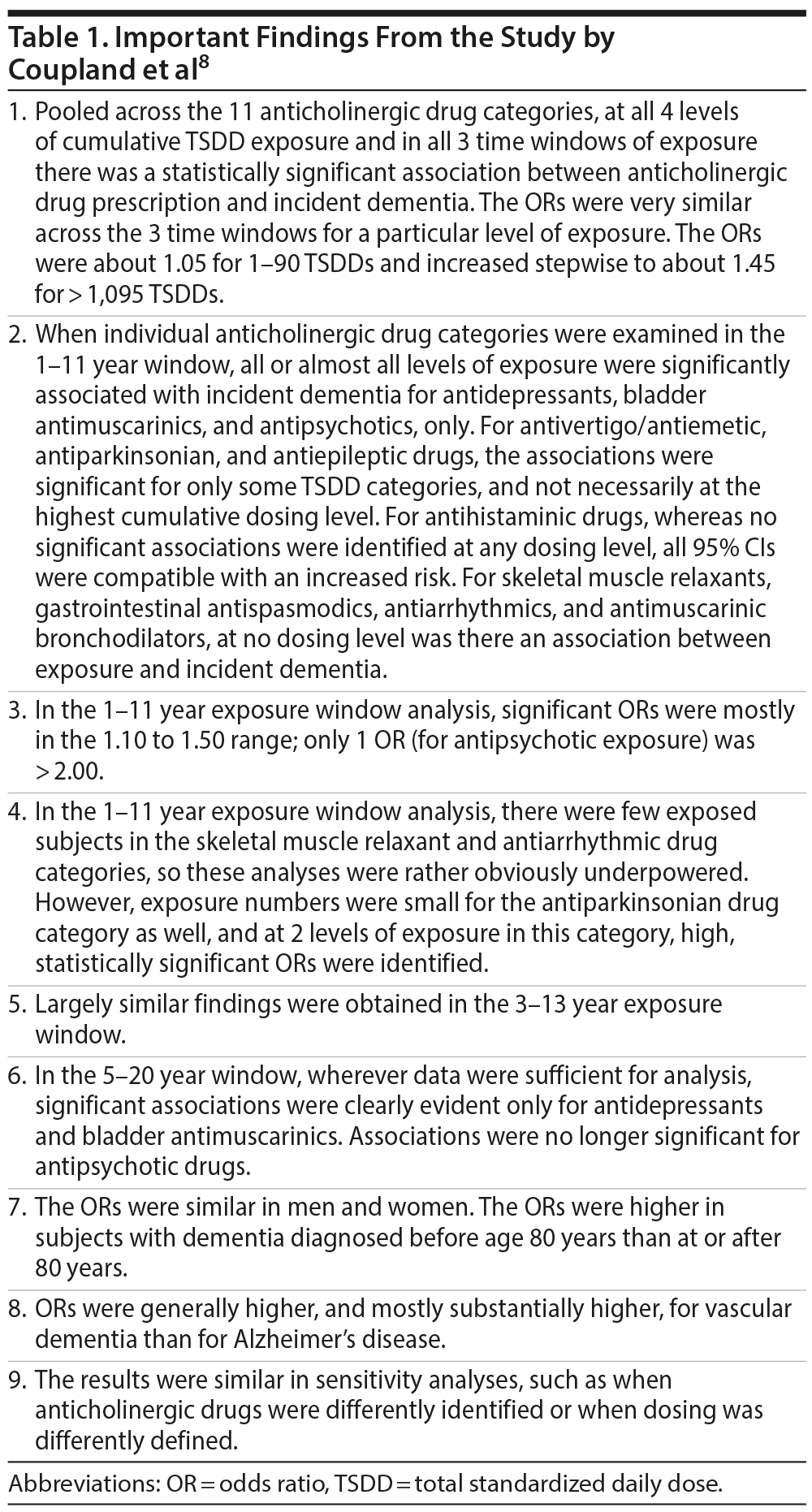
Important findings from the study are presented in Table 1. The authors8 observed that, regardless of what the window of exposure was, the population attributable fraction of dementia associated with total anticholinergic exposure was about 9%-10%. Whereas they accepted that their observational study was not able to evaluate causality, their abstract asserted that their findings “highlight the importance of reducing exposure to anticholinergic drugs in middle-aged and older people.”
General Comments
The issue at stake is whether or not anticholinergic drugs play a causal role in dementia. If they do not play a causal role, their use by patients will merely serve as a marker of risk. If they do play a causal role, then exposure to these drugs is an important modifiable risk factor for dementia, given the reported population attributable fraction of 9%-10%.8
The study8 was well designed and well conducted. The authors adjusted for a large number of risk factors for dementia; importantly, they also adjusted for diagnosis, implying that the analysis factored in subjects who, despite having the indication (eg, depression), did not use an anticholinergic treatment for the indication. The authors addressed protopathic bias by studying exposure in different time windows, including windows that well antedated the date of identification of dementia. Finally, for all time windows, in the main analysis that pooled data across anticholinergic drug categories, there appeared to be a dose-dependent relationship between exposure and risk; that is, higher cumulative TSDDs were associated with higher ORs for incident dementia. The authors seemed convinced; they concluded that “anticholinergic drugs should be prescribed with caution in middle-aged and older people.”
The Elephants in the Room
As already explained in an earlier section, anticholinergic drugs produce state-dependent impairment of cognition. However, to suggest that the use of these drugs can lead to dementia is a different matter, altogether; there are too many elephants in the room, provided that one knows where to look.
The first elephant is the matter of inconsistency in findings across drug categories. If anticholinergic activity is responsible for an increased risk of dementia, then it should be responsible for an increased risk regardless of the category of the drug. In this study,8 all drugs in each category were selected with a priori delimitation of strong anticholinergic activity. Yet, there was clear evidence of increased risk for only 3 out of the 11 drug categories: the antidepressant, antipsychotic, and bladder antimuscarinic drugs (Table 1). This seems to suggest that having anticholinergic activity is insufficient in itself and that some additional quality is necessary to make the drug neurotoxic, or even that anticholinergic activity is an irrelevant common denominator for certain drug categories that are each associated with increased risk through one or more different mechanisms.
As a side note, there were few subjects in the skeletal muscle relaxant and antiarrhythmic drug analyses, so perhaps the lack of statistical significance may merely have been because these analyses were underpowered. However, there were few subjects in the antiparkinsonian drug analyses, as well; nevertheless, some of the ORs were statistically significant in the antiparkinsonian analyses. Furthermore, the analyses for antimuscarinic bronchodilators, for example, included large numbers of subjects, yet almost all the ORs were nonsignificant.
This leads to the second elephant. Could confounding by indication have been responsible for the significant risks identified in specific drug groups? This is indeed possible; statistical adjustment can never be perfect, and residual confounding will always be a specter in analyses of data from nonrandomized studies. Adverse neuroanatomical changes have long been known in the brains of patients with depression32 and schizophrenia,33 and this may explain why antidepressant and antipsychotic drugs were significantly associated with an increased risk of dementia.
Now here is an interesting point. After controlling for age (which is a common risk factor for overactive bladder disease as well as dementia), as the authors8 did, there does not appear to be a logical confound to explain why bladder antimuscarinics should be associated with an increased risk of dementia. On the one hand, white matter disease in the brain has been suggested to explain at least some cases of overactive bladder.34 On the other hand, bladder antimuscarinics were associated with an increased risk of dementia at all dosing levels in all exposure windows in all analyses, unadjusted and adjusted. Given the consistency of the findings, perhaps future research could examine bladder antimuscarinics more closely, but it could be difficult to find appropriate controls.
This leads to the third elephant. An exposure of even as little as 1-90 TSDDs of bladder antimuscarinics, antidepressants, and antivertigo/antiemetics sufficed to increase the risk of dementia in 2 of 3 exposure time windows; for antipsychotic drugs, a 1-90 TSDD exposure was associated with significantly increased risk in 1 exposure window. For exposure in such low doses (and for such brief periods) to predispose to the widespread brain changes of dementia at a time interval of up to 20 years later begs the identification of a credible mechanism. It takes years (and not 1-90 days) of exposure to physical inactivity, overweight, hypertension, diabetes, or other risk factors for the exposure to translate into significant dementia risk. For brief exposure to increase the risk would perhaps mean that the toxic effect of anticholinergic exposure would need to be similar to that of traumatic brain injury. This concern cannot be easily dismissed.
The final elephant is the finding that the ORs were numerically higher, and (mostly) substantially so, for the association of anticholinergic exposure with vascular dementia as compared with Alzheimer’s disease. This flies against the animal study findings, referred to in an earlier section, that suggested that anticholinergic drugs may increase neuroinflammation, amyloid and tau deposit, and changes that predispose to Alzheimer’s disease. This also flies against any speculation involving the consequences of chronic cholinergic depletion. Rather, this demands an explanation for why even brief (1-90 days) anticholinergic exposure can result in changes that culminate in vascular dementia. In this context, an autopsy study found no relationship between 10-year antemortem anticholinergic exposure and atherosclerosis or cerebral macroinfarcts; in fact, there was an inverse relationship between anticholinergic exposure and cerebral microinfarcts.18
It may also be noted that replication of the results of previous studies in the field, summarized in an earlier section, does not support a causal argument if only because a marker will remain a marker and not a cause no matter in how many studies it is identified. Finally, the population attributable fraction of 9%-10% is so large that one must have better quality evidence before drawing firm conclusions. Case-control studies, no matter how large, can never establish causality.
Summary
The available data do not support a unitary hypothesis that an anticholinergic mechanism is even non-causally associated with an increased risk of dementia. However, the data do suggest that exposure to some but not all anticholinergic drug categories is a marker for dementia. For these drug categories, the data are insufficient to support a causal mechanism; in fact, aspects of the data actually suggest that a causal mechanism is unlikely.
Parting Notes
Anticholinergic drugs may be associated with an increased risk of delirium in the elderly, though this risk may at least partly be mediated by confounding variables.35 Anticholinergic drugs have also been associated with an increased risk of falls and fractures in elderly patients.36 Finally, in patients with dementia, anticholinergic drug use may be associated with an increased risk of hospitalization.37 So, because anticholinergic burden is also associated with state-dependent cognitive impairments, deprescribing anticholinergics, wherever possible, could be a good idea, especially in demographically and medically vulnerable populations. However, risk of future dementia is not a justifiable reason for deprescription; at least, not based on present evidence.
Published online: August 6, 2019.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Islam MM, Iqbal U, Walther B, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47(3-4):181-191. PubMed CrossRef
2.He Q, Chen X, Wu T, et al. Risk of dementia in long-term benzodiazepine users: evidence from a meta-analysis of observational studies. J Clin Neurol. 2019;15(1):9-19. PubMed CrossRef
3.Lee J, Jung SJ, Choi JW, et al. Use of sedative-hypnotics and the risk of Alzheimer’s dementia: a retrospective cohort study. PLoS One. 2018;13(9):e0204413. PubMed CrossRef
4.Lee CW, Lin CL, Sung FC, et al. Antidepressant treatment and risk of dementia: a population-based, retrospective case-control study. J Clin Psychiatry. 2016;77(1):117-122, quiz 122. PubMed CrossRef
5.Kodesh A, Sandin S, Reichenberg A, et al. Exposure to antidepressant medication and the risk of incident dementia [published online May 29, 2019]. Am J Geriatr Psychiatry. PubMed CrossRef
6.Duan Y, Grady JJ, Albertsen PC, et al. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340-348. PubMed CrossRef
7.Andrade C. How to read a research paper: an exercise in critical thinking in the context of an epidemiologic study on tamsulosin and the risk of dementia. J Clin Psychiatry. 2018;79(6):18f12660. PubMed CrossRef
8.Coupland CAC, Hill T, Dening T, et al. Anticholinergic drug exposure and the risk of dementia: a nested case-control study [published online June 24, 2019]. JAMA Intern Med. PubMed CrossRef
9.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34(8):1307-1350. PubMed CrossRef
10.Papenberg G, Bäckman L, Fratiglioni L, et al. Anticholinergic drug use is associated with episodic memory decline in older adults without dementia. Neurobiol Aging. 2017;55:27-32. PubMed CrossRef
11.Tsoutsoulas C, Mulsant BH, Kumar S, et al. Anticholinergic burden and cognition in older patients with schizophrenia. J Clin Psychiatry. 2017;78(9):e1284-e1290. PubMed CrossRef
12.Chatterjee S, Bali V, Carnahan RM, et al. Anticholinergic burden and risk of cognitive impairment in elderly nursing home residents with depression [published online May 28, 2019]. Res Social Adm Pharm. PubMed CrossRef
13.Blanco-Silvente L, Castells X, Saez M, et al. Discontinuation, efficacy, and safety of cholinesterase inhibitors for Alzheimer’s disease: a meta-analysis and meta-regression of 43 randomized clinical trials enrolling 16 106 patients. Int J Neuropsychopharmacol. 2017;20(7):519-528. PubMed CrossRef
14.Chhatwal JP, Schultz AP, Hedden T, et al. Anticholinergic amnesia is mediated by alterations in human network connectivity architecture [published online September 2, 2018]. Cereb Cortex. 2018. PubMed CrossRef
15.Wurtman RJ. How anticholinergic drugs might promote Alzheimer’s disease: more amyloid-β and less phosphatidylcholine. J Alzheimers Dis. 2015;46(4):983-987. PubMed CrossRef
16.Yoshiyama Y, Kojima A, Itoh K, et al. Does anticholinergic activity affect neuropathology? implication of neuroinflammation in Alzheimer’s disease. Neurodegener Dis. 2015;15(3):140-148. PubMed CrossRef
17.Di Bari M, Di Pinto G, Reale M, et al. Cholinergic system and neuroinflammation: implication in multiple sclerosis. Cent Nerv Syst Agents Med Chem. 2017;17(2):109-115. PubMed CrossRef
18.Gray SL, Anderson ML, Hanlon JT, et al. Exposure to strong anticholinergic medications and dementia-related neuropathology in a community-based autopsy cohort. J Alzheimers Dis. 2018;65(2):607-616. PubMed CrossRef
19.Ancelin ML, Artero S, Portet F, et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455-459. PubMed CrossRef
20.Carrière I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317-1324. PubMed CrossRef
21.Jessen F, Kaduszkiewicz H, Daerr M, et al. Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci. 2010;260(suppl 2):S111-S115. PubMed CrossRef
22.Cai X, Campbell N, Khan B, et al. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013;9(4):377-385. PubMed CrossRef
23.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401-407. PubMed CrossRef
24.Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ. 2018;361:k1315. PubMed CrossRef
25.Campbell NL, Lane KA, Gao S, et al. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy. 2018;38(5):511-519. PubMed CrossRef
26.Yang YW, Liu HH, Lin TH, et al. Association between different anticholinergic drugs and subsequent dementia risk in patients with diabetes mellitus. PLoS One. 2017;12(4):e0175335. PubMed CrossRef
27.Sheu JJ, Tsai MT, Erickson SR, et al. Association between anticholinergic medication use and risk of dementia among patients with Parkinson’s disease [published online June 28, 2019]. Pharmacotherapy. PubMed CrossRef
28.Andrade C. Confounding. Indian J Psychiatry. 2007;49(2):129-131. PubMed CrossRef
29.Andrade C, Bhakta SG, Singh NM. Controversy revisited: selective serotonin reuptake inhibitors in paediatric depression. World J Biol Psychiatry. 2006;7(4):251-260. PubMed CrossRef
30.Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: meta-review of meta-analyses. J Clin Psychiatry. 2017;78(8):e1047-e1051. PubMed CrossRef
31.Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052-e1056. PubMed CrossRef
32.Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder. meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675-690. PubMed CrossRef
33.Haijma SV, Van Haren N, Cahn W, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129-1138. PubMed CrossRef
34.Sakakibara R, Panicker J, Fowler CJ, et al. Is overactive bladder a brain disease? the pathophysiological role of cerebral white matter in the elderly. Int J Urol. 2014;21(1):33-38. PubMed CrossRef
35.Pasina L, Colzani L, Cortesi L, et al. Relation between delirium and anticholinergic drug burden in a cohort of hospitalized older patients: an observational study. Drugs Aging. 2019;36(1):85-91. PubMed CrossRef
36.Machado-Duque ME, Castaño-Montoya JP, Medina-Morales DA, et al. Drugs with anticholinergic potential and risk of falls with hip fracture in the elderly patients: a case-control study. J Geriatr Psychiatry Neurol. 2018;31(2):63-69. PubMed CrossRef
37.Watanabe S, Fukatsu T, Kanemoto K. Risk of hospitalization associated with anticholinergic medication for patients with dementia. Psychogeriatrics. 2018;18(1):57-63. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top