Clinical Problem
Ms L, a 38-year-old woman, has been diagnosed with an episode of major depression. She experienced her first episode of depression 5 years earlier and remitted with sertraline (150 mg/d). Sertraline was tapered and withdrawn after 18-20 months of uneventful maintenance therapy. She remained well, without medication, for about 3 years. She developed her second episode of depression about 5 months ago. The present episode has not responded to adequate trials of sertraline and venlafaxine (225 mg/d; her current medication). She is reluctant to augment venlafaxine with an atypical antipsychotic drug because of the risk of weight gain. Given that some studies have found antidepressant benefits with nonsteroidal anti-inflammatory drug (NSAID) augmentation, might the addition of an NSAID to her current antidepressant be a viable treatment strategy for her?
Introduction
A large body of evidence suggests that depression is associated with inflammatory changes in the brain and in the periphery and that immune activation, prostaglandin synthesis, and proinflammatory cytokine production may be involved in the mechanisms of depression through direct effects on monoamine levels, dysregulation of the hypothalamic-pituitary-adrenal axis, abnormal microglial cell activation, impaired neuroplasticity, and structural and functional brain changes.1-4 If this is true, then anti-inflammatory drugs may improve depression outcomes by attenuating the neuroinflammatory changes5 or by other mechanisms, such as increased norepinephrine and serotonin levels.6 Data from animal models show that NSAIDs in monotherapy7,8 and in combination with conventional antidepressants5 indeed attenuate indices of inflammation and depression. What do the clinical data show?
Clinical Benefits With Anti-Inflammatory Drugs in Depression
Diverse strands of evidence indicate possible clinically relevant antidepressant benefits with NSAIDs. For example, data extracted from five 6-week randomized controlled trials (RCTs) of NSAIDs in 1,497 patients with osteoarthritis showed that ibuprofen (2,400 mg/d), naproxen (1,000 mg/d), and celecoxib (200 mg/d) were each associated with significantly lower depression ratings than placebo; the greatest benefits were recorded with celecoxib.9 It is not clear, however, to what extent the better depression outcomes were due to better pain control. In this context, the large (N = 2,528) Alzheimer’s Disease Anti-inflammatory Prevention Trial, conducted in cognitively normal elderly subjects, found that neither celecoxib (400 mg/d) nor naproxen (440 mg/d) influenced depression scores, even in the subgroup of subjects who were depressed at baseline.10 It therefore appears that NSAIDs in monotherapy do not improve depression when pain is not the indication for their prescription.
In a small (N = 24), uncontrolled, open-label study in depressed patients who had not responded to at least 4 weeks of selective serotonin reuptake inhibitor (SSRI) treatment, Mendlewicz et al11 obtained response and remission rates of 52% and 43%, respectively, after 4 weeks of SSRI augmentation with aspirin (160 mg/d). Marked improvement in the responder subgroup was apparent as early as within a week of initiation of the aspirin augmentation.
The best evidence available on the subject is for celecoxib. This evidence is examined in the next section.
Celecoxib RCTs: A Meta-Analysis
Faridhosseini et al12 described a meta-analysis of the use of celecoxib as an antidepressant augmentation agent. These authors searched electronic databases, reference lists, and other sources and identified 5 placebo-controlled RCTs of celecoxib for the treatment of unipolar (4 RCTs) or bipolar (1 RCT) depression in adults. Only data from the 4 unipolar depression RCTs13-16 were included in the meta-analysis; one15 of these 4 RCTs had not been published as a full paper. Three studies were conducted in Iran,14-16 and 1, in Germany.13 The antidepressant that was augmented was sertraline (50-200 mg/d) in 2 RCTs, fluoxetine (40 mg/d) in 1, and reboxetine (4-10 mg/d) in the last. The dose of celecoxib was 200 mg/d in 1 RCT and 400 mg/d in the rest.
The sample size was 40 in each RCT; the pooled sample size was 160. The mean ages of the patients in the RCTs were 35-45 years, where information on this variable was available. Heterogeneity in the different analyses was low. Only 2 of the 4 RCTs were judged to be at low risk of bias.
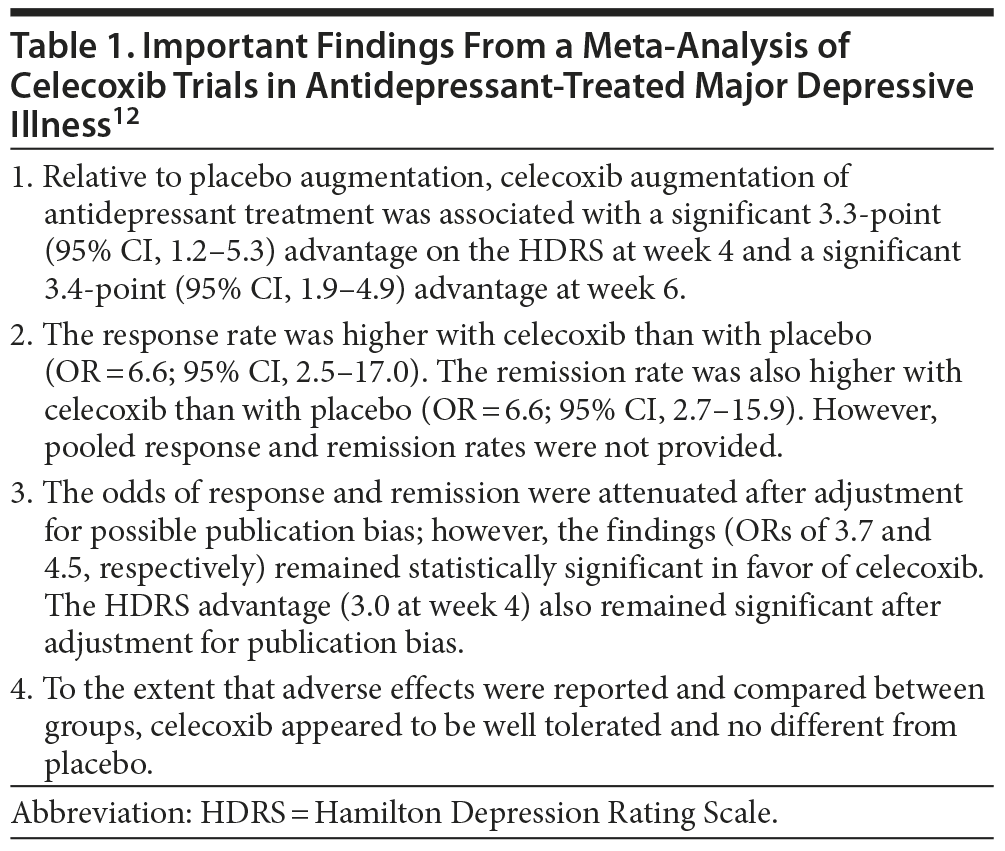
Important findings from the meta-analysis12 are summarized in Table 1. Essentially, the meta-analysis showed that 4-6 weeks of treatment with celecoxib (200-400 mg/d) was associated with significantly greater reduction in depression ratings and with significantly greater response as well as remission rates in depressed patients receiving antidepressant medication. Adverse effects did not differ between celecoxib and placebo groups, but the RCTs were underpowered for these outcomes. Very similar results were obtained in another meta-analysis on the subject.17
Can the results of the meta-analysis12 encourage antidepressant augmentation with celecoxib or other NSAIDs in major depressive illness? No, for several reasons. Faridhosseini et al12 did not include the mostly negative RCT of Nery et al18 in their meta-analysis because this RCT was conducted in bipolar patients (N = 28) experiencing a depressed (n = 24) or mixed (n = 4) episode. Nery et al18 had randomized patients to 6 weeks of treatment with celecoxib (400 mg/d) or placebo. They found that celecoxib was superior to placebo only at the end of 1 week, and only in patients who completed the whole trial. Two patients dropped out due to celecoxib-induced rash. The findings of this study18 are a small counterweight to the results of the meta-analysis.12
There are other and more important reasons why NSAID augmentation cannot as yet be recommended to depressed patients. Three14-16 of the 4 meta-analyzed RCTs came from a geographically localized region, and 115 was not peer-reviewed. The pooled sample (N = 160) in the meta-analysis was too small for confident clinical recommendations to be possible. Importantly, none of the 4 RCTs in the meta-analysis selected patients for prior antidepressant refractoriness; therefore, anecdotal data19 notwithstanding, the findings of the meta-analysis cannot be generalized to antidepressant-refractory patients. At best, the results of the meta-analysis suggest that celecoxib augmentation may improve short-term response in antidepressant-treated depressed patients.
Several other imponderables also need to be resolved. For example, are benefits with celecoxib limited to patients in whom peripheral markers of inflammation are demonstrably elevated? Or are there other predictors of response to celecoxib? How long should a celecoxib trial last, and for how long should the patient continue to take celecoxib after successful treatment? Finally, given that celecoxib has been associated with adverse medical outcomes (as discussed in a later section), what is the long-term safety profile of celecoxib in depressed patients?
NSAIDs and Possible Worsening of Depression Outcomes
Laboratory data exist to suggest that cyclooxygenase-2 (COX-2) inhibitors may increase lipid peroxidation, decrease the levels of important antioxidants, damage mitochondria, and otherwise aggravate the cellular pathophysiology of depression.20 Drugs such as ibuprofen may antagonize the action of SSRI antidepressants on neuroinflammatory mechanisms.21 In this connection, some data seem to suggest that NSAIDs may worsen depression outcomes and predispose to antidepressant resistance.22 For example, in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, use of NSAIDs or analgesics was associated with significantly decreased chances of response to citalopram; use of vitamins, in contrast, had no effect on citalopram outcomes.21 Other observational studies also noted worse antidepressant outcomes in NSAID-treated patients, but the findings attenuated or were no longer significant after adjusting for confounding.23,24 Furthermore, non-NSAID (opiate) analgesics were also associated with poorer antidepressant outcomes, and NSAIDs were associated with poorer cognitive-behavioral therapy outcomes as well23; these findings indicate that the most likely explanation for the data is that the burden of medical illness (for which NSAIDs are prescribed) is what probably attenuates antidepressant responsiveness.22
NSAIDs and Medical Risks
NSAIDs are known to increase the risk of gastrointestinal bleeding, and this risk is heightened with concurrent treatment with SSRI and other antidepressants that inhibit the reuptake of serotonin.25,26 Next, depression is associated with an increased risk of ischemic heart disease events,27 and NSAIDs may further increase this risk. For example, a meta-analysis of 25 studies showed that most of the commonly used NSAIDs were associated with a dose-dependent increase in the risk of myocardial infarction28; some NSAIDs may additionally increase the risk of stroke.29 COX-2 inhibition by NSAIDs may also worsen high blood pressure and exacerbate stable congestive heart failure.30 These and other adverse effects of NSAIDs must be weighed before considering NSAID augmentation of antidepressants in depression.
Summing up
Limited data exist to suggest that celecoxib (200-400 mg/d) improves short-term treatment outcomes in antidepressant-treated major depressive disorder. However, an advantage with NSAID augmentation in antidepressant-refractory patients remains to be demonstrated. Furthermore, NSAID augmentation of antidepressants is associated with medical risks. NSAID augmentation may therefore be an uncertain and experimental option for the patient described at the beginning of this article.
Parting Notes
NSAIDs have been studied in psychiatry in contexts ranging from the prevention of amnestic deficits associated with electroconvulsive therapy31-33 to the treatment of Alzheimer’s disease.34 Readers who are interested in the subject may wish to consult 2 recent reviews35,36 that examined the use of anti-inflammatory treatments in psychiatric disorders. The possible use of NSAIDs in schizophrenia was examined in an earlier article in this column.37
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Miller AH, et al. Biol Psychiatry. 2009;65(9):732-741. PubMed doi:10.1016/j.biopsych.2008.11.029
2. Leonard BE. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:261-267. PubMed doi:10.1016/j.pnpbp.2013.10.018
3. Han QQ, Yu J. Neurosci Bull. 2014;30(3):515-523. PubMed doi:10.1007/s12264-013-1439-3
4. Rosenblat JD, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23-34. PubMed doi:10.1016/j.pnpbp.2014.01.013
5. Maciel IS, et al. PLoS ONE. 2013;8(9):e77227. PubMed doi:10.1371/journal.pone.0077227
6. Johansson D, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(1):143-148. PubMed doi:10.1016/j.pnpbp.2012.06.003
7. Kurhe Y, et al Neurochem Res. 2014;39(7):1395-1402. PubMed doi:10.1007/s11064-014-1322-2
8. Santiago RM, et al. J Neural Transm. 2014;121(6):671-682. PubMed doi:10.1007/s00702-014-1159-5
9. Iyengar RL, et al. Am J Med. 2013;126(11):e11-e18. PubMed doi:10.1016/j.amjmed.2013.02.037
10. Fields C, et al; Am J Geriatr Psychiatry. 2012;20(6):505-513. PubMed doi:10.1097/JGP.0b013e318227f4da
11. Mendlewicz J, et al. Int Clin Psychopharmacol. 2006;21(4):227-231. PubMed doi:10.1097/00004850-200607000-00005
12. Faridhosseini F, et al. Hum Psychopharmacol. 2014;29(3):216-223. PubMed doi:10.1002/hup.2401
13. Müller N, et al. Mol Psychiatry. 2006;11(7):680-684. PubMed doi:10.1038/sj.mp.4001805
14. Akhondzadeh S, et al. Depress Anxiety. 2009;26(7):607-611. PubMed doi:10.1002/da.20589
15. Hashemian F, et al. Klin Psikofarmakol B. 2011;21:S183-S184.
16. Abbasi SH, et al. J Affect Disord. 2012;141(2-3):308-314. PubMed doi:10.1016/j.jad.2012.03.033
17. Na KS, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79-85. PubMed doi:10.1016/j.pnpbp.2013.09.006
18. Nery FG, et al. Hum Psychopharmacol. 2008;23(2):87-94. PubMed doi:10.1002/hup.912
19. Chen CY, et al. Gen Hosp Psychiatry. 2010;32(6):e7-e9. PubMed doi:10.1016/j.genhosppsych.2010.07.001
20. Maes M. Metab Brain Dis. 2012;27(4):405-413. PubMed doi:10.1007/s11011-012-9326-6
21. Warner-Schmidt JL, et al. Proc Natl Acad Sci U S A. 2011;108(22):9262-9267. PubMed doi:10.1073/pnas.1104836108
22. Shelton RC. Am J Psychiatry. 2012;169(10):1012-1015. PubMed doi:10.1176/appi.ajp.2012.12070924
23. Gallagher PJ, et al. Am J Psychiatry. 2012;169(10):1065-1072. PubMed doi:10.1176/appi.ajp.2012.11091325
24. Uher R, et al. Psychol Med. 2012;42(10):2027-2035. PubMed doi:10.1017/S0033291712000190
25. Andrade C, et al. J Clin Psychiatry. 2010;71(12):1565-1575. PubMed doi:10.4088/JCP.09r05786blu
26. Andrade C. J Clin Psychiatry. 2012;73(12):e1475-e1477. PubMed doi:10.4088/JCP.12f08248
27. Glassman AH, Shapiro PA. Am J Psychiatry. 1998;155(1):4-11. PubMed
28. Varas-Lorenzo C, et al. Pharmacoepidemiol Drug Saf. 2013;22(6):559-570. PubMed doi:10.1002/pds.3437
29. Varas-Lorenzo C, et al. Pharmacoepidemiol Drug Saf. 2011;20(12):1225-1236. PubMed doi:10.1002/pds.2227
30. Amer M, et al. Cardiol Rev. 2010;18(4):204-212. PubMed doi:10.1097/CRD.0b013e3181ce1521
31. Rao SK, et al. Biol Psychiatry. 2002;51(9):770-773. PubMed doi:10.1016/S0006-3223(01)01219-7
32. Andrade C, et al. J Psychiatr Res. 2008;42(10):837-850. PubMed doi:10.1016/j.jpsychires.2007.08.009
33. Andrade C, et al. J Neural Transm. 2008;115(7):1063-1070. PubMed doi:10.1007/s00702-008-0063-2
34. Jaturapatporn D, et al. Cochrane Database Syst Rev. 2012;2:CD006378. PubMed
35. Müller N. Psychiatr Danub. 2013;25(3):292-298. PubMed
36. Fond G, et al. Acta Psychiatr Scand. 2014;129(3):163-179. PubMed doi:10.1111/acps.12211
37. Andrade C. J Clin Psychiatry. 2014;75(7):e707-e709. doi:10.4088/JCP.14f09292

 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.



