There are no randomized controlled trials of antidepressant drugs to treat depression, or to prevent relapse into depression, during pregnancy; therefore, the safety of antidepressant drug exposure during pregnancy is based on evidence from case-control or cohort studies. Many of these observational studies, during the past decade, examined the risk of autism spectrum disorder (ASD) in exposed offspring. Different studies using different methods and examining different periods of antidepressant exposure before and during pregnancy obtained different results. Studies with adverse outcomes were highlighted in the mass media, whereas those with reassuring outcomes were mostly ignored. Meta-analyses were conducted to reconcile the findings of the different studies and determine the magnitude of the effect size. In the last year or so, at least 6 such meta-analyses examined the effects of antidepressant exposure during pregnancy on the risk of ASD in the offspring. The meta-analyses set different study selection criteria and employed different methods of analysis to address different objectives. The findings across meta-analyses have been reasonably consistent. Antidepressant exposure during pregnancy is associated with an increased risk of ASD in the offspring. The risk is decreased after adjusting for confounding variables and is mostly no longer statistically significant after adjusting for maternal mental illness. Additionally, antidepressant exposure is associated with an increased risk of ASD in the offspring even when exposure is limited to the preconception period, when the drugs cannot have a physiological effect on the fetus. These findings suggest that maternal mental illness is an important determinant of the risk of ASD associated with antidepressant exposure during pregnancy.


ABSTRACT
There are no randomized controlled trials of antidepressant drugs to treat depression, or to prevent relapse into depression, during pregnancy; therefore, the safety of antidepressant drug exposure during pregnancy is based on evidence from case-control or cohort studies. Many of these observational studies, during the past decade, examined the risk of autism spectrum disorder (ASD) in exposed offspring. Different studies using different methods and examining different periods of antidepressant exposure before and during pregnancy obtained different results. Studies with adverse outcomes were highlighted in the mass media, whereas those with reassuring outcomes were mostly ignored. Meta-analyses were conducted to reconcile the findings of the different studies and determine the magnitude of the effect size. In the last year or so, at least 6 such meta-analyses examined the effects of antidepressant exposure during pregnancy on the risk of ASD in the offspring. The meta-analyses set different study selection criteria and employed different methods of analysis to address different objectives. The findings across meta-analyses have been reasonably consistent. Antidepressant exposure during pregnancy is associated with an increased risk of ASD in the offspring. The risk is decreased after adjusting for confounding variables and is mostly no longer statistically significant after adjusting for maternal mental illness. Additionally, antidepressant exposure is associated with an increased risk of ASD in the offspring even when exposure is limited to the preconception period, when the drugs cannot have a physiological effect on the fetus. These findings suggest that maternal mental illness is an important determinant of the risk of ASD associated with antidepressant exposure during pregnancy.
J Clin Psychiatry 2017;78(8):e1047-e1051
https://doi.org/10.4088/JCP.17f11903
© Copyright 2017 Physicians Postgraduate Press, Inc.
Introduction
When many studies on a specific subject are available, the published data from studies with similar design are often combined and summarized through meta-analysis. This is particularly helpful when the results of the different studies are inconclusive or contradictory, or when researchers want to know what the averaged effect size is.1,2 There is often more than one published meta-analysis addressing the same research question, and these meta-analyses are sometimes similar in their date of publication and the studies summarized. When this happens, the findings of the meta-analyses are usually similar. However, it is not unusual for contemporary meta-analyses to differ or even contradict each other.
Meta-Analyses That Disagree
In 3 meta-analyses published in 2013, one concluded that depression does not increase the risk of diabetes mellitus and vice versa,3 and the others concluded that each condition increased the risk of the other.4,5 More recently, one meta-analysis concluded that the use of ketamine in electroconvulsive therapy (ECT) anesthesia improved antidepressant outcomes,6 whereas the other concluded that it did not.7
Meta-analyses may disagree for different reasons. For example, the authors may have set different inclusion and exclusion criteria for study selection, or they may have analyzed the data in different ways. When meta-analyses disagree, readers need help in deciding what to conclude. This is where meta-reviews become important; as an example, Sinha et al8 presented a meta-review that sought to reconcile disparate findings and conclusions of several systematic reviews on the safety of ECT during pregnancy.
Does Antidepressant Exposure During Pregnancy Increase the Risk of Autism in the Offspring?
Many cohort and case-control studies have examined whether antidepressant exposure during pregnancy increases the risk of autism spectrum disorder (ASD) in the offspring; the data have emerged from Denmark, Sweden, Canada, China, the Netherlands, and the United States. The results of these studies, and the interpretation of the results, have not been consistent. As a result, attempts have been made to reconcile the disagreements through meta-analysis. During approximately the last year, at least 6 important meta-analyses have examined the question9-14; 2 of the meta-analyses,9,10 in fact, were published in the same journal within a few months of each other.
All 6 meta-analyses found a significantly increased risk of ASD in the offspring of women with study-defined exposure to antidepressants in general, or selective serotonin reuptake inhibitors (SSRIs) in particular, during pregnancy. Whereas 5 of the meta-analyses9-13 suggested that confounding by indication (explained below) cannot be ruled out, the sixth and among the most recent meta-analysis14 asserted that there is a significant association between prenatal SSRI exposure and ASD and that the practice of SSRI use during pregnancy should be reviewed. The title of this meta-analysis was itself a warning of sorts: "Maternal SSRI Exposure Increases the Risk of Autistic Offspring."
The 6 meta-analyses included different studies, excluded different studies, and examined the data with different specific objectives and in different ways; therefore, although there was considerable overlap with regard to the primary studies included, the meta-analyses were far from being duplications of each other. Given the potential for generation of uncertainty in the minds of readers, especially considering that the press and other mass media tend to publicize primary studies that demonstrate a positive association of risk and neglect research that finds no association of risk, the present article sought to provide a meta-review of the 6 meta-analyses listed above with a view to summarize the findings of the meta-analyses, present a consistent explanation for the findings, and consider how the findings may influence clinical practice.
Confounding by Indication
As an explanatory note, pregnant women and their physicians know that, wherever possible, most medications should be avoided during pregnancy, especially during the first trimester. Therefore, if a women receives a prescription for an SSRI or other antidepressant during pregnancy, it is probably because she and her physician consider that the illness is sufficiently severe or that the risk of relapse is sufficiently high to justify treatment with the antidepressant. So, it may be the dysfunctional symptoms and the behaviors associated with the illness, or the genetic and environmental factors that contribute to the illness, that drive the risk of ASD in the offspring rather than the use of the antidepressant, per se. This is known as confounding by indication. That is, it is the indication for which the antidepressant is prescribed that drives the risk rather than the antidepressant itself.
Confounding by indication and ways to overcome the problem through the use of propensity score matching, sibling controls, and other methods have been discussed elsewhere, but the problem can never be overcome in observational studies, and the randomized controlled study gold standard will perhaps never be considered ethical during pregnancy.15-18
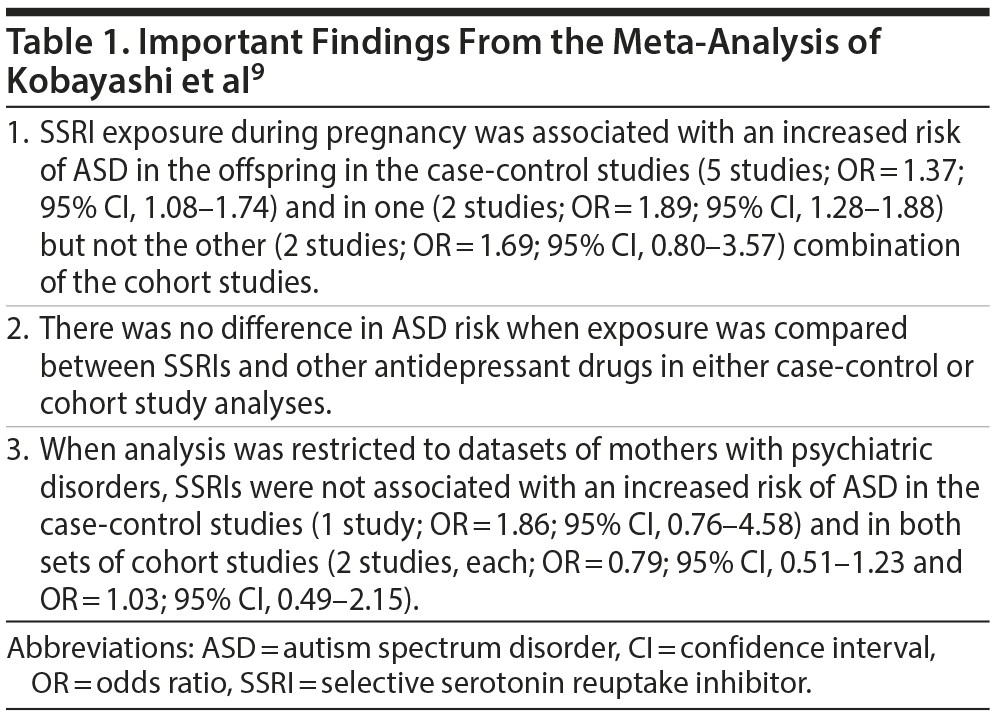
Meta-Analysis: Kobayashi et al
These authors9 identified 7 case-control studies and 4 cohort studies through their literature search. Because many of these studies had examined the same primary data source, resulting in considerable data overlap, the list was pruned to 5 case-control studies and 3 cohort studies. Because 2 of these 3 cohort studies had overlapping data and because there was no a priori reason to favor one over the other, the cohort study analysis was conducted twice, with first one and then the other overlapping study included.
Important findings from this meta-analysis are presented in Table 1. In summary, whereas SSRI exposure during pregnancy was associated with an increased risk of ASD in the offspring in the overall analyses, the significance was lost in analyses restricted to mothers with psychiatric disorders. The authors therefore concluded that maternal psychiatric conditions are a major confounding factor in the association between SSRI exposure and ASD risk.
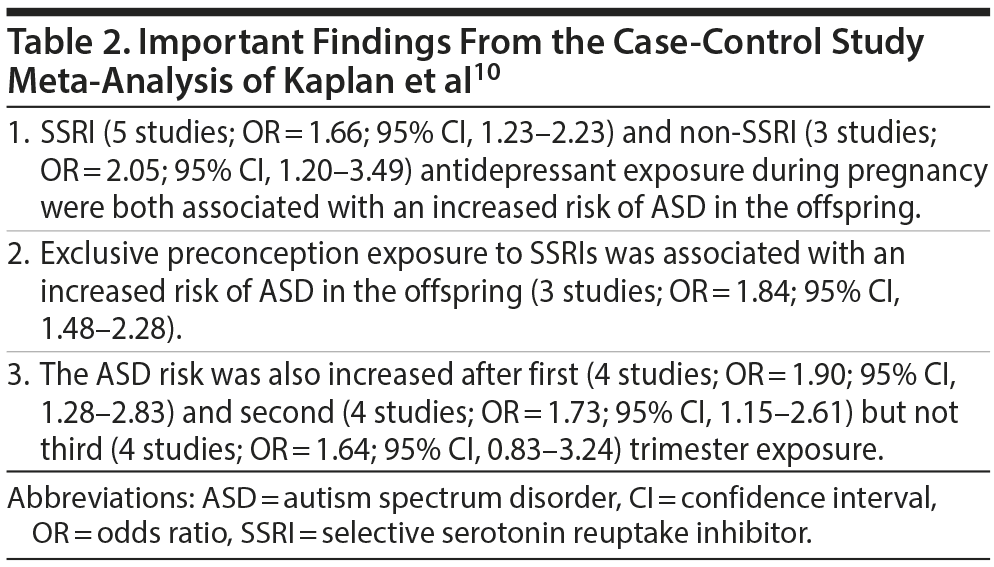
Meta-Analysis: Kaplan et al
These authors10 identified 6 case-control studies and 4 cohort studies through their literature search. One case-control study was excluded from the quantitative analysis because its data were largely a subset of another study. The data from the cohort studies were not meta-analyzed because the authors considered that outcome reporting was not uniform with regard to the antidepressant exposure windows.
The main findings from this meta-analysis are presented in Table 2. In summary, SSRI and non-SSRI antidepressant exposure during pregnancy were both associated with an increased risk of ASD in the offspring. The increased risk (not significant for third trimester exposure) was almost identical, regardless of trimester of exposure. Importantly, the increased risk was almost identical even for exclusive preconception exposure. Given that preconception exposure cannot affect the fetus, this finding strongly suggests that confounding by indication drives the increased risk. The authors10 therefore concluded that maternal psychopathology may contribute bias in investigations of ASD risk after antidepressant exposure during pregnancy.
Meta-Analysis: Brown et al
These authors11 identified 8 relevant studies but meta-analyzed only 4 case-control and 2 cohort studies that had unique datasets. Important findings from this meta-analysis are presented in Table 3. In summary, in unadjusted analyses, SSRI exposure anytime during pregnancy as well as first trimester exposure were both associated with increased risk of ASD in the offspring. The magnitude and significance of the findings diminished in adjusted analyses. In analyses that controlled for maternal mental illness, only first trimester exposure in only the case-control studies was associated with a significant increase in risk. The authors concluded that it is unclear whether the association between first trimester SSRI exposure and ASD (that was present even after adjustment for maternal mental illness) is a true association or a consequence of residual confounding.
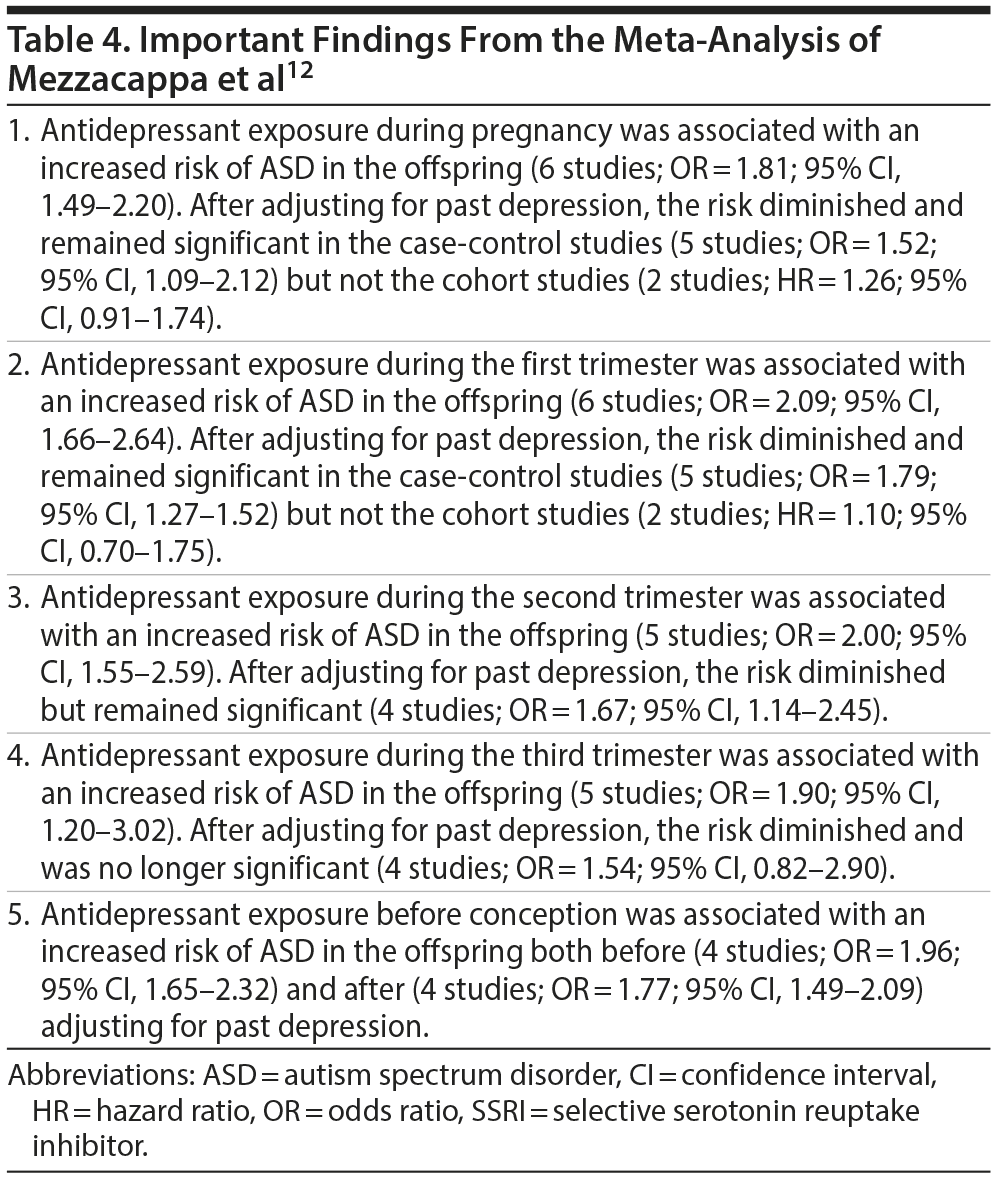
Meta-Analysis: Mezzacappa et al
These authors12 identified 7 case-control studies and 3 cohort studies. Important findings from this meta-analysis are presented in Table 4. In summary, they found that antidepressant exposure during pregnancy as well as during the different trimesters was associated with an increased risk of ASD in the offspring. The risks diminished after adjusting for past history of (maternal) depression but mostly remained significant (except in the cohort studies and in the third trimester analysis). Importantly, preconception antidepressant exposure was also associated with increased risk of the same magnitude both before and after adjusting for past maternal depression. The authors concluded that maternal psychiatric disorders in treatment before pregnancy (rather than exposure to antidepressants during pregnancy) could be driving the risk of ASD in the offspring.
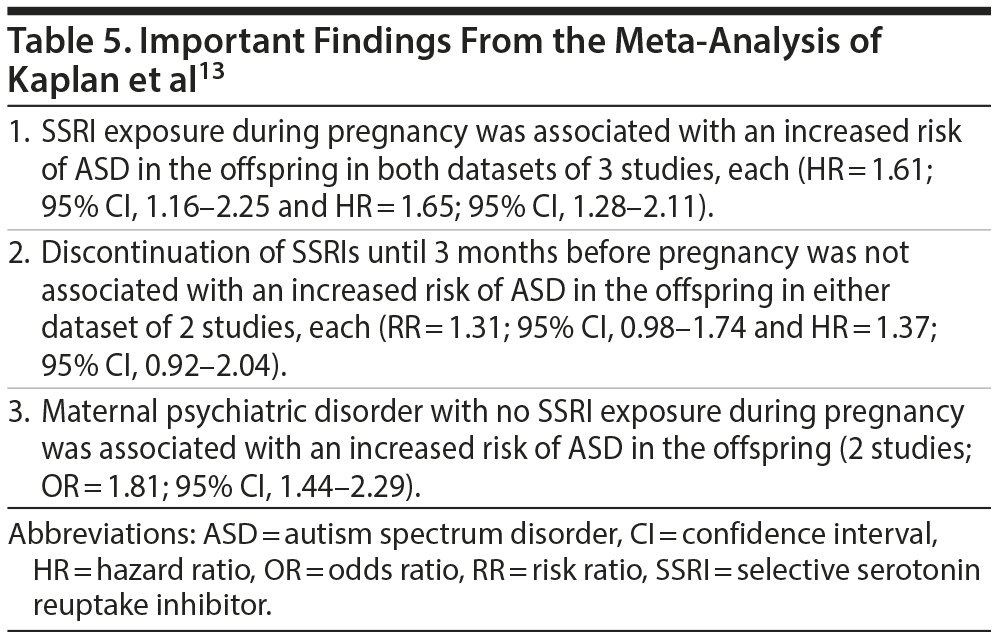
Meta-Analysis: Kaplan et al
These authors13 specifically examined cohort studies and identified 4 that met their study selection criteria. Because the datasets of 2 studies largely overlapped, some analyses were conducted separately with one and then the other study included. Important findings from the meta-analysis are presented in Table 5. In summary, SSRI exposure during pregnancy was associated with an increased risk of ASD in the offspring, but maternal psychiatric disorder in the absence of SSRI exposure was also associated with an increased risk. Preconception exposure to SSRIs was not associated with an increased risk. The authors concluded that the findings suggest that confounding by indication (maternal psychiatric illness) drives the risk of ASD in the offspring.
Meta-Analysis: Andalib et al
These authors14 identified 6 case-control and cohort studies and provided no explanation for why they excluded studies that had been identified by the other meta-analyses, many of which would have been available to them at the time of manuscript submission and revision. This meta-analysis was also the least enterprising of the 6 meta-analyses; it merely examined the overall risk of ASD associated with antenatal SSRI exposure. The finding was significant (6 studies, OR, 1.83; 95% CI, 1.59-2.10). The only additional observation of interest was that the 3 Danish studies in the meta-analysis contributed about 75% of the weightage in their analysis. The conclusions of the authors seemed clear from the title of their article itself: that maternal SSRI exposure increases (use of the active verb implies causality) the risk of autistic offspring.
Comments
The 6 meta-analyses observed that the included studies were mostly of good quality and that there was little heterogeneity in most of the analyses. Although the meta-analyses accessed much the same literature database, they differed substantially in the studies that they considered appropriate for inclusion, in their approaches to the analysis of data, and in the studies that they included in the separate analyses. Thus, there was little redundancy.
A common limitation of the meta-analyses is that although most set primary and secondary objectives, and some also defined sensitivity and subgroup analyses, none corrected for multiple hypothesis testing; besides this note, listing the strengths and limitations of individual meta-analyses is not the purpose of this article, which is to identify consistent themes across the meta-analyses. Readers interested in a more critical appraisal of the literature in the field may consult the discussion sections of certain of the meta-analyses, especially those of Kaplan et al10 and Mezzacappa et al.12
Despite the differences in the methods of the meta-analyses, the findings were reasonably consistent. In general, antidepressant exposure during pregnancy was associated with increased risk of ASD in the offspring. However, the findings diminished in magnitude and significance in analyses that adjusted for confounding variables and were mostly nonsignificant in analyses that controlled for maternal mental illness. Furthermore, antidepressants were associated with an increased risk even when exposure was limited to the preconception period, when the drugs could not have possibly affected the fetus.
There is little doubt that SSRI exposure during pregnancy is a marker for an increased risk of ASD in the offspring. However, the broad inference that can reasonably be drawn from the findings summarized above, and the conclusion that was endorsed by 5 of the 6 meta-analyses,9-13 is that maternal mental illness is a confound that may drive the increased risk. The only dissenting meta-analysis14 was the one that only examined overall risks without considering risks in subgroups (characterized by maternal mental illness) or specific exposure periods (preconception). The conclusions of this dissenting meta-analysis should therefore be regarded as being weaker than those of the other meta-analyses.
Importantly, if illness symptoms and behaviors contribute to the increased risk, then use of antidepressants may actually reduce the risk. However, this can never be identified through observational studies because of the problem related to confounding by indication.
Implications
The findings of these meta-analyses do not discourage the use of SSRIs during pregnancy if the condition being treated causes significant dysfunction, is associated with serious negative implications (eg, suicidal risk), or is associated with negative behaviors that impact maternal or fetal well-being. In such cases, the risk of ASD in the offspring should not be the sole consideration when considering treatment with SSRIs during pregnancy. Nevertheless, decision-making should be individualized, and the risk-benefit ratio discussed between physician and client in a shared decision-making process.
Parting Note
Much of the data in the different meta-analyses originated from Scandinavian countries, notably Denmark. One therefore needs to consider the extent to which the findings can be validly generalized to the rest of the world.
Published online: September 26, 2017.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Streiner DL. Using meta-analysis in psychiatric research. Can J Psychiatry. 1991;36(5):357-362. PubMed CrossRef
2. Tharyan P. The relevance to meta-analysis, systematic reviews and the Cochrane Collaboration to clinical psychiatry. Indian J Psychiatry. 1998;40(2):135-148. PubMed
3. Hasan SS, Clavarino AM, Mamun AA, et al. Population impact of depression either as a risk factor or consequence of type 2 diabetes in adults: a meta-analysis of longitudinal studies. Asian J Psychiatr. 2013;6(6):460-472. PubMed CrossRef
4. Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74(1):31-37. PubMed CrossRef
5. Rotella F, Mannucci E. Diabetes mellitus as a risk factor for depression: a meta-analysis of longitudinal studies. Diabetes Res Clin Pract. 2013;99(2):98-104. PubMed CrossRef
6. Li DJ, Wang FC, Chu CS, et al. Significant treatment effect of add-on ketamine anesthesia in electroconvulsive therapy in depressive patients: a meta-analysis. Eur Neuropsychopharmacol. 2017;27(1):29-41. PubMed CrossRef
7. McGirr A, Berlim MT, Bond DJ, et al. Adjunctive ketamine in electroconvulsive therapy: updated systematic review and meta-analysis. Br J Psychiatry. 2017;210(6):403-407. PubMed CrossRef
8. Sinha P, Goyal P, Andrade C. A meta-review of the safety of electroconvulsive therapy in pregnancy. J ECT. 2017;33(2):81-88. PubMed CrossRef
9. Kobayashi T, Matsuyama T, Takeuchi M, et al. Austism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol. 2016;65:170-178. PubMed CrossRef
10. Kaplan YC, Keskin-Arslan E, Acar S, et al. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol. 2016;66:31-43. PubMed CrossRef
11. Brown HK, Hussain-Shamsy N, Lunsky Y, et al. The association between antenatal exposure to selective serotonin reuptake inhibitors and autism: a systematic review and meta-analysis. J Clin Psychiatry. 2017;78(1):e48-e58. PubMed CrossRef
12. Mezzacappa A, Lasica PA, Gianfagna F, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr. 2017;171(6):555-563. PubMed CrossRef
13. Kaplan YC, Keskin-Arslan E, Acar S, et al. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies [published online ahead of print July 21, 2017]. Br J Clin Pharmacol. PubMed CrossRef
14. Andalib S, Emamhadi M, Yousefzadeh-Chabok S, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review [published online June 20, 2017]. Eur Psychiatry. 2017. CrossRef
15. Andrade C. How to read a research paper: reading between and beyond the lines. Indian J Psychiatry. 2011;53(4):362-366. PubMed CrossRef
16. Andrade C. Antidepressant use in pregnancy and risk of autism spectrum disorders: a critical examination of the evidence. J Clin Psychiatry. 2013;74(9):940-941. PubMed CrossRef
17. Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017;78(2):e162-e165. PubMed CrossRef
18. Andrade C. Offspring outcomes in studies of antidepressant-treated pregnancies depend on the choice of control group. J Clin Psychiatry. 2017;78(3):e294-e297. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top