Objective: We investigated the relationship between antidepressant use and the risk of subsequent dementia development.
Method: A population-based retrospective case-control analysis was conducted using the Taiwan National Health Insurance Research Database. From patients enrolled in the National Health Insurance program between 2005 and 2011, we identified 2 subsets: 5,394 cases, who had major depression in 1997-2004 and subsequently were diagnosed with dementia (ICD-9-CM code 290) in 2005-2011, and 5,232 controls, who had major depression in 2005-2011 but no dementia history. The proportional distributions of antidepressant use and comorbidities in the dementia case and nondementia control groups were compared. Univariable and multivariable logistic regression analyses were used to estimate the odds ratios (ORs) and 95% CIs for the association between dementia and antidepressant use.
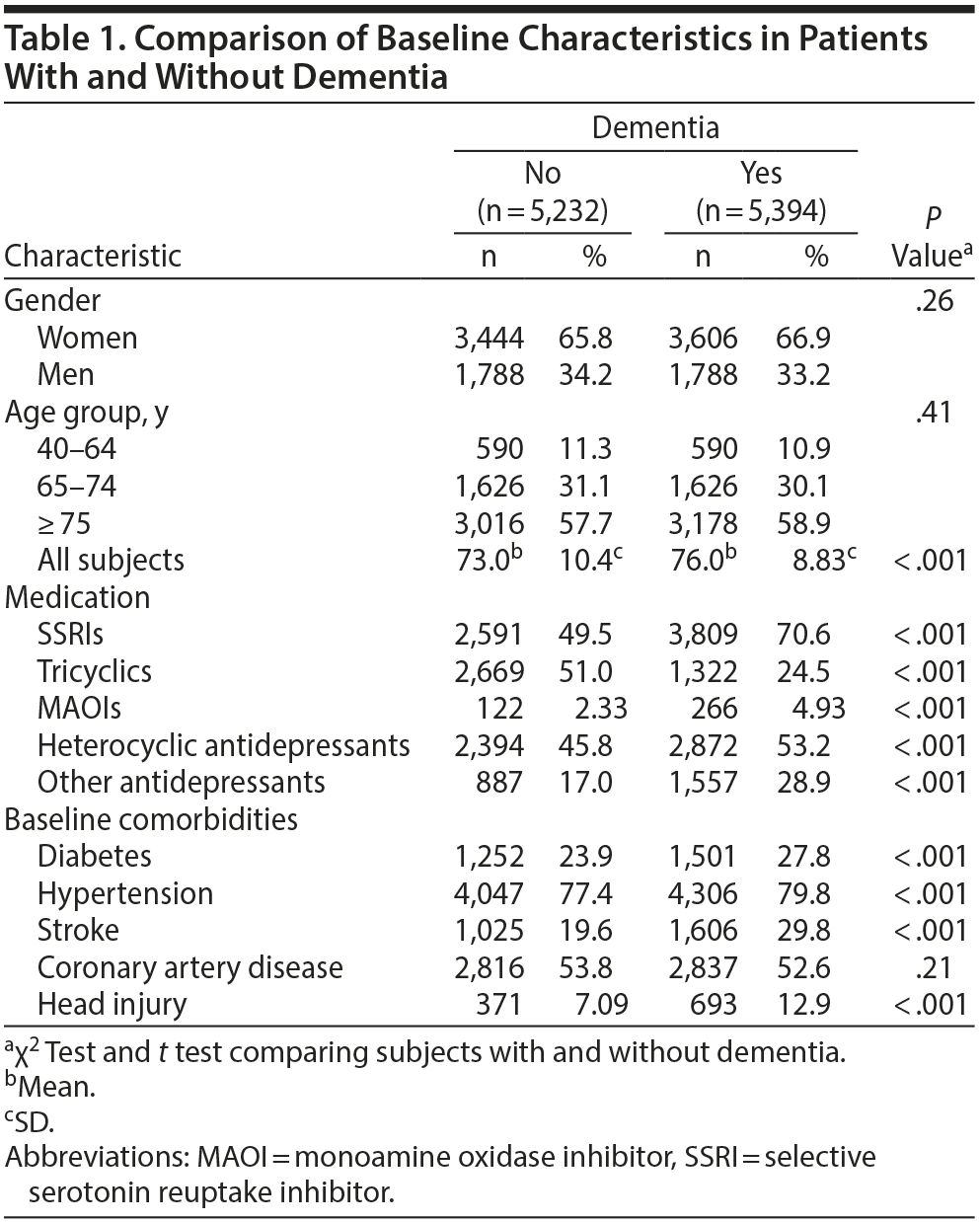
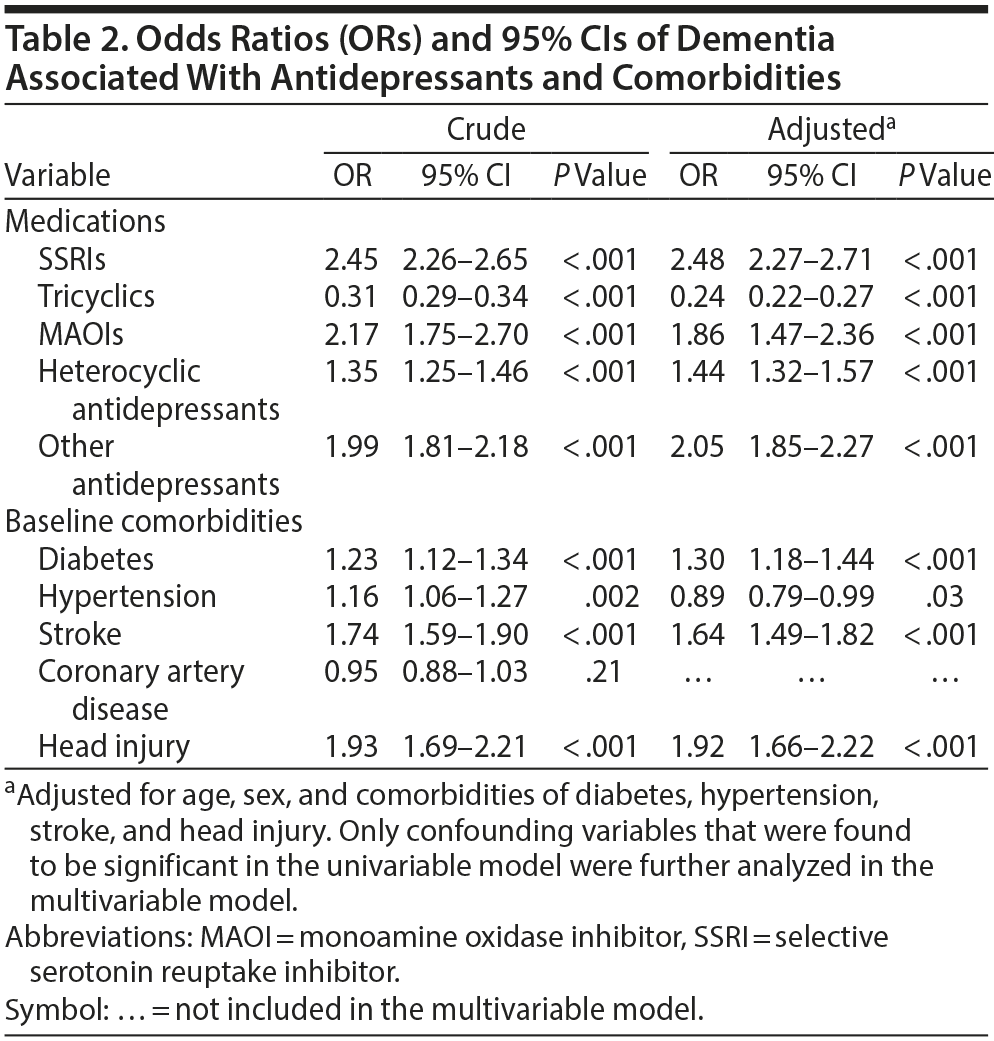
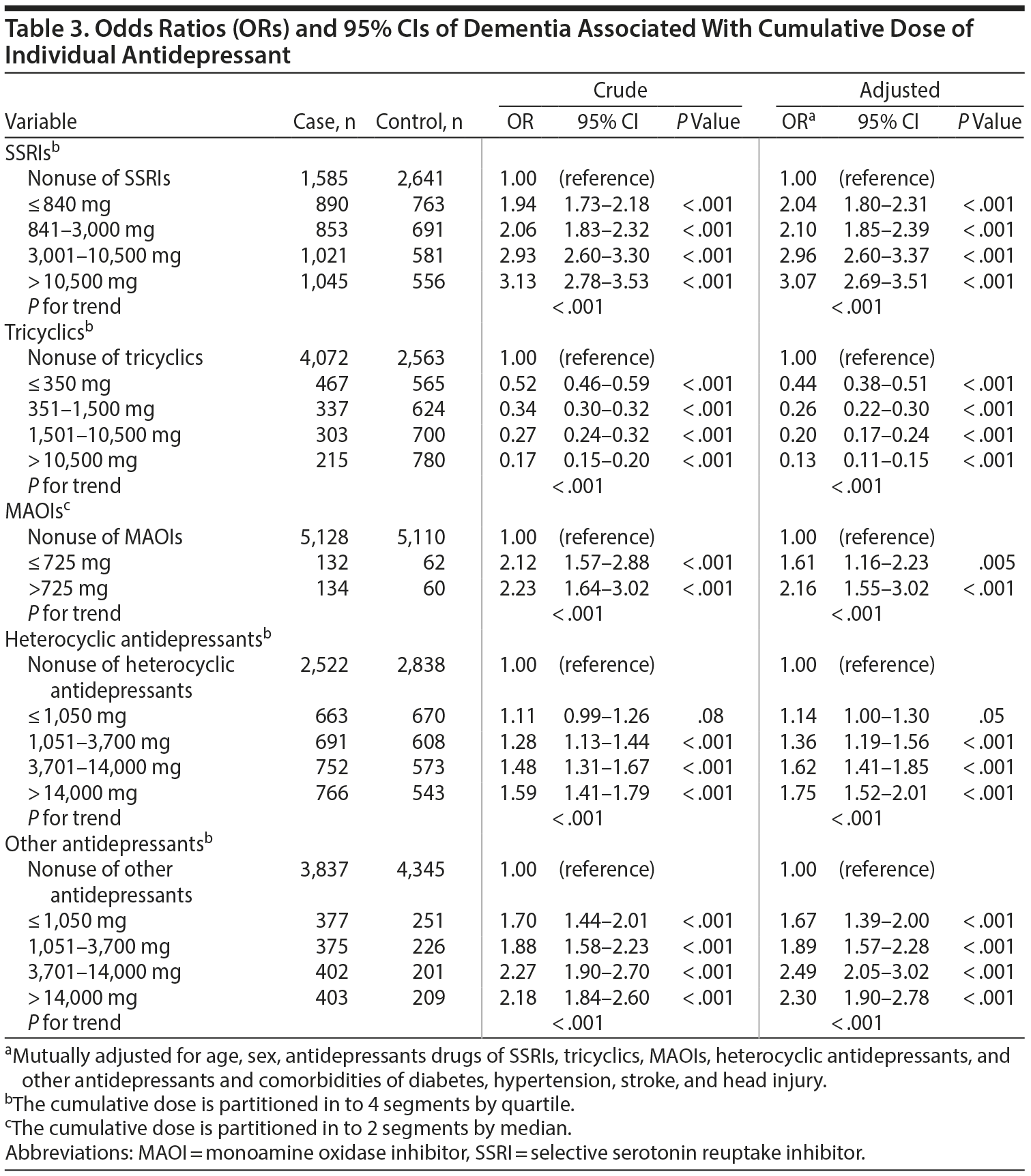
Results: The dementia patients were more likely to have diabetes, hypertension, stroke, and head injury. The adjusted OR for dementia was 0.24 (95% CI, 0.22-0.27) in patients using tricyclics . By contrast, the use of selective serotonin reuptake inhibitors (SSRIs) (OR = 2.48; 95% CI, 2.27-2.71), monoamine oxidase inhibitors (MAOIs) (OR = 1.86; 95% CI, 1.47-2.36), heterocyclic antidepressants (OR = 1.44; 95% CI, 1.32-1.57), and other antidepressants (OR = 2.05; 95% CI, 1.85-2.27) was associated with an increased risk of dementia. Furthermore, as the cumulative dose was increased, tricyclic antidepressants reduced the risk of dementia, whereas SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants increased the risk of dementia.
Conclusions: The incidence of dementia in patients is associated with antidepressant medication use. Treatment with tricyclic antidepressants was associated with a reduced risk of dementia, whereas treatment with SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants was associated with an increased risk of dementia.

Antidepressant Treatment and Risk of Dementia:
A Population-Based, Retrospective Case-Control Study
ABSTRACT
Objective: We investigated the relationship between antidepressant use and the risk of subsequent dementia development.
Method: A population-based retrospective case-control analysis was conducted using the Taiwan National Health Insurance Research Database. From patients enrolled in the National Health Insurance program between 2005 and 2011, we identified 2 subsets: 5,394 cases, who had major depression in 1997-2004 and subsequently were diagnosed with dementia (ICD-9-CM code 290) in 2005-2011, and 5,232 controls, who had major depression in 2005-2011 but no dementia history. The proportional distributions of antidepressant use and comorbidities in the dementia case and nondementia control groups were compared. Univariable and multivariable logistic regression analyses were used to estimate the odds ratios (ORs) and 95% CIs for the association between dementia and antidepressant use.
Results: The dementia patients were more likely to have diabetes, hypertension, stroke, and head injury. The adjusted OR for dementia was 0.24 (95% CI, 0.22-0.27) in patients using tricyclics . By contrast, the use of selective serotonin reuptake inhibitors (SSRIs) (OR = 2.48; 95% CI, 2.27-2.71), monoamine oxidase inhibitors (MAOIs) (OR = 1.86; 95% CI, 1.47-2.36), heterocyclic antidepressants (OR = 1.44; 95% CI, 1.32-1.57), and other antidepressants (OR = 2.05; 95% CI, 1.85-2.27) was associated with an increased risk of dementia. Furthermore, as the cumulative dose was increased, tricyclic antidepressants reduced the risk of dementia, whereas SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants increased the risk of dementia.
Conclusions: The incidence of dementia in patients is associated with antidepressant medication use. Treatment with tricyclic antidepressants was associated with a reduced risk of dementia, whereas treatment with SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants was associated with an increased risk of dementia.
J Clin Psychiatry 2016;77(1):117-122
dx.doi.org/10.4088/JCP.14m09580
© Copyright 2016 Physicians Postgraduate Press, Inc.
aCenter for Drug Abuse and Addiction, China Medical University Hospital, Taichung, Taiwan
bCollege of Medicine, China Medical University, Taichung, Taiwan
cManagement Office for Health Data, China Medical University Hospital, Taichung, Taiwan
dGraduate Institute of Clinical Medical Science and School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan
eDepartment of Radiation Oncology, China Medical University Hospital, Taichung, Taiwan
fDepartment of Nuclear Medicine and PET Center, China Medical University Hospital, Taichung, Taiwan
*Corresponding author: Chia-Hung Kao, MD, Graduate Institute of Clinical Medical Science and School of Medicine, College of Medicine, China Medical University, No. 2, Yuh-Der Rd, Taichung 404, Taiwan ([email protected]).
Dementia is a disabling clinical syndrome that is characterized by a progressive deterioration of cognition associated with impairment of the ability to engage in daily activities.1 In a previous study,2 the prevalence of dementia in mainland China, Hong Kong, and Taiwan was estimated to be 3.8% (95% CI, 2.6%-4.9%). Dementia currently affects approximately 36 million people worldwide, inflicting a substantial burden on patients, their families, and society.3 The current treatment for dementia is limited. Understanding the risk factors of dementia is essential for preventing this disease.4
Previous studies5-16 have demonstrated the relationship between depression and dementia. Depression may be both a prodrome and a risk factor for dementia.4 Some types of depressive illness, such as early onset depression before the age of 65 years and recurrent depression, may constitute long-term risk factors for the development of dementia, whereas the onset of more recent depressive symptoms may reflect a prodromal phase of dementia.10,12 Molecular mechanisms that underlie the pathogenesis of major depression, such as chronic inflammation and hyperactivation of the hypothalamic-pituitary-adrenal axis, might be involved in the pathogenesis of Alzheimer’s disease.6 In addition, studies10,17 have suggested that long-term treatment with antidepressants reduces the risk of developing some types of dementia, depending on the type of depressive disorder.
Because dementia affects large populations as well as the quality of life of patients, verifying the association between antidepressants and subsequent development of dementia is crucial. Few epidemiologic studies have investigated this relationship. To evaluate the possibility that antidepressant use reduces the risk of dementia, we compared the histories of antidepressant use in patients with dementia and those without dementia by using data from the Taiwan National Health Insurance Research Database (NHIRD).
METHOD
Data Sources
The National Health Insurance (NHI) program was established in Taiwan in March 1995. The National Health Research Institutes (NHRI) compiles NHI reimbursement data and stores them in the NHIRD to facilitate research. The NHIRD provides detailed information on the health care services used by each patient, including outpatient visits, hospitalizations, and medication prescriptions. The records indicate that the NHI program covers approximately 99% of the 23.74 million residents of Taiwan and has established contracts with 97% of hospitals and clinics in Taiwan.18 To protect the privacy of all people registered in the program, the NHRI encrypts and converts the identification numbers of all people on whom data are recorded in the NHIRD into research files. Our study was approved from full review by an institutional research ethics committee (CMU-REC-101-012). The diagnoses and procedures were coded in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) format.
Patient Selection
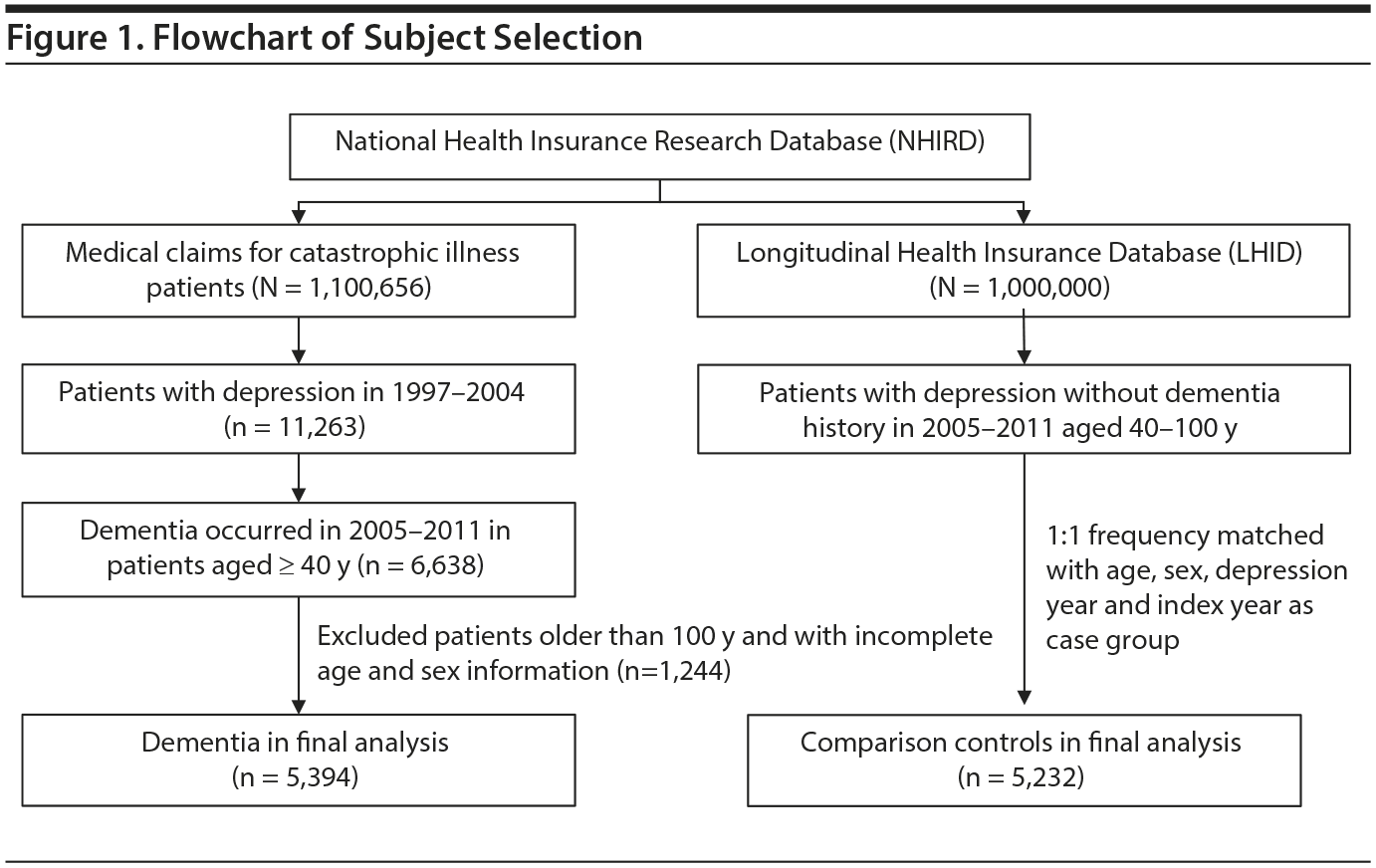
The patients were identified from 2 subsets of the NHIRD. We identified patients aged ≥ 40 years with major depression (ICD-9-CM codes 296.2, 296.3, 300.4, 311) who were newly diagnosed with dementia (ICD-9-CM code 290) between 2005 and 2010 from the Registry of Catastrophic Illnesses Patient Database of the NHIRD, and the first-time dementia diagnosis date served as the index date. In Taiwan, a clinical physician must confirm the diagnosis before a patient applies for a catastrophic illness certificate. Finally, we designated 5,394 dementia patients for whom complete age and sex information was available as the dementia group. Control patients were identified from the Longitudinal Health Insurance Database 2000 (LHID 2000), which contains claims data on 1 million people randomly sampled from NHIRD enrollment files in 2000. The NHRI reported no significant differences in sex, age, or health care costs between the cohorts in the LHID 2000 and all insurance enrollees. For each dementia patient, we randomly selected 1 patient with major depression without dementia from the same period and applied the same exclusion criteria and frequency matched with the dementia group on age (grouped with an interval of 15 years), sex, year of major depression diagnosis, and the year of index date (Figure 1).

- Patients with major depression are usually treated with antidepressants, but the risk of subsequent dementia development is unclear.
- Using tricyclic antidepressants rather than nontricyclic antidepressants to treat patients with major depression may lower their risk of developing dementia.
Variables of Interest
The baseline comorbidity history for each patient was determined by using the claims data. We designated several well-known risk factors of dementia, namely diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401-405), stroke (ICD-9-CM codes 430-438), coronary artery disease (ICD-9-CM codes 410-414), and head injury (ICD-9-CM codes 850-854, 959.01), as comorbidities. Antidepressants available in Taiwan, namely, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), heterocyclic antidepressants, and others (bupropion, venlafaxine, and mirtazapine), were analyzed. Medication use records were retrieved from ambulatory and inpatient claims data. According to total treatment duration (in days) and the quantity of antidepressants, we calculated the cumulative dose of each type of antidepressant for each user. For each type of antidepressant, the cumulative dose was divided into 2 levels according to the median or 4 levels according to the quartile.
Statistical Analysis
We first compared the proportional distributions of the demographic status, antidepressant use, and comorbidities of the dementia cases with those of the nondementia controls. Univariable and multivariable logistic regression analyses were conducted to estimate the odds ratios (ORs) and 95% CIs for the association between dementia and antidepressant use. We performed a multivariable analysis in which we adjusted for age, sex, and comorbidities, namely, diabetes, hypertension, stroke, and head injury. All analyses were conducted using SAS statistical software (version 9.2 for Windows; SAS Institute, Inc; Cary, North Carolina), and all statistical tests were performed at a 2-tailed significance level of .05.
RESULTS
The case group comprised 5,394 patients with newly diagnosed dementia, and the control group comprised 5,232 people without dementia. Of the dementia patients, 66.9% were women and 58.9% were ≥ 75 years of age (Table 1). The mean ages of the dementia patients and nondementia controls were 76.0 (± 8.83) and 73.0 (± 10.4) years, respectively. The proportions of SSRI, MAOI, heterocyclic antidepressant, and other antidepressant use were significantly higher in the case group than in the control group. However, tricyclic antidepressant use was significantly lower in the dementia group than in the nondementia group. The dementia group exhibited a statistically significantly higher likelihood of having diabetes, hypertension, stroke, and head injury. After we adjusted for confounders determined to be significant according to the results of the crude analysis, multivariable logistic regression analysis revealed that the adjusted OR for dementia was 0.24 (95% CI, 0.22-0.27) in patients using tricyclic antidepressants (Table 2). However, SSRIs (OR = 2.48; 95% CI, 2.27-2.71), MAOIs (OR = 1.86; 95% CI, 1.47-2.36), heterocyclic antidepressants (OR = 1.44; 95% CI, 1.32-1.57), and other antidepressants (OR = 2.05; 95% CI, 1.85-2.27) were significantly associated with an increased risk of dementia. Furthermore, we estimated the risk of dementia by cumulative dose for antidepressants use (Table 3). A dose-dependent risk of dementia was observed in patients using SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants, with the ORs progressively increasing as the cumulative dose was increased (over a third quartile). Compared with nontricyclic antidepressant users, those who took tricyclic antidepressants experienced a decrease in the risk of dementia from 0.44 (95% CI, 0.38-0.51) in those receiving cumulative doses ≤ 350 mg to 0.13 in those receiving cumulative doses > 10,500 mg (95% CI, 0.11-0.15) (trend test, P < .001).
DISCUSSION
The results indicated that the incidence of dementia in Taiwanese patients is associated with a history of antidepressant treatment. The risk of dementia increased as the cumulative dose of SSRIs, MAOIs, heterocyclic antidepressants, and other antidepressants was increased, and it decreased when the patients received tricyclic antidepressants.
Our results are consistent with those of a previous Danish study,19 which revealed that continued long-term treatment with older (ie, tricyclic) antidepressants was associated with a reduced rate of dementia in patients treated in psychiatric health care settings, whereas continued treatment with other types of antidepressant was not. Tricyclic antidepressants, clomipramine, and imipramine exert anti-inflammatory and neuroprotective effects in the central nervous system by modulating glial activation.20 Another study21 demonstrated that imipramine prevented cognitive decline and amyloid-β accumulation in a mouse model of Alzheimer’s disease, partly through tumor necrosis factor-α inhibition. Future studies are required to confirm our observation that tricyclic antidepressants play a role in preventing the development of dementia.
Although the study was well designed to ensure that included subjects were diagnosed with major depression, it is unclear whether the antidepressants were always being prescribed to target major depression versus some other condition (ie, possibly pain syndromes or insomnia). If so, it may be that those subjects receiving nontricyclic antidepressants had more severe episodes of major depressive disorder (and those on tricyclic antidepressants had less severe episodes of major depressive disorder, but more pain-related syndromes) and that it was the severity of the major depression, not the medication, that accounted for the study’s findings. The dose-dependent results speak against this. We postulate that the higher rates of dementia in the nontricyclic antidepressants group may reflect the risk factor of major depression itself and/or that there is something specific about the nontricyclic antidepressants that could place patients at higher risk for developing dementia. These possibilities warrant further research.
The strengths of this study include the use of population-based data and the evaluation of NHIRD records rather than data obtained from patient-reported data on drug use. The high accuracy and validity of diagnoses of cardiology-related and autoimmune diseases in the NHIRD have been verified,22 suggesting that the ICD-9-CM codes used in the NHIRD are valid and accurate.
However, this study had several limitations. First, the NHIRD lacks essential data, such as detailed demographic information on smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of systemic diseases. These are all potential risk factors for the development of dementia, and each factor is indirectly associated with major depression. Therefore, we were unable to correlate dementia with inactivity or malnutrition because the NHIRD does not contain such lifestyle data. Like other countries, major depression tends to be underdiagnosed or at least undercharted in Taiwan; yet, unlike other countries, antidepressant medications are not easily prescribed by psychiatrists in Taiwan unless the patients are diagnosed with major depression or related psychotic disorders. Therefore, the data on antidepressant medication in our study is reliable and tightly associated with the major depression diagnosis. However, because the NHIRD comprises data on a highly representative sample of the general population of Taiwan, and because the insurance reimbursement policy is universal, these factors are unlikely to have affected major depression and dementia distribution in the sample group. Second, because major depression might influence comorbidities, such as hypertension, we used univariable and multivariable models to eliminate confounding factors attributable to major depression. However, evidence derived from a retrospective cohort study is generally of lower quality than that obtained from randomized trials, because a retrospective case-control study design is subject to several biases stemming from adjustments made for confounding variables. Although we applied a meticulous study design that adequately controlled for known confounding factors, a potential key limitation of this study was the possibility that a bias caused by unknown confounders was present. Third, the diagnoses recorded in the NHI claims are primarily used for administrative billing purposes and have not been verified for scientific purposes. We were unable to contact patients to inquire about the severity of their major depression because the anonymity of all beneficiaries listed in the NHIRD is guaranteed. We were also unable to consider antidepressants issued before 1996. This omission may have led to the underestimation of cumulative dosages and may have subsequently weakened the observed associations. However, the data we obtained on the diagnoses of dementia and the prescription of antidepressants were reliable.
The study results indicated that dementia incidence is associated with a history of antidepressant use, and tricyclic antidepressants are dose dependently associated with a reduced risk of the subsequent development of dementia. However, future large, population-based studies or large-scale randomized clinical trials are required to confirm these findings before any definitive conclusions can be drawn.
Submitted: October 8, 2014; accepted July 7, 2015.
Drug names: bupropion (Aplenzin, Wellbutrin, and others), clomipramine (Anafranil and others), imipramine (Tofranil and others), mirtazapine (Remeron and others), venlafaxine (Effexor and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Author contributions: The individual contributions of the authors are listed as follows. Conception and design: Drs Lee and Kao. Administrative support: Ms Lin and Dr Sung. Data collection and assembly: Drs Lee, Liang, and Kao. Data analysis and interpretation: Drs Lee and Kao. Manuscript writing: all authors. Final approval of manuscript: all authors. The guarantor of the article assumes responsibility for the integrity of the work as a whole: Dr Kao.
Financial disclosure: Drs Lee, Sung, Liang, and Kao and Ms Lin have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This work was supported by study projects (DMR-102-014 and DMR-102-023) in China Medical University Hospital; Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), Health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TD-B-111-03, Taiwan); and International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303). No additional external funding was received for this study.
Role of the sponsor: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information: The National Health Insurance Research Database belongs to the government of Taiwan. This large computerized database is derived from claim data of the National Health Insurance Administration (formerly Bureau of National Health Insurance), Ministry of Health and Welfare (formerly Department of Health), Taiwan, maintained by the National Health Research Institutes, Taiwan, and provided to scientists in Taiwan for research purposes. Information on how to access the database can be found at http://nhird.nhri.org.tw/en/index.html.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. van der Flier WM, Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76(suppl 5):v2-v7. PubMed doi:10.1136/jnnp.2005.082867
2. Wu YT, Lee HY, Norton S, et al. Prevalence studies of dementia in mainland China, Hong Kong and Taiwan: a systematic review and meta-analysis. PLoS ONE. 2013;8(6):e66252. PubMed doi:10.1371/journal.pone.0066252
3. Ferri CP, Prince M, Brayne C, et al; Alzheimer’s Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112-2117. PubMed doi:10.1016/S0140-6736(05)67889-0
4. da Silva J, Gonçalves-Pereira M, Xavier M, et al. Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry. 2013;202(3):177-186. PubMed doi:10.1192/bjp.bp.111.101931
5. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331. PubMed doi:10.1038/nrneurol.2011.60
6. Caraci F, Copani A, Nicoletti F, et al. Depression and Alzheimer’s disease: neurobiological links and common pharmacological targets. Eur J Pharmacol. 2010;626(1):64-71. PubMed doi:10.1016/j.ejphar.2009.10.022
7. Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27-34. PubMed doi:10.1212/WNL.0b013e3181e62124
8. Goveas JS, Espeland MA, Woods NF, et al. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 2011;59(1):57-66. PubMed doi:10.1111/j.1532-5415.2010.03233.x
9. Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35(6):776-781. PubMed doi:10.1046/j.1440-1614.2001.00967.x
10. Kessing LV. Depression and the risk for dementia. Curr Opin Psychiatry. 2012;25(6):457-461. PubMed doi:10.1097/YCO.0b013e328356c368
11. Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affect Disord. 2003;73(3):261-269. PubMed doi:10.1016/S0165-0327(02)00004-6
12. Lenoir H, Dufouil C, Auriacombe S, et al. Depression history, depressive symptoms, and incident dementia: the 3C Study. J Alzheimers Dis. 2011;26(1):27-38. PubMed
13. Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry. 2011;68(9):970-977. PubMed doi:10.1001/archgenpsychiatry.2011.86
14. Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530-538. PubMed doi:10.1001/archpsyc.63.5.530
15. Aznar S, Knudsen GM. Depression and Alzheimer’s disease: is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis. 2011;23(2):177-193. PubMed
16. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493-498. PubMed doi:10.1001/archgenpsychiatry.2011.1481
17. Kessing LV, Søndergård L, Forman JL, et al. Antidepressants and dementia. J Affect Disord. 2009;117(1-2):24-29. PubMed doi:10.1016/j.jad.2008.11.020
18. Cheng TM. Taiwan’s National Health Insurance System: High Value for the Dollar. In: Okma KGH, Crivelli L, eds. Six Countries, Six Reform Models: The Healthcare Reform: Experience of Israel, The Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Singapore: World Scientific Publishing Co; 2010:171-204.
19. Kessing LV, Forman JL, Andersen PK. Do continued antidepressants protect against dementia in patients with severe depressive disorder? Int Clin Psychopharmacol. 2011;26(6):316-322. PubMed doi:10.1097/YIC.0b013e32834ace0f
20. Hwang J, Zheng LT, Ock J, et al. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55(5):826-834. PubMed doi:10.1016/j.neuropharm.2008.06.045
21. Chavant F, Deguil J, Pain S, et al. Imipramine, in part through tumor necrosis factor alpha inhibition, prevents cognitive decline and beta-amyloid accumulation in a mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2010;332(2):505-514. PubMed doi:10.1124/jpet.109.162164
22. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
This PDF is free for all visitors!
Save
Cite