ABSTRACT
Objective: To evaluate Positive and Negative Syndrome Scale (PANSS) categorical response rates, time course of response, and symptom subdomains of response with the combination oral agent KarXT (xanomeline–trospium) in the treatment of schizophrenia.
Methods: Post hoc analysis was conducted for EMERGENT-1 (NCT03697252), a 5-week, inpatient, placebo-controlled, phase 2 study of acute psychosis in patients who met DSM-5 criteria for schizophrenia. The EMERGENT-1 study was conducted between September 2018 and August 2019. Categorical thresholds of response used were PANSS total score reductions of ≥ 20%, ≥ 30%, ≥ 40%, and ≥ 50% between baseline and study end. Number needed to treat (NNT) for each categorical threshold was calculated. The proportion of KarXT- and placebo-treated patients achieving each response threshold at weeks 2, 4, and 5 was assessed. Marder 5-factor analysis of PANSS assessed response with KarXT across symptom domains.
Results: A total of 83 patients in the KarXT group and 87 patients in the placebo group were included in the modified intent-to-treat analysis. Response rates with KarXT ranged from 59.0% for a ≥ 20% threshold to 15.7% for a ≥ 50% threshold. All response rates with KarXT were significantly higher than in the placebo arm (P < .05), with NNTs ranging from 3 (≥ 20% improvement) to 11 (≥ 50% improvement). KarXT was associated with a significantly higher response rate relative to placebo as early as 2 weeks for ≥ 20% (P = .0001) and ≥ 30% (P = .0022) thresholds and at 4 weeks for the ≥ 40% (P = .0049) and ≥ 50% (P = .0041) thresholds. Each of the Marder 5 factors showed significant differences favoring KarXT over placebo (P < .05) by 2 weeks and continuing through week 5 (endpoint Cohen d effect sizes, 0.48−0.66).
Conclusions: KarXT provided clinically meaningful responder rates on PANSS total score compared with placebo at each response threshold, providing further support of the successful primary and secondary endpoints. Response was demonstrated as early as 2 weeks relative to placebo. KarXT demonstrated improvements vs placebo in all 5 factors (positive symptoms, negative symptoms, disorganized thought, uncontrolled hostility, and anxiety/depression).
Trial Registration: ClinicalTrials.gov identifier: NCT03697252
J Clin Psychiatry 2022;83(3):21m14316
To cite: Weiden PJ, Breier A, Kavanagh S, et al. Antipsychotic efficacy of xanomeline−trospium: post hoc analysis of Positive and Negative Syndrome Scale categorical response rates, time course of response, and symptom domains of response in a phase 2 study. J Clin Psychiatry. 2022;83(3):21m14316.
To share: https://doi.org/10.4088/JCP.21m14316
© 2022 The Authors. Published by Physicians Postgraduate Press, Inc. This is an open access article under the CC BY-ND license.
aKaruna Therapeutics, Boston, Massachusetts
bDepartment of Psychiatry, Indiana University School of Medicine, Indianapolis, Indiana
cKavanagh Statistical Consulting, Apex, North Carolina
*Corresponding author: Peter J. Weiden, MD, Karuna Therapeutics, 99 High St, Floor 26, Boston, MA 02110 ([email protected]).
All current antipsychotic drugs used to treat schizophrenia have direct dopamine D2-receptor blocking activity and, thus, share a common mechanism of action.1 Although they are usually effective in controlling positive symptoms and preventing relapse, there is little evidence that current antipsychotics substantially improve negative or cognitive symptoms.2 Further, direct D2 dopamine antagonism is associated with a range of problems, such as antipsychotic-induced parkinsonism, prolactin elevation, and risk of tardive dyskinesia. For these reasons, there have been long-standing efforts to find pharmacologic treatments that offer antipsychotic efficacy without direct antagonism of the dopamine D2 receptor.
Muscarinic receptors have shown promise as therapeutic targets for antipsychotic drug development dating back to the 1990s, when xanomeline, an M1/M4-preferring muscarinic receptor agonist initially developed for cognitive symptoms of Alzheimer’s disease, was unexpectedly found to reduce psychotic symptoms associated with dementia.3 This observation prompted further work evaluating xanomeline’s antipsychotic properties. In preclinical studies, xanomeline had no direct affinity for dopamine receptors and its antipsychotic activity was mediated by central muscarinic receptors.4,5 In a small, randomized, double-blind, proof-of-concept study in acutely psychotic patients with schizophrenia, xanomeline showed symptom improvement as measured by Positive and Negative Syndrome Scale (PANSS) total score in xanomeline-treated patients compared with those receiving placebo.6 However, further development of xanomeline was hampered because of unwanted side effects commonly associated with muscarinic receptor agonists, sometimes referred to as “procholinergic” side effects (eg, nausea, vomiting, and diarrhea).7 These tolerability problems are a well-known class effect of muscarinic receptor agonists and are believed to be the result of activation of peripheral muscarinic receptors.
The combination oral agent KarXT (xanomeline–trospium) was developed to address the problem of unwanted stimulation of peripheral muscarinic receptors by xanomeline while preserving its muscarinic receptor agonist effects in the central nervous system (CNS). KarXT combines xanomeline with trospium, a US Food and Drug Administration–approved peripheral muscarinic receptor antagonist commonly used for overactive bladder. Trospium does not cross the blood-brain barrier, so it counteracts xanomeline’s peripheral muscarinic receptor agonism without impacting xanomeline’s activity in the CNS.8 Relative to xanomeline alone, KarXT has been reported to be associated with fewer and less severe procholinergic side effects.9
In a recent phase 2 study, KarXT successfully met its primary efficacy and safety objectives as an investigational treatment for patients with schizophrenia.10 Briefly, the primary results showed a statistically significant difference in change from baseline to week 5 in PANSS total score (−17.4 points for KarXT vs −5.9 points for placebo; 95% confidence interval [CI], −16.1 to −7.1; P < .001). The results for most of the secondary endpoints, including PANSS positive and negative symptom subscales and Clinical Global Impression–Severity (CGI-S) endpoints, also favored the KarXT group. Safety results are detailed in the primary publication but are briefly summarized here. The adverse event (AE) profile of KarXT was consistent with the procholinergic AEs of a muscarinic agonist, such as xanomeline, and the peripheral anticholinergic AEs were consistent with those of trospium (eg, constipation and dry mouth).11 All procholinergic/anticholinergic AEs were rated as mild or moderate (Common Terminology Criteria for Adverse Events grade 1 or 2), and none resulted in early discontinuation from the clinical trial.10 These encouraging results form the basis of an ongoing phase 3 program investigating KarXT for the treatment of acute psychosis in schizophrenia.
Until the phase 3 data are available, the phase 2 study remains the primary source of efficacy information for KarXT. The primary publication focuses on the prospective statistical hierarchy of analysis that is required in registration studies. Here, we present additional analysis focused on domains of response and categorical outcomes that may help guide clinical decision-making.12 Categorical outcomes are anchored to the expectations of treatment efficacy, duration of exposure, and the severity of the symptoms of the disorder being studied. The latter are often calibrated against the anticipated efficacy within the treatment population to assess magnitude of clinical response.13 For short-term treatment of psychosis in patients with schizophrenia, it is common to choose 1 or more thresholds of symptom response achieved (eg, ranging from ≥ 20% improvement to ≥ 50% improvement in aggregate symptoms) between the start and end of the treatment period.14
Here, we provide additional, previously unpublished results of secondary and post hoc efficacy analyses to answer the following questions: (1) What are the categorical response rates associated with KarXT treatment assessed by PANSS total scores from baseline to the end of the study? (2) What is the clinical magnitude of these responses using a number needed to treat (NNT) analysis based on differences between KarXT and placebo at endpoint? (3) What is the time to reach these responses using PANSS assessments at 2 and 4 weeks? (4) What are the symptom domains of response beyond just positive and negative symptoms assessed using a 5-factor PANSS instead of the original 3 PANSS subscales?
METHODS
Study Design
This was a post hoc analysis from a phase 2, randomized, double-blind trial of KarXT vs placebo (EMERGENT-1; ClinicalTrials.gov identifier NCT03697252) for acutely psychotic adults with schizophrenia.10 The EMERGENT-1 study was conducted between September 2018 and August 2019. Details of the trial methods, population, safety, and the prespecified primary and secondary endpoints have been previously published.10 Briefly, after a 7-day screening period, participants were randomized 1:1 to receive either oral KarXT or matched placebo twice daily for 5 weeks of inpatient treatment. The dosing schedule of KarXT (mg xanomeline/mg trospium) was flexible, starting with 50 mg/20 mg twice daily and increasing to a maximum of 125 mg/30 mg twice daily.
A central institutional review board approved the study protocol and amendments. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. All patients provided written informed consent prior to participation.
Study Population
At study entry, patients aged 18–60 years with a primary diagnosis of schizophrenia based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), were enrolled.15 Other key inclusion criteria included recent worsening of positive symptoms warranting hospitalization, a PANSS total score > 80, and a CGI-S score of 4 (moderately ill) or higher. Patients with a primary disorder other than schizophrenia within the 12 months preceding screening, a history of treatment resistance to antipsychotic medications, or a decrease in the PANSS total score ≥ 20% between screening and baseline were excluded.
Efficacy Measures
The PANSS was the primary assessment used for efficacy outcomes of the study. PANSS is a 30-item symptom severity measure typically used in treatment studies of schizophrenia (Supplementary Table 1).16 PANSS assessments occurred at baseline, week 2, week 4, and week 5 (or last assessment for early discontinuation). The primary prespecified outcome was the KarXT–placebo difference in change from baseline to week 5 in PANSS total score. Secondary prespecified outcomes included PANSS positive and negative subscales and CGI-S outcomes. The CGI-S is a 7-point scale that requires the investigator to rate the severity of the patient’s illness relative to the investigator’s prior experience with patients with the same diagnosis.17
Categorical response definitions. For the present post hoc analysis, a series of prespecified percentage improvements in PANSS total score between baseline and endpoint was used to assess categorical response. For this analysis, a total of 4 thresholds were chosen: ≥ 20%, ≥ 30%, ≥ 40%, and ≥ 50% reduction in PANSS total score at endpoint compared with baseline. These thresholds have been used in the literature reporting on categorical response outcomes from clinical trials of antipsychotic treatment in patients with schizophrenia.14,18
Time course of response. The time course of response was evaluated based on the PANSS total score obtained at each postbaseline assessment (2 weeks, 4 weeks, and 5 weeks), using the same 4 thresholds (≥ 20%, ≥ 30%, ≥ 40%, and ≥ 50% reduction) but considering earlier timepoints.
Symptom domains of response. A Marder 5-factor model of the PANSS19 was used to assess symptom domains of response. The 5 Marder factors consist of positive symptom, negative symptom, disorganized thought, uncontrolled hostility, and anxiety/depression factors (see Supplementary Table 2 for a list of items included in each factor).
Statistical Analyses
Analyses were performed for the modified intent-to-treat population (mITT), which was the prespecified population for the primary and secondary endpoints. The mITT population was defined as all randomized patients who received at least 1 dose of study medication, had a baseline PANSS assessment, and had at least 1 postbaseline PANSS assessment.
Responder and time course analyses. For all PANSS categorical response analyses, PANSS response rates transformed the original 1- to 7-item range to a 0- to 6-item range, according to recent recommendations for PANSS categorical outcomes.20,21 For this transformation, the original scores were simply reduced by 1 point for the purposes of analysis (ie, original scores of 1 were reset to 0, original scores of 2 were reset to 1, and so forth).
The primary efficacy endpoint in the study was the difference between the placebo arm and active-treatment arm in continuous change from baseline in PANSS total score at week 5, analyzed using a mixed model for repeated measures (MMRM) for group differences in the least-squares mean (LSM) change. For this post hoc analysis, logistic regression models were used to compare PANSS response rates in each treatment group, adjusting for factors of age, sex, and treatment group. Differences between KarXT and placebo were estimated using odds ratios, 95% CIs, and nominal P values. The NNT at 5 weeks was calculated as 1 divided by the difference in the PANSS responder rates for KarXT and placebo. Missing data were imputed using last-observation-carried-forward methodology.
Symptom domains of response. For response based on a PANSS 5-factor analysis, change from baseline in each of the 5 factors was analyzed using MMRM, with the observed change-from-baseline score at each visit as the response, including treatment group, visit, and the interaction of treatment group and visit as fixed effects and baseline score, site, age, and sex as covariates. Differences between KarXT and placebo were estimated using LSM, standard errors, 95% CIs, and nominal P values. Effect size was determined using Cohen d calculations based on the LSM estimates.
RESULTS
Patients
A total of 182 patients were randomized to KarXT or placebo; 83 patients in the KarXT group and 87 patients in the placebo group were included in the mITT analysis. Baseline characteristics for the study population have been reported10 and are shown in Supplementary Table 3; they were similar between the 2 treatment groups and are fairly representative of demographics of other US-based inpatient studies for the treatment of schizophrenia.22 Mean PANSS total scores at baseline were 97.3 ± 9.34 points in the KarXT group and 96.6 ± 8.39 points in the placebo group.
Categorical Response Rates
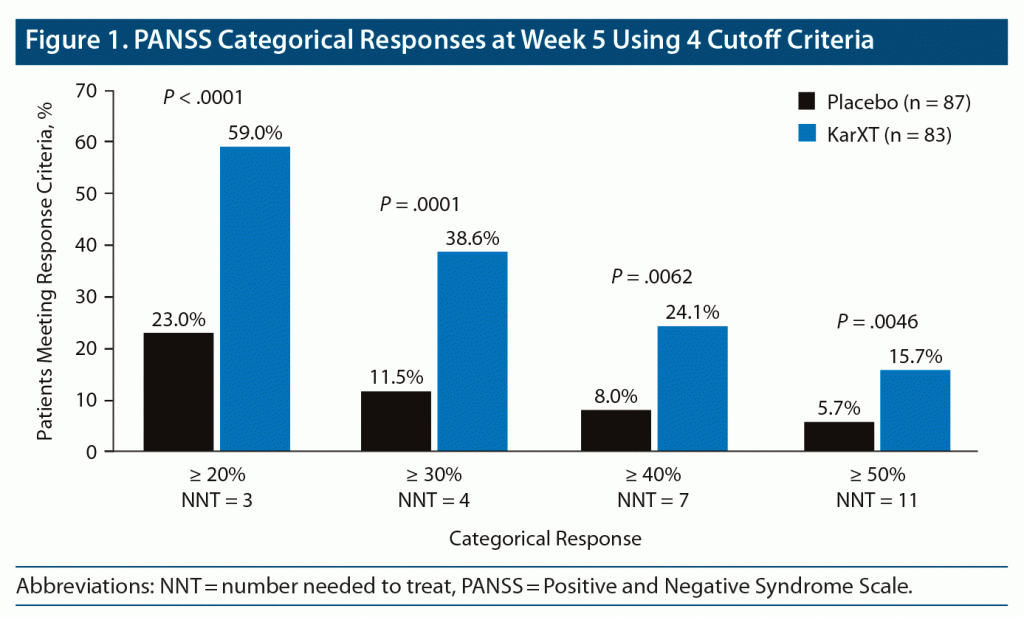
Figure 1 shows the responder rates for the KarXT and placebo groups at the week 5 study endpoint across all 4 PANSS response threshold categories. The proportion of KarXT patients meeting the categorical response rate criteria at the week 5 study endpoint ranged from 59.0% (n = 49) using the ≥ 20% threshold to 15.7% (n = 13) for the ≥ 50% threshold. Regardless of the response threshold evaluated, the proportion of KarXT patients responding was consistently higher than the proportion of placebo patients (nominal P < .05 for all response criteria). As the response threshold was increased, a smaller number of patients in both the KarXT and placebo groups met the response criteria, as expected. The corresponding NNTs (95% CI) for the number of patients needed to achieve a PANSS response at week 5 were NNT = 3 (3–5) for ≥ 20%, NNT = 4 (3–7) for ≥ 30%, NNT = 7 (4–20) for ≥ 40%, and NNT = 11 (6–145) for ≥ 50% improvement in PANSS total score between baseline and week 5.
Time Course of Response
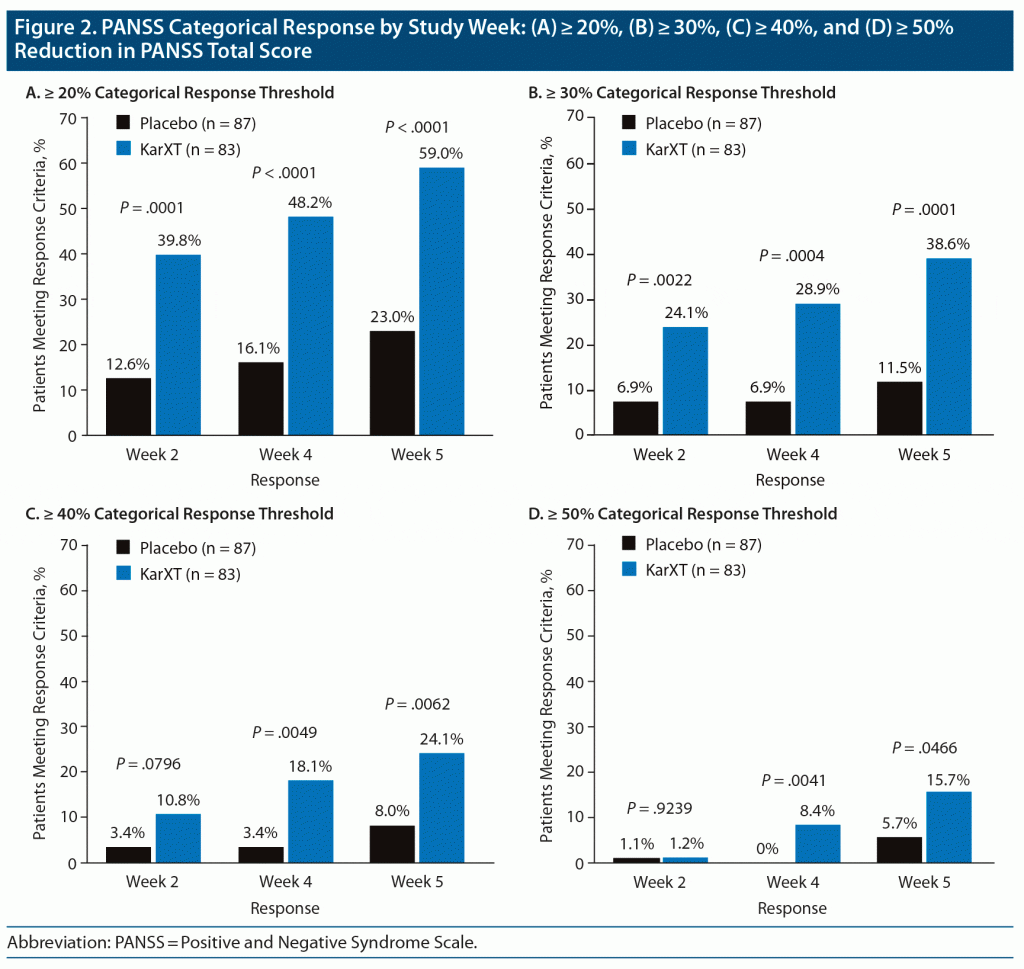
The time course for achievement of response based on each of the 4 response criteria is shown in Figure 2. Comparing the response of KarXT with that of the placebo group, the ≥ 20% and ≥ 30% threshold criteria showed significant differences favoring KarXT by week 2, whereas the ≥ 40% and ≥ 50% thresholds did not reach P < .05 until week 4.
Response on PANSS 5-Factor (Marder) Analysis
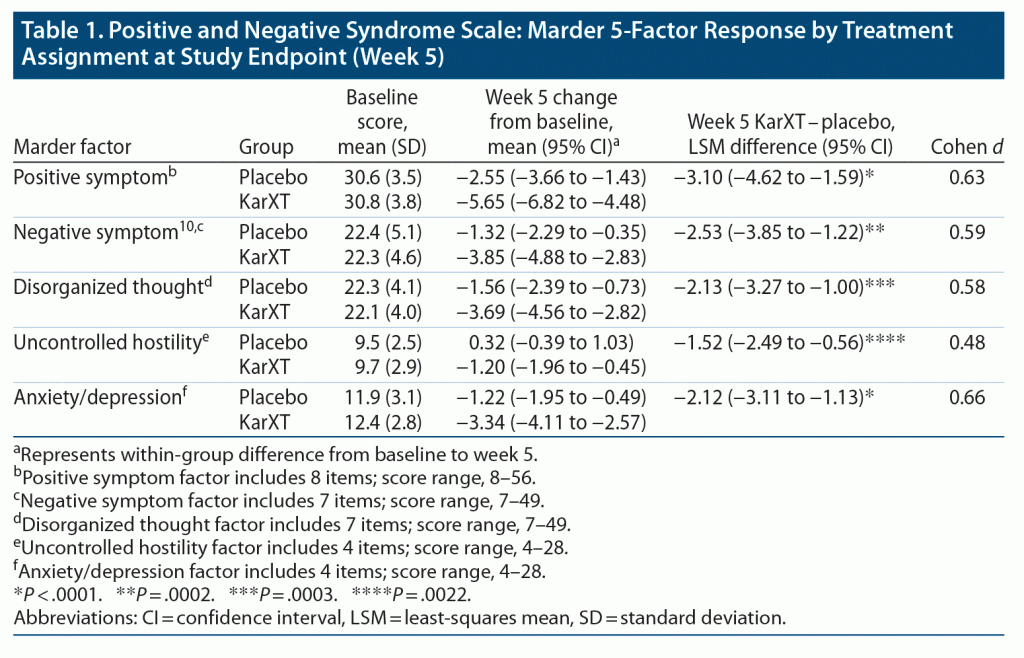
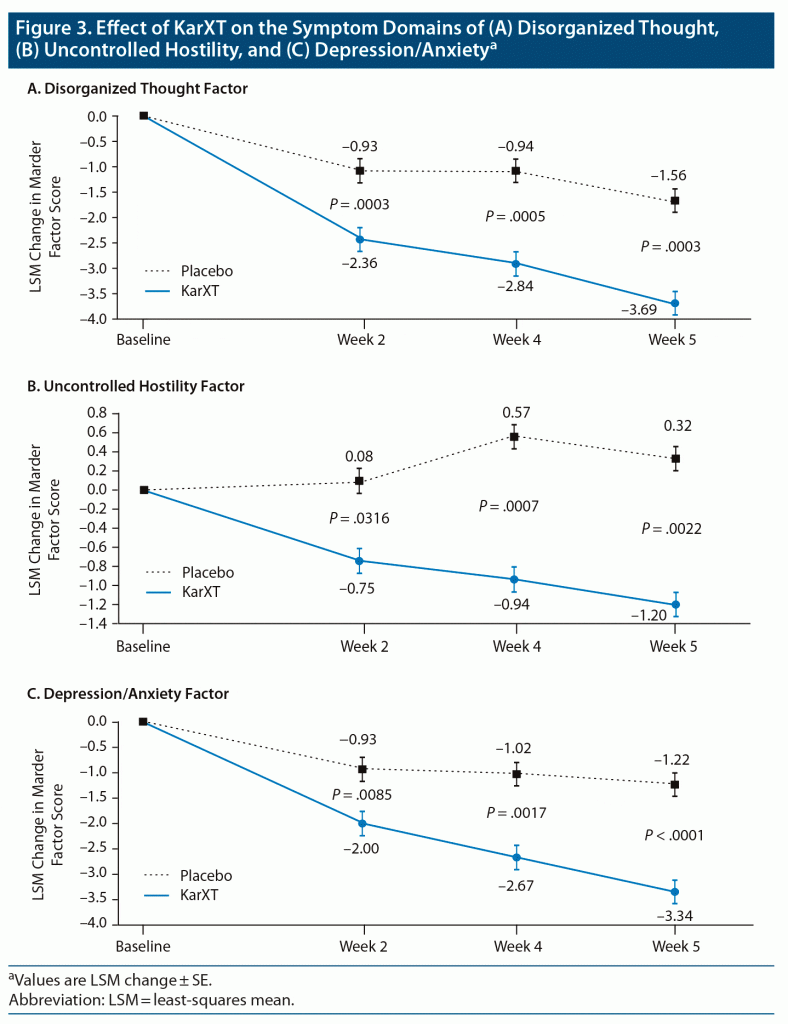
Patients in the KarXT group showed significant improvement over placebo from baseline to week 5 in all 5 PANSS Marder factors (Table 1). The effect size differences at week 5 ranged from 0.48 to 0.66. The between-group differences between KarXT and placebo were significant starting at week 2 for all 5 factors (Figure 3, Supplementary Figure 1).
DISCUSSION
The additional analysis presented here supports the primary and secondary endpoints of the recently published paper on the efficacy and safety of KarXT (xanomeline–trospium) in treating acute psychosis in patients with schizophrenia.10 In summary, in this initial phase 2 study of KarXT for the treatment of patients with schizophrenia, KarXT showed a consistent pattern of statistical superiority compared with placebo on the likelihood of categorical treatment response, the time course of response, and all 5 PANSS factors.
As expected, the magnitude of response depended on the threshold criteria used. The lowest commonly accepted PANSS threshold for response is a 20% reduction of PANSS total symptoms.14 Using these criteria, about 6 of 10 KarXT study patients met response criteria at week 5. Using the highest commonly accepted PANSS threshold of ≥ 50% reduction of symptoms,14 about 1 of 7 patients receiving KarXT met response criteria at week 5. The NNT analysis provides a way to estimate the impact of KarXT treatment relative to placebo.13 Lower NNTs denote more effective treatments. As expected, lower response thresholds resulted in lower NNTs, with the ≥ 20% threshold associated with the lowest NNT of 3, whereas the ≥ 50% threshold was associated with the highest NNT of 11. For the time course of improvement, clinically meaningful differences between KarXT and placebo were observed within 4 weeks for all 4 PANSS response criteria and within 2 weeks for 3 of the 4 PANSS response criteria in our analysis.
A 5-factor model of PANSS is now widely recognized as a more informative way to evaluate antipsychotic response to symptom domains than the original 3-subscale approach from the initial publication of PANSS,23,24 which does not differentiate many of the clinically important domains, such as anxiety or depression, hostility, or cognitive symptoms. Most 5-factor models include these subdomains. The 5-factor analysis chosen here, known as Marder factors,17,18,19 is widely used and reported in secondary analyses to provide response information on symptom subdomains.19,25–27 Expanding the PANSS domains from 3 to 5 is helpful for understanding the pattern and types of symptom domains associated with KarXT treatment response. This may be of particular interest given KarXT’s muscarinic receptor mechanism of action rather than direct dopamine receptor affinity, as with all currently marketed antipsychotic drugs.4,5 Three of the 5-factor PANSS items were not reported in the primary and secondary analysis from this trial: disorganized thought, uncontrolled hostility, and anxiety/depression factors. The patterns of response to KarXT in all 3 of these factors were similar to those initially reported for the positive and negative symptom factors of the PANSS total. One caveat is that these 5 factors might not be independent of one another. In particular, for studies in acute schizophrenia, many PANSS items used across factors may be influenced by positive symptoms.28 What might be scored as negative symptom items might be secondary to psychotic symptoms and will improve alongside positive symptoms. Therefore, the improvements observed in the 5-factor PANSS analysis are able to assess the more enduring and long-term nature of primary negative symptoms.
The main limitation of these results is that they are from a single, well-controlled phase 2 study and require replication. As the first efficacy study of a new investigational treatment for patients with schizophrenia, these findings should be considered preliminary. To confirm and extend these results, an active phase 3 program is underway, which includes 2 additional placebo-controlled trials of KarXT of similar design (EMERGENT-2, ClinicalTrials.gov identifier NCT04659161; EMERGENT-3, ClinicalTrials.gov identifier NCT04738123). As a single study, the results cannot be used to infer that KarXT is effective for schizophrenia, which needs to await completion of an ongoing phase 3 program. Furthermore, there was no active control arm, so the study cannot be used to compare KarXT with any other marketed or investigational antipsychotics. KarXT has not been studied in any head-to-head trials with other antipsychotic drugs; caution is needed when comparing the results of this trial to any other trial. As with all short-term trials, the 5-week duration limits the understanding of a longer-term response trajectory and durability of response, which will be addressed with data from longer-term follow-up studies included in the ongoing phase 3 studies of KarXT.
In summary, the analysis presented here shows that in this phase 2 trial, treatment with KarXT was associated with significant categorical responses over placebo across all PANSS threshold definitions evaluated, with demonstrated efficacy beginning as early as 2 weeks into treatment. Corresponding NNTs ranged from 3 for the lowest threshold definition of ≥ 20% reduction in total symptoms by 5 weeks to an NNT of 11 for the highest threshold of ≥ 50% reduction. Using a 5-factor PANSS analysis, KarXT improved all 5 factors at the first timepoint of assessment (2 weeks into treatment), showing that symptom improvements extend broadly beyond positive and negative symptoms and include cognitive, hostility, and affective domain symptoms, as well. If confirmed by the ongoing phase 3 studies, KarXT may represent a new class of antipsychotic drugs based on muscarinic receptor agonism.
Submitted: November 8, 2021; accepted January 31, 2022.
Published online: May 11, 2022.
Relevant financial relationships: Drs Weiden, Miller, Brannan, and Paul are employees of and hold equity in Karuna Therapeutics. Dr Breier provides consulting services to Karuna Therapeutics and Perception Neuroscience and holds equity in Karuna Therapeutics. Ms Kavanagh provides consulting services for Karuna Therapeutics, UCB Pharma, Novartis Gene Therapies, Worldwide Clinical Trials, PharPoint Research, and Nesos Inc.
Funding/support: This research was sponsored by Karuna Therapeutics and the Wellcome Trust (award 208970/Z/17/Z). Funding for additional statistical support was provided by Karuna Therapeutics.
Role of the sponsor: The sponsor was involved in the design and conduct of the study; collection, analysis, and interpretation of data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Previous presentation: Part of the data in this manuscript were previously presented as a poster at the Schizophrenia International Research Society Virtual Congress, April 17−21, 2021 (Poster T54).
Acknowledgments: The authors thank the study patients and the investigators for their participation. Medical writing and editorial support were provided by Austin Ulrich, PharmD, and Jeni Crockett-Holme, BA, of Dragonfly Editorial (Tipp City, Ohio) and Shannon Davis, BA, and Matthew Jacobson, MALS, CMPP, of Apollo Medical Communications (Guilford, Connecticut) and funded by Karuna Therapeutics. Mr Jacobson and Ms Davis have no conflicts of interest to disclose.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- KarXT (xanomeline–trospium) is a central muscarinic receptor agonist without direct affinity for dopamine receptors.
- KarXT demonstrated efficacy in schizophrenia in a 5-week, phase 2, inpatient study, providing evidence that muscarinic receptor agonists may be a new approach to treating schizophrenia.
- This article reports additional PANSS outcomes, including categorical response rates, time course of response, and improvement across broader symptom domains.
References (28)

- McCutcheon RA, Pillinger T, Mizuno Y, et al. The efficacy and heterogeneity of antipsychotic response in schizophrenia: a meta-analysis. Mol Psychiatry. 2021;26(4):1310–1320. PubMed CrossRef
- McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia: an overview. JAMA Psychiatry. 2020;77(2):201–210. PubMed CrossRef
- Bodick NC, Offen WW, Shannon HE, et al. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11(suppl 4):S16–S22. PubMed
- Andersen MB, Fink-Jensen A, Peacock L, et al. The muscarinic M1/M4 receptor agonist xanomeline exhibits antipsychotic-like activity in Cebus apella monkeys. Neuropsychopharmacology. 2003;28(6):1168–1175. PubMed CrossRef
- McKinzie D, Fischler K, Meyer T, et al. Xanomeline’s activity in rodent models of psychosis: role of central muscarinic receptors and augmentation by risperidone and aripiprazole. Presented at: American Society for Clinical Pathology Annual Meeting. Virtual; October 27–29, 2021.
- Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033–1039. PubMed CrossRef
- Felder CC, Porter AC, Skillman TL, et al. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001;68(22–23):2605–2613. PubMed CrossRef
- Staskin D, Kay G, Tannenbaum C, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64(9):1294–1300. PubMed CrossRef
- Miller AC, Paul SM, Breier A. Xanomeline plus trospium: a novel strategy to enhance pro-muscarinic efficacy and mitigate peripheral side effects. Presented at: American Society for Clinical Pathology Annual Meeting; May 28–31, 2019; Scottsdale, AZ.
- Brannan SK, Sawchak S, Miller AC, et al. Muscarinic cholinergic receptor agonist and peripheral antagonist for schizophrenia. N Engl J Med. 2021;384(8):717–726. PubMed CrossRef
- Staskin D, Sand P, Zinner N, et al; Trospium Study Group. Once daily trospium chloride is effective and well tolerated for the treatment of overactive bladder: results from a multicenter phase III trial. J Urol. 2007;178(3 Pt 1):978–983, discussion 983–984. PubMed CrossRef
- Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111–123. PubMed CrossRef
- Citrome L, Du Y, Weiden PJ. Assessing effectiveness of aripiprazole lauroxil vs placebo for the treatment of schizophrenia using number needed to treat and number needed to harm. Neuropsychiatr Dis Treat. 2019;15:2639–2646. PubMed CrossRef
- Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Association; 2013.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:218–222.
- Correll CU, Kishimoto T, Nielsen J, et al. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. 2011;33(12):B16–B39. PubMed CrossRef
- Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538–546. PubMed CrossRef
- Obermeier M, Schennach-Wolff R, Meyer S, et al. Is the PANSS used correctly? a systematic review. BMC Psychiatry. 2011;11(1):113. PubMed CrossRef
- Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr Bull. 2010;36(3):461–462. PubMed CrossRef
- Brannan SK, Breier A, Weiden PJ, et al. The M1/M4 agonist xanomeline in combination with trospium is effective for acute treatment of schizophrenia: PANSS responder and PANSS 5-factor analyses of a phase 2 placebo-controlled inpatient trial. Presented at: Schizophrenia International Research Society Virtual Congress; April 17–21, 2021.
- Wallwork RS, Fortgang R, Hashimoto R, et al. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1-3):246–250. PubMed CrossRef
- Lindenmayer JP, Grochowski S, Hyman RB. Five factor model of schizophrenia: replication across samples. Schizophr Res. 1995;14(3):229–234. PubMed CrossRef
- Citrome L, Risinger R, Cutler AJ, et al. Effect of aripiprazole lauroxil in patients with acute schizophrenia as assessed by the Positive and Negative Syndrome Scale: supportive analyses from a Phase 3 study. CNS Spectr. 2018;23(4):284–290. PubMed CrossRef
- Citrome L, Meng X, Hochfeld M. Efficacy of iloperidone in schizophrenia: a PANSS five-factor analysis. Schizophr Res. 2011;131(1–3):75–81. PubMed CrossRef
- Ismail Z, Peters-Strickland T, Miguelez M, et al. Aripiprazole once-monthly in the treatment of acute psychotic episodes in schizophrenia: post hoc analysis of Positive and Negative Syndrome Scale Marder factor scores. J Clin Psychopharmacol. 2017;37(3):347–350. PubMed CrossRef
- Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophr Bull. 1985;11(3):440–452. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite