Objective: The study objective was to examine whether and when antipsychotic-induced weight gain in first episode psychosis (FEP) stabilizes over a 12-month exposure to the same antipsychotic in a sample of previously untreated FEP patients.
Methods: In this prospective naturalistic outcome study, 109 patients diagnosed with non-affective or affective psychosis (DSM-IV) were treated with the same antipsychotic medication (olanzapine n = 45, risperidone n = 39, or aripiprazole n = 25) throughout the first year of treatment. Body weight was measured and body mass index calculated at baseline and 1, 2, 3, 6, 9, and 12 months. Additional weight data over the second year were available, making extending the comparison for a second year possible.
Results: Linear mixed model analysis showed a significant main effect of time (Type III test P < .001) after adjusting for baseline weight values. Post hoc pairwise comparisons showed that incremental weight changes subsequent to month 6 were insignificant, suggesting weight stabilization by month 9. No significant difference (P = .243) between groups or time ×— group interaction (P = .111) was observed. Similar findings were obtained with BMI. A follow-up analysis, of a subsample who continued treatment with the same antipsychotic for an additional 12 months (n = 57), confirmed weight stabilization in the second year. There was no significant main effect of time (P = .641), group (P = .539), or time ×— group interaction (P = .250).
Conclusions: Antipsychotic-induced weight gain occurs mostly in the first few months of treatment. Preventive interventions concurrent to second-generation antipsychotic treatment initiation in medication-naive FEP patients might be warranted.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
• Implement evidence-based interventions to prevent antipsychotic-induced weight gain at the beginning of treatment
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in August 2019 and is eligible for AMA PRA Category 1 Credit™ through October 31, 2021. The latest review of this material was August 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: The study objective was to examine whether and when antipsychotic-induced weight gain in first episode psychosis (FEP) stabilizes over a 12-month exposure to the same antipsychotic in a sample of previously untreated FEP patients.
Methods: In this prospective naturalistic outcome study, 109 patients diagnosed with non-affective or affective psychosis (DSM-IV) were treated with the same antipsychotic medication (olanzapine n = 45, risperidone n = 39, or aripiprazole n = 25) throughout the first year of treatment. Body weight was measured and body mass index calculated at baseline and 1, 2, 3, 6, 9, and 12 months. Additional weight data over the second year were available, making extending the comparison for a second year possible.
Results: Linear mixed model analysis showed a significant main effect of time (Type III test P < .001) after adjusting for baseline weight values. Post hoc pairwise comparisons showed that incremental weight changes subsequent to month 6 were insignificant, suggesting weight stabilization by month 9. No significant difference (P = .243) between groups or time ×— group interaction (P = .111) was observed. Similar findings were obtained with BMI. A follow-up analysis, of a subsample who continued treatment with the same antipsychotic for an additional 12 months (n = 57), confirmed weight stabilization in the second year. There was no significant main effect of time (P = .641), group (P = .539), or time ×— group interaction (P = .250).
Conclusions: Antipsychotic-induced weight gain occurs mostly in the first few months of treatment. Preventive interventions concurrent to second-generation antipsychotic treatment initiation in medication-naive FEP patients might be warranted.
J Clin Psychiatry 2019;80(5):18m12717
To cite: Mustafa S, Joober R, Iyer S, et al. Early stabilization of weight changes following treatment with olanzapine, risperidone, and aripiprazole: a 12-month naturalistic study of first episode psychosis. J Clin Psychiatry. 2019;80(5):18m12717.
To share: https://doi.org/10.4088/JCP.18m12717
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDouglas Institute, Montréal, Quebec, Canada
bDepartment of Psychiatry, McGill University, Montréal, Quebec, Canada
*Corresponding author: Ashok Malla, MBBS, FRCPC, 6625, boulevard LaSalle, Montréal, QC, H4H 1R3, Canada ([email protected]).
Second-generation antipsychotics (SGAs) are a key component for the treatment of first episode psychosis (FEP). However, antipsychotic-induced weight gain (AIWG) is a serious side effect of SGAs. Early exposure to antipsychotics appears to be an important risk factor for AIWG, making FEP patients potentially especially susceptible.1-3
It has been suggested that AIWG would be highest with olanzapine, lower with risperidone, and lowest with aripiprazole.4 However, in a recent study,5 we found no difference in the weight gained per month of exposure to olanzapine, risperidone, or aripiprazole when prescribed as initial antipsychotics to FEP patients in an early intervention service. In the latter study, the discontinuation rate was high (72%), which is in line with previous studies,6 and the average time to discontinuation was approximately 7 months; this made it difficult to longitudinally follow AIWG for each antipsychotic.
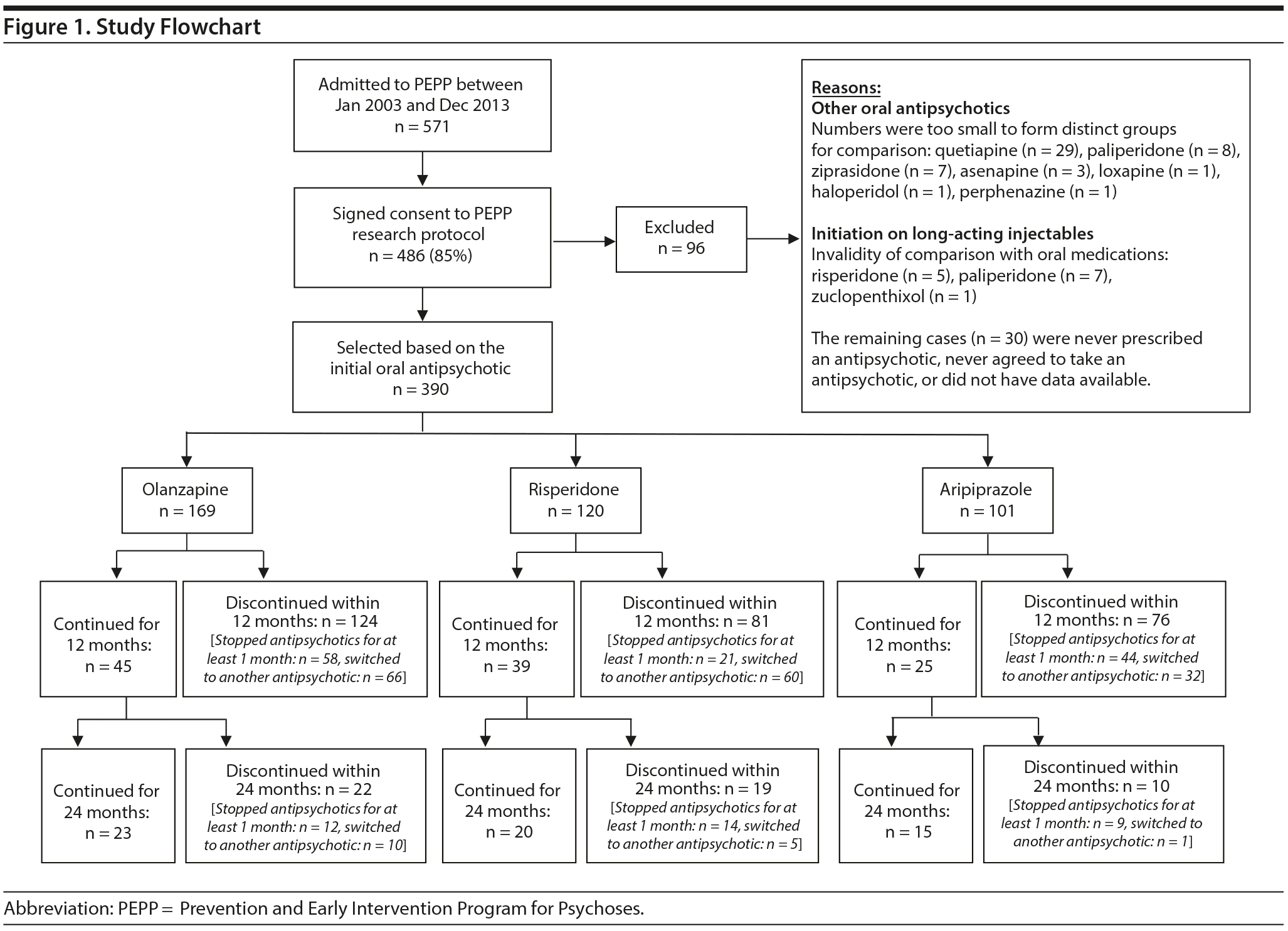
The present study is, therefore, based on a subsample in which only the cases who did not discontinue the initial antipsychotic were selected. Our aim was to test whether and when weight gain stabilizes over the first year of treatment of FEP patients using a continuous prescription of olanzapine, risperidone, or aripiprazole and, if so, whether there are differences between the 3 SGAs.
METHODS
Setting and Inclusion and Exclusion Criteria
This study was conducted at the Prevention and Early Intervention Program for Psychoses (PEPP), Montreal, Quebec, Canada. PEPP-Montreal is the only specialized early intervention service in a catchment area of 300,000 in southwest Montreal, which treats all new cases of affective or non-affective psychotic disorders, making the pool of patients from which the sample for the current study is derived, close to one of treated incidence. Program eligibility criteria are a DSM-IV diagnosis of a first episode of schizophrenia spectrum or affective psychosis, 14-35 years of age, IQ higher than 70, prior exposure to antipsychotic medication of 30 days or less, and absence of substance-induced psychosis or neurologic disorders/head injuries. Comorbid substance abuse is not an exclusion criterion. The research protocol was explained to all participants, and written informed consent was obtained from patients older than 18 years. For younger patients, consent was signed by a parent or guardian and assent was provided by the patient. The present data are derived from a larger longitudinal study of FEP for which ethics approval was obtained from the Douglas Mental Health University Institute’s Research Ethics Board.

- Clinicians should be aware of the risk of very early weight gain associated with 3 commonly used antipsychotics when prescribed to previously untreated first episode psychosis patients.
- As most first episode psychosis patients need antipsychotic therapy, treatment should include interventions to limit weight gain from the first day of treatment irrespective of the specific antipsychotic prescribed.
Medication Protocol
The present study is part of a prospective naturalistic outcome study with a flexible medication protocol that has been previously described in detail.5 No randomization process was performed. Choice of antipsychotic was at the discretion of the treating psychiatrist following program guidelines recommending the use of low dose SGAs but also taking into consideration patient needs, preferences, and medication tolerance. Patients responding to and tolerating SGAs prescribed prior to PEPP entry were continued on the same SGAs, whereas those who were not were initiated on or switched to a different SGA upon PEPP admission.
In Canada, prescribing olanzapine to FEP patients is not overtly restricted; however, clinicians have come to avoid using olanzapine in the last few years as first choice since alternatives such as aripiprazole have become available. The present data involve patients admitted to the program for treatment over the 10-year period between 2003 to 2013. Initially, only olanzapine, risperidone, and quetiapine were available and publicly funded, whereas aripiprazole was introduced in 2009. “Safety First” is a 2010 PEPP-developed guideline that discusses with the patient drugs such as aripiprazole as being potentially less adverse on body weight and metabolic indices based on the evidence available at the time.
Sample
Since the inception of the program (January 2003) until the start of this analysis (January 2015), 571 patients had completed assessments over a 12-month follow-up. Of these, 486 (85%) consented to the research protocol and were then grouped based on their first antipsychotic prescribed at entry to the treatment program. Only patients (total 390) initiated on oral olanzapine (n = 169), risperidone (n = 120), or aripiprazole (n = 101) were selected because the remaining oral SGAs were not prescribed frequently enough to form distinct groups for comparison. Of the 390 selected cases, 109 patients were prescribed the same antipsychotic consistently since entry until the end of 1 year and constitute the sample for this study: olanzapine (n = 45), risperidone (n = 39), and aripiprazole (n = 25). A flowchart is presented in Figure 1. The proportion of participants staying on the same medication following initial prescription was not different across groups (χ22 = 1.89, P = .388). The initial antipsychotic was discontinued by either not being taken for at least 1 month (n = 123 out of 390; 32%) or being switched to another antipsychotic (n = 158 of 390; 41%). In case of switching, the time on the initial antipsychotic was not different between olanzapine, risperidone, or aripiprazole (F2,155 = 1.086, P = .340). In addition, there was no difference in the per month weight gain on each drug (F2,118 = 2.154, P = .121).
Sixty-two (57%) of the participants were outpatients at the point of entry to the program and the initial assessment, whereas 47 (43%) were inpatients. The latter is part of the same early intervention service, and clinicians follow patients across the 2 settings (for details, see Iyer et al7 and Pira et al8). A stay at the hospital emergency department of less than 24 hours was considered outpatient and more than 24 hours as inpatient.
Assessments
Demographic and clinical data were collected. These included diagnosis using the Structured Clinical Interview for DSM-IV Axis I Disorders9; duration of untreated psychosis, using the Circumstances of Onset and Relapse Schedule10; visible minority status, based on Statistics Canada definition; symptoms, using the Scale for the Assessment of Positive Symptoms,11 Scale for the Assessment of Negative Symptoms,12 and Brief Psychiatric Rating Scale13; and functioning, using the Social and Occupational Functioning Assessment Scale.14
Adherence to medication was assessed on a monthly basis. Research staff interviewed patients and families (when available) and reviewed case managers’ clinical reports to acquire adherence data. Adherence was recorded on a 5-point scale reflecting the percentage of time a patient was taking his/her antipsychotic as prescribed: never = 0%, very infrequent = 1%-25%, sometimes = 26%-50%, quite often = 51%-75%, or always = 76%-100%. Patients were rated as fully adherent if they took their antipsychotic dosage 76%-100% of the time. We have previously shown this method to be as reliable as pill counting.15
Body weight in kilograms was measured at baseline and months 1, 2, 3, 6, 9, and 12. Height was also measured, and body mass index (BMI) in kg/m2 was calculated from weight and height data. Change in body weight and BMI from baseline and from other time points was the main outcome variable of this study. Additional weight data were available for months 15, 18, 21, and 24, which made it possible to extend the comparison for the second year.
Data Analysis
Longitudinal mixed effects models, using all available observations without imputation, with restricted maximum likelihood-based inference and adjusting for baseline scores were used for the primary analysis in this study (SPSS, version 23; IBM, Armonk, New York). We tested time as the within-subjects variable, group/type of antipsychotic as the between-subjects variable, and time-by-group interactions while controlling for baseline outpatient-inpatient status in addition to baseline values of the dependent variable. Pairwise comparisons were performed by post hoc tests with Bonferroni adjustment in case a significant main effect was observed. Since this was an exploratory study with a post hoc analysis, no power calculation was performed a priori. This was an intention-to-treat analysis with P values less than or equal to .05 considered significant. All statistical tests were 2-tailed.
RESULTS
Sample Description
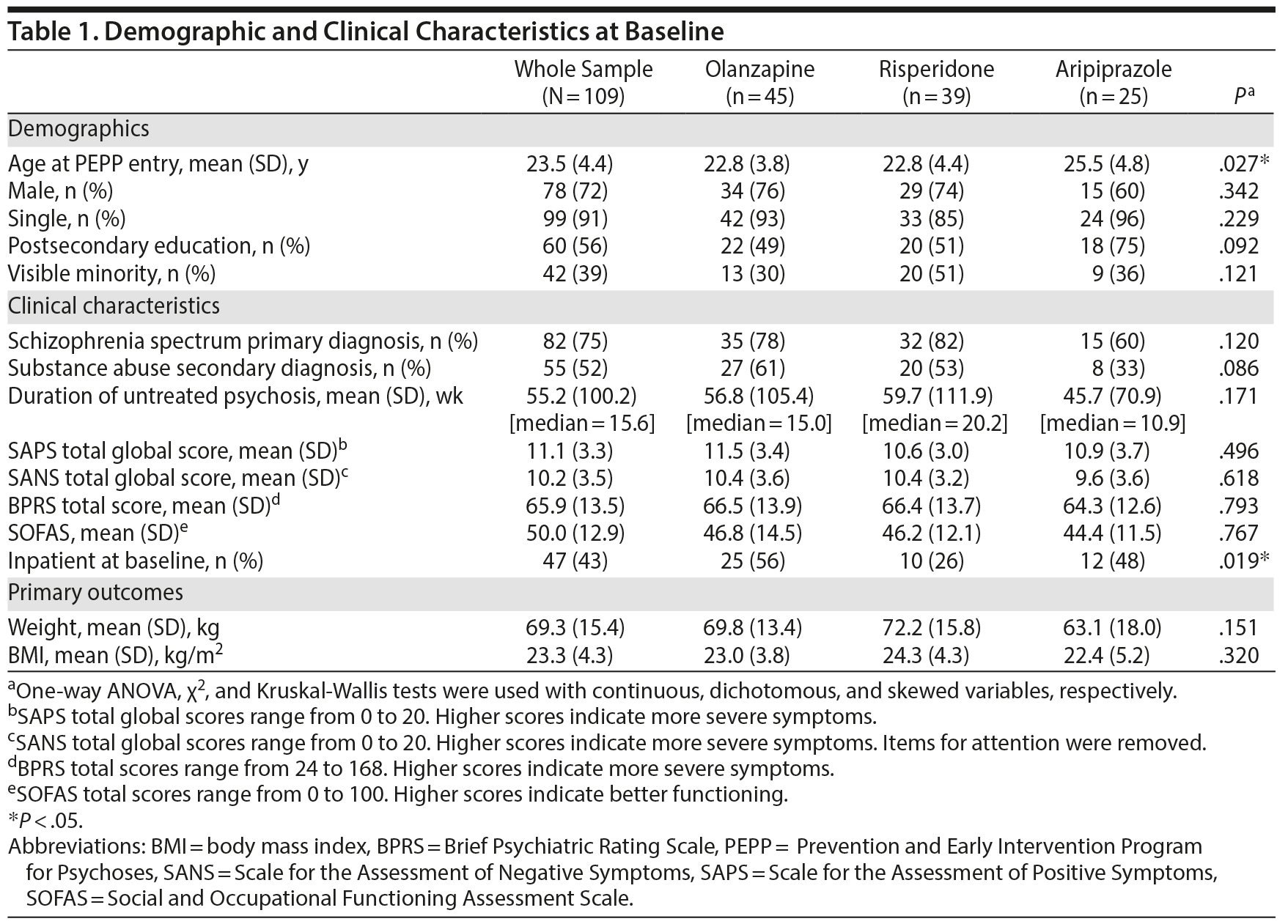
Demographic and clinical characteristics of the study sample and comparison between subgroups at baseline are shown in Table 1. There were no differences between the 3 groups on any variable except age and hospitalization at baseline.
Antipsychotic mean (± SD) daily doses were 10.8 (6.3) mg for olanzapine, 2.1 (1.0) mg for risperidone, and 9.3 (4.7) mg for aripiprazole. Most of the participants (86%, n = 93/108) took antipsychotic medication before entering PEPP for a mean duration of 14.2 (SD = 18.1) days and a median of 10 days. The majority (75%, n = 82/109) of patients were fully adherent to taking medication as prescribed (76%-100% of the time), with no significant difference between the 3 SGAs (χ22 = 0.555, P = .758).
Primary Outcomes
Weight. For the overall population, mean (SD) weight at baseline was 69.3 (15.4) kg. Body weight increased at the rate of 0.7 (SD = 0.6) kg per month on average. Mean weight gain at the end of 1-year follow-up was 9.5 (SD = 7.6) kg, representing a 14.1% average percent weight gain (SD = 11.4). Significant weight gain (≥ 7%) during the 1 year was observed in 70% of patients (n = 59 of 84, remaining data were missing). Seven individuals (8%) showed weight loss (−2.8 [1.1] kg).
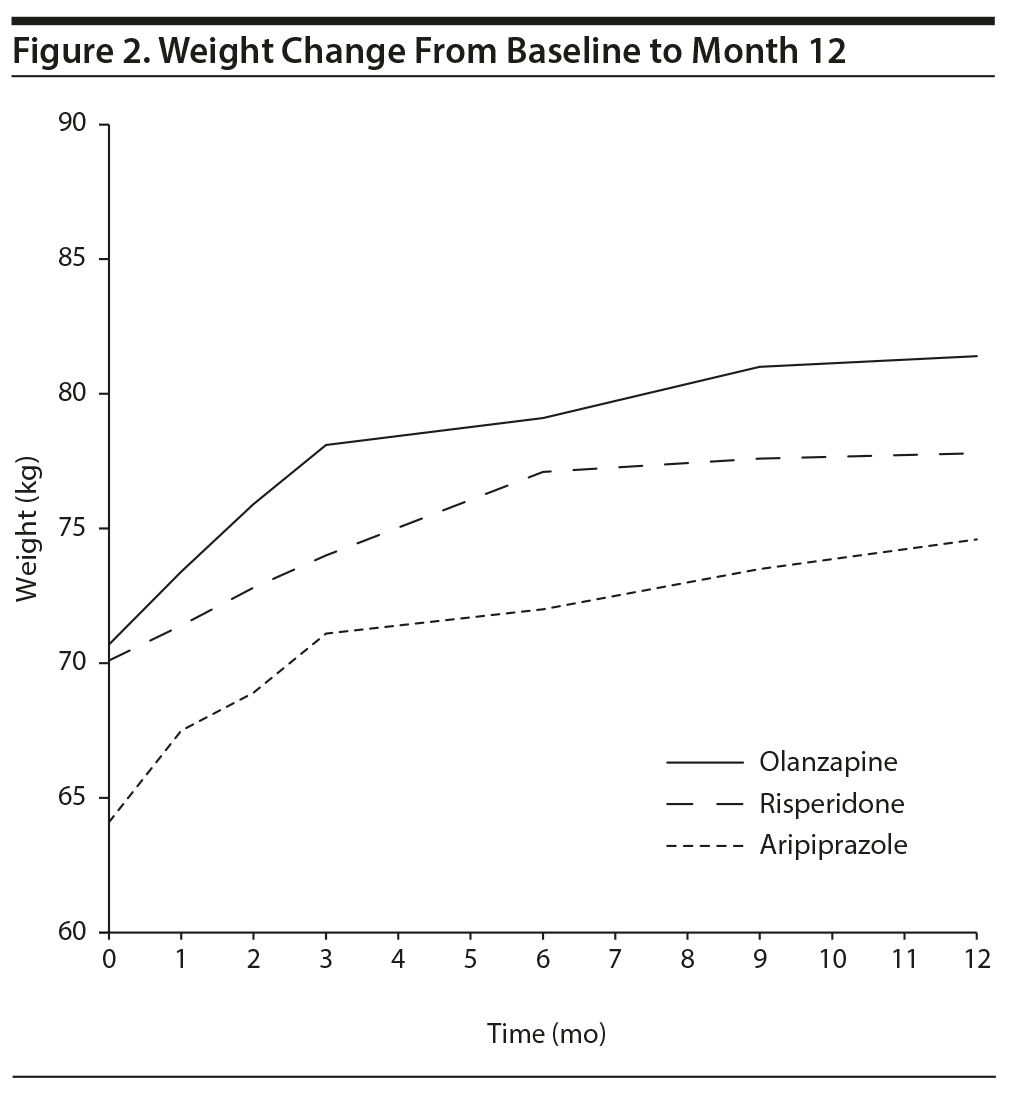
With adjustment for baseline weight and patients’ outpatient-inpatient status, the linear mixed model analysis showed a significant main effect of time (Type III test P value < .001). Post hoc pairwise comparisons revealed a significant increase in body weight at each time point compared to baseline (all P < .001). However, weight changes at month 9 and subsequent time points were not statistically different from month 6 (all P > .050), suggesting weight gain stabilization. No significant difference (P = .243) between groups (olanzapine, risperidone, and aripiprazole) or time ×— group interaction (P = .111) was observed (Figure 2).
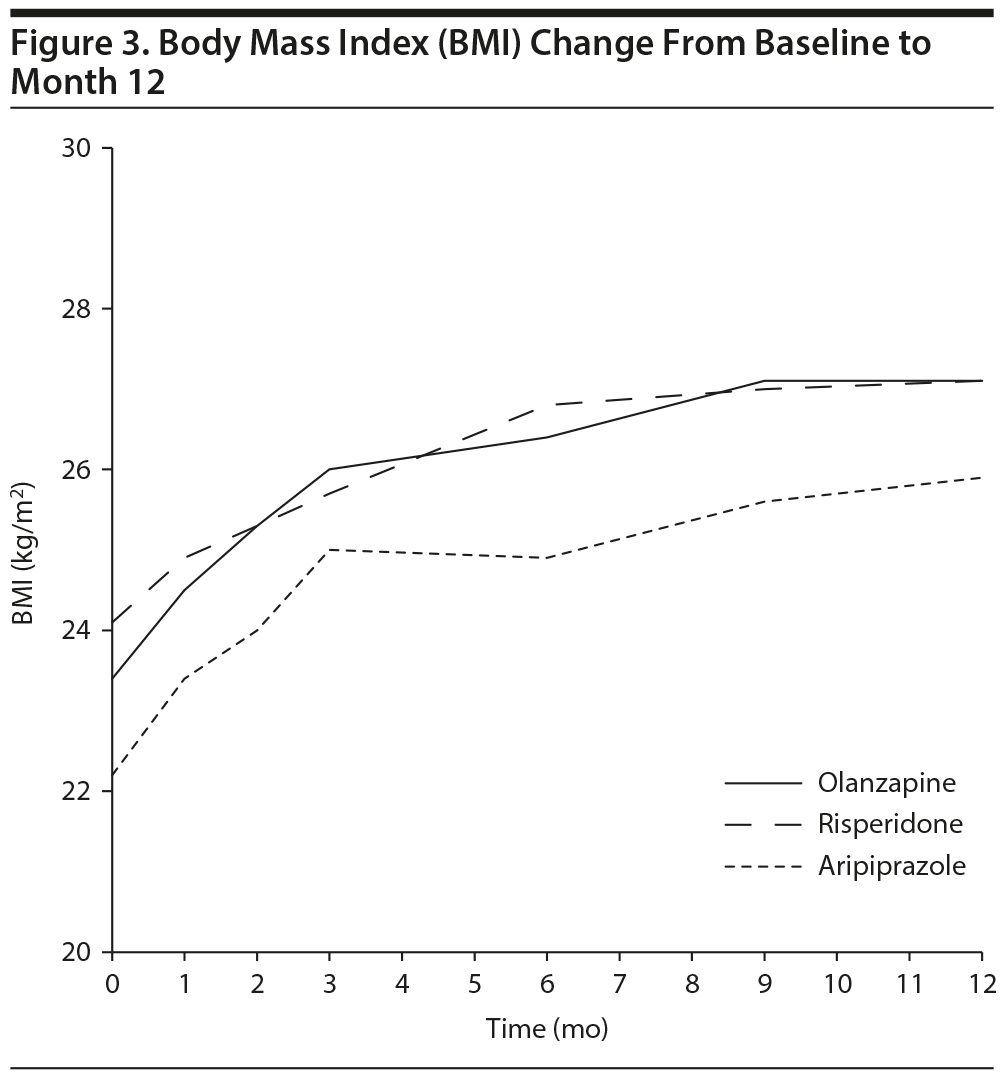
BMI. Consistent with weight findings, a significant main effect of time (Type III test P value < .001) was found after adjusting for baseline BMI and hospitalization. BMI significantly increased at each time point compared to baseline (all P < .001). BMI at month 6 was not significantly different from that at month 9 (P = .218) or month 12 (P = .121), suggesting BMI stabilization. There were no significant differences between groups (P = .441) or time ×— group interactions (P = .493) (Figure 3).
24-Month Subgroup Analysis
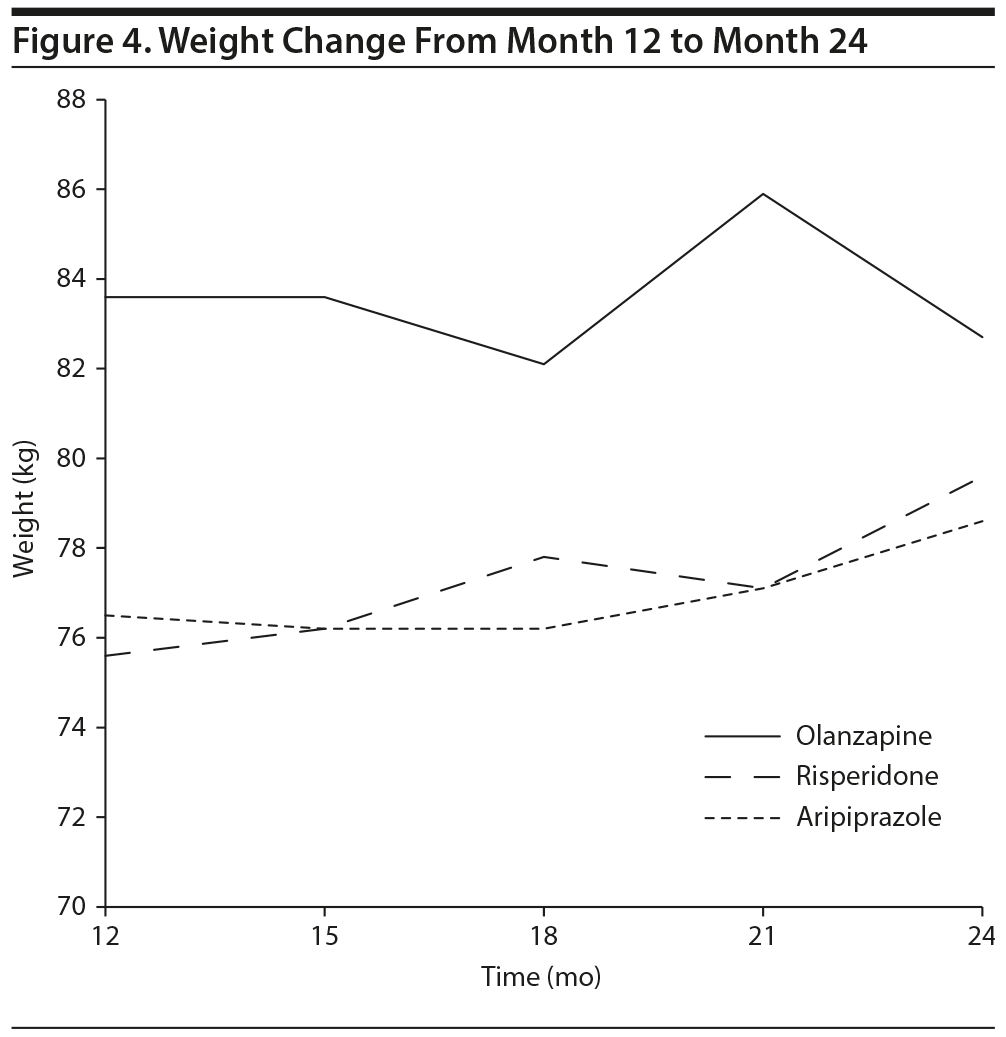
Fifty-seven (52%) of the 109 study participants remained on the same SGA (olanzapine [n = 23], risperidone [n = 20], and aripiprazole [n = 14]) up to month 24. A follow-up analysis, month 12 to 24 weight change, was performed on this subgroup. There was no significant main effect of time (P = .641), group (P = .539), or time ×— group interaction (P = .250). This suggests the stabilization of weight gain during the first year and the lack of difference between the 3 tested antipsychotics (Figure 4).
DISCUSSION
The present study confirms that SGAs in essentially treatment-naive FEP patients (median exposure to antipsychotic medication, 10 days) are associated with significant weight gain beginning with the first month of treatment.1,16 On average, a 9.5-kg increase in weight was observed by the end of 1 year of treatment, all weight measurements along the year were significantly higher than baseline, and the majority of patients (70%) showed a clinically significant weight gain (≥ 7%) along this time span. However, this weight gain stabilized before the end of the first year of treatment. This was detected as nonsignificant differences between weight measurements at month 6 compared to those at months 9 and 12, suggesting weight gain stabilization by month 9 of treatment. To our knowledge, this is the first study to report such an optimistic finding, which, regretfully, could be neither confirmed nor contradicted by previous findings given the scarcity of longitudinal AIWG studies in FEP in the literature.
In this analysis, we could not detect any difference in weight gain between olanzapine, risperidone, or aripiprazole. This is contrary to a recent (2016) meta-analysis of differential effects of antipsychotics on AIWG in FEP reporting olanzapine to cause the highest weight gain, although weight gain with risperidone and aripiprazole was also found to be significant.17 However, our results are in agreement with Perez-Iglesias et al (2008),18 who did not find difference in the magnitude of weight gain after 1 year of treatment with haloperidol, olanzapine, or risperidone in drug-naive patients, despite the presence of differences in weight gain patterns. Similarly, Vázquez-Bourgon et al (2018)19 found no difference in 1-year body weight or BMI changes of drug-naive non-affective FEP patients randomly assigned to aripiprazole, ziprasidone, or quetiapine. The authors reported an 8.5-kg mean weight gain following 1 year of treatment with aripiprazole, which is comparable to our current and previous findings.1
When we repeated the analyses on BMI changes, the results were the same as with weight change. The results suggesting stabilization of weight gain and BMI changes in the first year were also confirmed with a subsample of patients who continued the initial antipsychotic for 24 months. In this subsample, the patients’ weight was stable from month 12 to month 24 of treatment. This sustenance of the stability of weight in the second year suggests the findings of the study of early stabilization of weight gain are likely to be robust.
Our findings, if replicated, have significant clinical implications. The finding that most weight gain happened in the first 6 months suggests this to be a critical period for intervention aimed at preventing this side effect until the plateau phase is reached. This would imply that, concurrent to prescribing antipsychotic medications, initiating interventions to minimize weight gain would be advisable irrespective of the antipsychotic medication prescribed. A number of psychosocial and pharmacologic interventions have been proposed in this regard. For example, a recent meta-analysis20 found that combining metformin and lifestyle intervention shows significant effect in reducing AIWG. These interventions would also include close monitoring of diet and exercise and behavioral interventions to assist with such monitoring.21
Our finding of lack of difference between the 3 antipsychotics tested may at first suggest that treatment can be initiated with any of these drugs, given their equal effectiveness in FEP.6 However, our observations do not include any measures of metabolic disturbance (eg, triglycerides, fasting glucose, glycated hemoglobin), which may be more sensitive measures of long-term risk of metabolic syndrome and cardiovascular disease.22 It is also possible that differences between the 3 antipsychotics are present in patients who were not continued on the same medication for a variety of reasons, including efficacy and patient’s preference.
The rapidity of weight gain in FEP patients may increase risk of nonadherence and account for high rates of discontinuation of the first antipsychotic prescribed.5 Previous reports have suggested that patients discontinue medications for a variety of reasons including, but not limited to, side effects such as weight gain, despite significant improvement in symptoms.23,24 On the other hand, patients are also likely to continue medication when they experience an improvement in psychotic symptoms5 while others stop them when they experience very early improvement in negative symptoms.25 Our results suggest that choosing any particular antipsychotic medication at the very beginning of treatment of FEP may not necessarily decrease or increase the risk of nonadherence or medication discontinuation.
Limitations
One limitation of this study is the exclusion of those who discontinued, when discontinuation could well be the result of intolerability including weight gain, thus potentially biasing the results. Admittedly, patients who continued on the same medication are a special group who had better clinical outcomes such as positive and negative remission and less weight gain as previously reported.5 However, we found no difference in the time to switching or the weight gained before switching between the 3 antipsychotics. Another limitation is the small sample size resulting from high rates of discontinuation of the first antipsychotic prescribed.5 Another limitation of this study is that the assignment to each antipsychotic was not random. However, naturalistic designs are known for their capability to extract evidence from real life data. Further, the fact that the patients were on the same SGA for the entire year enabled comparison of the 3 SGAs being tested despite the lack of randomized design and high discontinuation rate of SGAs. Moreover, baseline comparisons showed that baseline parameters had no bearing on the choice of the initial antipsychotic or the outcomes of the study. Given the small sample size, we were unable to explore any sex differences in the risk for weight gain and for any such differences in patterns of stability of weight gain. The relatively limited exposure to antipsychotic medications in the patient population from which these data were drawn is a major strength of this study.
CONCLUSION
SGAs do cause a statistically and clinically significant increase in body weight and BMI in drug-naive FEP patients. This adverse effect starts as early as the first month of treatment. However, the weight gain observed seems to stabilize by the ninth month of treatment and remains stable until the end of the second year. This suggests that there may be a relatively small window of opportunity in which pairing interventions to reduce risk of weight gain with initiation of SGA treatment is likely to help offset the early rapid significant increase in body weight.
Submitted: December 24, 2018; accepted May 17, 2019.
Published online: August 27, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, metformin is not approved by the US Food and Drug Administration for the prevention of antipsychotic-induced weight gain.
Financial disclosure: Dr Joober is a speaker and/or consultant for Pfizer, Janssen, Bristol-Myers Squibb, Sunovion, Mylan, Otsuka, Lundbeck, Shire, and Purdue; has received grants from Janssen, Bristol-Myers Squibb, Otsuka, Lundbeck, AstraZeneca, and HLS; and has received royalties from Henry Stewart Talks, all of which are unrelated to the present article. Dr Lepage has received grants from Otsuka Lundbeck Alliance and Janssen and personal fees from Otsuka Canada, Lundbeck Canada, Janssen, MedAvante-Prophase, and Amplexor, all of which are unrelated to the present article. Dr Malla has received honoraria for speaking at conferences sponsored by Otsuka and Lundbeck (Canada and Global) and consulting with Otsuka and Lundbeck. Drs Mustafa, Iyer, and Shah have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This study is part of a longitudinal project on outcome in first episode psychosis and early intervention. No specific funding was obtained for this aspect of the project. Funding for the larger project has been received from multiple sources including Canadian Institutes of Health Research (CIHR), National Institute of Mental Health (US; MH093303), and investigator-initiated studies funded by Bristol-Myers Squibb, AstraZeneca, and Pfizer. In addition, Dr Malla is funded by the Canada Research Chairs Program; Dr Iyer received a CIHR New Investigator Award; Drs Iyer, Shah, and Joober received a Fonds de Recherche du Québec—Santé (FRQS) Clinician-Scientist Award; and Dr Lepage received an FRQS Research Chair award.
Role of the sponsor: The funding agencies had no role in the conduct or publication of the study.
Previous presentation: Presented as a short oral communication at the World Psychiatric Association (WPA) congress, Mexico City, Mexico, September 26-30, 2018.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1.Malla A, Mustafa S, Rho A, et al. Therapeutic effectiveness and tolerability of aripiprazole as initial choice of treatment in first episode psychosis in an early intervention service: a one-year outcome study. Schizophr Res. 2016;174(1-3):120-125. PubMed CrossRef
2.Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773. PubMed CrossRef
3.Alvarez-Jiménez M, González-Blanch C, Crespo-Facorro B, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs. 2008;22(7):547-562. PubMed CrossRef
4.Almandil NB, Liu Y, Murray ML, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139-150. PubMed CrossRef
5.Mustafa S, Joober R, Lepage M, et al. Predictors of “all-cause discontinuation” of initial oral antipsychotic medication in first episode psychosis. Schizophr Res. 2018;201:287-293. PubMed CrossRef
6.Whale R, Harris M, Kavanagh G, et al. Effectiveness of antipsychotics used in first-episode psychosis: a naturalistic cohort study. BJPsych Open. 2016;2(5):323-329. PubMed CrossRef
7.Iyer S, Jordan G, MacDonald K, et al. Early intervention for psychosis: a Canadian perspective. J Nerv Ment Dis. 2015;203(5):356-364. PubMed CrossRef
8.Pira S, Durr G, Pawliuk N, et al. Determinants of hospitalization status upon entry into treatment and its impact on outcome in first episode psychosis: b45. Early Interv Psychiatry. 2012;6(S1):41.
9.First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Hilsenroth MJ, Segal DL, eds. Comprehensive Handbook of Psychological Assessment, Volume 2 Personality Assessment. Hoboken, NJ: John Wiley & Sons, Inc; 2004.
10.Norman RM, Malla AK, Verdi MB, et al. Understanding delay in treatment for first-episode psychosis. Psychol Med. 2004;34(2):255-266. PubMed CrossRef
11.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, IA: University of Iowa, Iowa City; 1984.
12.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: Department of Psychiatry, College of Medicine, The University of Iowa; 1983.
13.Ventura J, Lukoff D, Nuechterlein K, et al. Manual for the expanded Brief Psychiatric Rating Scale. Int J Methods Psychiatr Res. 1993;3(3):227-244.
14.Goldman HH, Skodol AE, Lave TR. Revising Axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry. 1992;149(9):1148-1156. PubMed CrossRef
15.Cassidy CM, Rabinovitch M, Schmitz N, et al. A comparison study of multiple measures of adherence to antipsychotic medication in first-episode psychosis. J Clin Psychopharmacol. 2010;30(1):64-67. PubMed CrossRef
16.Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200. PubMed CrossRef
17.Tek C, Kucukgoncu S, Guloksuz S, et al. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. 2016;10(3):193-202. PubMed CrossRef
18.Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naׯve population. Schizophr Res. 2008;99(1-3):13-22. PubMed CrossRef
19.Vázquez-Bourgon J, Pérez-Iglesias R, Ortiz-Garc×a de la Foz V, et al. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naׯve patients with a first-episode of non-affective psychosis. Psychopharmacology (Berl). 2018;235(1):245-255. PubMed CrossRef
20.Zheng W, Zhang Q-E, Cai D-B, et al. Combination of metformin and lifestyle intervention for antipsychotic-related weight gain: a meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2019;52(1):24-31. PubMed CrossRef
21.Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2008;193(2):101-107. PubMed CrossRef
22.Balõtšev R, Haring L, Koido K, et al. Antipsychotic treatment is associated with inflammatory and metabolic biomarkers alterations among first-episode psychosis patients: a 7-month follow-up study. Early Interv Psychiatry. 2019;13(1):101-109. PubMed CrossRef
23.Miller BJ, Bodenheimer C, Crittenden K. Second-generation antipsychotic discontinuation in first episode psychosis: an updated review. Clin Psychopharmacol Neurosci. 2011;9(2):45-53. PubMed CrossRef
24.Leclerc E, Noto C, Bressan RA, et al. Determinants of adherence to treatment in first-episode psychosis: a comprehensive review. Br J Psychiatry. 2015;37(2):168-176. PubMed CrossRef
25.Steger KA, Cassidy C, Rabinovitch M, et al. Impact of symptom resolution on medication adherence in first episode psychosis. Psychiatry Res. 2012;196(1):45-51. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite