Objective: Antipsychotic drugs are known to increase mortality among patients with dementia. Many patients receive concomitant treatment with other psychotropic agents. The aim of this retrospective cohort study was to investigate the impact of benzodiazepines and antidepressants on the risk of death in patients with dementia initiating antipsychotic drug treatment.
Methods: Nationwide registry data on all incident dementia cases among individuals aged 65 years and older in Denmark between 2009 and 2013 for which antipsychotic treatment was initiated were used. The 180-day mortality was evaluated by crude and adjusted hazard ratios (HRs, including adjustment for somatic and psychiatric comorbidity, other prescription drugs, nursing home residency, and time since diagnosis), comparing periods of antipsychotic treatment with periods of concomitant treatment with benzodiazepines or antidepressants.
Results: Among 41,494 incident dementia cases, antipsychotic treatment was initiated for 10,291 (24.8%). After 3,140 people were excluded due to recent antipsychotic drug use or hospitalization, 7,151 people were included in the analysis. The total follow-up time during current antipsychotic treatment was 1,146 person-years, and 831 died during antipsychotic treatment. Compared with antipsychotic treatment alone, the risk of death increased during antipsychotic treatment in combination with benzodiazepines (adjusted HR = 2.19; 95% CI, 1.83-2.63), while there was a decreased risk of death during antipsychotic treatment in combination with antidepressants (adjusted HR = 0.61; 95% CI, 0.50-0.74).
Conclusions: The diverse impact of concomitant use of benzodiazepines and antidepressants on mortality may be due to a direct drug-related effect. Alternatively, the findings could reflect differential mortality associated with different indications for therapy. Although the results cannot prove causality, and there may be residual confounding, clinicians should be cautious when considering the combination of antipsychotics and benzodiazepines in patients with dementia.

ABSTRACT
Objective: Antipsychotic drugs are known to increase mortality among patients with dementia. Many patients receive concomitant treatment with other psychotropic agents. The aim of this retrospective cohort study was to investigate the impact of benzodiazepines and antidepressants on the risk of death in patients with dementia initiating antipsychotic drug treatment.
Methods: Nationwide registry data on all incident dementia cases among individuals aged 65 years and older in Denmark between 2009 and 2013 for which antipsychotic treatment was initiated were used. The 180-day mortality was evaluated by crude and adjusted hazard ratios (HRs, including adjustment for somatic and psychiatric comorbidity, other prescription drugs, nursing home residency, and time since diagnosis), comparing periods of antipsychotic treatment with periods of concomitant treatment with benzodiazepines or antidepressants.
Results: Among 41,494 incident dementia cases, antipsychotic treatment was initiated for 10,291 (24.8%). After 3,140 people were excluded due to recent antipsychotic drug use or hospitalization, 7,151 people were included in the analysis. The total follow-up time during current antipsychotic treatment was 1,146 person-years, and 831 died during antipsychotic treatment. Compared with antipsychotic treatment alone, the risk of death increased during antipsychotic treatment in combination with benzodiazepines (adjusted HR = 2.19; 95% CI, 1.83-2.63), while there was a decreased risk of death during antipsychotic treatment in combination with antidepressants (adjusted HR = 0.61; 95% CI, 0.50-0.74).
Conclusions: The diverse impact of concomitant use of benzodiazepines and antidepressants on mortality may be due to a direct drug-related effect. Alternatively, the findings could reflect differential mortality associated with different indications for therapy. Although the results cannot prove causality, and there may be residual confounding, clinicians should be cautious when considering the combination of antipsychotics and benzodiazepines in patients with dementia.
J Clin Psychiatry 2020;81(4):19m12828
To cite: Nørgaard A, Jensen-Dahm C, Gasse C, et al. Association of benzodiazepines and antidepressants with 180-day mortality among patients with dementia receiving antipsychotic pharmacotherapy: a nationwide registry-based study. J Clin Psychiatry. 2020;81(4):19m12828.
To share: https://doi.org/10.4088/JCP.19m12828
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDanish Dementia Research Centre, Department of Neurology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
bNational Centre for Register-based Research, Aarhus University, Aarhus, Denmark
cDepartment of Affective Disorders, Aarhus University Hospital Psychiatry, Aarhus, Denmark
dCentre for Integrated Register-based Research at Aarhus University (CIRRAU), Aarhus, Denmark
*Corresponding author: Ane Nørgaard, MD, PhD, Department of Neurology, Danish Dementia Research Centre, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, 2100 Copenhagen Ø, Denmark ([email protected]).
Neuropsychiatric symptoms in dementia are common and a major burden on patients and caregivers. Antipsychotics are frequently prescribed even though non-pharmacologic approaches are recommended as first-line management of neuropsychiatric symptoms.1-3 Antipsychotic drug treatment may be indicated in selected patients, but the drugs are associated with several side effects, such as sedation, extrapyramidal symptoms, stroke, myocardial infarction, hip fracture, and, most importantly, an increased risk of death.4-7 Thus, a risk-benefit assessment should always be included prior to prescribing antipsychotics for patients with dementia.
Aside from antipsychotics, patients with dementia are also prescribed other psychotropic drugs, including benzodiazepines and related drugs (BZDs) and antidepressants. Patients with dementia are often frail and more susceptible to side effects from psychotropic drugs.8 The patterns of psychotropic drug prescription have changed during the last decade, probably due to altered prescription guidelines and warnings against the indiscriminate use of antipsychotics.3 We9 previously found that, among dementia patients treated with an antipsychotic drug, 75% also received an agent from another psychotropic drug class during the treatment period. Consequently, potential psychotropic drug interactions may further increase the risk of adverse events and death.10 None of the previous large-scale studies4-6,11 on mortality hazards associated with antipsychotic drug use have studied this potential, altered risk. One study12 investigated the association between use of multiple psychotropic drugs and mortality in older adults and found an overall association between concomitant prescriptions and increased mortality. However, at present it is unclear whether concomitant treatment with BZDs or antidepressants is associated with an increased risk of death when compared with antipsychotic treatment alone.
Observational study designs are crucial to investigate possible negative health outcomes in dementia patients treated with multiple psychotropic drug classes. Danish nationwide registries possess a unique opportunity to investigate prescription patterns in a cohort of dementia patients with complete follow-up. The aim of the present study was to investigate the risk of death among new users of antipsychotics also being treated with BZDs and/or antidepressants and to compare this with the risk of death among patients treated with only antipsychotics.
METHODS
The study was designed as a registry-based nationwide cohort study. In Denmark, all individuals are assigned a civil registration number at birth or upon becoming a permanent resident. This number makes it possible to link data from the Danish health registries at an individual level.13 The registries and data contained within have been described previously.9
Study Population
Patients with incident dementia were identified among all Danish residents aged 65 years and older. Incident cases of dementia comprised individuals registered with a first-time discharge diagnosis of dementia (diagnostic codes are available in Supplementary Table 1) from a Danish hospital or at an outpatient visit and/or individuals who had filled at least 1 prescription for an antidementia drug (Anatomical Therapeutic Chemical [ATC] code N06D) between January 1, 2009, and December 31, 2013.
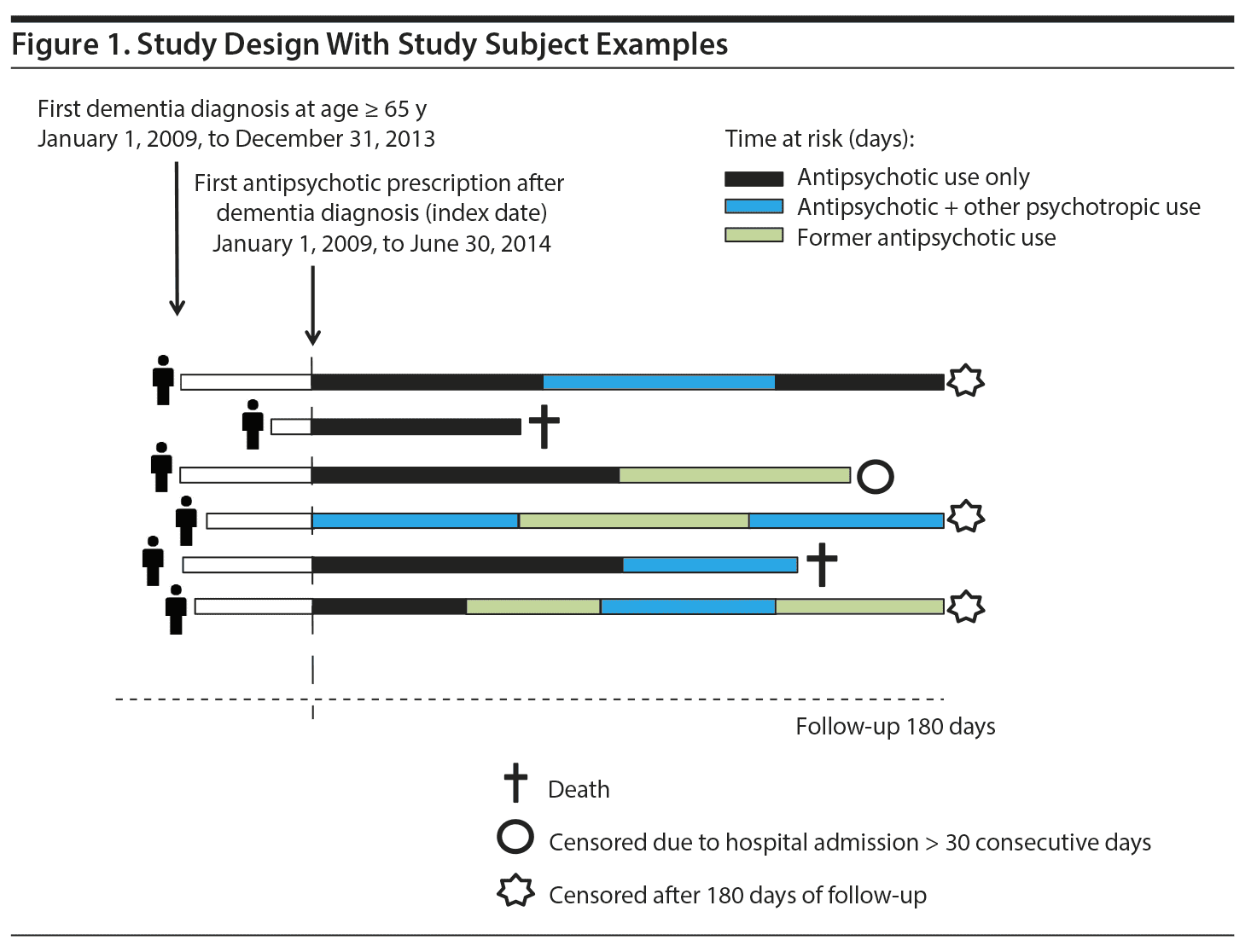
Figure 1 illustrates the study design. Individuals were included in the cohort at the time of the first filled antipsychotic (ATC N05A, excluding lithium: ATC N05AN) prescription (index date) after first dementia diagnosis/antidementia drug prescription between January 1, 2009, and June 30, 2014. The first exclusion criterion was use of antipsychotics 180 days prior to the index date (washout period). This new user design was chosen to avoid bias due to inclusion of prevalent users.14 The second exclusion criterion was admission to hospital for > 30 consecutive days preceding the index date because information about drug use during hospitalization is not available in the national registries.

- Patients with dementia are often treated with antipsychotics in combination with other psychotropics; however, little is known about the safety of this practice.
- This study found an increased mortality when antipsychotics were combined with benzodiazepines but a lower mortality when antipsychotics and antidepressants were combined.
- The findings suggest that combination treatment with antipsychotics and benzodiazepines should be used with caution in patients with dementia.
Exposure
Psychotropic drug exposure was defined as use of antipsychotics, BZDs (N05B, N05C), and antidepressants (N06A); see Supplementary Table 2 for ATC codes. As previously described,9 the duration of each prescription was calculated using the number of defined daily doses (DDDs) per prescription, resulting in an estimated treatment period for every filled prescription. The DDD is defined as the assumed average maintenance dose per day for a drug used for its main indication in adults. However, when used in older adults, the recommended dose is often lower than 1.0 DDD, and research has confirmed this for subgroups of psychotropic drugs.15 For the main analysis, 0.5 DDD was set as the assumed daily intake for antipsychotics, anxiolytics (N05B), and tricyclic antidepressants. For hypnotics/sedatives (N05C) and all other antidepressants (eg, selective serotonin reuptake inhibitors [SSRIs]), the assumed daily intake was set at 1.0 DDD. A grace period of 14 days was added to all prescriptions, meaning that individuals who did not fill a new prescription within 14 days after end of treatment were considered as discontinuing treatment. Individuals with any period of filled prescriptions for other psychotropic drug classes, in addition to an antipsychotic during the 180-day follow-up, were classified as concomitant users. During follow-up, each individual was able to contribute with time at risk to specific treatment groups.
Mortality
The outcome of interest was 180-day, all-cause mortality during current use of antipsychotics in combination with BZDs versus current antipsychotic therapy and during current use of antipsychotics in combination with an antidepressant versus current antipsychotic therapy.
Covariates
Time since dementia diagnosis at index date was used as the marker of dementia severity. Comorbidity (Charlson Comorbidity Index16,17 score) was assessed through registered diagnoses at discharge or at an outpatient visit before the index date. Psychiatric diagnoses registered before the first dementia diagnoses were used to assess psychiatric comorbidity and included as a covariate. Thus, patients with a history of psychiatric illness were not excluded. To assess comorbidity beyond registered diagnoses in hospital registries, we used data on the total number of drugs used other than psychotropic drugs.18 The number of prescription drugs (chemical substance level, ATC level 5) used within 3 months was included as a time-varying covariate. The first count was applied over a 3-month period before the index date. Furthermore, any admission to hospital in the 6 months prior to the index date was assessed. Use of antipsychotics 10 years before the time of dementia diagnosis was assessed as a proxy for conditions such as anxiety and insomnia as well as psychotic depression and primary psychotic disorders treated with antipsychotics. Antidepressant drug use 1 year before the index date was assessed as a proxy for anxiety and depression treated in the primary care sector. The calendar year of the first antipsychotic prescription was included to assess possible changes in health care practice during the study period. Information on nursing home residency registered before the index date was extracted from Statistics Denmark. Please see Supplementary Table 3 for detailed definitions of covariates.
Statistical Methods
Individuals were followed from the date of the first filled antipsychotic prescription (index date) until emigration, admission to hospital for > 30 consecutive days during follow-up (due to missing information on drug use during admission), or completion of 180 days of follow-up, whichever came first. The mortality rates with 95% confidence intervals (CIs) were calculated per 100 person-years. An extended Cox regression model was performed to calculate hazard ratios (HRs) for the 180-day risk of death. The time since index date was used as the underlying time scale. The full model included the following covariates (defined at index date): age, sex, living status (nursing home resident versus home living), calendar year of first antipsychotic prescription, time since diagnosis, Charlson Comorbidity Index score (continuous), psychiatric comorbidity prior to dementia diagnosis (dichotomous), use of antidepressants 1 year before index date, and hospital admission 6 months prior to index date (dichotomous). Both the cumulative number of days with antipsychotic treatment and total number of drugs used were coded as time-dependent variables. The analysis was also stratified for short- versus long-term exposure to combination therapy (> 60 or ≤ 60 days of antipsychotic treatment in combination with other psychotropic drugs).
Sensitivity analyses were performed to test the robustness of the model. An actual consumption of lower or higher doses of psychotropic drugs could change the exposure periods and thus the estimates for risk of death. To test the model, exposure was also calculated based on an assumed intake of 0.25 DDD of antipsychotics per day and 1.0 DDD per day. Some patients may have been treated with combinations of psychotropic medication as part of a palliative regimen. Because end-of-life care often includes psychotropic drugs prescribed for subcutaneous administration, we performed a sensitivity analysis that excluded all injectable drugs.
Data analysis was performed using SAS statistical software, version 9.4 (Cary, North Carolina; SAS Institute, Inc; 2013).
Ethical Approval
The study was approved by the Danish Data Protection Agency (ID: 2007-58-0015/30-0667), Statistics Denmark, and the Danish Health and Medicines Authority (ID: 6-8011-907/1). Danish law did not require ethics committee approval or informed patient consent.
RESULTS
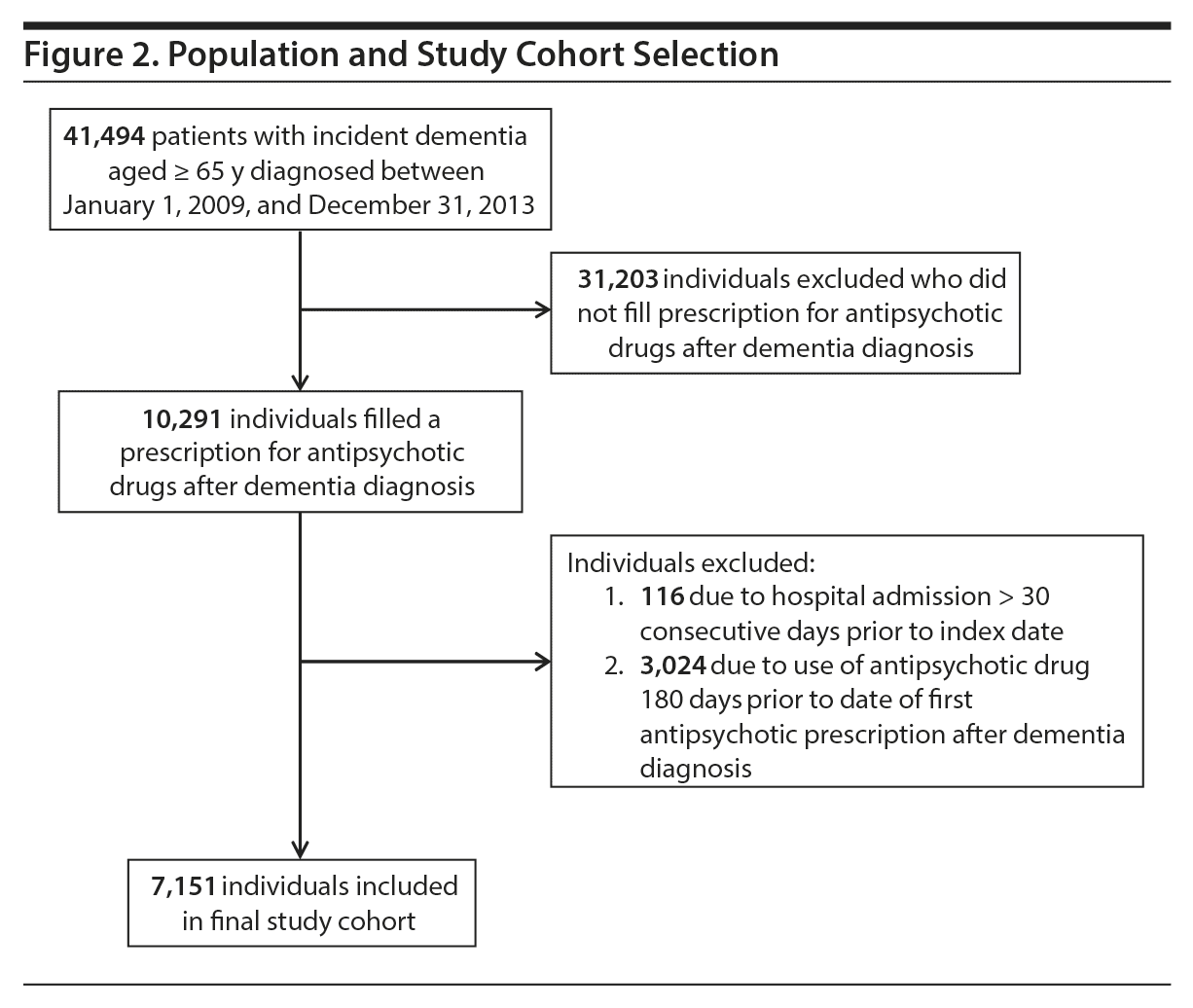
We identified 41,494 people with incident dementia aged 65 years and older between 2009 and 2013, of whom 10,291 (24.8%) initiated antipsychotic drug treatment after a dementia diagnosis and thus were included in the study. We excluded 116 individuals due to hospital admissions lasting more than 30 consecutive days preceding the index date. Another 3,024 individuals were excluded due to antipsychotic drug use 180 days prior to the first antipsychotic prescription after diagnosis.
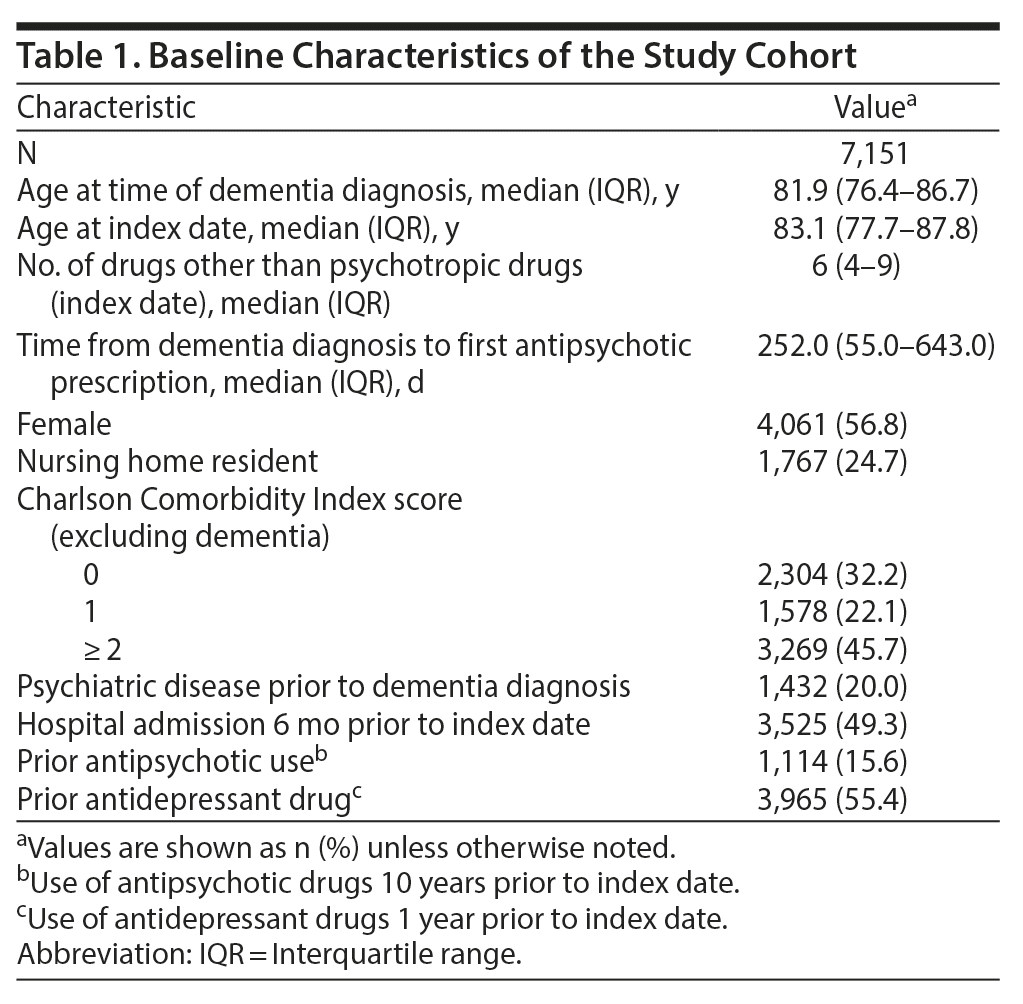
This resulted in 7,151 individuals with complete follow-up information (see Figure 2 for population and Table 1 for baseline characteristics). Almost half of the individuals in the study had been hospitalized within the 6 months prior to antipsychotic treatment. The median time from dementia diagnosis to first antipsychotic prescription was 252 days (interquartile range, 55-643), and 12% (884) filled the first prescription within 7 days after a hospitalization. The individual could have at least 1 period with antipsychotic treatment during follow-up, and on average, each individual had 2 to 3 separate treatment periods during the 180-day follow-up. The total follow-up time was 2,912.6 person-years, with follow-up time during current antipsychotic treatment representing 1,146.0 person-years.
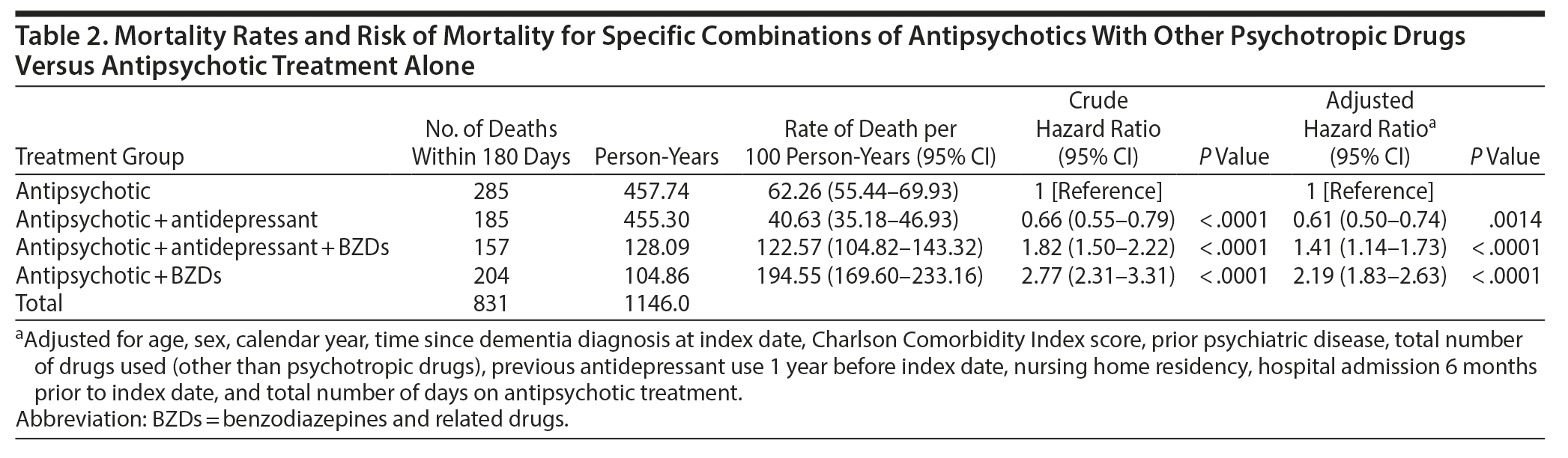
During the initial 180 days after the first antipsychotic prescription, 1,787 people died (25.0%), with 831 (46.5%) of the deaths occurring during current antipsychotic treatment periods. Within the first 60 days after index date, 643 people died during current antipsychotic treatment. The median duration of each treatment period that included at least 1 antipsychotic drug was 13 (interquartile range: 8-27) days. Table 2 presents time at risk and rates of death for specific combinations of antipsychotics with other psychotropic drug classes in specific treatment groups. Use of antipsychotics in combination with BZDs was associated with more than twice the rate of death (HR = 2.19, 95% CI, 1.83-2.63) when compared with antipsychotic treatment alone. On the other hand, treatment periods with the combination of antipsychotic and antidepressant drugs showed a 39% decreased mortality rate. For periods with antipsychotic use in combination with both BZDs and antidepressants, we found a lower mortality rate than for the periods with antipsychotics and BZDs but that was still higher than for antipsychotic treatment alone. Stratifying the analyses for duration of exposure to combination therapy showed that the risk of death was mainly present during the first 60 days of treatment.
When antipsychotic treatment was discontinued, we found an overall unaltered mortality rate (periods of former antipsychotic treatment) when compared with antipsychotic treatment alone (HR = 0.99; 95% CI, 0.86-1.14). However, further drug class analyses showed that an increased mortality remained for patients treated with BZDs during antipsychotic treatment breaks. In contrast, patients treated with antidepressants during antipsychotic treatment breaks had a lower mortality compared with antipsychotic use alone.
Sensitivity analyses of a higher or lower antipsychotic dose per day found that HRs were slightly lower when we assumed 0.25 DDD per day of antipsychotic treatment and slightly higher when we assumed an intake of 1.0 DDD per day. When we excluded injectable drugs, the estimates decreased modestly, yielding an HR of 1.85 (CI, 1.50-2.28) for the combination of antipsychotic and BZDs. However, the overall conclusion from the main analysis did not change.
DISCUSSION
This nationwide study of 7,151 individuals with dementia initiating antipsychotic treatment found an increased risk of death during the 180-day follow-up when antipsychotic drug use was combined with BZDs compared with antipsychotic treatment alone. In contrast, combined prescription of antipsychotics and antidepressants was associated with a decreased risk of death.
Several international studies5,19 have shown a greater risk of death for patients with dementia initiating antipsychotic therapy. However, comedication with other psychotropic drugs was not included in the analyses. Risk comparison between antipsychotic drug classes and other psychotropic drug classes has also been presented, suggesting that not only antipsychotics but also BZDs and antidepressants may yield a risk for patients compared with non-use.4,20 Our findings suggest that certain combinations increase the risk of death among antipsychotic users even further than previously addressed in the literature.
Concomitant prescription of psychotropic drugs has been addressed in patients with schizophrenia. Two observational studies21,22 found an increased mortality among patients with schizophrenia using psychotropic drug combinations, ie, combinations of antipsychotics with benzodiazepines. These findings may reflect possible side effects due to drug interactions. Therefore, it is worrying that we find the same risk profile for patients with dementia during antipsychotic and BZD regimens.
The more than 2-fold increase in mortality associated with combining antipsychotics and BZDs may be explained by a direct effect of BZDs on mortality during treatment. If there is a direct effect, it could be mediated by common side effects associated with BZDs, such as sedation and respiratory depression, potentially leading to pneumonia. These side effects may in some cases be reinforced when BZDs are combined with antipsychotics. Alternatively, the differential mortality in the 2 groups with and without BZD use may be connected to patient-related factors, such as type of neuropsychiatric symptoms or comorbidity, that is, confounding by indication. Antipsychotics and BZDs in combination may be used to treat certain, and maybe more severe, neuropsychiatric symptoms, which in and of themselves are associated with increased mortality. We found that 12% of the study population filled the first antipsychotic prescription shortly after a hospitalization, suggesting that they initiated treatment during their hospital stay. Delirium is a common syndrome in dementia and frequent among hospitalized patients, indicating the importance of doing further analyses on this subgroup.23
The median number of days from the dementia diagnosis to first antipsychotic prescription was 252. This observation suggests that focusing on non-pharmacologic approaches to prevent and manage neuropsychiatric symptoms may be crucial during this period of time. We found the highest risk during the first 60 days after the initial antipsychotic prescription, implying that these patients are particularly vulnerable to adverse events relatively shortly after initiation. This implication suggests that clinicians should consider the risk and benefits before starting antipsychotics and particularly combination therapy of antipsychotics and BZDs. Further, this finding highlights that patients should be monitored closely during the first 60 days, including careful evaluation of treatment effect, side effects, and potential drug interactions. Interestingly, we found a decreased risk of death during combinations of antipsychotic and antidepressant treatment. There are various potential mechanisms to explain this decreased risk. First, antidepressants may possibly have a direct protective effect on mortality. Second, the profile of the patients may differ between those treated with antipsychotics alone and those treated with antipsychotics and antidepressants in combination. There is ongoing off-label use of both antipsychotics and antidepressants. A randomized controlled trial24 compared the effect of an SSRI with that of an antipsychotic in treating neuropsychiatric symptoms in hospitalized patients with dementia. The drugs had similar efficacy; however, side effects occurred more frequently among patients treated with an antipsychotic. Some patients with depressive symptoms and agitation or anxiety are treated with an antidepressant in combination with an antipsychotic drug. Clinicians may also choose to combine antipsychotic treatment with an antidepressant to use a lower dose of the antipsychotic drug, thus yielding a lower risk profile for these patients.
It is not clear whether the increased risk of death with antipsychotic use continues after discontinued treatment. Some studies11,19 on antipsychotics and the risk of death in patients with dementia have used a follow-up of 180 days after initiation of study medication, but did not differentiate between ongoing and discontinued treatment. Our results suggest that there may be an extended risk-of-death window even after discontinuation of antipsychotic treatment or treatment breaks (former antipsychotic use). This risk seems to be modified, however, by the initiation or continuation of BZDs or antidepressant treatment. The dementia antipsychotic withdrawal trial (DART-AD)25 investigated mortality in dementia patients discontinuing antipsychotic monotherapy using a randomized placebo-controlled study design and found a better survival rate among those who discontinued treatment compared with those who continued antipsychotic treatment. Further research should address this issue to improve patient care during and after antipsychotic treatment.
The major strength of the present study is the use of nationwide registry data with complete prescription data and diagnoses from the secondary health care sector. The dementia diagnosis has been validated, showing that 86% of individuals registered with dementia were diagnosed correctly.26 The detailed longitudinal data made it possible to assess real-life prescription patterns in the study design. This is in contrast with many other studies, which have used only information about initiation of antipsychotic treatment in their analyses. Our results are important, as a substantial number of patients change treatment during the 180-day follow-up.
This study has important limitations. The nature of an observational study design implies that the results should be interpreted with caution. This study indicates an association between exposure and outcome, but causality cannot be established. It is possible that residual confounding remains. We did, however, control for important confounders in the multivariate analysis. The registry-based data used in this study enable complete population data but cannot provide information on important confounders such as dementia severity and degree of behavioral symptoms. We addressed this limitation by adding time from dementia diagnosis to antipsychotic prescription. It is not possible to determine, through registry data, the actual amount of drug dosages consumed, but several sensitivity analyses were performed to assess a possible effect on our results. Our study was based on certain assumptions, presenting the risk of misclassification of exposure. We based our assumptions on the Danish prescription guidelines for the elderly and individuals with dementia. Further, antipsychotics are known to carry a high-risk profile for some dementia subtypes, such as Lewy body dementia. The registry data did not allow investigation of drug use and risks among patients with specific dementia diagnoses because the validity of dementia subtypes is low.26 The specific cause of death was not included in this study, as the Danish Register of Causes of Death is not sufficiently valid for such analyses. Finally, the generalizability of the present study is limited to patients with a registered dementia diagnosis and patients who used an antidementia drug.
CONCLUSION
In this nationwide observational study of incident dementia patients on antipsychotic treatment, the combination of antipsychotics with BZDs was associated with an increased risk of death, while the combination of antipsychotics with antidepressants reduced the risk of death. Further research should investigate the background for combining antipsychotic treatment with BZDs and the mechanisms behind the increased mortality. When antipsychotic treatment is judged necessary, there is reason to be cautious when prescribing combinations of antipsychotics and BZDs until further evidence exists. For patients with severe behavioral symptoms, clinical guidelines may be needed to address risk-benefit assessment for therapy with combinations of antipsychotics and other psychotropic drugs.
Submitted: March 13, 2019; accepted December 17, 2019.
Published online: June 2, 2020.
Potential conflicts of interest: The authors have no potential conflicts of interest to declare.
Funding/support: The Danish Dementia Research Centre is supported by grants from the Danish Ministry of Health (file no. 2007-12143-112/59506 and file no. 0901110/34501). Dr Nørgaard was funded by the Research Fund of Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark.
Role of the sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplementary material: See accompanying pages.
REFERENCES
1. Barnes TRE, Banerjee S, Collins N, et al. Antipsychotics in dementia: prevalence and quality of antipsychotic drug prescribing in UK mental health services. Br J Psychiatry. 2012;201(3):221-226. PubMed CrossRef
2. Martinez C, Jones RW, Rietbrock S. Trends in the prevalence of antipsychotic drug use among patients with Alzheimer’s disease and other dementias including those treated with antidementia drugs in the community in the UK: a cohort study. BMJ Open. 2013;3(1):e002080. PubMed CrossRef
3. Nørgaard A, Jensen-Dahm C, Gasse C, et al. Time trends in antipsychotic drug use in patients with dementia: a nationwide study. J Alzheimers Dis. 2016;49(1):211-220. PubMed CrossRef
4. Huybrechts KF, Rothman KJ, Silliman RA, et al. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183(7):E411-E419. PubMed
5. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786. PubMed CrossRef
6. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576, quiz 1623. PubMed CrossRef
7. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943. PubMed CrossRef
8. Hajjar ER, Hanlon JT, Artz MB, et al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother. 2003;1(2):82-89. PubMed CrossRef
9. Nørgaard A, Jensen-Dahm C, Gasse C, et al. Psychotropic polypharmacy in patients with dementia: prevalence and predictors. J Alzheimers Dis. 2017;56(2):707-716. PubMed CrossRef
10.Pasqualetti G, Tognini S, Calsolaro V, et al. Potential drug-drug interactions in Alzheimer patients with behavioral symptoms. Clin Interv Aging. 2015;10:1457-1466. PubMed
11.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632. PubMed
12.Johnell K, Jonasdottir Bergman G, Fastbom J, et al. Psychotropic drugs and the risk of fall injuries, hospitalisations and mortality among older adults. Int J Geriatr Psychiatry. 2017;32(4):414-420. PubMed CrossRef
13.Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 suppl):12-16. PubMed CrossRef
14.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. PubMed CrossRef
15.Rikala M, Hartikainen S, Saastamoinen LK, et al. Measuring psychotropic drug exposures in register-based studies—validity of a dosage assumption of one unit per day in older Finns. Int J Methods Psychiatr Res. 2013;22(2):155-165. PubMed CrossRef
16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. PubMed CrossRef
17.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288-1294. PubMed CrossRef
18.Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154(9):854-864. PubMed CrossRef
19.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341. PubMed CrossRef
20.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445. PubMed CrossRef
21.Baandrup L, Gasse C, Jensen VD, et al. Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study. J Clin Psychiatry. 2010;71(2):103-108. PubMed CrossRef
22.Tiihonen J, Suokas JT, Suvisaari JM, et al. Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry. 2012;69(5):476-483. PubMed CrossRef
23.Ryan DJ, O’ Regan NA, Caoimh Rד, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3(1):e001772. PubMed CrossRef
24.Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry. 2007;15(11):942-952. PubMed CrossRef
25.Ballard C, Hanney ML, Theodoulou M, et al; DART-AD investigators. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8(2):151-157. PubMed CrossRef
26.Phung TKT, Andersen BB, Høgh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24(3):220-228. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!
Save
Cite