Are Selective Serotonin Reuptake Inhibitors Associated With Hepatocellular Carcinoma in Patients With Hepatitis C?

ABSTRACT
Background and Aims: Selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed for patients with chronic hepatitis C virus (HCV) infection. Research suggests that serotonin promotes the development and growth of hepatocellular carcinoma (HCC). We tested the hypothesis whether exposure to SSRIs is associated with an increased risk of HCC in HCV patients.
Method: Patients who entered the United States Veterans Affairs (VA) Hepatitis C Clinical Case Registry in 2000 to 2009 were analyzed. During the 8 years of follow-up, 36,192 patients filled at least 1 SSRI prescription. Cases of HCC were identified by diagnosis codes (ICD-9 155.0). Multivariable Cox regression analyses estimated adjusted HCC hazard ratios (HRs) for SSRI-exposed versus SSRI-unexposed subjects and categories of average SSRI doses.
Results: The annual incidence of HCC in the VA registry cohort of 109,736 patients was 0.5% and significantly greater in the 8% with cirrhosis at baseline (HR = 5.2; 95% CI, 4.7-5.7). There was no evidence for significant interactions between the effect of SSRI-exposure and cirrhosis. Baseline characteristics of the exposed (n = 36,192) and unexposed (n = 73,544) subjects were similar. The median (interquartile range [IQR]) follow-up period after SSRI-exposure began was 44 (20-74) months with 18 (3-49) months between the first and last prescription. The median average SSRI dose during follow-up expressed as a fraction of initial recommended doses for depression was 0.94 (IQR, 0.5 to 1.3). The risk of HCC was not significantly increased after SSRI exposure (HR = 0.96; 95% CI, 0.87-1.05) or with increasing SSRI doses.
Conclusions: Analysis of a large cohort of HCV patients did not support the hypothesis that SSRIs increase the risk of developing HCC.
J Clin Psychiatry 2014;75(10):e1122-e1126
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: November 8, 2013; accepted March 6, 2014 (doi:10.4088/JCP.13m08877).
Corresponding author: Eric Dieperink, MD, Minneapolis VAHCS, One Veterans Drive, Minneapolis, MN 55417 ([email protected]).
Liver cancer is the fifth most common cancer in men worldwide (523,000 cases/year; 7.9% of all cancers) and the seventh most common cancer in women (226,000 cases/year; 6.5% of all cancers) and carries a high mortality rate.1 Primary hepatocellular carcinoma (HCC) is the most common form of liver cancer, and approximately 80% of HCC cases are associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections. Many studies have investigated risk factors for developing HCC. Cirrhosis, HBV and HCV viremia, coinfection with HIV, heavy use of alcohol, metabolic syndrome, and male gender have been positively associated with HCC. However, many individuals with hepatitis B or C infection never develop cirrhosis or HCC; therefore, host genetic factors such as variants of tumor necrosis factor or glutathione S-transferase, other regulatory hormones, and environmental factors have been postulated to play a role in the development of HCC.1-3
Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine neurotransmitter, biochemically derived from tryptophan, and is found in 90% of enterochromaffin cells of the gastrointestinal tract. Lower concentrations are found in platelets and in the central nervous system of animals and humans.4 Serotonin is synthesized by enteric nerves and is metabolized to 5-hydroxyindoleacetic acid (5-HIAA) by the liver and then excreted by the kidneys.5 Functionally, serotonin plays a role in both motor and sensory functioning of the gut and is linked to a variety of emotions in the central nervous system.
There are several lines of evidence supporting a direct and indirect role for serotonin in the development and growth of HCC. In vitro studies find serotonin to be a mitogen promoting hepatocyte regeneration, and thus it may play a direct role in the development of HCC. Lesurtel and colleagues6 describe serotonin as a potential broad promoter of HCC affecting disease incidence and HCC growth. Liang and colleagues7 demonstrated that serotonin promotes the proliferation of serum-deprived HCC cells via up-regulation of FOXO3a, a transcription factor at the interface of crucial cellular processes. Soll et al8 have recently shown that serotonin promotes the growth of human HCC.
Serotonin may also indirectly promote the development of HCC because of its involvement in the development of fibrosis and steatohepatitis. For example, serotonin fosters development of liver fibrosis by stimulating stellate cells.9 Serotonin binds to its receptor 5-HT2B expressed in activated hepatic stellate cells, produces transforming growth factor β1 (TGF-β1) in diseased livers, and suppresses hepatocyte proliferation.10 In a murine model of diet-induced steatohepatitis, researchers showed that serotonin increases reactive oxygen species and lipid peroxides, which leads to inflammation and mitochondrial damage and ultimately hepatocyte damage.11 These preclinical data suggest that serotonin may contribute to the development and/or growth of HCC in humans; however, no data are available from clinical samples.
Determining whether the use of SSRIs is associated with HCC is important as many patients who are at risk for HCC have psychiatric comorbidities and are prescribed antidepressants. Psychiatric and substance use disorders are common among HCV-infected individuals. Studies show that over 85% of veterans with HCV have a history of either a psychiatric or substance use disorder,12 and antidepressants are frequently used to treat these problems. In a study of 783 consecutive patients with HCV seen at the Portland Veterans Affairs Medical Center who filled out psychiatric self-report questionnaires, 63% had depression and 38% were taking an antidepressant, most commonly an SSRI.13 In addition, patients with cirrhosis are often depressed, and many are likely taking antidepressants.14 As SSRIs are frequently prescribed for patients with HCV and cirrhosis and as preclinical data suggest an association between serotonin and HCC, we sought to determine whether there is an association between SSRI use and HCC in patients with chronic hepatitis C.

- Clinicians should be aware that hepatocellular carcinoma commonly develops in patients with chronic hepatitis C, particularly in those with existing cirrhosis.
- Current evidence indicates that selective serotonin reuptake inhibitors do not increase the risk of hepatocellular carcinoma (HCC), and HCC risk should not be a significant concern for prescribing clinicians.
METHOD
Study Cohort
A Department of Veterans Affairs (VA) Clinical Case Registry of veterans with HCV provided a limited set of data files (outpatient prescription records, outpatient visit diagnoses, hospital discharge diagnoses, laboratory tests, and patient demographics) electronically extracted from VA medical records for patients who were registered in 2000 through 2009.15 A randomly assigned patient identification number was included to link records in each file. The institutional review board at the Minneapolis Veterans Affairs Health Care System approved the study protocol.
Beginning in 2000, the study cohort included all patients who had 1 or more consecutive years with at least 1 VA health care encounter and 2 prescription fills, and at least 1 year of baseline data without a prescription for an SSRI or a prior inpatient or outpatient diagnosis of liver cancer (ICD-9 code 155.0, 155.1, or 155.2). We excluded anyone who did not have a laboratory record of a positive ribonucleic acid test for HCV at any time.
Case Finding of HCC
Cases of HCC were identified by a primary hospital discharge ICD-9 diagnosis code of 155.0 (primary malignant neoplasm of the liver), 1 secondary inpatient diagnosis plus an outpatient diagnosis on different days, or 2 outpatient or 2 secondary inpatient diagnoses on different days. After cases were identified by the specific HCC code of 155.0, the earliest date of a 155.0 or a 155.2 (unspecified neoplasm of liver) code was taken as the case date. In the absence of HCC, follow-up was terminated on the date of the subject’s last VA health care encounter or at the end of the period of observation in December 2009. This censoring criterion excludes time after death as we did not have access to vital status records to obtain dates of death.
Definition of SSRI Exposure
Exposure began on the date the first prescription for an antidepressant with SSRI activity (citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, nefazodone, paroxetine, sertraline, trazodone if more than 150 mg/d, and venlafaxine) was dispensed after the 1-year SSRI-free baseline period. Not knowing whether there was a lag period before any SSRI-related HCC would be diagnosed, the exposure period continued until the end of the follow-up period even when SSRI prescriptions ceased. The prescribed daily dose for each SSRI prescription was calculated as the quantity of pills dispensed times the pill strength in milligrams divided by the dispensed days of supply. To standardize the prescribed daily doses of different SSRIs, the calculated prescribed daily dose for each prescription was divided by the recommended initial daily dose of the SSRI for treating depression. Then to calculate the standardized average daily dose, the sum of the standardized prescribed daily doses from all of a subject’s SSRI prescriptions, ie, the cumulative exposure index, was divided by the defined SSRI follow-up period. Thus, any gaps between SSRI prescription supplies during the follow-up period and discontinuation reduced the calculated standardized average daily dose. An SSRI possession ratio was calculated as the sum of all SSRI days of supply dispensed before a subject’s last SSRI prescription, divided by the time elapsed between the subject’s first and last SSRI prescription.
Data Analysis
Continuous data are described as the mean (standard deviation) or median (interquartile range, IQR). Characteristics of the exposed and unexposed groups are summarized without P values because nearly all differences between these large groups including very small ones were statistically significant. Cumulative incidence curves were estimated as 1 minus the Kaplan-Meier survival without HCC curve.
Hazard ratios (HRs, exposed to unexposed) were estimated by Cox regression analysis of times to HCC with incident SSRI exposure (yes or no) as a time-varying variable. Estimates were adjusted via multivariable Cox regression that included several available baseline measures that may be related to the risk of HCC including age, gender, year of entry into the study cohort, a diagnosis of cirrhosis, hepatitis B, human immunodeficiency virus, alcohol abuse, other liver diseases, liver transplant, other liver cancer, other cancer, and obesity. Adjustments were also made for use of a number of medications including ribavirin/interferon, lactulose, chemotherapy, immunosuppressants, testosterone, migraine medications that act via serotonin receptors, tricyclic antidepressants, and trazodone in doses less than 150 mg/d. Not knowing what the SSRI-HCC induction period might be, a second analysis of the dichotomous SSRI-exposure estimated separate HRs for the first 6-month period after exposure began, the next 2-year period, and the period after 2.5 years using a discrete time period Cox regression model.16 A third regression analysis replaced the dichotomous SSRI exposure with categories of the standardized average daily dose using the lowest doses as the reference group.
RESULTS
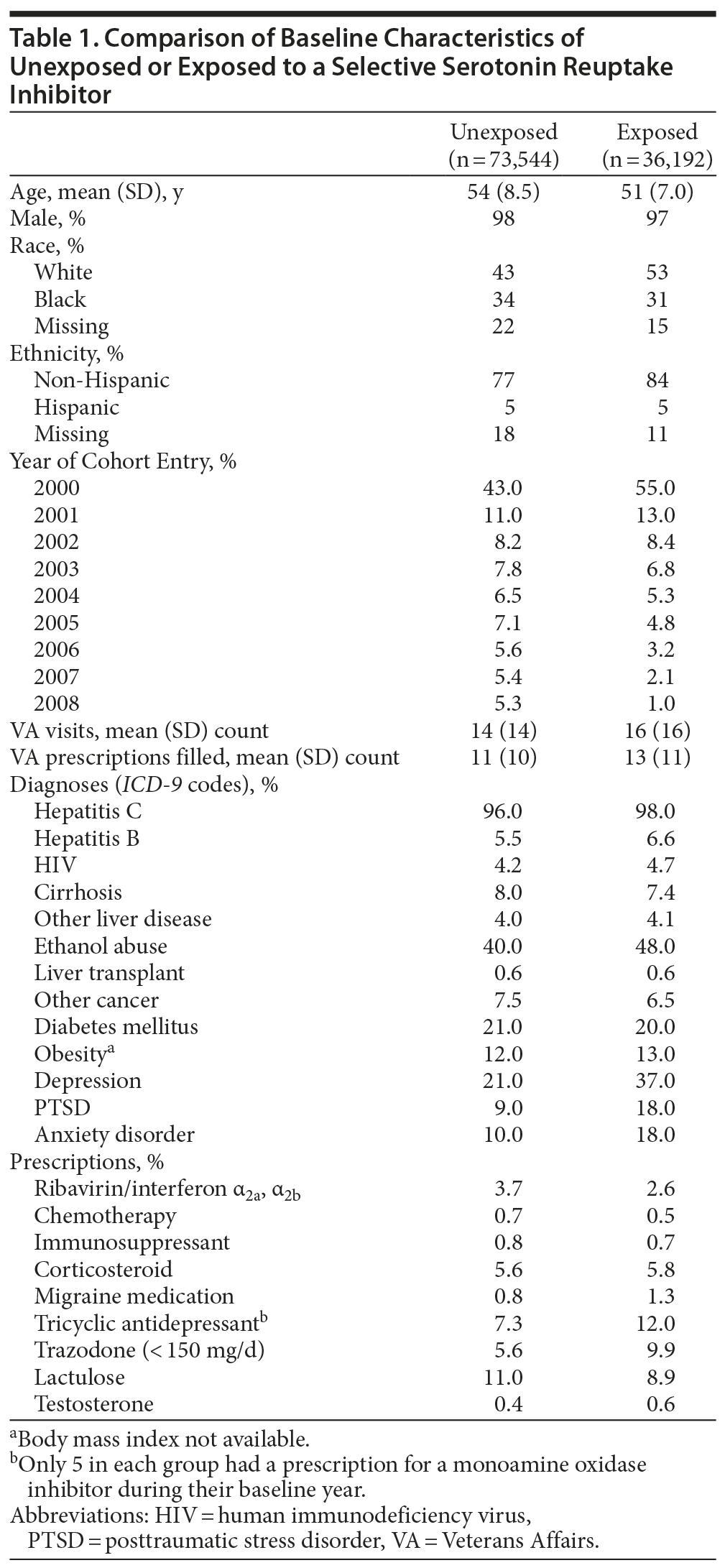
The exposed (n = 36,192) and unexposed (n = 73,544) groups are compared in Table 1. About 8% of each group had a diagnosis of liver cirrhosis—the major known HCC risk factor—recorded during their baseline year. The percentages with other known risk factors for HCC including alcohol abuse were also similar for both groups. A greater percentage of the group exposed to SSRIs had a diagnosis of depression and/or PTSD during the baseline year.
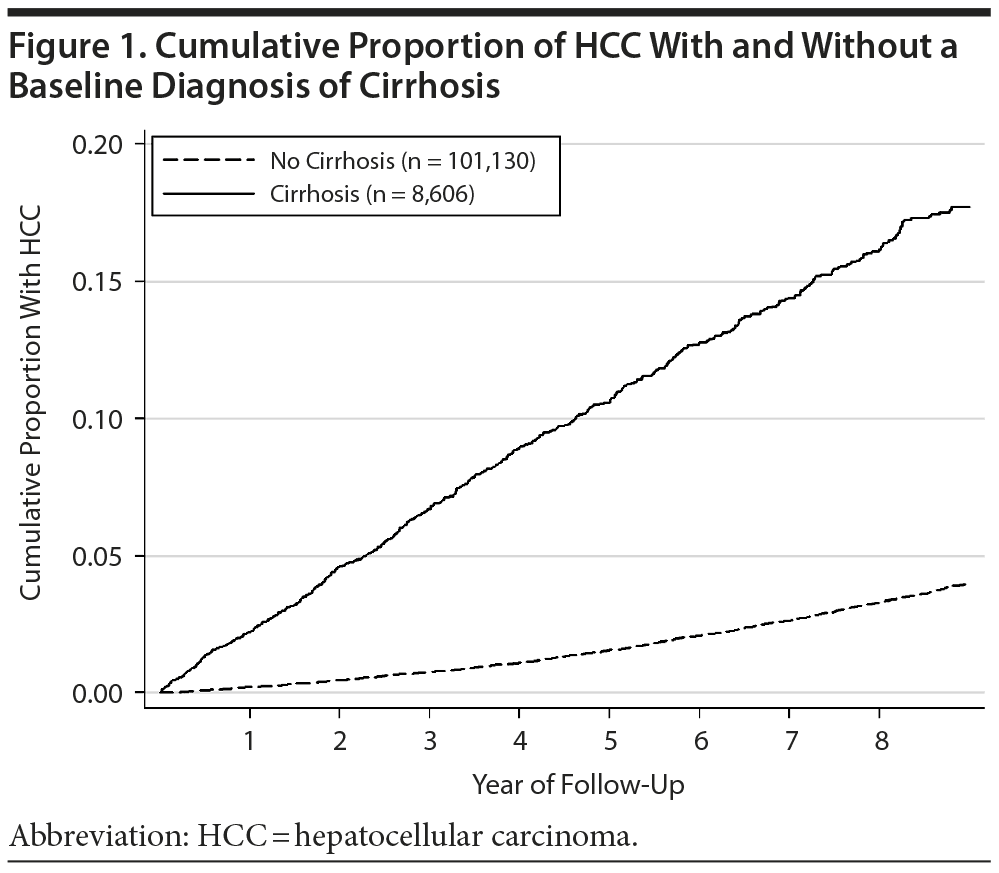
The overall annual incidence of HCC in the entire study cohort was approximately 0.5%. During the 363,605 total years of unexposed follow-up time, there were 1,790 new HCC diagnoses (unadjusted incident rate 0.49/100 person-years), and there were 728 during the 141,213 years of exposed follow-up time (unadjusted incident rate 0.52/100 person-years). The cumulative incidence of HCC in the subgroups with and without a baseline diagnosis of cirrhosis is shown in Figure 1. As expected, the incidence of HCC was substantially greater in the subgroup that had a baseline diagnosis of cirrhosis (HR = 5.2; 95% CI, 4.7-5.7).
The median time from the end of the baseline period to SSRI exposure was 18 (IQR, 7.3-38) months with a median period of follow-up after the subjects’ first SSRI prescription of 44 (IQR, 20-74) months. The median time between subjects’ first and last SSRI prescriptions was 18 (IQR, 3-49) months with a median SSRI possession ratio during this time of 0.80 (IQR, 0.45-1.00). Citalopram (40%), sertraline (22%), fluoxetine (13%), and paroxetine (10%) were the most commonly prescribed SSRIs in the study cohort. The majority were exposed to only 1 (65%) or 2 (22%) different SSRIs.
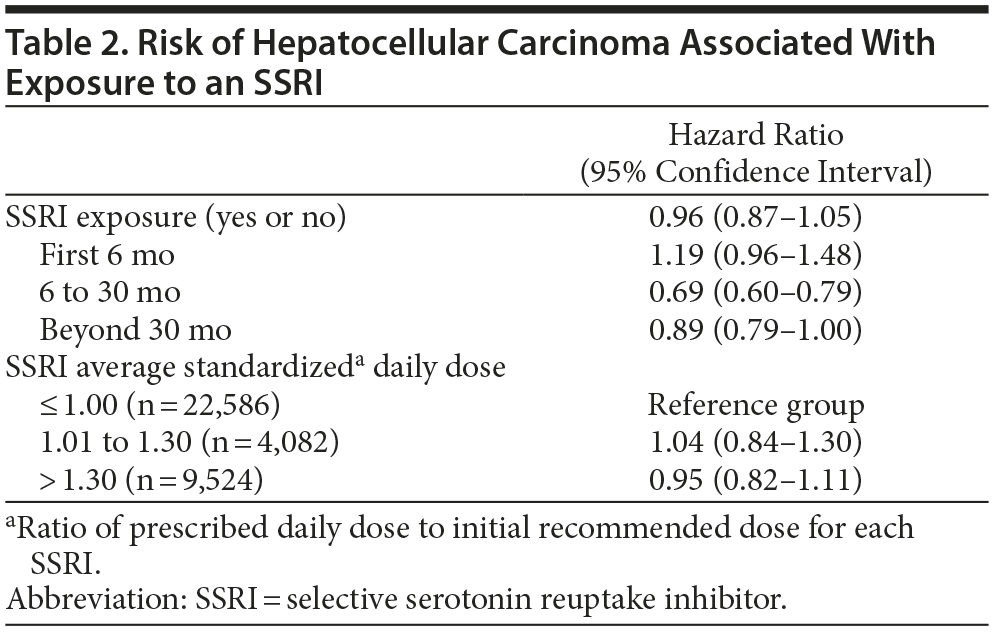
The estimated HRs for HCC associated with SSRI exposure are summarized in Table 2. The 33% (n = 36,192) of the hepatitis C cohort with any amount of exposure to an SSRI during as many as 8 years of follow-up did not have an increased risk of HCC (HR = 0.96; 95% CI, 0.87-1.05). Furthermore, there were no significant increases in risk of HCC found in any of the 3 discrete periods of follow-up including those subjects who were followed for at least 30 months who had the longest median duration of SSRI exposure, 39 (IQR, 12-65) months. The HRs for developing HCC in subjects with SSRI exposure in the groups with or without cirrhosis during the baseline year were nearly identical, 1.00 versus 0.98, respectively. The median standardized average daily SSRI dose during the period after exposure began was 0.94 (IQR, 0.5-1.3). The risk of HCC did not increase in the groups exposed to an average standardized daily dose between 1.0 and 1.3 or more than 1.3 compared to the group with an average standardized daily dose of 1.0 or less, ie, a dose equal to or less than the initial recommended SSRI dose for treating depression.
DISCUSSION
Our study did not find an increased risk of HCC in patients with hepatitis C who were treated with SSRI antidepressants compared to patients who were not. Although the level of exposure in many patients may not have been sufficient to cause HCC, there was no association between the highest observed average daily doses and the incidence of HCC. However, this study cannot exclude the possibility of an increased risk of HCC with more intense exposure to SSRIs.
The duration of follow-up after SSRI exposure began may have been too short to cover the period of possible tumor induction and clinical diagnosis. Most patients with hepatitis C have advanced fibrosis or cirrhosis for decades before HCC occurs. Further study is necessary to determine whether more prolonged SSRI exposure contributes to HCC, especially in patients who have a greater risk due to cirrhosis.
We are unaware of any other studies in clinical populations investigating the potential impact of SSRIs on the promotion of HCC. In contrast, there are some clinical studies in other cancers, some showing an increased risk of cancer with SSRIs and others showing a protective effect. For example, previous case-control studies of SSRIs and breast cancer found an increase in risk,17 whereas in colorectal cancer, there were decreased odds of developing cancer in those who regularly took an SSRI.18 Later studies and a meta-analysis found no association between serotonin and breast cancer.19
Hepatocellular carcinoma is one of the few cancers increasing in frequency in the United States. This is mainly due to the large number of patients with hepatitis C who have had the illness for more than 2-3 decades and are now developing cirrhosis of the liver, which puts them at risk for HCC at a rate of 2%-5% per year.2 Preliminary preclinical evidence implicated serotonin as a possible factor directly or indirectly influencing the development and growth of HCC. Friedman and colleagues20 screened a multitude of pharmaceuticals including SSRIs for possible carcinogenic effects in a large nested case-control study and followed subjects for more than 12 years. They reported that most antidepressants showed an association with some form of cancer and concluded that nortriptyline and related tricyclic drugs would merit further investigation with regard to their possible carcinogenic effect. Fluoxetine and paroxetine were found to have a positive association with testicular cancer, but no association to liver cancer was reported.
As expected, the incidence of HCC was substantially greater in patients with cirrhosis at baseline, which shows that our data are robust and confirms that cirrhosis is an important risk factor for the development of HCC.1,2 However, the presence of cirrhosis in a small percentage of this patient cohort with hepatitis C did not modify the finding of a lack of an association between the exposure to SSRIs and HCC. Additional studies of higher risk patients with cirrhosis are needed.
Our study has several limitations. As all patients in the study were veterans and most were male, the results may not generalize to other populations. In addition, VA medical records may not completely reflect the care veterans receive as some are using services outside the VA health care system. The 8% rate of cirrhosis in this cohort of patients chronically infected with hepatitis C is somewhat lower than might be expected. Davila et al21 reported a cirrhosis prevalence of 10% using the same database (VA Hepatitis C Clinical Case Registry). The difference between the studies is most likely explained by our more restrictive inclusion criteria based on prior SSRI prescriptions. In addition, we used only 1 year of baseline data to identify the presence of cirrhosis assuming any under-coding or mis-coding would be similar in the exposed and unexposed groups. This may have limited our ability to test for interaction between the effects of cirrhosis and SSRI exposure. Longer follow-up as well as collecting data such as fibrosis scores and surrogate markers of cirrhosis would quite likely have increased capture of cirrhosis; however, these data are unavailable in the data set. Given the low prevalence of cirrhosis, the incidence of HCC in the study cohort is consistent with published rates,21 and thus the data regarding HCC are very likely reasonably accurate. Finally, some patients quite likely developed cirrhosis during the study period, but we were unable to analyze the influence of this transition on outcome. However, as there was no difference in the development of HCC between groups, it is likely that the transition did not influence the primary outcome.
To our knowledge, this is the only study to date investigating the possible association between the use of SSRI antidepressants and the risk of HCC in patients with chronic hepatitis C. Given the extensive use and potential benefits of SSRIs in patients with hepatitis C and concomitant psychiatric disorders, our study suggests that an increased risk of HCC should not be a major concern for patients and clinicians. However, further studies are important given the high rate of antidepressant use in this population, concern over preclinical studies suggesting a negative impact of serotonin on HCC, and the limitations of this study.
Drug names: citalopram (Celexa and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), nortriptyline (Pamelor, Aventyl, and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), trazodone (Oleptro and others), venlafaxine (Effexor and others).
Author affiliations: Departments of Medicine (Drs Pocha, Knott, and Rector) and Psychiatry (Dr Dieperink), Veterans Health System Minneapolis and the University of Minnesota Medical School.
Potential conflicts of interest: None reported.
Funding/support: Supported by the research service of the Veterans Health System and the Center for Chronic Disease Outcomes Research that was supported by VA Health Service Research & Development grant HFP-98-011.
Role of the sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclaimer: The views expressed herein are the authors and do not necessarily represent the Veterans Health Administration.
Previous presentation: International Liver Congress of the European Association for the Study of Liver Diseases; April 24-28, 2013; Amsterdam, the Netherlands.
Additional information: The Clinical Case Registry of Veterans with HCV is owned by the Department of Veterans Affairs (VA) and is an extract of the VA electronic medical record that contains data regarding the medical care of HCV-infected veterans seen at all VA medical facilities in the United States. The de-identified data that were extracted for this analysis reside at the Minneapolis VA Health Care System. Access to these data files is restricted to VA employees who have approved protocols.
REFERENCES
1. Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44(suppl 19):96-101. PubMed doi:10.1007/s00535-008-2258-6
2. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1. PubMed doi:10.1053/j.gastro.2011.12.061
3. Zhang YJ, Chen Y, Ahsan H, et al. Silencing of glutathione S-transferase P1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005;221(2):135-143. PubMed doi:10.1016/j.canlet.2004.08.028
4. Sulaiman P, Joseph B, Kaimal SB, et al. Decreased hepatic 5-HT1A receptors during liver regeneration and neoplasia in rats. Neurochem Res. 2008;33(3):444-449. PubMed doi:10.1007/s11064-007-9452-4
5. Lesurtel M, Soll C, Graf R, et al. Role of serotonin in the hepato-gastroIntestinal tract: an old molecule for new perspectives. Cell Mol Life Sci. 2008;65(6):940-952. PubMed doi:10.1007/s00018-007-7377-3
6. Lesurtel M, Soll C, Humar B, et al. Serotonin: a double-edged sword for the liver? Surgeon. 2012;10(2):107-113. PubMed doi:10.1016/j.surge.2011.11.002
7. Liang C, Chen W, Zhi X, et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12(1):14. PubMed doi:10.1186/1476-4598-12-14
8. Soll C, Jang JH, Riener MO, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51(4):1244-1254. PubMed doi:10.1002/hep.23441
9. Ruddell RG, Oakley F, Hussain Z, et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am J Pathol. 2006;169(3):861-876. PubMed doi:10.2353/ajpath.2006.050767
10. Ebrahimkhani MR, Oakley F, Murphy LB, et al. Stimulating healthy tissue regeneration by targeting the 5-HTâ‚‚B receptor in chronic liver disease. Nat Med. 2011;17(12):1668-1673. PubMed doi:10.1038/nm.2490
11. Nocito A, Dahm F, Jochum W, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133(2):608-618. PubMed doi:10.1053/j.gastro.2007.05.019
12. el-Serag HB, Kunik M, Richardson P, et al. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476-482. PubMed doi:10.1053/gast.2002.34750
13. Nelligan JA, Loftis JM, Matthews AM, et al. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: results from a retrospective chart review. J Clin Psychiatry. 2008;69(5):810-816. PubMed doi:10.4088/JCP.v69n0514
14. Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593-600. PubMed doi:10.1016/j.dld.2005.01.020
15. Veterans Health Administration, Department of Veterans Affairs. Directive 2011-026: Clinical case registry (ccr) software: maintenance and clinical staff Support. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2420. Updated June 23, 2011. Accessed April 2, 2014.
16. Singer JD, Willett JB. Extending the Cox Regression Model. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford Elsevier Press; 2003:543-606. doi:10.1093/acprof:oso/9780195152968.003.0015
17. Fulton-Kehoe D, Rossing MA, Rutter C, et al. Use of antidepressant medications in relation to the incidence of breast cancer. Br J Cancer. 2006;94(7):1071-1078. PubMed doi:10.1038/sj.bjc.6603017
18. Coogan PF, Strom BL, Rosenberg L. Antidepressant use and colorectal cancer risk. Pharmacoepidemiol Drug Saf. 2009;18(11):1111-1114. PubMed doi:10.1002/pds.1808
19. Eom CS, Park SM, Cho KH. Use of antidepressants and the risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;136(3):635-645. PubMed doi:10.1007/s10549-012-2307-y
20. Friedman GD, Udaltsova N, Chan J, et al. Screening pharmaceuticals for possible carcinogenic effects: initial positive results for drugs not previously screened. Cancer Causes Control. 2009;20(10):1821-1835. PubMed doi:10.1007/s10552-009-9375-2
21. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93. PubMed doi:10.7326/0003-4819-154-2-201101180-00006