Objective: To evaluate efficacy, safety, and tolerability of long-acting injectable antipsychotic aripiprazole once-monthly 400 mg (AOM 400) as maintenance treatment for bipolar I disorder (BP-I).
Methods: In a double-blind, placebo-controlled, 52-week randomized withdrawal study conducted from August 2012 to April 2016, patients with a DSM-IV-TR diagnosis of BP-I currently experiencing a manic episode were stabilized sequentially on oral aripiprazole and AOM 400 and then randomized to AOM 400 or placebo. The primary end point was time from randomization to recurrence of any mood episode. Other end points included proportion of patients with recurrence of any mood episode and recurrence by mood episode type.
Results: Of 266 randomized patients, 64 (48.1%) of 133 in the AOM 400 group and 38 (28.6%) of 133 in the placebo group completed the study. AOM 400 significantly delayed the time to recurrence of any mood episode compared with placebo (hazard ratio: 0.45; 95% CI, 0.30 to 0.68; P < .0001). Significantly fewer patients (P < .0001) experienced recurrence of any mood episode with AOM 400 (35/132; 26.5%) compared with placebo (68/133; 51.1%), with the effects observed predominantly on manic episodes (P < .0001). Patients were not depressed at study entry, and between-group differences in depressive episodes were not significant (P < .864). The treatment-emergent adverse events (incidence > 5%) that were reported at higher rates with AOM 400 than placebo were weight increase, akathisia, insomnia, and anxiety.
Conclusions: AOM 400 delayed the time to and reduced the rate of recurrence of mood episodes and was generally safe and well tolerated. These findings support the use of AOM 400 for maintenance treatment of BP-I.
Trial Registration: ClinicalTrials.gov identifier: NCT01567527
Efficacy and Safety of Aripiprazole Once-Monthly in the Maintenance Treatment of Bipolar I Disorder:
A Double-Blind, Placebo-Controlled, 52-Week Randomized Withdrawal Study

ABSTRACT
Objective: To evaluate efficacy, safety, and tolerability of long-acting injectable antipsychotic aripiprazole once-monthly 400 mg (AOM 400) as maintenance treatment for bipolar I disorder (BP-I).
Methods: In a double-blind, placebo-controlled, 52-week randomized withdrawal study conducted from August 2012 to April 2016, patients with a DSM-IV-TR diagnosis of BP-I currently experiencing a manic episode were stabilized sequentially on oral aripiprazole and AOM 400 and then randomized to AOM 400 or placebo. The primary end point was time from randomization to recurrence of any mood episode. Other end points included proportion of patients with recurrence of any mood episode and recurrence by mood episode type.
Results: Of 266 randomized patients, 64 (48.1%) of 133 in the AOM 400 group and 38 (28.6%) of 133 in the placebo group completed the study. AOM 400 significantly delayed the time to recurrence of any mood episode compared with placebo (hazard ratio: 0.45; 95% CI, 0.30 to 0.68; P < .0001). Significantly fewer patients (P < .0001) experienced recurrence of any mood episode with AOM 400 (35/132; 26.5%) compared with placebo (68/133; 51.1%), with the effects observed predominantly on manic episodes (P < .0001). Patients were not depressed at study entry, and between-group differences in depressive episodes were not significant (P < .864). The treatment-emergent adverse events (incidence > 5%) that were reported at higher rates with AOM 400 than placebo were weight increase, akathisia, insomnia, and anxiety.
Conclusions: AOM 400 delayed the time to and reduced the rate of recurrence of mood episodes and was generally safe and well tolerated. These findings support the use of AOM 400 for maintenance treatment of BP-I.
Trial Registration: ClinicalTrials.gov identifier: NCT01567527
J Clin Psychiatry 2017;78(3):324-331
https://doi.org/10.4088/JCP.16m11201
© Copyright 2017 Physicians Postgraduate Press, Inc.
aUniversity Hospitals Case Medical Center, Case Western Reserve School of Medicine, Cleveland, Ohio
bOtsuka Pharmaceutical Development & Commercialization, Inc, Princeton, New Jersey
cH. Lundbeck A/S, Valby, Denmark
*Corresponding author: Joseph R. Calabrese, MD, University Hospitals Case Medical Center, 10524 Euclid Ave, Cleveland, OH 44106 ([email protected]).
Bipolar I disorder (BP-I) is characterized by manic, depressive, and mixed mood episodes interspersed with periods of remission.1 Long-term pharmacologic treatment is required to prevent relapse of symptoms.1-3 Patients often experience persistent symptoms leading to social and functional impairment.4-7 Episodes of mania are recurrent and commonly associated with unfavorable outcomes, including poorer cognitive performance and greater number of hospitalizations.8 The chronic nature of the disorder and the negative consequences of unremitted or recurrent symptoms underscore the need for effective long-term treatment.
The highly recurrent nature of bipolar disorder can be very difficult to manage. Compounds that possess prophylactic efficacy during maintenance therapy are the mainstay treatment for BP-I; however, their use is limited by side effect burden and the need for therapeutic blood monitoring.1,9,10 Multiple studies support the use of atypical antipsychotics in BP-I.1,11 The efficacy and safety of oral aripiprazole, as monotherapy and adjunctive therapy, in both acute and maintenance treatment of BP-I, have been established in randomized controlled studies.2,12-16
Poor adherence to treatment is a significant problem in BP-I, resulting in suboptimal outcomes.5,17-19 Nonadherence rates can be as high as 79%.20-22 Long-acting injectable (LAI) antipsychotics have the potential to improve medication adherence.23 Currently, risperidone injection every 2 weeks is the only LAI atypical antipsychotic approved by the US Food and Drug Administration for the maintenance treatment of BP-I.24 In a randomized study,25 risperidone LAI (RLAI) significantly delayed the time to recurrence of mood episodes versus placebo in patients with BP-I. Aripiprazole once-monthly 400 mg (AOM 400), an LAI formulation of aripiprazole, has demonstrated efficacy in schizophrenia in multiple randomized clinical studies.26-29 Findings from these studies, the established efficacy of oral aripiprazole in BP-I, and the potential for improved adherence formed the basis for evaluating AOM 400 as maintenance treatment of BP-I.
The primary objective of this study was to evaluate the efficacy of AOM 400 in preventing recurrence of any mood episode in patients with BP-I. Safety and tolerability of AOM 400 were also evaluated.
METHODS
Study Design
This multicenter, double-blind, placebo-controlled, randomized withdrawal study (ClinicalTrials.gov: NCT01567527) assessed time to recurrence of any mood episode in patients with BP-I who had maintained stability on AOM 400 for ≥ 8 consecutive weeks, having also received ≥ 3 injections. The trial was conducted from August 2012 to April 2016 at 103 sites in 7 countries (Canada, Japan, Republic of Korea, Poland, Romania, Taiwan, and the United States) in compliance with the International Conference of Harmonization and Good Clinical Practice consolidated guideline.30 The protocol was approved by an institutional review board or independent ethics committee, as appropriate. Informed consent was obtained from patients or their guardians or legal representatives.
The trial consisted of 4 treatment phases (Supplementary eFigure 1). During the first 2 phases (conversion to oral aripiprazole, 4-6 weeks; and oral aripiprazole stabilization, 2-8 weeks), patients were converted to oral aripiprazole monotherapy, if not currently receiving it (target dose, 15-30 mg/day), and then assessed for stability. During the subsequent single-blind AOM 400 stabilization phase (12-28 weeks), patients received AOM 400 injections every 4 weeks. Patients who met a priori stability criteria for ≥ 8 consecutive weeks were randomized at a 1:1 ratio to 52 weeks of double-blind treatment with AOM 400 or placebo in the maintenance phase (randomization stratified by region: United States and Canada, Europe, Japan, and other Asian countries). At the completion of 52 weeks, patients were invited to enroll in an open-label extension study.
Stability was defined as meeting all of the following criteria: outpatient status, Young Mania Rating Scale (YMRS)31 total score ≤ 12, Montgomery-Asberg Depression Rating Scale (MADRS)32 total score ≤ 12, and no active suicidality (defined as score ≥ 4 on MADRS item 10 or “yes” on question 4 or 5 of the Columbia Suicide Severity Rating Scale [C-SSRS]).33 Patients were randomly assigned via an interactive voice or web response system. In both AOM 400 stabilization and randomized phases, a single decrease to 300 mg was permitted for tolerability, as was a single return to 400 mg, if required. To maintain the blind, study drug was administered by unblinded site personnel, independent from those conducting the trial. Patient evaluations occurred every 2 weeks for the first 28 weeks and every 4 weeks thereafter.

- Long-acting injectable antipsychotics have the potential to improve medication adherence, a challenge in bipolar I disorder (BP-I).
- The established efficacy and safety of oral aripiprazole in BP-I led to the evaluation of aripiprazole once-monthly 400 mg (AOM 400) in BP-I.
- In a 52-week randomized controlled trial, AOM 400 significantly delayed the time to recurrence of mood episodes and was generally safe and well tolerated as maintenance treatment for BP-I.
Patients
Study participants were men or women, inpatient or outpatient at entry, aged 18 to 65 years, with a diagnosis of BP-I according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)34 criteria and confirmed by the Mini-International Neuropsychiatric Interview.35 Eligible patients had experienced ≥ 1 previous manic or mixed episode with manic symptoms of sufficient severity to require hospitalization, treatment with a mood stabilizer, or treatment with an antipsychotic agent. Patients were required to be currently experiencing a manic episode (per DSM-IV-TR criteria) with YMRS total score ≥ 20. Those who experienced ≥ 9 episodes in the past year (rapid cycling) were excluded.
The use of cytochrome P450 (CYP) 3A4 or CYP2D6 inhibitors or CYP3A4 inducers was not allowed during the study. Benzodiazepine use was allowed at ≤ 2 mg/day lorazepam equivalent, but not within 8 hours of a rating scale assessment. Anticholinergics were allowed at ≤ 4 mg/day benztropine or equivalent but not within 12 hours of a rating scale assessment.
Efficacy Endpoints
The primary efficacy end point was time from randomization to recurrence of any mood episode, which was defined as meeting any of the following criteria: hospitalization for any mood episode; YMRS total score ≥ 15; MADRS total score ≥ 15; Clinical Global Impressions for Bipolar Disorder-Severity (CGI-BP-S) scale36 overall score > 4; serious adverse event (AE) of worsening BP-I; discontinuation due to lack of efficacy or an AE of worsening of BP-I; clinical worsening with the need for addition of a mood stabilizer, antidepressant treatment, antipsychotic medication, or increase in benzodiazepine dose above the highest permitted dose; or active suicidality (score ≥ 4 on MADRS item 10 or “yes” answer to question 4 or 5 on the C-SSRS).
The key secondary efficacy end point was the proportion of patients meeting criteria for recurrence of any mood episode. Additional end points included change from randomization in YMRS and MADRS total scores. For the randomized phase, baseline was defined as the last visit in the AOM 400 stabilization phase.
Safety
All AEs were coded based on the Medical Dictionary for Regulatory Activities. A treatment-emergent AE (TEAE) was defined as an AE that occurred after the start of treatment or an AE that continued from baseline of one phase and became serious; was drug related; or resulted in death, discontinuation, interruption, or reduction of dosage during the subsequent phase. Suicidality was assessed using C-SSRS. Injection site pain was evaluated using the patient-reported Visual Analog Scale and investigator ratings. Extrapyramidal symptoms were reported as the change from baseline in Simpson-Angus Scale (SAS37; used in countries other than Japan), Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS38; used in Japan), Abnormal Involuntary Movement Scale (AIMS),39 and Barnes Akathisia Rating Scale (BARS)40 scores. Standard safety measurements included clinical laboratory tests, vital signs, electrocardiogram (ECG), body weight, and physical examination. In addition, serum prolactin concentrations were monitored. Central laboratories designated by the sponsor were used for all laboratory tests and ECG review.
Statistical Analyses
Sample size estimates assumed that ≥ 55% of placebo-treated patients and ≤ 30% of AOM 400-treated patients would experience a recurrence. With an estimated attrition rate of 25%, 1:1 randomization of 238 subjects would provide at least 90% power to detect a 25% between-group difference in the proportion of patients having a recurrence using Fisher exact test at .049.
The safety sample included all patients who received ≥ 1 injection in that phase, and the efficacy sample included those who received ≥ 1 injection and had ≥ 1 postbaseline efficacy assessment in that phase.
Time to recurrence and time to discontinuation were plotted as Kaplan-Meier curves and analyzed using the log-rank test, and hazard ratios (HRs; AOM 400 vs placebo) and their 95% CIs were estimated using a Cox proportional hazards model with treatment as term. To assess the sensitivity of the primary efficacy analysis due to potentially informative withdrawal (missing not at random), worst-case analysis, Kaplan-Meier multiple imputations, and worst-comparison analysis using multiple imputation were performed (see Supplementary eFigure 2 for details). The proportion of patients with recurrence of any mood episode was analyzed using Fisher exact test. Changes from baseline in YMRS and MADRS scores were analyzed using mixed model repeated measures (MMRM), with treatment, region, trial week, and treatment-by-week interaction as terms, as well as the covariates of baseline-score-by-week interaction.
All statistical analyses used Statistical Analysis Software, version 9.4 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Patients
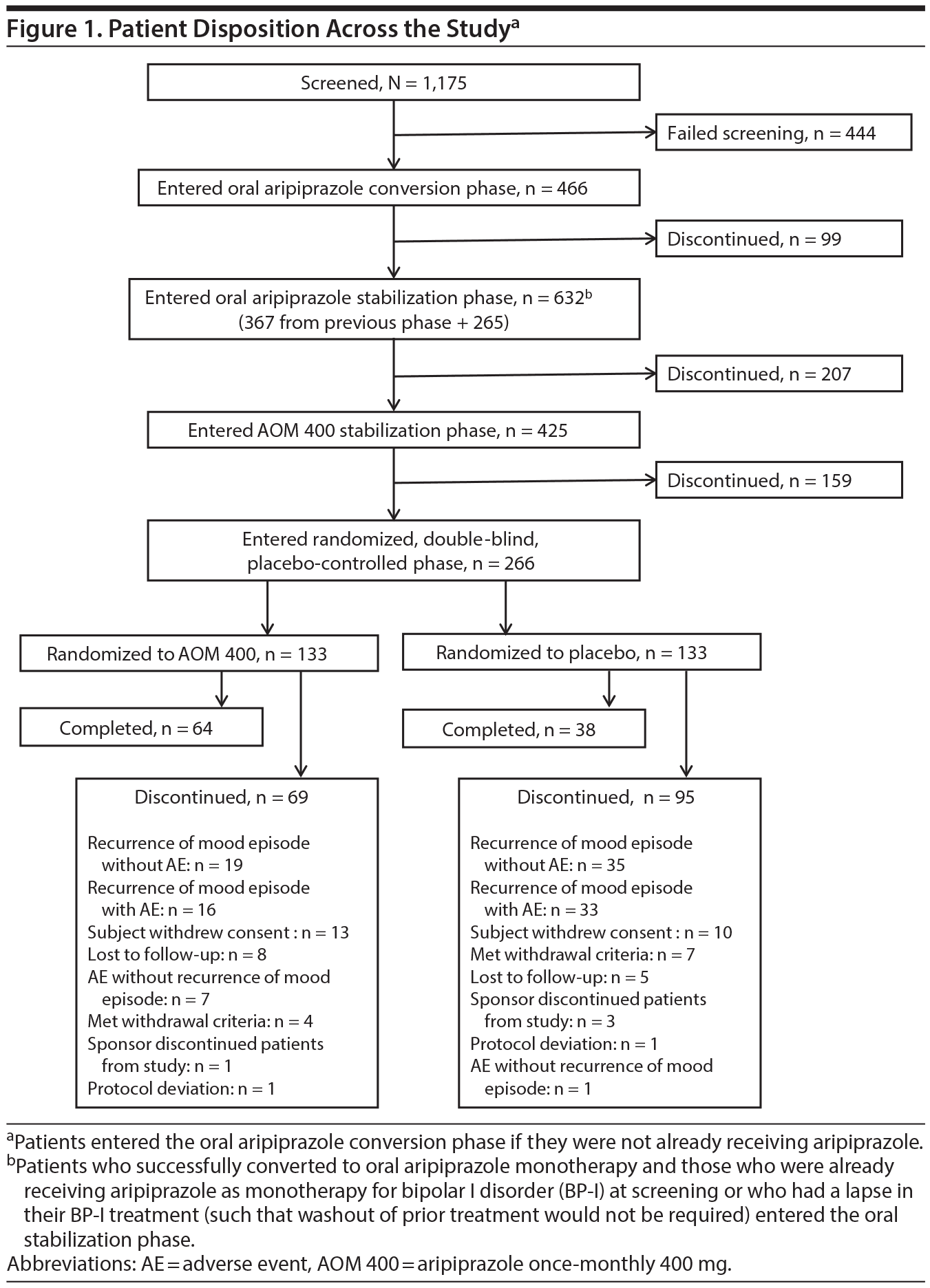
Of 1,175 patients screened, 731 met eligibility criteria. Figure 1 details the patient disposition during the 4 treatment phases. A total of 266 patients entered the double-blind withdrawal phase and were randomly assigned to AOM 400 (n = 133) or placebo (n = 133). Of these, 102 (38.3%) completed the study, 48.1% in the AOM 400 group and 28.6% in the placebo group. The most common reasons for discontinuation were recurrence of any mood episode without AE (AOM 400, 19 [14.3%]; placebo, 35 [26.3%]) and recurrence of any mood episode with AE (AOM 400, 16 [12.0%]; placebo, 33 [24.8%]).
Demographic and disease characteristics for AOM 400 and placebo groups are reported in Supplementary eTable 1.
Efficacy
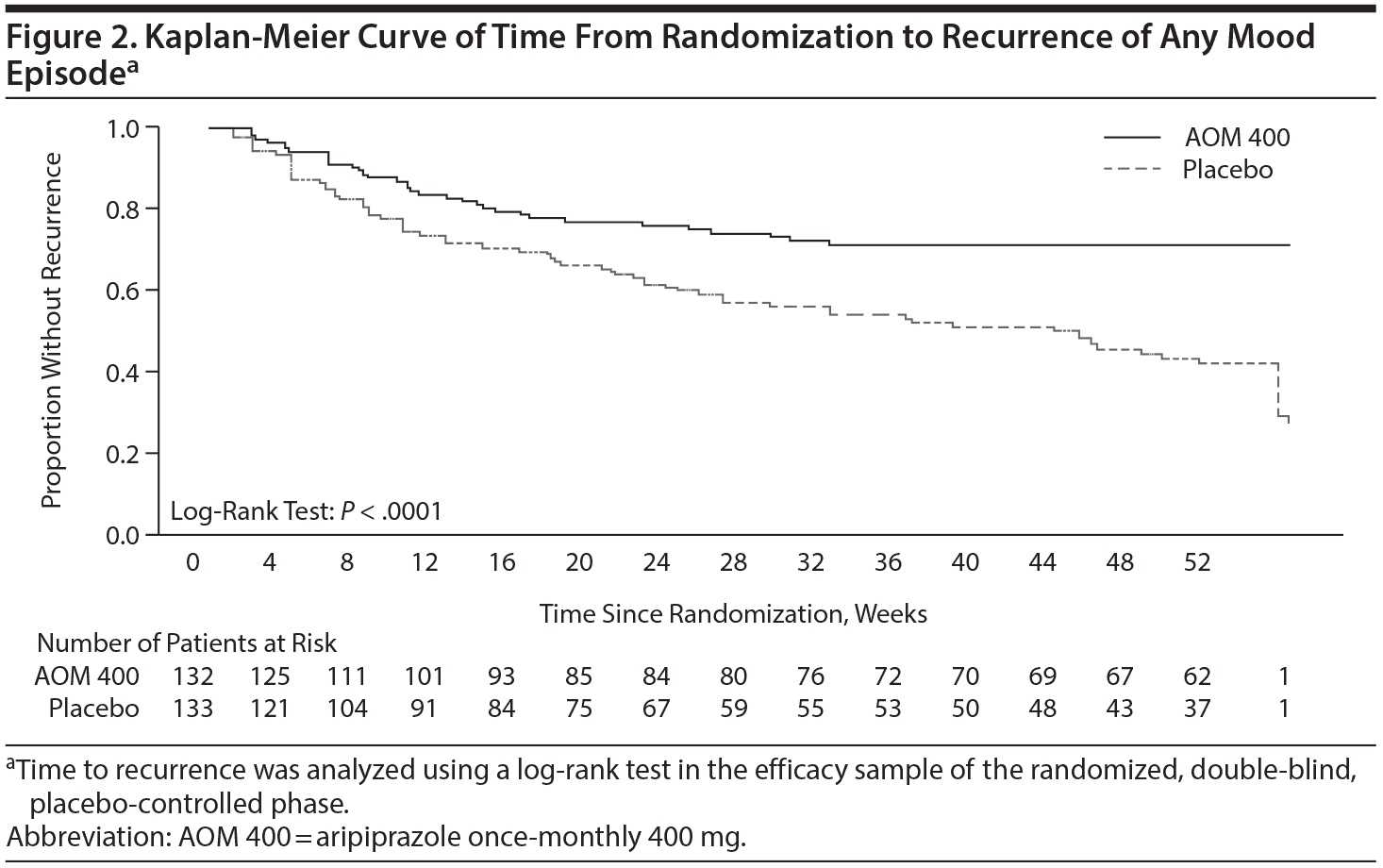
Time to recurrence of any mood episode was significantly delayed with AOM 400 compared with placebo (log-rank test P < .0001; Figure 2). The risk of recurrence of any mood episode over 1 year was reduced by approximately half with AOM 400 compared with placebo (HR = 0.45; 95% CI, 0.30 to 0.68). Sensitivity analyses confirmed the robustness of the primary efficacy end point result (Supplementary eFigure 2).
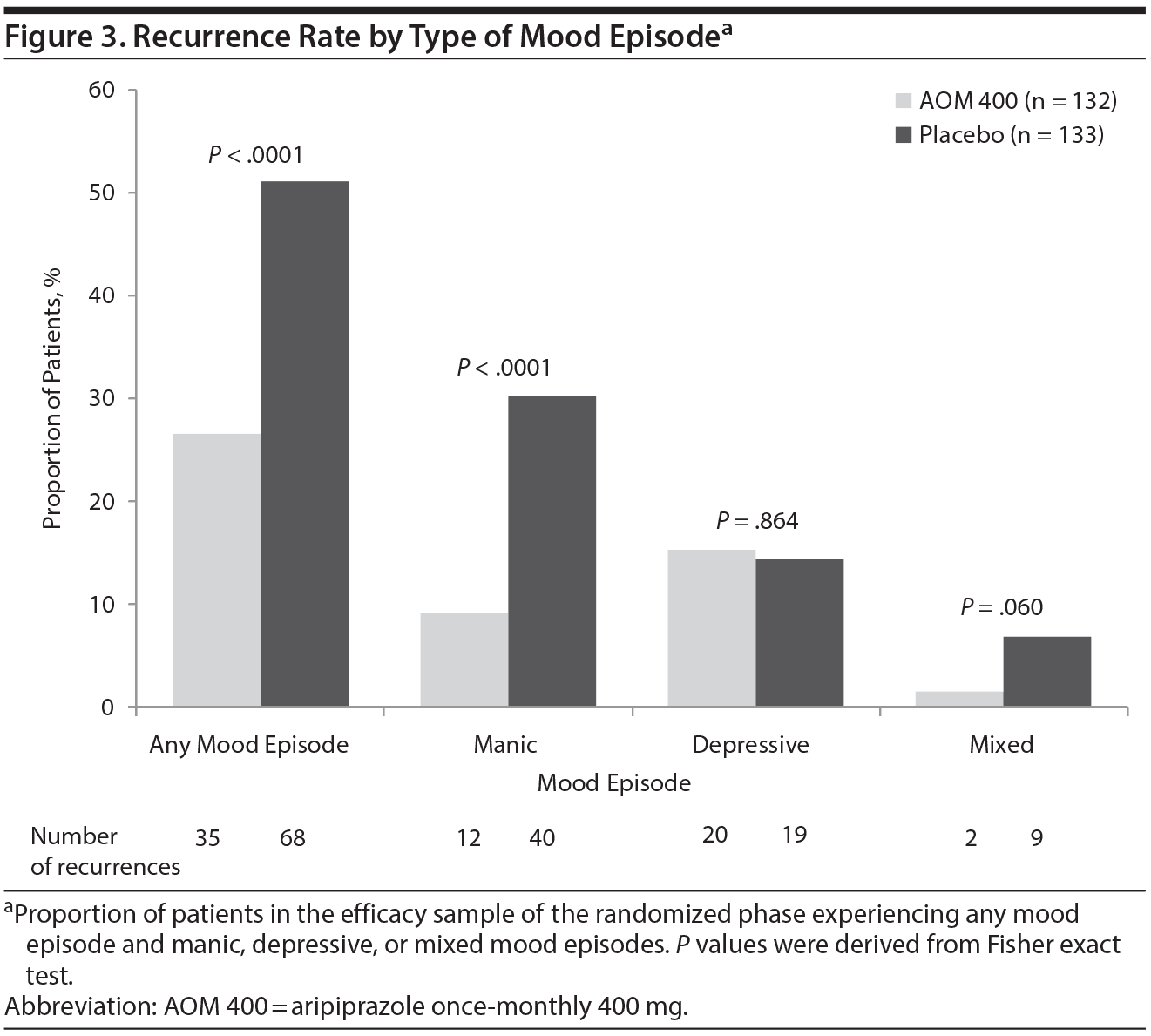
The proportion of patients with recurrence of any mood episode in the randomized phase was significantly lower (Fisher exact test P < .0001) in the AOM 400 group (35/132; 26.5%) than in the placebo group (68/133; 51.1%; Figure 3). When treatment groups were assessed by type of mood episode, the difference between treatment groups was statistically significant for recurrence of manic episodes (52 episodes total; P < .0001). While the number of mixed-mood recurrences was small (11 episodes total), the relative proportion of patients with mixed episodes (AOM 400, 1.5% vs placebo, 6.8%; Fisher exact test P = .06) was similar to that observed for manic recurrences. There was no difference between treatments for recurrence of depressive episodes (39 episodes total; Fisher exact test P = .864).
Mean (standard deviation [SD]) YMRS scores in AOM 400 and placebo groups were 3.95 (6.08) and 7.99 (9.03) at week 52, respectively. Adjusted mean change in YMRS total score from baseline to week 52 significantly favored AOM 400 over placebo (-3.09; 95% CI, -5.01 to -1.17; P = .002; MMRM), with consistently significant differences between treatment groups starting at week 14. The mean MADRS total score varied by < 1 point in each group during the randomized phase; no statistical differences in change from baseline were observed between the treatment groups (AOM 400 vs placebo, -0.04; 95% CI, -1.1 to 0.97; P = .935; MMRM).
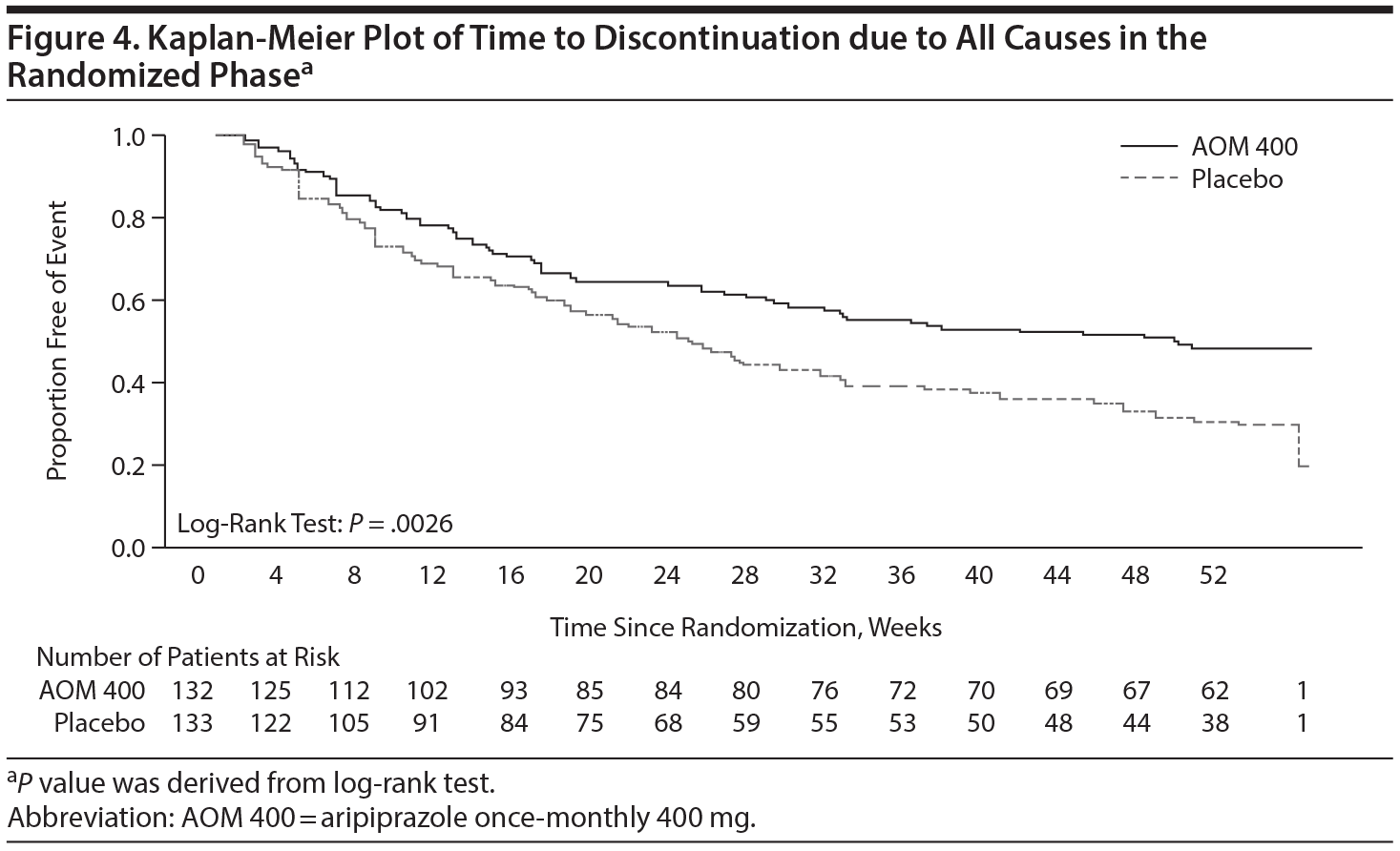
Median time to discontinuation from all causes was significantly longer with AOM 400 treatment (345 days) compared with placebo (170 days, log-rank test P = .0026; Figure 4). The risk of discontinuation over 1 year was reduced by almost 40% with AOM 400 treatment versus placebo (HR = 0.62; 95% CI, 0.46 to 0.85).
Safety and Tolerability
In the randomized phase, patients received injections every 4 weeks, for a total of up to 13 injections. Of 132 patients who received ≥ 1 injection of AOM 400 in the randomized phase, 113 (85.6%) received an initial dose of 400 mg and 19 (14.4%) received an initial dose of 300 mg; at each injection visit, >80% of patients received a dose of 400 mg. Mean dose of the last injection was 380.3 mg. Concomitant use of anticholinergics (AOM 400, 18.0%; placebo, 14.3%) and benzodiazepines (AOM 400, 27.8%; placebo, 24.1%) was similar between groups.
During the AOM 400 stabilization phase (single-blind AOM 400), 291 (68.5%) of 425 patients experienced TEAEs; of these, 8.5% had ≥ 1 serious TEAE, 6.6% had ≥ 1 severe TEAE, and 8.7% had ≥ 1 TEAE resulting in treatment discontinuation. The most frequently occurring TEAEs (≥ 5%) were akathisia (17.4%), weight increase (11.1%), insomnia (9.6%), anxiety (7.1%), restlessness (5.6%), fatigue (5.2%), and nasopharyngitis (5.2%). Mean (SD) increase in weight from baseline to the last visit in AOM stabilization phase was 1.0 kg (4.0 kg), with potentially clinically relevant weight gain at any time during the randomized phase observed in 44 (10.9%) patients.
In the randomized phase, 208 (78.5%) of 265 patients experienced ≥ 1 TEAE: 101 (76.5%) of 132 in the AOM 400 group and 107 (80.5%) of 133 in the placebo group (Table 1). The majority of TEAEs were mild or moderate in intensity. Serious TEAEs occurring in ≥ 1 patient, all related to the underlying disease, occurred at a lower rate with AOM 400 than placebo (bipolar disorder, 0.8% vs 2.3%, respectively; BP-I, 1.5% vs 2.3%; major depression, 0.0% vs 1.5%; and mania, 1.5% vs 7.5%). Two deaths were reported, 1 in the oral stabilization phase (myocardial infarction) and 1 in the randomized phase (AOM 400 group, anoxic brain injury and respiratory failure secondary to asthma), neither of which was considered drug related by the investigator.
Treatment-emergent extrapyramidal symptoms and extrapyramidal symptom-related AEs are reported in Table 1. There was minimal variation from baseline and between groups in extrapyramidal symptoms as assessed by SAS, BARS, DIEPSS, or AIMS total scores. Mean (SD) increase in weight from baseline to week 52 was 1.3 kg (5.9 kg) and 1.5 kg (6.1 kg) in the AOM 400 and placebo groups, respectively, with a numerically higher proportion of patients in the AOM 400 group (18.0% vs 12.9% for placebo) experiencing potentially clinically significant weight gain (increase ≥ 7% from baseline; Table 1). No clinically meaningful changes in the mean levels of fasting serum glucose, lipids, or prolactin were observed within or between groups. One patient in each group had a prolactin concentration more than twice the upper limit of normal during the randomized phase. Additionally, mean C-SSRS suicidal ideation intensity total scores showed minimal change from baseline, which was not considered clinically meaningful. Injection site pain assessed using patient-reported Visual Analog Scale scores and investigator ratings of injection site reactions remained low and decreased with subsequent injections.
DISCUSSION
There are multiple studies showing the efficacy of oral atypical antipsychotics in the treatment of BP-I11; however, literature on the use of LAIs in BP-I is scarce. The benefits of a once-monthly injection of aripiprazole (AOM 400) as maintenance treatment for BP-I were shown in the present double-blind, placebo-controlled, randomized withdrawal study. In patients with BP-I who had a manic episode at study enrollment, AOM 400 delayed the time to recurrence of mood episodes, primarily manic episodes, without increasing depressive episodes. Significantly fewer patients experienced any mood episode during 52 weeks with AOM 400 treatment compared with placebo. Effectiveness of AOM 400 was further supported by the improvement in mania symptom severity as assessed by YMRS. AOM 400 treatment was generally safe and well tolerated, as evidenced by the prolonged time to discontinuation and lower rate of discontinuation due to AEs versus placebo.
These efficacy results are consistent with the known efficacy of oral aripiprazole monotherapy in the maintenance treatment of BP-I.13 The present efficacy results also are consistent with those of RLAI given every 2 weeks, the only LAI atypical antipsychotic currently approved for maintenance treatment of BP-I.24,41 In contrast to aripiprazole, which is a partial agonist at the D2 receptor, risperidone is a high affinity D2 receptor antagonist; thus, differences in side effect profiles may be anticipated and may differentially affect adherence.42
Adherence remains a significant challenge in the treatment of patients with BP-I.18,19 Nonadherence leads to unfavorable outcomes such as increased risks of recurrence, hospitalization, and suicide.23 LAI antipsychotics, by increasing treatment adherence, can potentially improve outcomes in patients with BP-I.43 A meta-analysis44 of randomized controlled trials demonstrated that the efficacy of LAI antipsychotics in BP-I was comparable with that of oral atypical antipsychotics. Available data on atypical LAI antipsychotics in BP-I are largely derived from controlled studies of RLAI44; thus, additional studies on the potential benefits of LAIs in bipolar disorder are needed, including comparisons with oral formulations.
Patients were required to have a manic episode at study entry. The YMRS thresholds used for determining manic episodes at study entry and for assessing recurrence are consistent with those used previously in the studies with oral aripiprazole and RLAI in BP-I.13,25 Because the polarity of the BP-I index episode typically predicts the polarity of relapse and recurrence,45 it was not surprising that the preponderance of mood events in the present study were manic. The present study shows robust evidence for the prophylactic efficacy of AOM 400 in preventing manic episodes. Patients had few-to-no depressive symptoms at study enrollment, the number of depressive episodes during the study was low, and a clinical benefit of AOM 400 in preventing depressive episodes was not evident. However, it should also be noted that maintenance treatment with AOM 400 did not result in an increase in depressive episodes. This finding contrasts with typical antipsychotics, which, although effective at reducing recurrence of manic episodes, are associated with increased number of depressive episodes.23,46,47
The tolerability profile of a therapeutic agent is important in guiding long-term treatment decisions. In the current study, AOM 400 was generally safe and well tolerated by patients with BP-I during long-term treatment with few discontinuations because of TEAEs. The overall discontinuation rate during the 26-week randomized withdrawal phase in a study evaluating oral aripiprazole for maintenance treatment of BP-I was 50%,13 similar to the 51.9% of patients on AOM 400 who discontinued during the longer 52-week randomized withdrawal phase of the present study. The majority of patients (> 80% at each injection visit) received the recommended dose of 400 mg. No new safety signals were noted, and observed TEAEs were mostly mild to moderate. Mean weight gain was low in the randomized phase and was similar between treatment groups. There were no clinically meaningful changes in extrapyramidal symptom scales, metabolic parameters, or vital signs. Prolactin elevation and the associated sexual dysfunction are troublesome consequences of antipsychotic treatment. Among the atypical antipsychotics, aripiprazole is known to be prolactin-sparing, whereas others, including risperidone, are considered to be prolactin-raising.42,48 The present study did not reveal any clinically meaningful alterations in prolactin levels with AOM 400 versus placebo, and there were few TEAEs related to prolactin or sexual dysfunction.
The study has some limitations. Stringent criteria were followed to assess stability and recurrence. The study’s randomized population included patients who had been previously stabilized on AOM 400 monotherapy, providing a population enriched in responders and those who tolerate AOM 400. Therefore, the clinical efficacy, tolerability, and safety of AOM 400 might be less robust in a clinical practice setting where patients were not previously exposed to aripiprazole or require adjunct treatment in addition to antipsychotics. Further, patients in the placebo group could have experienced AEs resulting from withdrawal of AOM 400 after having received it during the stabilization phase. Finally, patients were required to be experiencing a manic mood episode at study entry, which may limit the generalizability to other types of mood episodes.
CONCLUSIONS
AOM 400 demonstrated efficacy as maintenance treatment for BP-I by reducing the risk of recurrence of mood episodes. AOM treatment was generally safe and well tolerated. These findings support the role of AOM 400 as the first monthly LAI for the maintenance treatment of BP-I.
Submitted: September 8, 2016; accepted December 7, 2016.
Online first: January 31, 2017.
Drug names: aripiprazole (Abilify), aripiprazole once-monthly (Abilify Maintena), lorazepam (Ativan and others), risperidone (Risperdal and others).
Author contributions: All authors had full access to the data included in the paper, interpreted the data, critically reviewed drafts of the paper, and approved the final version for submission.
Potential conflicts of interest: Dr Calabrese has received federal funding from the US Department of Defense, Health Resources Services Administration, and National Institute of Mental Health and grant support from Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Cephalon (now Teva Pharmaceutical Industries), Dainippon Sumitomo Pharma, GlaxoSmithKline, Janssen, Eli Lilly, Intra-Cellular Therapies, Pfizer, H. Lundbeck A/S, Sunovion, and Takeda. He has also served as consultant/advisory board member/speaker for Abbott Laboratories, Allergan, AstraZeneca, Bristol-Myers Squibb, Cephalon (now Teva Pharmaceutical Industries), Dainippon Sumitomo Pharma, GlaxoSmithKline, Janssen, H. Lundbeck A/S, Merck, Otsuka, Pfizer, Repligen, Servier, Sunovion, Solvay, and Takeda. Drs Sanchez, Amatniek, Cox, Salzman, McQuade, Nyilas, and Carson; Mss Jin and Perry; and Mr Johnson are employees of Otsuka Pharmaceutical Development & Commercialization. Drs Such and Hertel are employees of H. Lundbeck A/S.
Funding/support: This study was funded by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck A/S.
Role of the sponsor: The study sponsor designed and conducted the study; collected, analyzed, and interpreted the data in consultation with the authors; and funded editorial support for writing the paper.
Acknowledgments: Manuscript writing support was provided by Vandana Sharma, PhD, at C4 MedSolutions (Yardley, PA), a CHC Group company, and funded by Otsuka Pharmaceutical Development & Commercialization and H. Lundbeck A/S.
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder, second edition. APA web site. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/bipolar.pdf. Accessed July 21, 2016.
2. Grunze H, Vieta E, Goodwin GM, et al; WFSBP Task Force on Treatment Guidelines for Bipolar Disorders. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry. 2013;14(3):154-219. PubMed doi:10.3109/15622975.2013.770551
3. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1-44. PubMed doi:10.1111/bdi.12025
4. Bortolato B, Miskowiak KW, Köhler CA, et al. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr Dis Treat. 2015;11:3111-3125. PubMed doi:10.2147/ndt.s76700
5. Martinez-Aran A, Scott J, Colom F, et al. Treatment nonadherence and neurocognitive impairment in bipolar disorder. J Clin Psychiatry. 2009;70(7):1017-1023. PubMed doi:10.4088/JCP.08m04408
6. Zimmerman M, Galione JN, Chelminski I, et al. Sustained unemployment in psychiatric outpatients with bipolar disorder: frequency and association with demographic variables and comorbid disorders. Bipolar Disord. 2010;12(7):720-726. PubMed doi:10.1111/j.1399-5618.2010.00869.x
7. Baune BT, Malhi GS. A review on the impact of cognitive dysfunction on social, occupational, and general functional outcomes in bipolar disorder. Bipolar Disord. 2015;17(suppl 2):41-55. PubMed doi:10.1111/bdi.12341
8. López-Jaramillo C, Lopera-Vásquez J, Gallo A, et al. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: implications for relapse prevention and treatment adherence. Bipolar Disord. 2010;12(5):557-567. PubMed doi:10.1111/j.1399-5618.2010.00835.x
9. Kemp DE. Managing the side effects associated with commonly used treatments for bipolar depression. J Affect Disord. 2014;169(suppl 1):S34-S44. PubMed doi:10.1016/S0165-0327(14)70007-2
10. Szmulewicz A, Samamé C, Caravotta P, et al. Behavioral and emotional adverse events of drugs frequently used in the treatment of bipolar disorders: clinical and theoretical implications. Int J Bipolar Disord. 2016;4(1):6. PubMed doi:10.1186/s40345-016-0047-3
11. Derry S, Moore RA. Atypical antipsychotics in bipolar disorder: systematic review of randomised trials. BMC Psychiatry. 2007;7:40. PubMed doi:10.1186/1471-244X-7-40
12. Kanba S, Kawasaki H, Ishigooka J, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole for the treatment of acute manic or mixed episodes in Asian patients with bipolar I disorder (the AMAZE study). World J Biol Psychiatry. 2014;15(2):113-121. PubMed doi:10.3109/15622975.2012.669047
13. Keck PE Jr, Calabrese JR, McIntyre RS, et al; Aripiprazole Study Group. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry. 2007;68(10):1480-1491. PubMed doi:10.4088/JCP.v68n1003
14. Keck PE Jr, Calabrese JR, McQuade RD, et al; Aripiprazole Study Group. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry. 2006;67(4):626-637. PubMed doi:10.4088/JCP.v67n0414
15. Sachs G, Sanchez R, Marcus R, et al; Aripiprazole Study Group. Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol. 2006;20(4):536-546. PubMed doi:10.1177/0269881106059693
16. Yatham LN, Fountoulakis KN, Rahman Z, et al. Efficacy of aripiprazole versus placebo as adjuncts to lithium or valproate in relapse prevention of manic or mixed episodes in bipolar I patients stratified by index manic or mixed episode. J Affect Disord. 2013;147(1-3):365-372. PubMed doi:10.1016/j.jad.2012.11.042
17. Perlis RH, Ostacher MJ, Miklowitz DJ, et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71(3):296-303. PubMed doi:10.4088/JCP.09m05514yel
18. Sajatovic M, Valenstein M, Blow FC, et al. Treatment adherence with antipsychotic medications in bipolar disorder. Bipolar Disord. 2006;8(3):232-241. PubMed doi:10.1111/j.1399-5618.2006.00314.x
19. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46, quiz 47-48. PubMed doi:10.4088/JCP.7090su1cj
20. Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. 2009;8:7. PubMed doi:10.1186/1744-859X-8-7
21. Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed doi:10.1177/2045125314540297
22. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18. PubMed
23. Gigante AD, Lafer B, Yatham LN. Long-acting injectable antipsychotics for the maintenance treatment of bipolar disorder. CNS Drugs. 2012;26(5):403-420. PubMed doi:10.2165/11631310-000000000-00000
24. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2016.
25. Quiroz JA, Yatham LN, Palumbo JM, et al. Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry. 2010;68(2):156-162. PubMed doi:10.1016/j.biopsych.2010.01.015
26. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617-624. PubMed doi:10.4088/JCP.11m07530
27. Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135-144. PubMed doi:10.1192/bjp.bp.113.134213
28. Ishigooka J, Nakamura J, Fujii Y, et al; ALPHA Study Group. Efficacy and safety of aripiprazole once-monthly in Asian patients with schizophrenia: a multicenter, randomized, double-blind, non-inferiority study versus oral aripiprazole. Schizophr Res. 2015;161(2-3):421-428. PubMed doi:10.1016/j.schres.2014.12.013
29. Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1-):498-504. PubMed doi:10.1016/j.schres.2015.07.007
30. International Conference on Harmonisation. E6: Good Clinical Practice: consolidated guideline. www.ich.org. Accessed July 26, 2016.
31. Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. PubMed doi:10.1192/bjp.133.5.429
32. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. PubMed doi:10.1192/bjp.134.4.382
33. Posner K, Brent D, Lucas C, et al. Columbia Suicide Severity Rating Scale. http://cssrs.columbia.edu/the-columbia-scale-c-ssrs/cssrs-for-research/. Accessed April 19, 2016.
34. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
35. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
36. Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159-171. PubMed doi:10.1016/S0165-1781(97)00123-6
37. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212:11-19. PubMed doi:10.1111/j.1600-0447.1970.tb02066.x
38. Kim JH, Jung HY, Kang UG, et al. Metric characteristics of the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS): a practical combined rating scale for drug-induced movement disorders. Mov Disord. 2002;17(6):1354-1359. PubMed doi:10.1002/mds.10255
39. Guy W. Abnormal Involuntary Movement Scale (AIMS). In: ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:534-537.
40. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676. PubMed doi:10.1192/bjp.154.5.672
41. Quiroz JA, Rusch S, Thyssen A, et al. Deltoid injections of risperidone long-acting injectable in patients with schizophrenia. Innov Clin Neurosci. 2011;8(6):20-28. PubMed
42. De Hert M, Detraux J, Peuskens J. Second-generation and newly approved antipsychotics, serum prolactin levels and sexual dysfunctions: a critical literature review. Expert Opin Drug Saf. 2014;13(5):605-624. PubMed doi:10.1517/14740338.2014.906579
43. Bates JA, Whitehead R, Bolge SC, et al. Correlates of medication adherence among patients with bipolar disorder: results of the Bipolar Evaluation of Satisfaction and Tolerability (BEST) study: a nationwide cross-sectional survey. Prim Care Companion J Clin Psychiatry. 2010;12(5):e1-e8. PubMed doi:10.4088/pcc.09m00883ye
44. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analyses of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):pyw038. PubMed doi:10.1093/ijnp/pyw038
45. Calabrese JR, Vieta E, El-Mallakh R, et al. Mood state at study entry as predictor of the polarity of relapse in bipolar disorder. Biol Psychiatry. 2004;56(12):957-963. PubMed doi:10.1016/j.biopsych.2004.09.022
46. Ahlfors UG, Baastrup PC, Dencker SJ, et al. Flupenthixol decanoate in recurrent manic-depressive illness: a comparison with lithium. Acta Psychiatr Scand. 1981;64(3):226-237. PubMed doi:10.1111/j.1600-0447.1981.tb00778.x
47. White E, Cheung P, Silverstone T. Depot antipsychotics in bipolar affective disorder. Int Clin Psychopharmacol. 1993;8(2):119-122. PubMed doi:10.1097/00004850-199300820-00007
48. Robinson DG, Gallego JA, John M, et al. A randomized comparison of aripiprazole and risperidone for the acute treatment of first-episode schizophrenia and related disorders: 3-month outcomes. Schizophr Bull. 2015;41(6):1227-1236. PubMed doi:10.1093/schbul/sbv125
This PDF is free for all visitors!
Save
Cite