Objective: Asenapine is a second-generation antipsychotic used to treat individuals with schizophrenia. This phase 3 study assessed efficacy and safety of HP-3070, an asenapine transdermal system (patch), in adults with schizophrenia.
Methods: In this inpatient study, a 3- to 14-day screening/single-blind run-in washout period was followed by a 6-week double-blind period wherein patients with acutely exacerbated schizophrenia (DSM-5 criteria) were randomized 1:1:1 and received HP-3070 7.6 mg/24 h (n = 204), HP-3070 3.8 mg/24 h (n = 204), or placebo (n = 206). Primary endpoint was change from baseline (CFB) in week 6 Positive and Negative Syndrome Scale (PANSS) total score versus placebo; key secondary endpoint was CFB in week 6 Clinical Global Impression-Severity of Illness score versus placebo. Safety endpoints included treatment-emergent adverse events (TEAEs) and dermal assessments.
Results: Each of the HP-3070 doses demonstrated significant improvement versus placebo at week 6 for the primary and key secondary endpoints. Differences in the least-squares mean (LSM) (95% CI; adjusted P) of CFB for PANSS total scores were −4.8 (−8.06 to −1.64; adjusted P = .003) and −6.6 (−9.81 to −3.40; adjusted P < .001) for 7.6 mg/24 h and 3.8 mg/24 h, respectively. HP-3070 was well tolerated, with a systemic safety profile consistent with sublingual asenapine. Incidence of application site TEAEs was higher for HP-3070 (14.2%, 7.6 mg/24 h; 15.2%, 3.8 mg/24 h) versus placebo (4.4%). Discontinuations due to application site reactions or skin disorders (urticaria, pruritus) were infrequent (≤ 0.5% per treatment group).
Conclusions: HP-3070 7.6 mg/24 h and 3.8 mg/24 h doses were efficacious and well tolerated. As the first transdermal antipsychotic patch available in the US, HP-3070 offers a novel treatment option for people with schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT02876900; EudraCT number: 2015-005134-21
ABSTRACT
Objective: Asenapine is a second-generation antipsychotic used to treat individuals with schizophrenia. This phase 3 study assessed efficacy and safety of HP-3070, an asenapine transdermal system (patch), in adults with schizophrenia.
Methods: In this inpatient study, a 3- to 14-day screening/single-blind run-in washout period was followed by a 6-week double-blind period wherein patients with acutely exacerbated schizophrenia (DSM-5 criteria) were randomized 1:1:1 and received HP-3070 7.6 mg/24 h (n = 204), HP-3070 3.8 mg/24 h (n = 204), or placebo (n = 206). Primary endpoint was change from baseline (CFB) in week 6 Positive and Negative Syndrome Scale (PANSS) total score versus placebo; key secondary endpoint was CFB in week 6 Clinical Global Impression-Severity of Illness score versus placebo. Safety endpoints included treatment-emergent adverse events (TEAEs) and dermal assessments.
Results: Each of the HP-3070 doses demonstrated significant improvement versus placebo at week 6 for the primary and key secondary endpoints. Differences in the least-squares mean (LSM) (95% CI; adjusted P) of CFB for PANSS total scores were −4.8 (−8.06 to −1.64; adjusted P = .003) and −6.6 (−9.81 to −3.40; adjusted P < .001) for 7.6 mg/24 h and 3.8 mg/24 h, respectively. HP-3070 was well tolerated, with a systemic safety profile consistent with sublingual asenapine. Incidence of application site TEAEs was higher for HP-3070 (14.2%, 7.6 mg/24 h; 15.2%, 3.8 mg/24 h) versus placebo (4.4%). Discontinuations due to application site reactions or skin disorders (urticaria, pruritus) were infrequent (≤ 0.5% per treatment group).
Conclusions: HP-3070 7.6 mg/24 h and 3.8 mg/24 h doses were efficacious and well tolerated. As the first transdermal antipsychotic patch available in the US, HP-3070 offers a novel treatment option for people with schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT02876900; EudraCT number: 2015-005134-21
J Clin Psychiatry 2021;82(1):20m13602
To cite: Citrome L, Walling DP, Zeni CM, et al. Efficacy and safety of HP-3070, an asenapine transdermal system, in patients with schizophrenia: a phase 3, randomized, placebo-controlled study. J Clin Psychiatry. 2021;82(1):20m13602.
To share: https://doi.org/10.4088/JCP.20m13602
© Copyright 2020 Physicians Postgraduate Press, Inc.
aPsychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York
bCNS Network, LLC, Garden Grove, California
cResearch and Development, Noven Pharmaceuticals, Inc., Jersey City, New Jersey
dHisamitsu Pharmaceutical Co., Inc., Chiyoda-ku, Tokyo, Japan
*Corresponding author: Leslie Citrome, MD, MPH, Department of Psychiatry and Behavioral Sciences, New York Medical College, 40 Sunshine Cottage Rd, Valhalla, NY 10595 ([email protected]).
Schizophrenia is a leading cause of disability worldwide.1 Despite the wide array of antipsychotic agents available, patients often remain unsatisfied with current treatment options, as evidenced by frequent changes in antipsychotic regimens, observed in 25%-50% of patients in past studies.2-5 These frequent treatment changes can be associated with increased risk of relapse, adverse effects, and further challenges with medication adherence, highlighting the need for additional schizophrenia treatment options.6-10 A transdermal treatment for schizophrenia is a novel option that may address some of the sources of dissatisfaction driving regimen adjustments.
HP-3070, an asenapine transdermal system (patch), has been approved by the US Food and Drug Administration for once-daily use in adults with schizophrenia.11,12 Asenapine, a second-generation antipsychotic, was initially available only as a twice-daily sublingual tablet.13 Complex dosing instructions and reported undesirable adverse effects including dysgeusia, oral hypoesthesia, and oral ulcers or blisters can make adherence to sublingual asenapine (SLA) challenging.13,14 Transdermal administration of asenapine may address several unmet needs in patients with schizophrenia, including improved adherence by reducing dosing frequency to once daily. Compared with oral treatments, transdermal delivery offers more consistent plasma drug concentrations because of steady drug delivery and the option to immediately cease dosing by simply removing the patch.15 Use of an asenapine transdermal system instead of SLA essentially eliminates the risk of dysgeusia and oral hypoesthesia and removes the need for food or drink restrictions.11,13 As the first transdermal antipsychotic patch approved for use in the United States,11,12 HP-3070 represents an important innovation for patients who require antipsychotic treatment but are unable or unwilling to take oral medications, averse to receiving injections, or simply looking for another treatment option.
Here, we report results from a phase 3, randomized, placebo-controlled study designed to assess the efficacy and safety of HP-3070 in adults with schizophrenia.
METHODS
Trial Design and Participants
This phase 3, multicenter, placebo-controlled, randomized, double-blind, inpatient study (ClinicalTrials.gov identifier: NCT02876900) was designed to demonstrate the efficacy and safety of HP-3070 transdermal patches in patients with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] criteria). Eligible patients were voluntarily hospitalized during the treatment period, first entering a 3- to 14-day screening/run-in period during which single-blind placebo patches were applied once daily and prior antipsychotic and other prohibited medications were washed out. No oral bridge with SLA was required. Patients with a decrease in Positive and Negative Syndrome Scale (PANSS) total score of ≥ 20% or who were noncompliant with patches were ineligible for the subsequent 6-week double-blind treatment period and 30-day follow-up. Eligible study participants were ≥ 18 years old, were experiencing an acute exacerbation of schizophrenia, had a Clinical Global Impression-Severity of Illness (CGI-S) scale score of ≥ 4 (moderate), and had a PANSS total score of ≥ 80, with scores of ≥ 4 in ≥ 2 of the PANSS items for conceptual disorganization, delusions, hallucinatory behavior, and unusual thought content at screening and baseline. Potential participants were excluded if they had been diagnosed with schizophrenia < 6 months prior to the screening visit, had a current DSM-5 diagnosis other than schizophrenia, or were known to be resistant/refractory to antipsychotic treatment, including asenapine. Prior experience with SLA was not required. The study protocol was approved by an independent ethics committee or review board at each study center. Written informed consent was obtained from all participants.
Interventions
During the 6-week double-blind treatment period, patients were randomized (1:1:1) to HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, or placebo. Patch application/removal was performed at approximately the same time daily. One active patch delivered an asenapine dose of 3.8 mg/24 h. To maintain blinding, patients receiving the HP-3070 3.8 mg/24 h dose received 1 active and 1 placebo patch, patients receiving HP-3070 7.6 mg/24 h received 2 active patches, and patients receiving placebo received 2 placebo patches. Patch application sites (abdomen, hip, upper arm, upper back, and upper chest) were rotated daily. Sites containing tattoos, scar tissue, infections, or other skin abnormalities were avoided.

- Numerous antipsychotic agents are available for schizophrenia treatment, although many patients cannot find a suitable treatment option.
- The asenapine transdermal system (HP-3070) treatment option can improve adherence with once-daily dosing and avoid undesirable effects or restrictions associated with sublingual asenapine such as dysgeusia and food or drink restrictions.
- The HP-3070 doses investigated were comparable to the 5 and 10 mg twice-daily sublingual asenapine doses investigated previously with similar efficacy. HP-3070 represents a new option for people with schizophrenia who are unsatisfied with their current treatment.
Dose Selection
The rationale supporting the 2 doses selected for this study was based on comparison of exposure (AUC0-24) between approved doses of SLA tablets and selected HP-3070 doses in the target population, and safety assessments for HP-3070.13 In a separate pharmacokinetic study, the mean areas under the curve during 24 hours (AUC0-24) following HP-3070 7.6 mg/24 h and 3.8 mg/24 h doses were similar to mean AUC0-24 following administration of twice-daily 10 mg and 5 mg SLA, respectively.16-18
Outcome Measures
The primary efficacy endpoint was change from baseline (CFB) to week 6 in PANSS total score.19 The key secondary endpoint was CFB to week 6 in CGI-S score.20 Other secondary endpoints included weekly change in PANSS total score and CGI-S score, weekly Clinical Global Impression-Improvement (CGI-I) scale score, and weekly proportion of PANSS and CGI-I responders.20 PANSS responders were defined as patients with ≥ 30% reduction in PANSS total score. CGI-I responders had a score of 1 (very much improved) or 2 (much improved).
Safety was assessed using standard measures (ie, spontaneously reported treatment-emergent adverse events [TEAEs], dermal assessments measuring skin irritation and patch discomfort, clinical laboratory assessments, vital signs, weight, and electrocardiogram results) and extrapyramidal symptom (EPS) assessments performed using the Abnormal Involuntary Movement Scale (AIMS),20 Barnes Akathisia Rating Scale (BARS),21 and Simpson-Angus Scale (SAS).22
Analysis Populations
The full analysis set (FAS), used for efficacy analyses, included all randomized patients who received ≥ 1 dose of study medication and had a baseline and ≥ 1 additional postbaseline assessment of PANSS total score. The safety analysis set (SAF), used for dermal evaluations and safety endpoints, included patients receiving ≥ 1 dose of study medication.
Statistical Methods
The primary efficacy variable was analyzed using a mixed-model repeated-measures (MMRM) analysis. The MMRM included CFB in PANSS total score as the repeated dependent variable, with country, treatment, visit (weeks 1-6), treatment-by-visit interaction, and baseline PANSS score as covariates. The key secondary endpoint was analyzed using the same method, with the baseline value of CGI-S included in the MMRM as a covariate. Additional details are provided in the Supplementary Methods.
A matched parallel gatekeeping procedure was used to control the overall type I error rate at the level of 0.05 for primary and key secondary objectives.23-25 Primary and key secondary efficacy hypotheses were grouped into 2 hierarchical families, each including multiple comparisons of HP-3070 7.6 mg/24 h versus placebo and HP-3070 3.8 mg/24 h versus placebo. Hypothesis testing was based on the truncated (truncation parameter γ = 0.9) Hochberg procedure for the primary endpoint (PANSS total score) and regular for the key secondary endpoint (CGI-S). CGI-S comparisons were conducted only for doses showing significance in the PANSS total score test.
The secondary efficacy endpoints CFB in PANSS total score and CGI-I score at each week were analyzed using the same MMRM method as for the primary and key secondary endpoints but were not included in the gatekeeping procedure. Secondary endpoints involving proportion of responders were tested using the Cochran-Mantel-Haenszel (CMH) method stratified by country, with the hypothesis test based on the general association statistic.
All statistical tests of treatment effects were performed at a 2-sided significance level of .05. Confidence intervals (CIs) were reported at 95%.
RESULTS
Participants
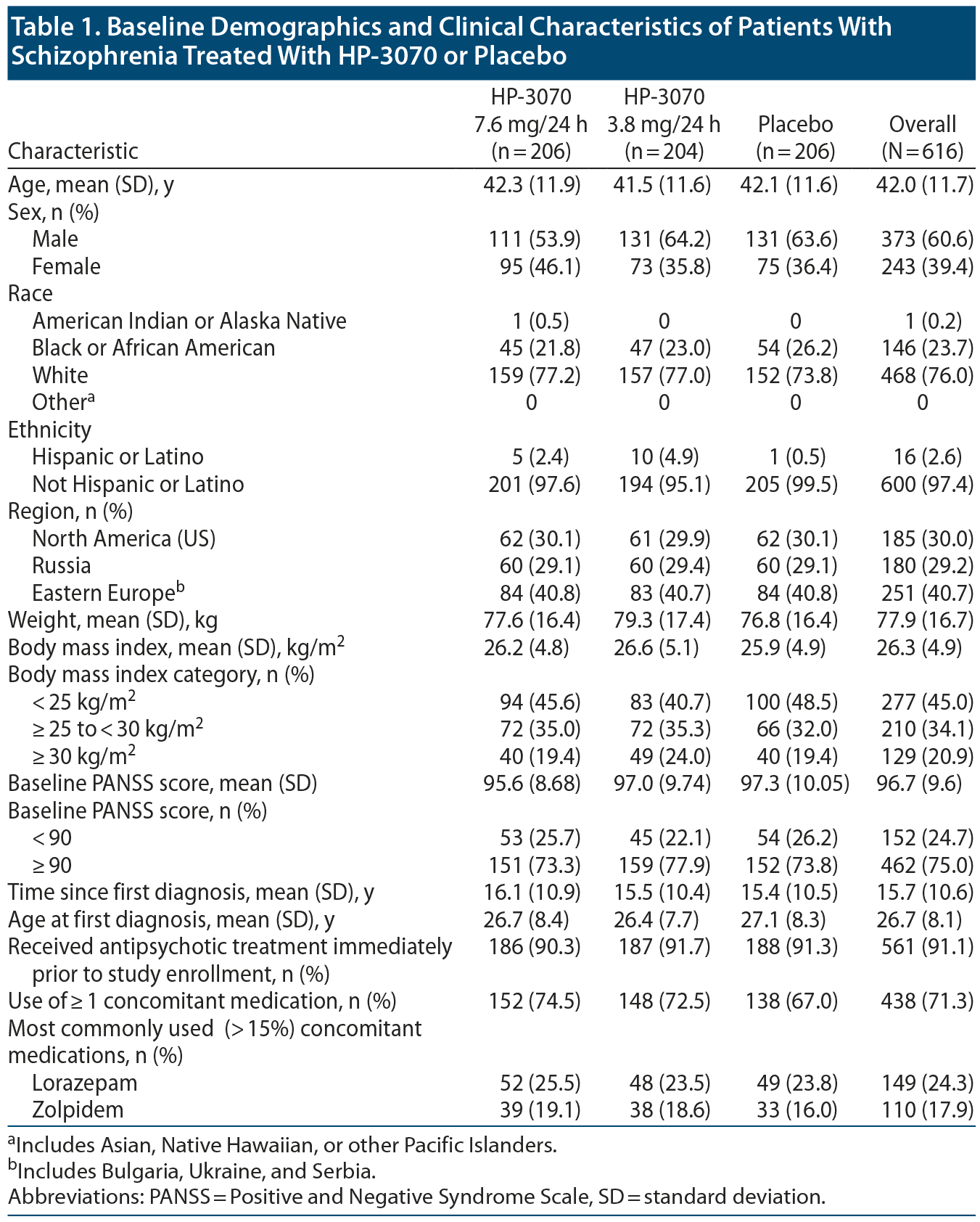
A total of 782 patients were screened for eligibility at 59 study sites in the United States, Russia, Ukraine, Bulgaria, and Serbia (Supplementary Figure 1). Eligible patients providing informed consent were randomized into HP-3070 7.6 mg/24 h (n = 206), HP-3070 3.8 mg/24 h (n = 205), and placebo groups (n = 206). The SAF included 204 patients in each HP-3070 treatment group and 206 in the placebo group. The FAS included 203, 201, and 203 patients from the HP-3070 7.6 mg/24 h, 3.8 mg/24 h, and placebo groups, respectively. Study discontinuations occurred in 46 (22.5%) and 38 (18.6%) patients from the HP-3070 7.6 mg/24 h and 3.8 mg/24 h groups, respectively, and in 44 (21.4%) placebo patients from the SAF population (Supplementary Figure 1).
There were no notable differences between HP-3070 and placebo treatment groups with respect to demographics and baseline disease characteristics (Table 1, Supplementary Table 1). The majority of patients were male (60.6%) and white (76.0%). Mean age was 42.0 years, and mean BMI, 26.3 kg/m2. Mean (SD) time since first diagnosis of schizophrenia was 15.7 (10.6) years, with 64.4% of patients having a diagnosis for ≥ 10 years. Concomitant medication use was slightly higher for HP-3070 groups (74.5% and 72.5% for 7.6 mg/24 h and 3.8 mg/24 h) versus placebo (67.0%) (Table 1). Treatment compliance was similar across groups, with 83.6% of patients using ≥ 80% and ≤ 120% of study medication (> 100% possible when a detached patch was replaced, occurring infrequently: 2.5% for 7.6 mg/24 h, 5.4% for 3.8 mg/24 h, 3.9% for placebo).
Primary Efficacy Endpoint: Week 6 PANSS CFB
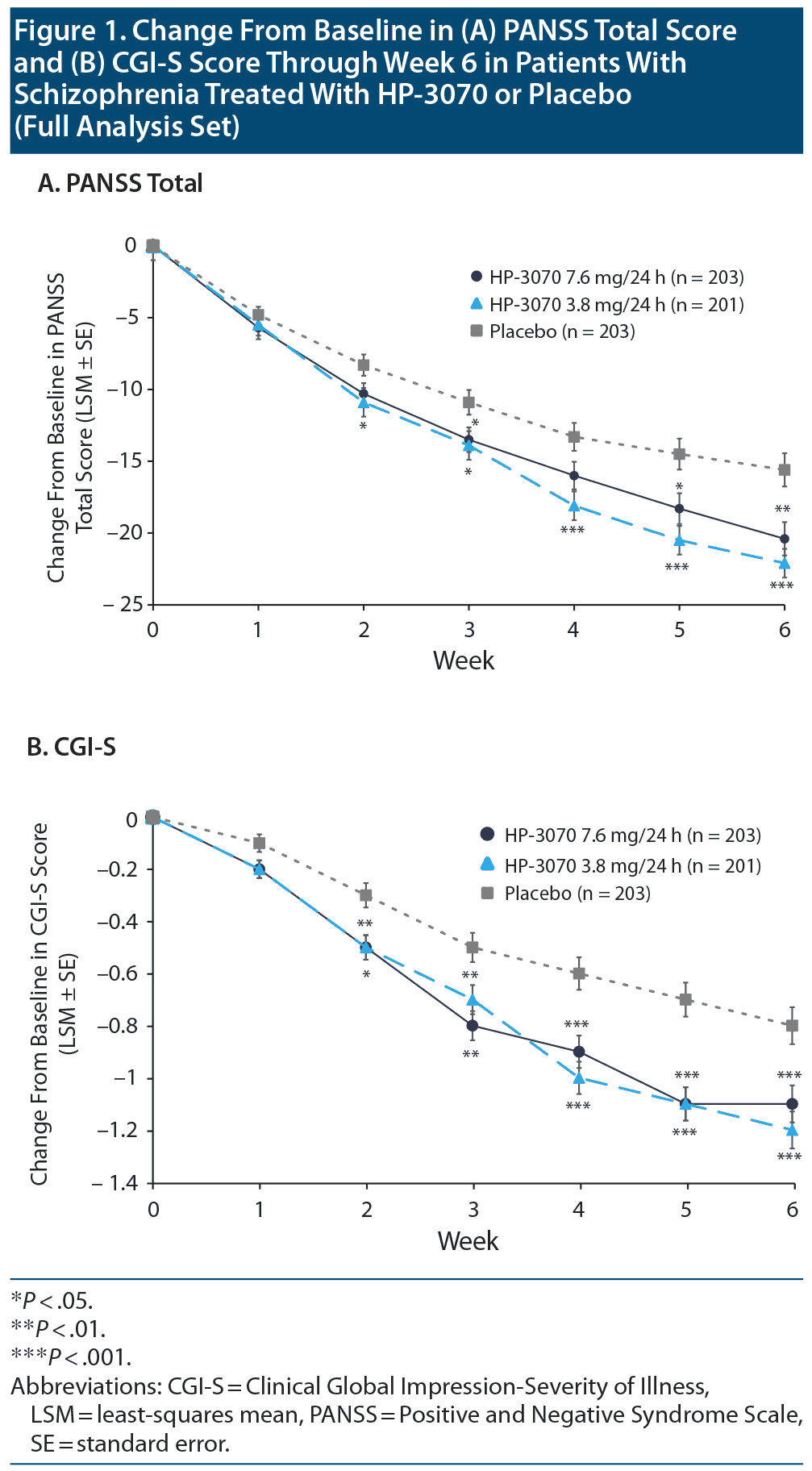
At baseline, similar mean (SD) PANSS total scores were observed for HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, and placebo groups (95.6 [8.68], 97.0 [9.74], and 97.4 [10.05]). After 6 weeks of treatment, differences in the least-squares mean (LSM) CFB in PANSS total score versus placebo were −4.8 (95% CI: −8.06 to −1.64; adjusted P = .003) and −6.6 (95% CI: −9.81 to −3.40; adjusted P < .001) for the HP-3070 7.6 mg/24 h and 3.8 mg/24 h groups (Figure 1A).
Key Secondary Efficacy Endpoint: Week 6 CGI-S CFB
Mean (SD) CGI-S scores were similar at baseline among the HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, and placebo groups (4.9 [0.50], 4.9 [0.54], and 4.9 [0.58]). The differences in LSM (SE) CFB in CGI-S score at week 6 versus placebo were −0.4 (0.100) (95% CI: −0.55 to −0.16; adjusted P < .001) for HP-3070 7.6 mg/24 h and −0.4 (0.099) (95% CI: −0.64 to −0.25; adjusted P < .001) for HP-3070 3.8 mg/24 h (Figure 1B). Multiple sensitivity analyses for primary and key secondary endpoints supported these findings (Supplementary Table 2).
Other Secondary Efficacy Endpoints
For PANSS total scores, a statistically significant difference in LSM estimate (SE) CFB versus placebo was observed for HP-3070 7.6 mg/24 h from week 3 through week 6, and for HP-3070 3.8 mg/24 h from week 2 through week 6 (Figure 1A).
For all treatments, percentage of patients demonstrating ≥ 30% improvement from baseline in PANSS total score (PANSS responders) increased with each successive weekly time point, with higher responder rates for HP-3070 7.6 mg/24 h and HP-3070 3.8 mg/24 h versus placebo; differences were statistically significant (P < .05 by CMH test) for both HP-3070 groups versus placebo at week 6 (Supplementary Table 3).
For CGI-S scores, a statistically significant difference in LSM estimate (SE) CFB versus placebo was observed for both HP-3070 doses from week 2 through week 6 (Figure 1B).
Regarding CGI-I, significant differences versus placebo were observed for the HP-3070 7.6 mg/24 h group from week 3 to week 6, and the HP-3070 3.8 mg/24 h group at weeks 2, 4, 5, and 6 (Supplementary Table 3). Higher rates of CGI-I responders were observed for HP-3070 7.6 mg/24 h and HP-3070 3.8 mg/24 h versus placebo, with statistically significant (P < .05 by CMH test) differences observed for both HP-3070 groups versus placebo for weeks 4-6 (Supplementary Table 3).
Safety Outcomes
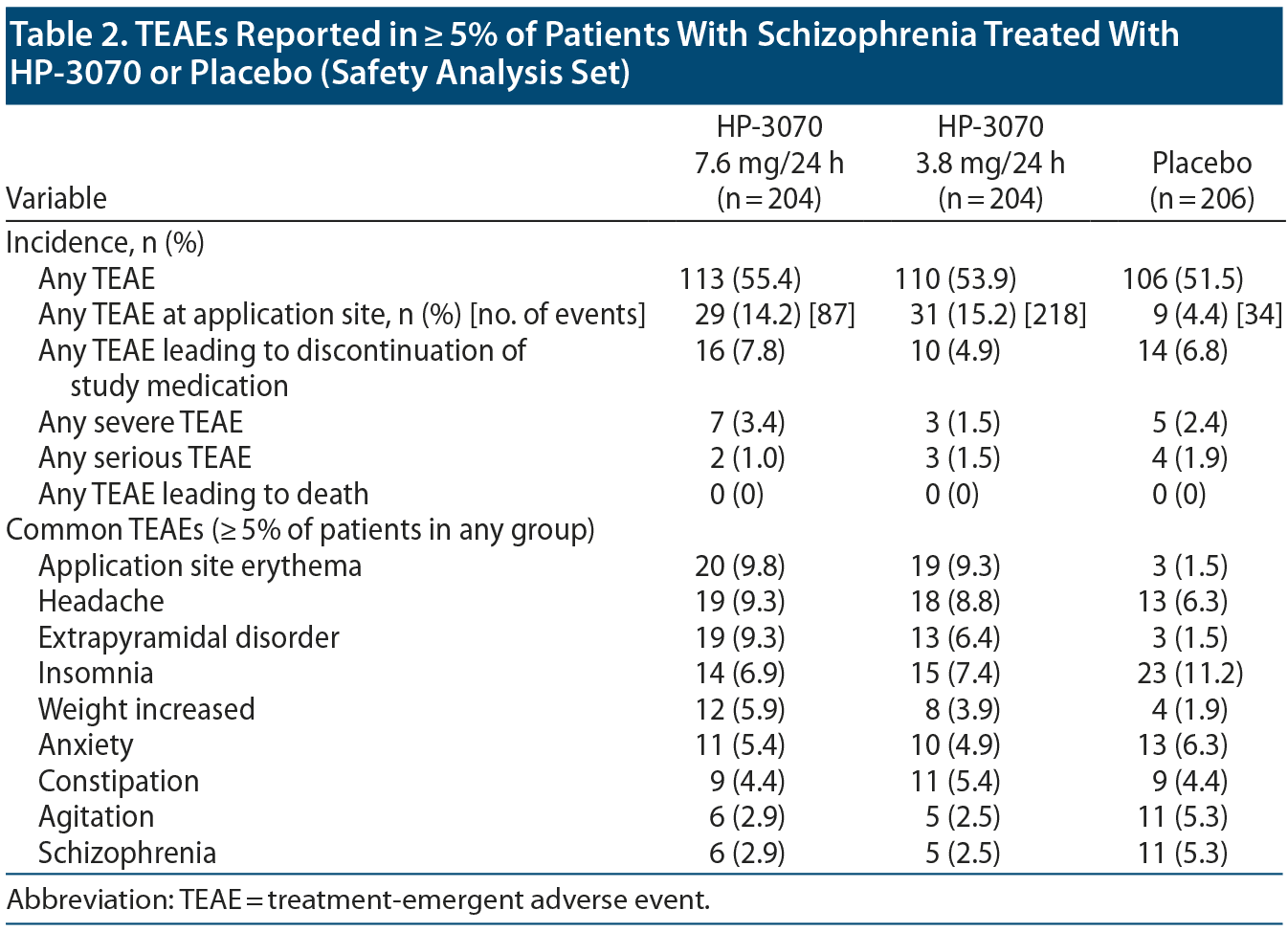
TEAEs were reported at similar rates among patients receiving HP-3070 7.6 mg/24 h (55.4%), HP-3070 3.8 mg/24 h (53.9%), and placebo treatment (51.5%) (Table 2). Most TEAEs were mild or moderate in intensity, while severe TEAEs were reported in 7 (3.4%), 3 (1.5%), and 5 (2.4%) patients in the HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, and placebo groups, respectively. No deaths occurred during the study, incidences of serious adverse events were similar across groups (< 2% of patients in each), and no serious adverse events were considered related to study treatment or occurred at patch application sites (Table 2).
TEAEs leading to discontinuation of study treatment occurred in 16 (7.8%), 10 (4.9%), and 14 (6.8%) patients in the HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, and placebo groups (Table 2). TEAEs most frequently leading to study discontinuation were schizophrenia in 3 (1.5%), 2 (1.0%), and 6 (2.9%) and akathisia in 3 (1.5%), 0 (0%), and 1 (0.5%) of patients in the HP-3070 7.6 mg/24 h, HP-3070 3.8 mg/24 h, and placebo groups, respectively. The most frequently reported TEAEs were application site erythema, headache, and extrapyramidal disorder, occurring more frequently with HP-3070 treatment versus placebo (Table 2). Of note, extrapyramidal disorder appeared to be dose sensitive, arising more frequently in patients receiving HP-3070 7.6 mg/24 h (9.3%) versus HP-3070 3.8 mg/24 h (6.4%) or placebo (1.5%) (Table 2). No episodes of dysgeusia occurred. A potential instance of oral hypoesthesia, reported as “numbness of the lower jaw on the right side,” occurred in 1 patient (7.6 mg/24 h group).
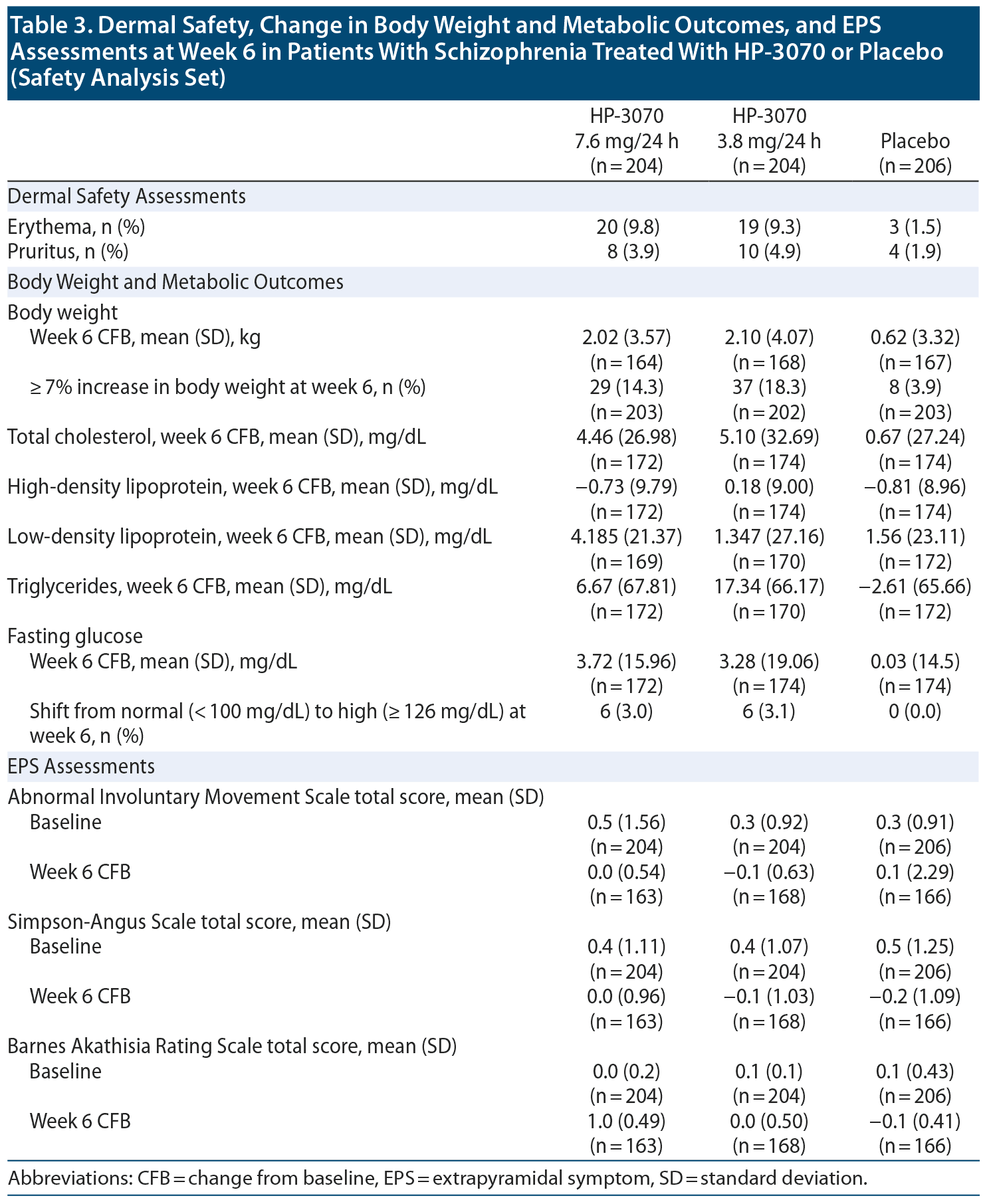
Dermal safety. The incidence of TEAEs at patch application sites was higher in the HP-3070 groups (14.2% and 15.2% of patients for 7.6 mg/24 h and 3.8 mg/24 h) versus placebo (4.4%). The most frequently reported patch application site reactions were erythema and pruritus, occurring in fewer than 10% and 5% of patients, respectively (Table 3). One HP-3070 7.6 mg/24 h-treated patient experienced severe application site erythema during week 2, which resolved without intervention, and the patient continued the study. All other patch application site events were mild or moderate in severity. Rates of discontinuation due to application site reactions or skin disorders were ≤ 0.5% across all groups.
Weight and metabolic outcomes. Occurrence of the adverse event increased weight appeared to be dose-related, reported in 12 (5.9%) and 8 (3.9%) patients receiving HP-3070 7.6 mg/24 h and 3.8 mg/24 h, respectively, and 4 (1.9%) placebo patients, with most cases considered related to study drug (Table 2). The proportion of patients experiencing ≥ 7% increase in body weight at week 6 was 14.3%, 18.3%, and 3.9%, respectively (Table 3). Mean CFBs at week 6 for total cholesterol, triglycerides, and fasting glucose were greater in the HP-3070 groups than placebo (Table 3).
Movement scales. No treatment differences were observed for the EPS assessments (AIMS, SAS, and BARS total scores) from baseline to week 6 for treated or placebo patients (Table 3).
DISCUSSION
The results of this phase 3, placebo-controlled, randomized study indicated that the HP-3070 asenapine transdermal system was both effective and well tolerated for treatment of adult inpatients with schizophrenia. After 6 weeks of treatment with HP-3070 7.6 mg/24 h or 3.8 mg/24 h, significant improvements from baseline in both PANSS total score and CGI-S score versus placebo were observed. Separation from placebo in PANSS, CGI-S, and CGI-I scores was observed as early as week 2 and generally maintained through week 6.
The systemic safety profile observed in this study was generally consistent with the known SLA safety profile. There were no instances of dysgeusia with HP-3070 treatment and 1 instance of oral hypoesthesia, whose mechanism is unclear, as the patch is applied to skin.13,26 Incidence and severity of application site reactions for HP-3070 were similar to or lower than those observed for transdermal patches for other neuropsychiatric conditions.10,27,28 A low rate of patch detachment was observed. HP-3070 3.8 mg/24 h-treated patients experienced fewer serious TEAEs and discontinuations due to TEAEs than 7.6 mg/24 h-treated patients.
The HP-3070 transdermal system investigated in this study offers a once-daily asenapine treatment option with efficacy similar to twice-daily SLA. The total daily asenapine exposures with the HP-3070 3.8 mg/24 h and 7.6 mg/24 h patches were designed to correspond to twice-daily 5 mg and 10 mg SLA, respectively.16 An intermediate option, 5.7 mg/24 h, is also available to increase dosing flexibility and corresponds to a 15 mg/d SLA dosage that is sometimes used in clinical practice.
Because of the corresponding daily exposures, the doses examined in our study can be considered comparable to the doses investigated in previous SLA studies. In the 2 positive 6-week, placebo-controlled studies of SLA in acute schizophrenia, 5-mg twice-daily treatment demonstrated significantly greater improvement in PANSS total scores versus placebo by week 3, continuing through week 6, consistent with the results observed in the current trial.14,29,30 In addition, a significantly greater percentage of patients treated with HP-3070 were classified as PANSS or CGI-I responders versus placebo, also seen in patients treated with 5 mg twice-daily SLA.29 Moreover, the percentages of CGI-I responders were similar in the SLA study and the current trial.29
With SLA, patients are advised to avoid food or liquids for 10 minutes after each twice-daily administration, necessitating that it be taken after other coprescribed pills are swallowed.13 In contrast, the pharmacokinetic profile of transdermal asenapine permits once-daily dosing with no restrictions on oral intake, potentially addressing some challenges associated with low medication adherence in individuals with schizophrenia, who, in addition to having difficulties with insight into their illness, often struggle with cognitive impairment, making complex medication regimens difficult.10 By alleviating some of these concerns, a transdermal asenapine patch may help address the known issue of frequent treatment changes in patients with schizophrenia.2-5 Overall, HP-3070 may be more acceptable to patients than SLA—in trials of SLA, more than half (54%) of asenapine-treated patients withdrew and discontinued study medication,30 versus < 24% of patients receiving HP-3070 in the current study.
Transdermal systems have historically offered an alternative to oral, sublingual, and intramuscular agents. The HP-3070 transdermal patch may be a good option in patients for whom the oral route is difficult, potentially because of difficulty swallowing or an altered ability to absorb oral products due to previous gastric bypass surgery, other concomitant oral medications prone to drug-drug interactions, etc. HP-3070 also offers an alternative for patients who simply prefer a patch. Two studies31,32 investigating preference for rivastigmine transdermal therapy for Alzheimer’s disease found that 80% of physicians and ≥ 70% of caregivers preferred transdermal administration over oral for ease of use, improved treatment compliance, simple dosing schedule, and convenience. Overall, rivastigmine studies have demonstrated transdermal treatment as effective as oral formulations and more tolerable, yielding better compliance rates in patients with dementia.10,31-33 A transdermal patch may also appeal to patients who have difficulty taking sublingual medications. For example, patients who experience unpleasant dysgeusia after taking SLA or find sublingual administration undesirable may want to consider this transdermal formulation. Availability of a wide array of formulation options can increase the possibility of finding a medication that is efficacious and tolerable and that the individual with schizophrenia is willing to adhere to.
A limitation of this study is that it was conducted in hospitalized patients with nurses completing daily application of the patch. While this design was selected to maximize the safety of participants and integrity of the study, real-world clinical use will be important to help inform the field about outpatient use, where patients or caregivers would apply the daily HP-3070 patch.
In conclusion, we have found that the HP-3070 asenapine transdermal drug delivery system appears to be efficacious, safe, and well tolerated. Given the known unmet needs in the treatment of schizophrenia, patients, caregivers, and health care providers are in search of new treatment options that can be individually optimized for patient use. As the first and only transdermal antipsychotic available in the United States, HP-3070 provides a novel and potentially preferred treatment formulation for individuals with schizophrenia.
Submitted: July 17, 2020; accepted October 21, 2020.
Published online: December 15, 2020.
Potential conflicts of interest: Dr Citrome has received non-financial support from Hisamitsu Pharmaceutical Co, Inc. and personal fees from Noven Pharmaceuticals, Inc, during the conduct of the study; personal fees from Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, BioXcel, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Eli Lilly, Forum, Genentech, Impel, Indivior, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Luye, Meiji, Merck, Medivation, Mylan, Neurocrine, Novartis, Noven, Osmotica, Otsuka, Pfizer, Indivior/Reckitt Benckiser, Reviva, Sage, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda, outside the submitted work; stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer purchased > 10 years ago; and royalties from Springer Healthcare (book), UpToDate (reviewer), and Wiley (Editor-in-Chief, International Journal of Clinical Practice). Dr Walling has received non-financial support from Hisamitsu Pharmaceutical Co, Inc, during the conduct of the study; grants from AbbVie, Acadia, Alkermes, Allergan, Avanir, Boehringer Ingelheim, CoMentis, Intra-Cellular Therapies, Janssen, Johnson & Johnson PRD, Lundbeck, Lupin, Novartis, Noven, Omeros, Otsuka, Pfizer, Roche, Sunovion, Takeda, and Zogenix and personal fees from Janssen and Otsuka, outside the submitted work. Dr Zeni has received non-financial support from Hisamitsu Pharmaceutical Co, Inc, during the conduct of the study; personal fees from Noven Pharmaceuticals, Inc, as an employee during the time that the work was performed; and personal fees from Sunovion Pharmaceuticals as a current employee, outside the submitted work. Drs Starling and Komaroff and Ms Park have received non-financial support from Hisamitsu Pharmaceutical Co, Inc, during the conduct of the study and personal fees from Noven Pharmaceuticals, Inc, outside the submitted work. Dr Terahara and Mr Kuriki have received non-financial support from Hisamitsu Pharmaceutical Co, Inc, and personal fees from Hisamitsu Pharmaceutical Co, Inc, outside the submitted work.
Funding/support: This study was funded by Hisamitsu Pharmaceutical Co, Inc.
Role of the sponsor: Although personnel at Hisamitsu Pharmaceutical Co, Inc. reviewed the manuscript, final approval for the decision to submit the manuscript was the sole decision of the authors.
Previous presentation: Presented at the American College of Neuropsychopharmacology Annual Meeting; Hollywood, Florida; December 9-13, 2018 ▪ Society of Biological Psychiatry Annual Meeting; Chicago, Illinois; May 16-18, 2019 ▪ American Psychiatric Association Annual Meeting, San Francisco, California; May 18-22, 2019 ▪ American Society of Clinical Psychopharmacology Annual Meeting; Scottsdale, Arizona; May 28-31, 2019 ▪ Psych Congress; San Diego, California; October 3-6, 2019 ▪ and Neuroscience Education Institute Congress; Colorado Springs, Colorado; November 7-10, 2019.
Acknowledgments: Medical writing and editorial assistance were provided by Michelle L. Jones, PhD, MWC, of PharmaWrite, LLC, and were funded by Hisamitsu Pharmaceutical Co, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines.”
Supplementary material: See accompanying pages.
REFERENCES
1.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. PubMed CrossRef
2. Faries DE, Ascher-Svanum H, Nyhuis AW, et al. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9(1):54. PubMed CrossRef
3. CrossRef Tsutsumi C, Uchida H, Suzuki T, et al. The evolution of antipsychotic switch and polypharmacy in natural practice: a longitudinal perspective. Schizophr Res. 2011;130(1-3):40-46. PubMed CrossRef
4. Weiden PJ, Simpson GM, Potkin SG, et al. Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry. 2003;64(5):580-588. PubMed CrossRef
5. CrossRef Citrome L. Interpreting and applying the CATIE results: with CATIE, context is key, when sorting out Phases 1, 1A, 1B, 2E, and 2T. Psychiatry (Edgmont). 2007;4(10):23-29. PubMed
6. Ayyagari R, Thomason D, Mu F, et al. Association of antipsychotic treatment switching in patients with schizophrenia, bipolar, and major depressive disorders. J Med Econ. 2020;23(2):204-212. PubMed CrossRef
7. Cerovecki A, Musil R, Klimke A, et al. Withdrawal symptoms and rebound syndromes associated with switching and discontinuing atypical antipsychotics: theoretical background and practical recommendations. CNS Drugs. 2013;27(7):545-572. PubMed CrossRef
8. Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879-911. PubMed CrossRef
9. bMedDassori AM, Copeland LA, Zeber JE, et al. Factors in second-generation antipsychotic switching patterns in a national sample of older veterans with schizophrenia. Psychiatr Serv. 2011;62(1):47-53. PubMed CrossRe
10.Citrome L, Zeni CM, Correll CU. Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry. 2019;80(4):e1-e10. PubMed CrossRef
11.Secuado (asenapine) transdermal system [package insert]. Tosu, Japan: Hisamitsu Pharmaceutical Co, Inc; 2019.
12.Noven Pharmaceuticals, Inc. US FDA approves SECUADO (asenapine) transdermal system, the first-and-only transdermal patch for the treatment of adults with schizophrenia [press release]. October 15, 2019. Accessed October 23, 2019.
13.Saphris (asenapine) sublingual tablets [package insert]. Irvine, CA: Allergan USA, Inc; 2017.
14.Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762-1784. PubMed CrossRef
15.Stevens JR, Justin Coffey M, Fojtik M, et al. The use of transdermal therapeutic systems in psychiatric care: a primer on patches. Psychosomatics. 2015;56(5):423-444. PubMed CrossRef
16.Castelli M, Suzuki K, Komaroff M, et al. Pharmacokinetic profile of asenapine transdermal system HP-3070: the first antipsychotic patch in the US [poster]. Presented at American Society for Clinical Psychopharmacology (ASCP) 2020 Virtual Annual Meeting, May 29-30, 2020.
17.Chapel S, Hutmacher MM, Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297-1308. PubMed CrossRef
18.Dogterom P, Timmer C, de Greef R, et al. Asenapine safety, tolerability, and pharmacokinetics after single and multiple doses in healthy volunteers. Clin Pharmacol Drug Dev. 2012;1(4):131-143. PubMed CrossRef
19.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
20.Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976:217-222.
21.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed CrossRef
22.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212:11-19. PubMed CrossRef
23.Chen X, Luo X, Capizzi T. The application of enhanced parallel gatekeeping strategies. Stat Med. 2005;24(9):1385-1397. PubMed CrossRef
24.Dmitrienko A, Tamhane AC. Gatekeeping procedures with clinical trial applications. Pharm Stat. 2007;6(3):171-180. PubMed CrossRef
25.Dmitrienko A, Tamhane AC, Wiens BL. General multistage gatekeeping procedures. Biom J. 2008;50(5):667-677. PubMed CrossRef
26.Citrome L. Asenapine review, part II: clinical efficacy, safety and tolerability. Expert Opin Drug Saf. 2014;13(6):803-830. PubMed
27.Emsam (selegiline transdermal system) [package insert]. Morgantown, WV: Mylan Specialty LP; 2020.
28.Neupro (rotigotine transdermal system) [prescribing information]. Smyrna, GA: UCB, Inc; 2020.
29.Kane JM, Cohen M, Zhao J, et al. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106-115. PubMed CrossRef
30.Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68(10):1492-1500. PubMed CrossRef
31.Pai MC, Aref H, Bassil N, et al. Real-world evaluation of compliance and preference in Alzheimer’s disease treatment. Clin Interv Aging. 2015;10:1779-1787. PubMed CrossRef
32.Winblad B, Kawata AK, Beusterien KM, et al. Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(5):485-491. PubMed CrossRef
33.Exelon patch (rivastigmine transdermal system) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2020.
This PDF is free for all visitors!
Save
Cite