Background: Inflammatory responses from chronic infection might affect the brain and increase the risk of depressive disorder. However, the temporal association between chronic infection (eg, tuberculosis [TB]) and incident depressive disorder has not been prospectively evaluated.
Objective: To determine the association of pulmonary tuberculosis (PTB) and anti-TB drugs with incident depressive disorder (ICD-9-CM codes 296.2x-296.3x, 300.4, and 311.x).
Method: From January 1, 2000, we identified adult patients with PTB from the Taiwan National Health Insurance Research Database. A control cohort without PTB, matched for age (± 5 years), sex, comorbidities, and income level, was selected for comparison. The 2 cohorts were followed until December 31, 2011, and observed for occurrence of depressive disorder.
Results: Of the 23,145 patients (4,629 study patients and 18,516 matched controls), 302 (1.3%) had depressive disorder during a mean follow-up period of 6.53 years, including 79 study patients (1.71%) and 223 controls (1.20%). After adjusting for age, sex, comorbidities, and income level in the Cox proportional hazards model, PTB was found to be an independent risk factor of incident depressive disorder (adjusted hazard ratio [HR], 1.74; 95% CI, 1.35-2.25). The risk of incident depressive disorder was significantly higher (adjusted HR, 2.54; 95% CI, 1.19-5.45) in patients with TB who received more than 60 defined daily doses (DDDs) of ethambutol, and the effect was dose-dependent.
Conclusions: PTB patients had a higher risk of incident depressive disorder, particular in those with an ethambutol dose of more than 60 DDDs. Depressive disorder should be sought in patients following tuberculosis.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
ABSTRACT
Background: Inflammatory responses from chronic infection might affect the brain and increase the risk of depressive disorder. However, the temporal association between chronic infection (eg, tuberculosis [TB]) and incident depressive disorder has not been prospectively evaluated.
Objective: To determine the association of pulmonary tuberculosis (PTB) and anti-TB drugs with incident depressive disorder (ICD-9-CM codes 296.2x-296.3x, 300.4, and 311.x).
Method: From January 1, 2000, we identified adult patients with PTB from the Taiwan National Health Insurance Research Database. A control cohort without PTB, matched for age (± 5 years), sex, comorbidities, and income level, was selected for comparison. The 2 cohorts were followed until December 31, 2011, and observed for occurrence of depressive disorder.
Results: Of the 23,145 patients (4,629 study patients and 18,516 matched controls), 302 (1.3%) had depressive disorder during a mean follow-up period of 6.53 years, including 79 study patients (1.71%) and 223 controls (1.20%). After adjusting for age, sex, comorbidities, and income level in the Cox proportional hazards model, PTB was found to be an independent risk factor of incident depressive disorder (adjusted hazard ratio [HR], 1.74; 95% CI, 1.35-2.25). The risk of incident depressive disorder was significantly higher (adjusted HR, 2.54; 95% CI, 1.19-5.45) in patients with TB who received more than 60 defined daily doses (DDDs) of ethambutol, and the effect was dose-dependent.
Conclusions: PTB patients had a higher risk of incident depressive disorder, particular in those with an ethambutol dose of more than 60 DDDs. Depressive disorder should be sought in patients following tuberculosis.
J Clin Psychiatry 2015;76(4):e505-e511
© Copyright 2015 Physicians Postgraduate Press, Inc.
Submitted: July 22, 2014; accepted November 18, 2014 (doi:10.4088/JCP.14m09403).
Corresponding author: Chung-Yeh Deng, PhD, National Yang-Ming University, 155, Section 2, Ni-Long St, Taipei, Taiwan 11221 ([email protected]).
Depressive disorder is a leading cause of disability worldwide.1 Accumulating evidence from human and animal studies indicates that activation of inflammatory reactions can induce symptoms of depressive disorder.2-4 Proinflammatory cytokines such as interferon-γ and tumor necrosis factor-α might affect development of depressive disorder by regulating neuronal excitability, synaptic transmission, synaptic plasticity, and neuronal survival.2,3,5
Tuberculosis (TB) is a chronic infectious disease that is prevalent throughout the world. A large proportion of TB patients have depressive disorder, which could hamper their adherence to TB treatment.6 However, few studies have investigated whether TB infection is associated with incident depressive disorder.6-8 Tuberculosis and depressive disorder share many risk factors (eg, poverty and homelessness)6 that could partially explain the high prevalence of comorbid depressive disorder in TB patients. In addition, certain anti-TB medications—isoniazid, ethambutol, and rifampin—were found to be associated with the development of mental illness.6 Therefore, to determine if TB infection is independently associated with incident depressive disorder, we conducted a nationwide, population-based cohort study of the risk of depressive disorder in people with and without TB.
METHOD
Data Source
In this nationwide cohort study, we analyzed patient data obtained from the National Health Insurance Research Database (NHIRD), which is managed by the Taiwan National Health Research Institutes. The NHIRD can be found at http://nhird.nhri.org.tw/en/index.htm and is provided to scientists for research purposes. The NHIRD contains health care data from more than 99% of the population in Taiwan.9 In the NHIRD, the accuracy of diagnoses of major diseases such as diabetes mellitus and cerebrovascular disease has been well validated.10,11 This study was approved by the institutional review board of Taipei City Hospital.
Study Subjects
In this cohort study, we selected subjects who were aged 20 years or older and were newly diagnosed with pulmonary TB (PTB) between January 1, 2000, and December 31, 2010. The diagnosis of new PTB required the presence of ICD-9-CM code 010, 011, 012, or 018 plus prescription of at least 2 anti-TB drugs (eg, isoniazid, ethambutol, rifampin, pyrazinamide).12 Patients who had received a diagnosis of depressive disorder (ICD-9-CM codes 296.2x-296.3x, 300.4, and 311.x) or bipolar disorder (ICD-9-CM codes 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, 296.8, 296.80, and 296.89) before the TB diagnosis were excluded.

- Pulmonary tuberculosis was an independent risk factor for incident depressive disorder.
- An ethambutol dose of more than 60 defined daily doses increased the risk of incident depressive disorder in a dose-dependent manner.
The control group was matched by age (± 5 years), sex, year of enrollment, income level, and comorbidities, including diabetes (ICD-9 code 250), coronary artery disease (ICD-9 code 411-414), congestive heart failure (ICD-9 code 428.0), cerebrovascular disease (ICD-9 code 430-437, excluding 432), cancer (ICD-9 code 140-208), and chronic kidney disease (ICD-9 code 580-587). Four controls were randomly selected for each PTB patient.13,14 A person was considered to have a comorbidity only if the condition occurred in an inpatient setting or 2 or more outpatient visits.15 Control subjects were excluded if they had received a diagnostic code for PTB or preexisting depressive or bipolar disorder before inclusion in the study. Both the exposure and control groups were followed until a diagnosis of depressive disorder, death, withdrawal from the National Health Insurance system, or December 31, 2011.
Variables and Measures
The outcome depressive disorder was defined as ICD-9-CM codes 296.2x-296.3x, 300.4, and 311.x16 plus a psychiatrist-assigned diagnosis. The prescribed daily dose of anti-TB drugs was expressed as defined daily dose (DDD) in this study, according to the Anatomic Therapeutic Chemical Classification/Defined Daily Doses (ATC/DDD) system.17 Income level, an indicator of the socioeconomic status of the study subjects, was calculated from the average monthly income (New Taiwan dollar) of the insured person and grouped into 3 levels: low (≤ NT$20,000) (US $635), intermediate (NT$20,000 to < NT$40,000) (US $635 to < $1,270), and high (≥ NT$40,000) (US ≥ $1,270).
Statistical Analysis
First, the demographic data of the study subjects were analyzed. Continuous data are presented as mean (SD), and the t test was used for comparisons between groups. Categorical data were analyzed by the Pearson χ2 test where appropriate.
To identify independent risk factors for depressive disorder, a Cox proportional hazards model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) after adjusting for age, sex, income level, and comorbidities, including diabetes, coronary artery disease, congestive heart failure, cerebrovascular disease, cancer, and chronic kidney disease. The assumption of proportional hazards was confirmed by plotting the graph of the survival function versus survival time and the graph of the log (−log [survival]) versus the log of survival time.
A Cox proportional hazards model was also used to evaluate the effects of anti-TB drugs on the development of depressive disorder among PTB patients. The multivariate-adjusted HR for depressive disorder was calculated, and a 2-tailed P value of less than .05 was considered to indicate statistical significance.
To examine the robustness of the main findings, sensitivity analyses were conducted after stratifying study subjects by sex, age, comorbidities, income level, and treatment regimen, according to the sample size of each subgroup. All data management and analyses were performed using the SAS 9.3 software package (SAS Institute; Cary, North Carolina).
RESULTS
Participant Selection
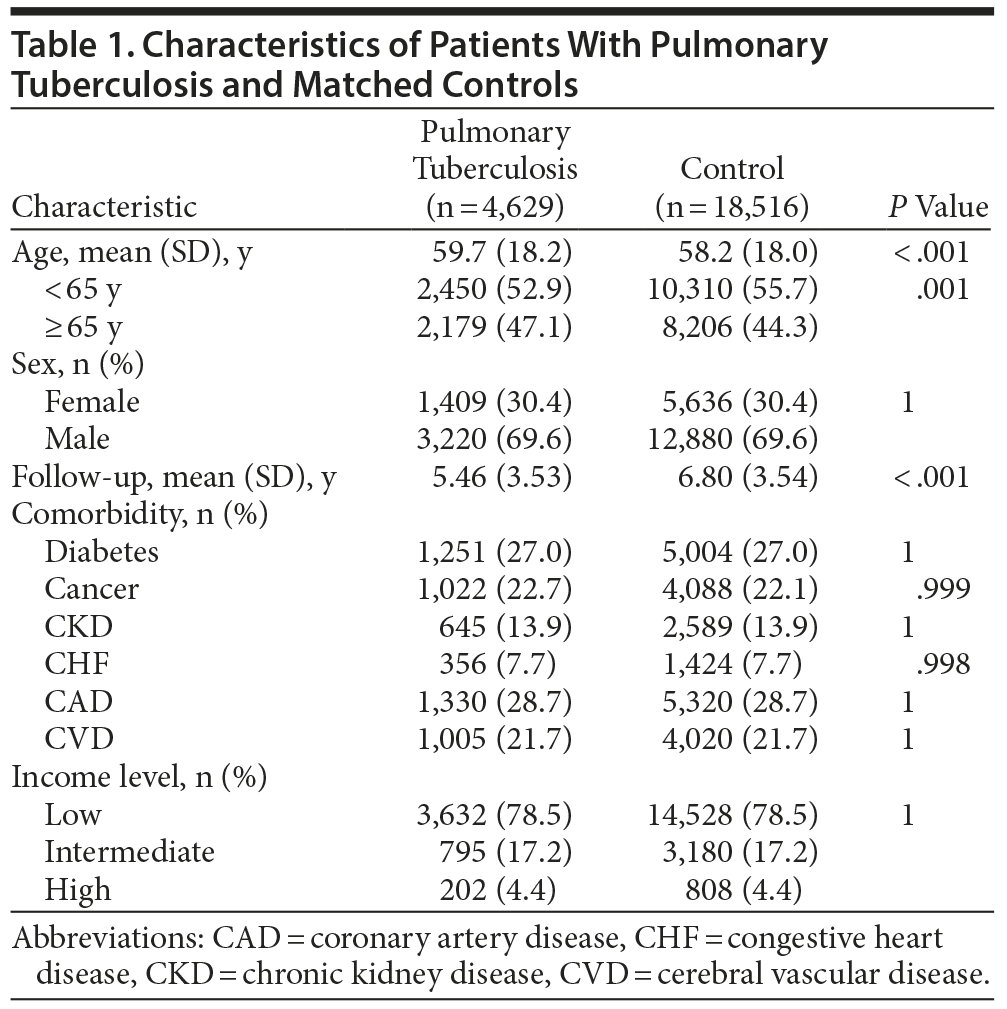
We identified 4,983 individuals who had received a new diagnosis of PTB during the period from January 1, 2000, through December 31, 2010. After excluding those younger than 20 years (n = 180), those with antecedent depressive disorder (n = 169), and those with antecedent bipolar disorder (n = 5), the remaining 4,629 patients were included in the PTB group. Another 18,516 subjects without PTB were randomly selected for the control group. The overall mean (SD) age was 59.7 (18.2) years, and 62.9% of the subjects in the PTB group were male. Mean (SD) follow-up time was 5.46 (3.53) years in the PTB cohort and 6.80 (3.54) years in the control group. The demographic characteristics and comorbidities of the 2 groups are shown in Table 1. The PTB patients were slightly older than the control group (59.7 vs 58.2 years). There were no significant differences in sex, comorbidities, or income level between the 2 groups.
Incidence Rate of Depressive Disorders in PTB and Control Group
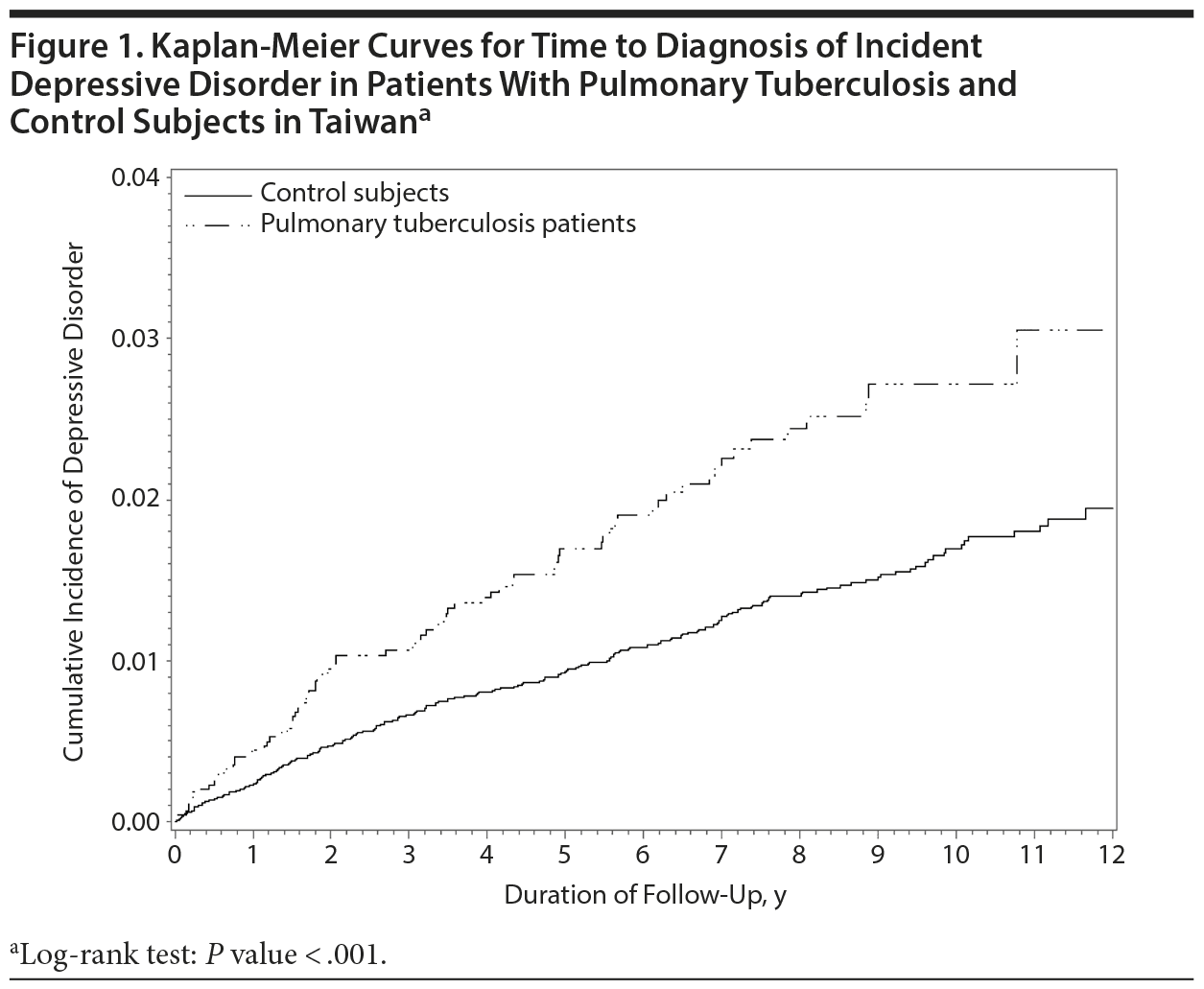
During the study follow-up period, 302 individuals had new onset of depressive disorder, including 79 study patients (1.71%) and 223 controls (1.20%). The incidence rate of depressive disorder per 1,000 person-years was 3.13 in the PTB patients and 1.77 in the control group (P < .001). The incidence risk ratio (IRR) of depressive disorder between the PTB and control groups was 1.76 (95% CI, 1.36-2.28; P < .001). Time to diagnosis of incident depressive disorder was significantly shorter in people with PTB than in the control group (P < .001, log rank test; Figure 1).
Among 4,629 PTB patients, 26 of 1,932 PTB patients treated ≤ 6 months and 53 of 2,698 PTB patients treated > 6 months had new onset of depressive disorder, which corresponds to the incidences of depressive disorder of 2.76 and 3.35 per 1,000 person-years, respectively. Comparing with control group, the incidence risk ratio of depressive disorder in PTB patients treated ≤ 6 months and > 6 months were 1.56 (95% CI, 1.04-2.34; P = .03) and 1.89 (95% CI, 1.40-2.55; P < .001), respectively.
Risks Factors for Depressive Disorder in Patients With and Without PTB
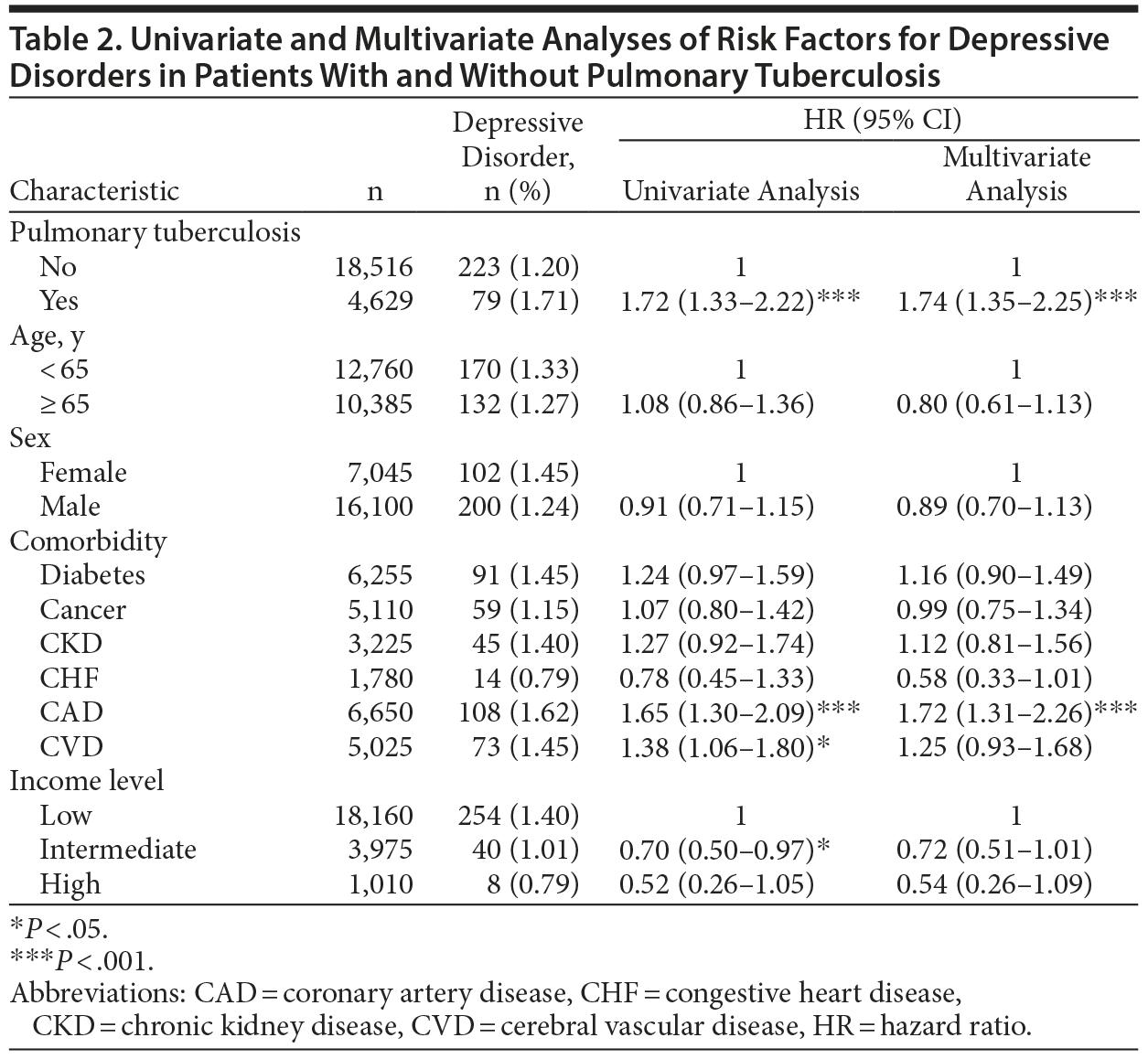
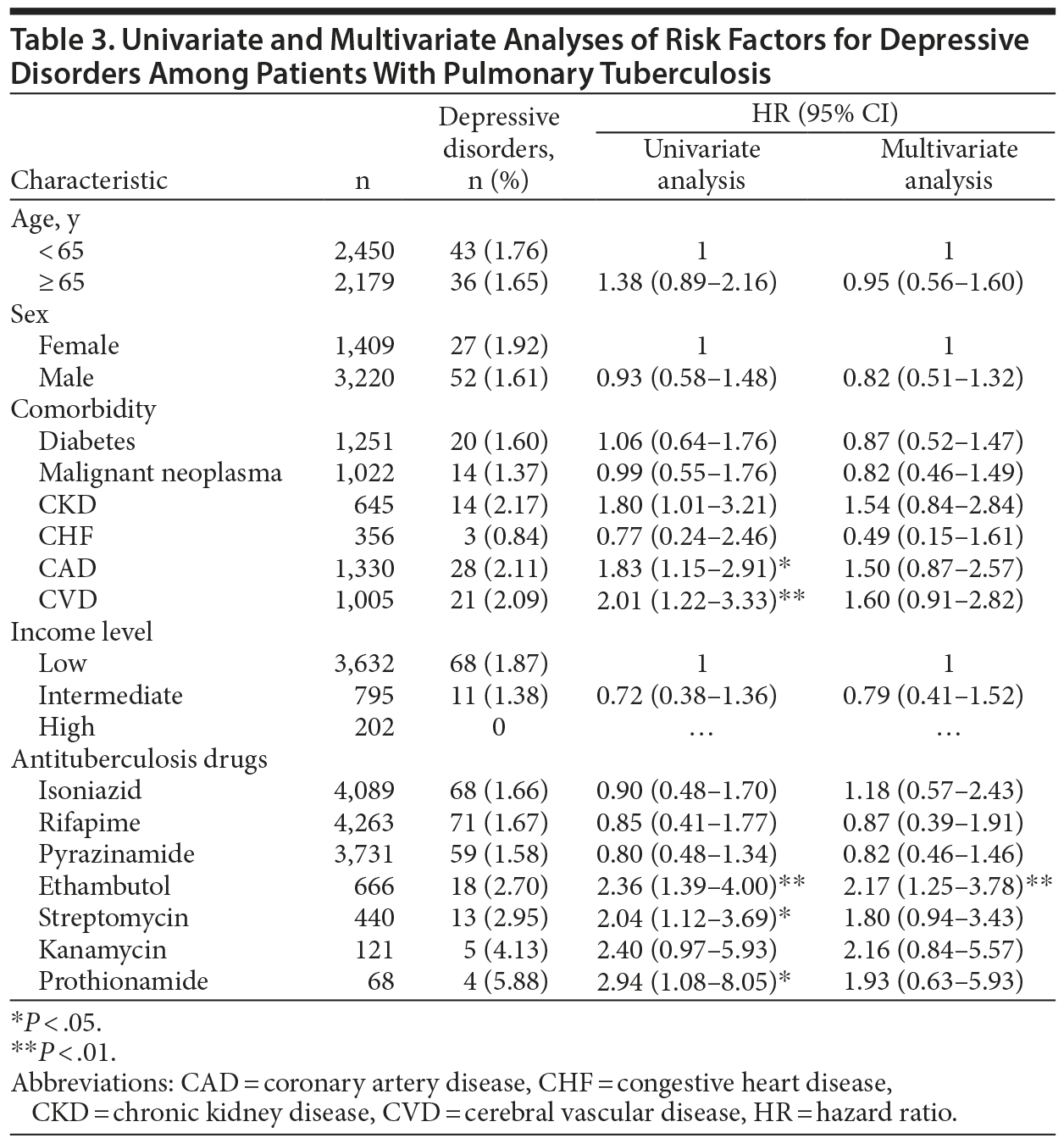
A Cox proportional hazards model was used to identify independent risk factors for depressive disorder. After we adjusted for age, sex, comorbidities, and income level, the risk of depressive disorder was significantly higher in the PTB group (adjusted HR [AHR], 1.74; 95% CI, 1.35-2.25; P < .001) than in the control group (Table 2). Additionally, coronary artery disease was independently associated with depressive disorder (AHR, 1.72; 95% CI, 1.31-2.26).
Sensitivity Analysis for the Association Between PTB and Depressive Disorders
Figure 2 shows the result of sensitivity analysis of the association between PTB and depressive disorder after patients were stratified by age group, sex, comorbidities, and income level. Pulmonary tuberculosis was a significant risk factor for depressive disorder in all patient subgroups except patients with cancer or congestive heart disease and those with an intermediate income level.
Effects of Anti-TB Drugs on Incident Depressive Disorder Within PTB Patients
In multivariate analysis of the effects of anti-TB drugs on incident depressive disorder within PTB patients, the risk of depressive disorder was significantly higher in patients taking ethambutol (AHR, 2.17; 95% CI, 1.25-3.78) than in those not receiving the drug, after adjusting for demographic factors, comorbidities, income level, and treatment regimen (Table 3).
Incidence Rate of Depressive Disorders in PTB Patients Receiving Different Defined Daily Doses of Ethambutol
Among the 79 PTB patients with new-incident depressive disorder, 61 did not receive ethambutol, 2 received 1 to 60 DDDs of ethambutol, 8 received 61 to 180 DDDs of ethambutol, and 8 received more than 180 DDDs of ethambutol. The corresponding incidence rates were 2.70, 2.03, 7.84, and 12.16 per 1,000 person-years.
Dose-Response Relationship Between Ethambutol and Risk of Depressive Disorder
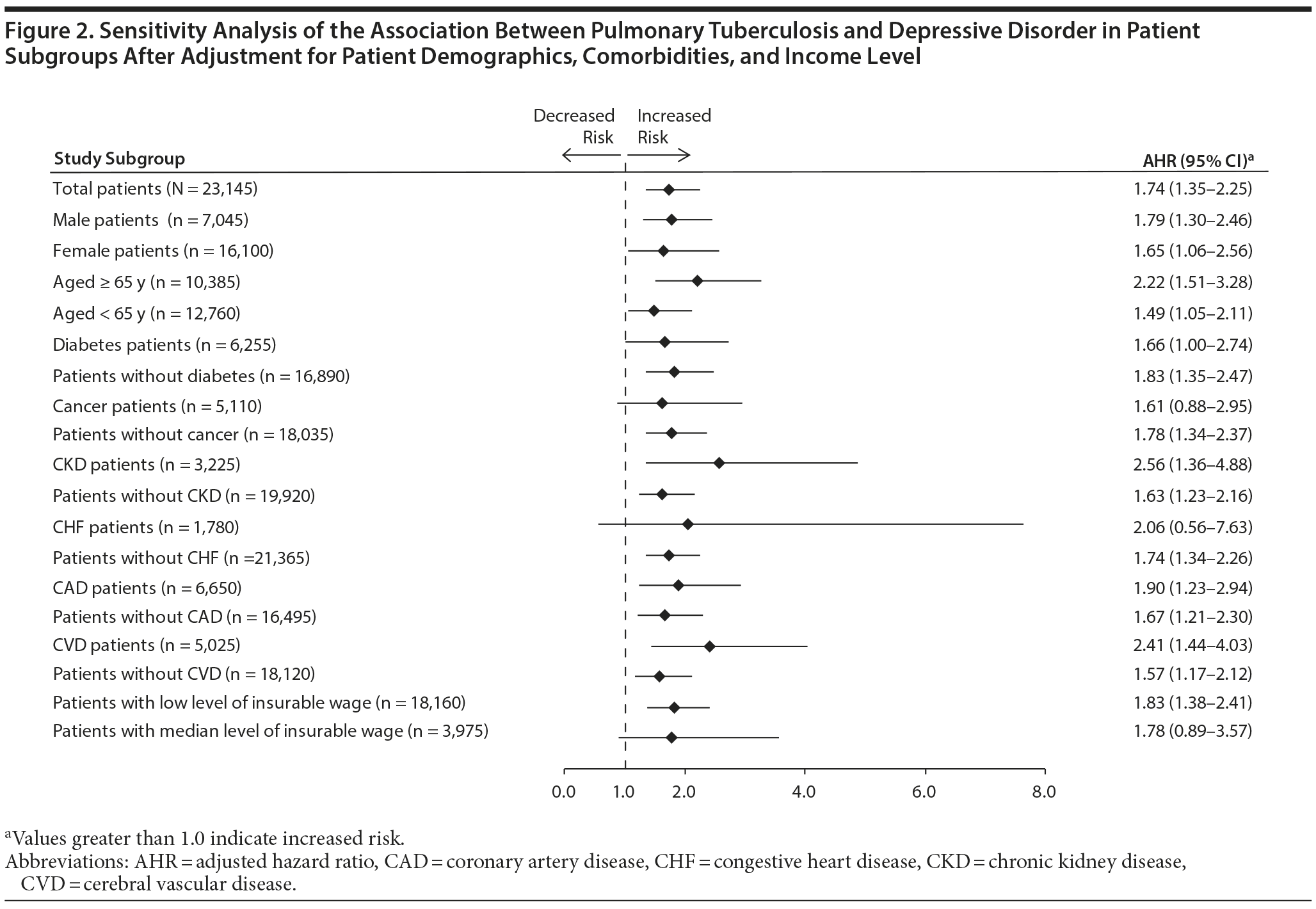
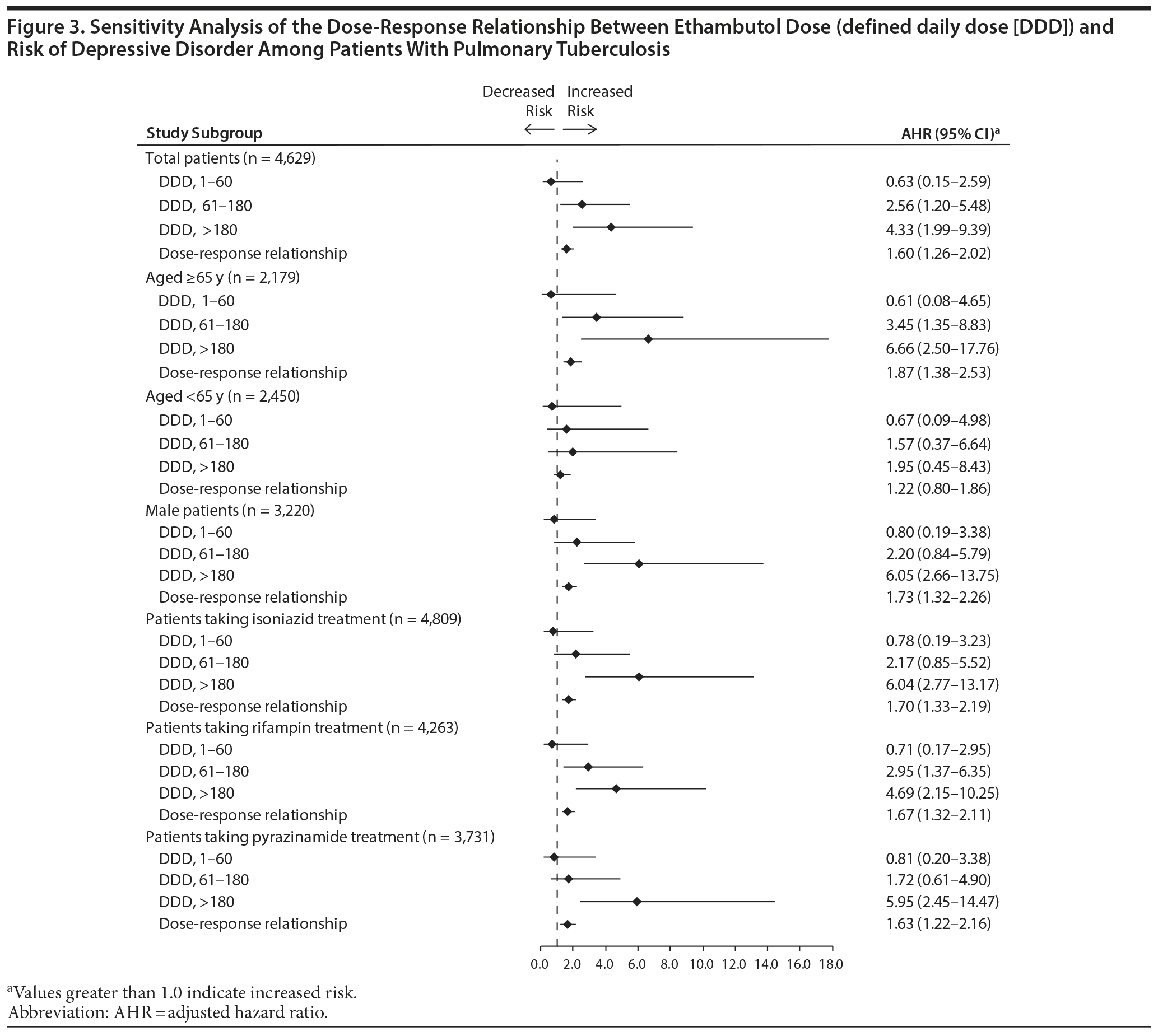
The dose-response relationship between ethambutol and risk of depressive disorder was evaluated in the above-mentioned groups. Figure 3 shows that the risk of depressive disorder was significantly higher among patients receiving 61 to 180 DDDs (AHR, 2.56; 95% CI, 1.20-5.48) and more than 180 DDDs (AHR, 4.33; 95% CI, 1.99-9.39) of ethambutol. The risk of developing a depressive disorder increased as ethambutol dose increased (AHR, 1.60; 95% CI, 1.26-2.02).
Sensitivity Analysis for the Dose-Response Relationship Between Ethambutol and Risk of Depressive Disorder
Figure 3 presents the results of sensitivity analysis for the dose-response relationship between ethambutol and incident depressive disorder in PTB patients after stratifying patients by age, sex, and treatment regimen. Cox regression analysis showed a significant dose-response relationship between ethambutol dose and incident depressive disorder in patients aged 65 years or older, men, and patients receiving isoniazid, rifampin, or pyrazinamide.
Risk of Depressive Disorder in TB Patients Who Did and Did Not Receive Ethambutol
The risk of incident depressive disorder in relation to ethambutol use was evaluated among PTB patients, as compared with the corresponding control groups. The Cox proportional hazards model revealed that, after adjusting for age, sex, comorbidities, and income level, the risk of incident depressive disorder was significantly increased both among PTB patients who had received ethambutol (AHR, 3.78; 95% CI, 2.08-6.89) and those who had not (AHR, 1.51; 95% CI, 1.12-2.00).
DISCUSSION
This is the first longitudinal study of the temporal association between PTB infection and incident depressive disorder. The results show that, as compared with controls, PTB patients had a higher risk for developing depressive disorder, after adjustments for demographic data, comorbidities, and income level. In addition, an ethambutol dose greater than 60 DDDs was associated with a dose-dependent increase in the risk of incident depressive disorder among PTB patients. However, the association of PTB infection and incident depressive disorder was significant even among PTB patients who did not take ethambutol.
Recently, the link between infection and later development of depressive disorder has received much attention.2,3 However, to our knowledge, this temporal association was investigated in only 2 longitudinal studies,18,19 both of which were nationwide, population-based studies. The findings suggest that the risk of incident depressive disorder is higher in people with a history of sepsis18 or herpes zoster infection.19 However, those studies defined history of infection according to crude diagnosis, without confirmation by treatment regimen,18,19 and 1 did not adequately control for potential confounders (eg, comorbidities and socioeconomic status).18 Clearly, more studies are needed.
After we controlled for comorbidities and income level, the risk of depressive disorder was higher after a PTB diagnosis in the present study. The association between PTB and incident depressive disorder was strong in analysis stratified by age, sex, comorbidities, and income level. Pulmonary tuberculosis increased the risk of incident depressive disorder in all subgroups except patients with cancer or congestive heart disease and those with intermediate incomes.
Activation of indoleamine 2,3-dioxygenase (IDO) might explain the increased risk of depressive disorder in patients after a PTB diagnosis. TB is a chronic inflammatory disease that results in the release of proinflammatory cytokines such as interleukin-1β, interferon-γ, and tumor necrosis factor-α. These proinflammatory cytokines can activate IDO, which degrades tryptophan, an essential amino acid that is the limiting factor in serotonin synthesis.2,3 Because IDO is expressed in the brain,2,3 the increased enzymatic activity of IDO may substantially decrease serotonin biosynthesis, resulting in the development of depressive disorder. Moreover, when the enzymatic activity of IDO increases, IDO degrades tryptophan into quinolinic acid, a neurotoxic metabolite that acts as an agonist to the N-methyl-d-aspartate (NMDA) glutamate receptor.2 The increased level of NMDA agonists in the brain can accelerate development of depressive disorder.20 A recent study supported this immune hypothesis: levels of quinolinic acid in the subgenual anterior cingulate cortex and anterior midcingulate cortex were significantly higher in patients with acute depression than in control patients.21
In the present study, patients receiving more than 60 DDDs of ethambutol had a higher risk of incident depressive disorder, and the relationship between ethambutol dose and incident depressive disorder was dose dependent, as indicated by analysis of patients grouped by ethambutol dose. After stratification according to age, sex, and treatment regimen, the dose-response relationship was statistically significant in patients aged 65 years or older, men, and patients taking isoniazid, rifampin, or pyrazinamide.
The presence of prolonged inflammation and more severity might explain the increased risk of incident depressive disorder in patients receiving more than 60 DDDs of ethambutol. Ethambutol is often prescribed in the first 2 to 6 months of TB treatment.22 However, the duration of ethambutol treatment might be extended by more than 6 months for drug-resistant mycobacterium TB (MTB) (eg, by 6 months for isoniazid-resistant MTB and by 12-18 months for rifampin-resistant MTB).23,24 In this study, we found that 13.3% of the patients receiving 61 to 180 DDDs of ethambutol and 16.8% of patients receiving more than 180 DDDs of ethambutol received second-line anti-TB drugs; however, only 11.7% of patients not taking ethambutol received such drugs. Second-line anti-TB drugs are often prescribed for drug-resistant TB, which can cause prolonged inflammation and thus might increase the risk of incident depressive disorder among PTB patients.
The present study has some limitations. First, detailed personal information (eg, physical disability) and socioeconomic status (eg, low education) was not available. Previous cross-sectional studies suggest that these factors are associated with depressive disorders.7,8 To minimize these confounding factors in the present study, major comorbidities were matched between PTB patients and the controls. In addition, income level was used to represent socioeconomic status, although this may not have resulted in adequate adjustment for unmeasured confounding. Second, this study included only patients who had contacts with health care facilities during the study follow-up period. As a result, if PTB or depressive patients had no hospital contacts, they were not included in this analysis. However, Taiwan has initiated universal health insurance to assure patients’ accessibility to health care since 199525; more than 99% of the population was covered under the program in Taiwan.9 Therefore, underdiagnosis of depressive disorder or PTB, if it occurred in this study, would not likely be a major bias in this analysis. Finally, the present study population was limited to ethnic Chinese. Whether the present findings can be generalized to other ethnic groups remains to be determined. The strengths of this study are that it was a nationwide PTB cohort with a comparable control group and was thus able to evaluate the impact of PTB infection on incident depressive disorder.
In conclusion, this was the first cohort study of the temporal association between PTB infection and depressive disorder. Our results suggest that PTB infection is an etiologic factor in subsequent depressive disorder. Moreover, PTB patients who received more than 60 DDDs of ethambutol had a dose-dependent increase in the risk of incident depressive disorder. Because depressive disorder is a modifiable illness that is amenable to treatment, clinicians should carefully monitor TB patients for symptoms of incident depressive disorder, with particular attention to those receiving treatment with ethambutol. The incident depressive disorder should be evaluated carefully in patients following tuberculosis.
Drug names: ethambutol (Myambutol and others), isoniazid (Laniazid, Rifater, and others), rifampin (Rimactane, Rifadin, and others).
Author affiliations: Section of Infectious Diseases (Dr Yen) and Department of Education and Research (Dr Hu), Taipei City Hospital; Community Medicine Research Center and Institute of Public Health (Dr Yen, Chung, Hu, Lai, Huang and Chou) and Institute of Hospital and Health Care Administration (Dr Deng), National Yang-Ming University; Institute of Health Policy and Management, National Taiwan University, (Miss Lin), Taipei City; Jianan Psychiatric Center, MOHW, Tainan (Dr Chung); and Division of Endocrinology and Metabolism, Department of Internal Medicine, Puli Branch of Taichung Veterans General Hospital, Nantou (Dr Lai), Taiwan.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Acknowledgments: The authors are grateful to the members of Research Office for Health Data, Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis.
REFERENCES
1. Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858. PubMed doi:10.1016/S0140-6736(07)61415-9
2. Dantzer R, O’ Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56. PubMed doi:10.1038/nrn2297
3. Rosenblat JD, Cha DS, Mansur RB, et al. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:23-34. PubMed doi:10.1016/j.pnpbp.2014.01.013
4. Goldstein BI, Kemp DE, Soczynska JK, et al. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70(8):1078-1090. PubMed doi:10.4088/JCP.08r04505
5. Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181-213. PubMed doi:10.1016/j.bbi.2010.10.015
6. Doherty AM, Kelly J, McDonald C, et al. A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry. 2013;35(4):398-406. PubMed doi:10.1016/j.genhosppsych.2013.03.018
7. Berg J, Nyamathi A, Christiani A, et al. Predictors of screening results for depressive symptoms among homeless adults in Los Angeles with latent tuberculosis. Res Nurs Health. 2005;28(3):220-229. PubMed doi:10.1002/nur.20074
8. Peltzer K, Naidoo P, Matseke G, et al. Prevalence of psychological distress and associated factors in tuberculosis patients in public primary care clinics in South Africa. BMC Psychiatry. 2012;12(1):89. PubMed doi:10.1186/1471-244X-12-89
9. Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906-1914. PubMed doi:10.1001/2012.jama.11975
10. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
11. Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104(3):157-163. PubMed
12. Ou SM, Liu CJ, Teng CJ, et al. Impact of pulmonary and extrapulmonary tuberculosis infection in kidney transplantation: a nationwide population-based study in Taiwan. Transpl Infect Dis. 2012;14(5):502-509. PubMed doi:10.1111/j.1399-3062.2012.00737.x
13. Wacholder S, McLaughlin JK, Silverman DT, et al. Selection of controls in case-control studies, 1: principles. Am J Epidemiol. 1992;135(9):1019-1028. PubMed
14. Beaumont JJ, Steenland K, Minton A, et al. A computer program for incidence density sampling of controls in case-control studies nested within occupational cohort studies. Am J Epidemiol. 1989;129(1):212-219. PubMed
15. Chu SY, Chen YJ, Liu CJ, et al. Increased risk of acute myocardial infarction in systemic sclerosis: a nationwide population-based study. Am J Med. 2013;126(11):982-988. PubMed doi:10.1016/j.amjmed.2013.06.025
16. Chen MH, Su TP, Chen YS, et al. Higher risk of developing major depression and bipolar disorder in later life among adolescents with asthma: a nationwide prospective study. J Psychiatr Res. 2014;49:25-30. PubMed doi:10.1016/j.jpsychires.2013.10.015
17. WHO Collaborating Center for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2014. http://www.whocc.no/atc_ddd_index/. Updated December 19, 2014. Accessed January 30, 2015.
18. Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812-820. PubMed doi:10.1001/jamapsychiatry.2013.1111
19. Chen MH, Wei HT, Su TP, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76(4):285-291. PubMed doi:10.1097/PSY.0000000000000051
20. Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets. 2007;6(2):101-115. PubMed doi:10.2174/187152707780363267
21. Steiner J, Walter M, Gos T, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8(1):94. PubMed doi:10.1186/1742-2094-8-94
22. Onyebujoh P, Zumla A, Ribeiro I, et al. Treatment of tuberculosis: present status and future prospects. Bull World Health Organ. 2005;83(11):857-865. PubMed
23. Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603-662. PubMed doi:10.1164/rccm.167.4.603
24. Cattamanchi A, Dantes RB, Metcalfe JZ, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48(2):179-185. PubMed doi:10.1086/595689
25. Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan: results from a natural experiment. JAMA. 1997;278(2):89-93. PubMed doi:10.1001/jama.278.2.89
Save
Cite
Advertisement
GAM ID: sidebar-top