Association of Zolpidem Use and Subsequent Increased Risk of Epilepsy: A Population-Based, Case-Control Study

ABSTRACT
Objective: To evaluate the impact of long-term zolpidem use on the subsequent risk of epilepsy.
Method: We used data from the National Health Insurance system of Taiwan to conduct a population-based case-control study. We identified 4,972 newly diagnosed epilepsy patients (ICD-9-CM code 345) for the period of 2005-2010 as cases. For each epilepsy case, 4 controls without a history of epilepsy were randomly selected from the rest of the population. Zolpidem was used as a predictor of epilepsy.
Results: Patients with epilepsy exhibited an adjusted odds ratio (OR) of 1.86 (95% CI, 1.70-2.03) and were, therefore, more strongly associated with zolpidem exposure than control patients were. The adjusted OR of epilepsy increased with the increase of mean zolpidem exposure (g/y). Compared with the OR of nonusers, the adjusted OR was 1.64 (95% CI, 1.44-1.86) for those who had taken < 1.0 g/y of zolpidem and 2.38 (95% CI, 2.06-2.74) for those who had taken ≥ 20.0 g/y of zolpidem. An adjusted OR of 3.55 (95% CI, 2.94-4.28) was noted to be associated with epilepsy when users had stopped taking the drug less than 7 days earlier. The estimated risk declined to an OR of 1.62 (95% CI, 1.47-1.78) when users had stopped taking the drug more than 90 days earlier.
Conclusions: This population-based, retrospective case-control study revealed a possible increase in epilepsy risk with zolpidem use, at either typical or supratherapeutic doses. These findings might stimulate public interest in safety issues regarding zolpidem use.
J Clin Psychiatry 2014;75(10):e1127-e1132
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: December 18, 2013; accepted April 9, 2014 (doi:10.4088/JCP.13m08953.
Corresponding author: Chia-Hung Kao, MD, Graduate Institute of Clinical Medicine Science and School of Medicine, College of Medicine, China Medical University, No. 2, Yuh-Der Rd, Taichung 404, Taiwan ([email protected]).
Sleep problems are common in the general population; consequently, hypnotic drugs or sleeping pills are widely prescribed in adults.1 Previous investigations have revealed that approximately 25% of people are dissatisfied with their sleep quality and approximately 10% meet the criteria for insomnia syndrome.2,3 Epilepsy is also a common brain disease worldwide, with a prevalence of 0.5%-1% in humans.4,5 Based on its pathogenesis in adulthood, epilepsy might have an idiopathic origin or be caused by 3 main diseases (head injury, stroke, and brain tumor), as symptomatic epilepsies.6 In the human brain, γ-aminobutyric acid (GABA)-ergic neurons play a critical role in regulating the cortical stability in both sleep problems and epilepsy. With their GABA-ergic effects, benzodiazepines were used for a long time to treat sleep problems and epileptic seizures.
Zolpidem is a nonbenzodiazepine GABA-ergic drug that can be prescribed for short-term treatment of insomnia.7,8 A study published in 1991 reported that 10 mg of zolpidem per night is an effective therapeutic dose for treating sleep disturbances of various origins.9 The drug has been the most popular prescription for sleep problems in Taiwan for more than a decade. However, since 2000, some reports revealed that overuse of zolpidem could happen in a medical care system and that sudden withdrawal of the drug may result in epileptic seizures.7,8 Meanwhile, long-term zolpidem users were recently reported as having 4 times or higher risk of death by various causes compared with nonusers in the United States,10 besides the common adverse effects of zolpidem such as nausea, vomiting, amnesia, headache, delirium, hallucinations, short-term memory loss, nightmares, and sleepwalking.11,12 On the basis of these data, we supposed that long-term use of zolpidem might change the cortical stability and increase locomotor activities of users, thereby enhancing the likelihood of adult epilepsy.7,8,13 Therefore, we conducted this study by using a Taiwanese, nationwide, population-based database to determine whether epilepsy risk increases subsequent to zolpidem use.
METHOD
Data Sources
This study used the reimbursement data of the Taiwanese National Health Insurance (NHI) system for obtaining medical claims from 1996 to 2010. More than 99% of the population was enrolled in this insurance program by 2008.14,15 The Taiwan Department of Health has authorized the National Health Research Institutes (NHRI) to manage and maintain the claims data and to establish the National Health Insurance Research Database (NHIRD), which draws data from several randomly selected claims databases that are available for research and administrative purposes.

- Long-term use of zolpidem, even in typical doses, might change users’ cortical stability and increase locomotor activities, thereby enhancing epilepsy.
- Patients with epilepsy had an adjusted odds ratio (OR) of 1.86 times more strongly associated with zolpidem exposure than control patients were. The OR of developing epilepsy increased with the increase of mean zolpidem exposure per year.
- Withdrawal effects of zolpidem play a role in epileptogenesis, and their avoidance requires considerable time to taper the dose when discontinuing the drug.
In this study, we used the NHRI database to obtain medical reimbursement claims data from 1996 to 2010 for one million randomly selected people representative of the entire population of Taiwan. We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to identify diseases. The NHRI scrambles the personal identification information of all insurants before releasing the database to users. This study was approved by the Ethics Review Board at China Medical University (CMU-REC-101-012).
Study Patients
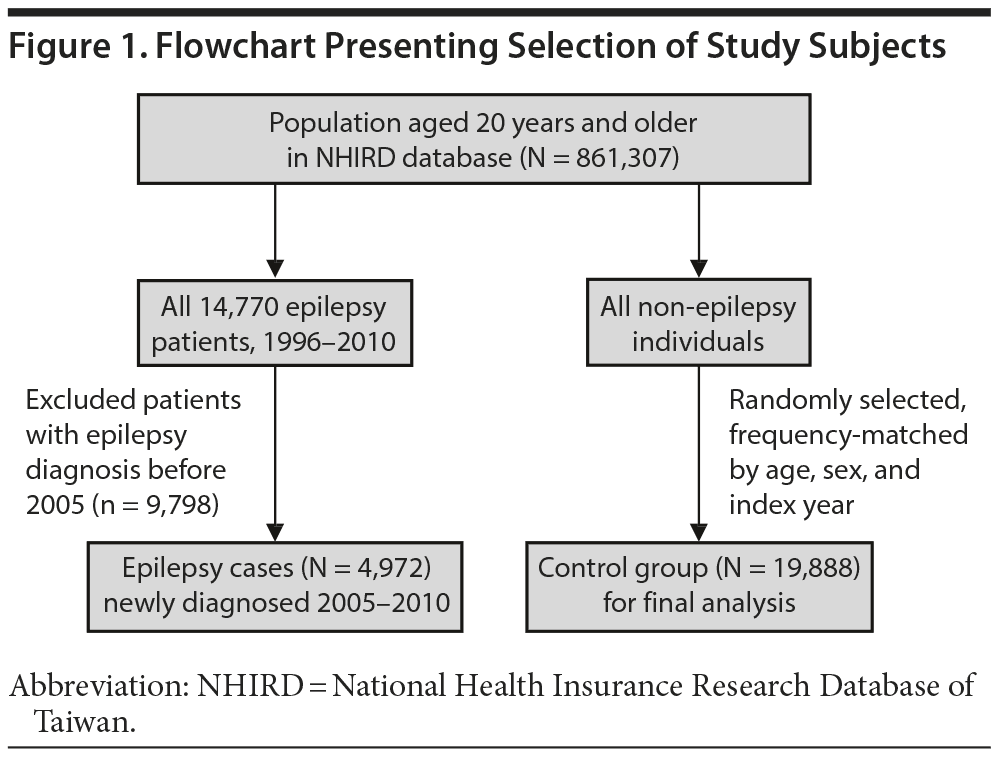
We used the claims data from 2005 to 2010 to conduct a population-based case-control study for investigating the risk of epilepsy associated with zolpidem use. Figure 1 shows the flowchart for selecting the study groups. Patients with epilepsy history prior to 2005 or aged < 20 years were excluded. We identified 4,972 newly diagnosed epilepsy patients (ICD-9-CM code 345) for the period of 2005-2010 as cases. The diagnosed date of epilepsy was used as the index date. For each epilepsy case, 4 controls without a history of epilepsy were randomly selected from the rest of the population, frequency-matched by sex, age, and index year. In this study, zolpidem was used as a predictor of epilepsy. The zolpidem medication history before the index date was estimated for each study patient. We calculated the annual mean exposure to zolpidem between the first exposure date and the index date for each study patient.
The other causes or risk factors for epilepsy were identified before the index date, including stroke (ICD-9-CM 430-438), head injury (ICD-9-CM 850-854, 959.01), brain tumor (ICD-9-CM 225, 191, 192, 194.3 and 194.4), sleep disorder (ICD-9-CM 307.4 and 780.5), diabetes (ICD-9-CM 250), hypertension (ICD-9-CM 401-405), and hyperlipidemia (ICD-9-CM 272).
Statistical Analyses
The distributions of demographic status, history of exposures to zolpidem, and other comorbidities were compared between epilepsy cases and controls. We used the χ2 test and Student t test to examine differences in categorical variables and continuous variables, respectively. We then used logistic regression analysis to calculate the odds ratios (ORs) of epilepsy presented with 95% confidence intervals (95% CIs) associated with zolpidem exposure and other diseases, compared with controls. The zolpidem exposure histories of the study patients were measured according to 2 categorical types, one to stratify the exposure into yes or no and one to categorize the exposure dose (none, < 1, 1-4, 5-9, or ≥ 10 g/y) based on quartile method to estimate the dose-response relationship between the zolpidem levels and the risk of epilepsy. Data analysis further revealed interactions between zolpidem exposure and individual comorbidities for the risk of epilepsy.
All data analyses were performed using the SAS 9.3 package (SAS Institute Inc, Cary, North Carolina), and P < .05 in 2-tailed tests was considered significant.
RESULTS
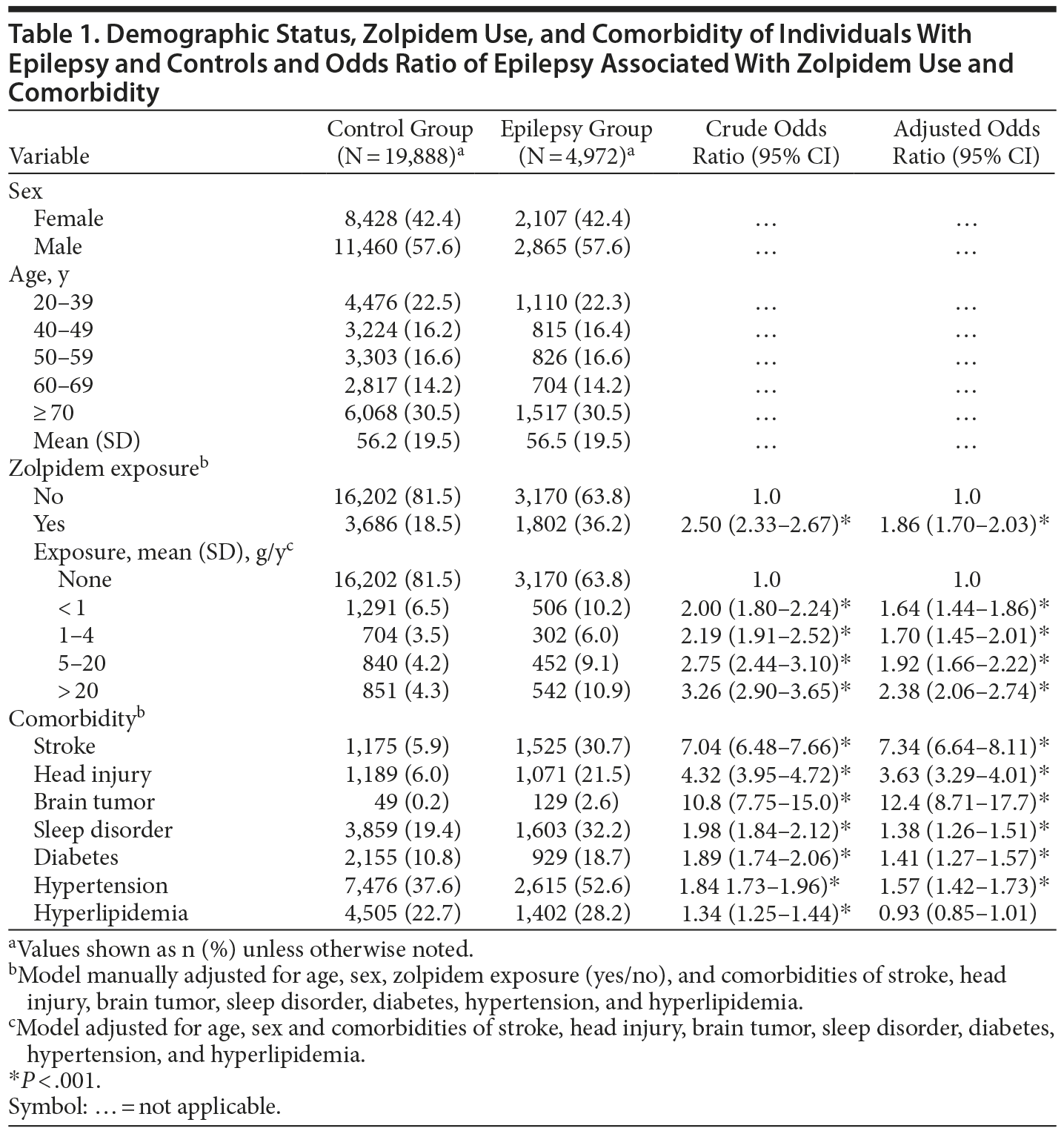
The mean zolpidem exposure time was 0.99 years in the epilepsy group and 0.70 years in the control group. Patients with epilepsy and controls were similar in distributions of sex and age, with similar mean ages of approximately 56 years (P = .36) (Table 1). The prevalence of zolpidem exposure was approximately 2-fold greater in the epilepsy patients than in the controls. The epilepsy group was also more likely than the control group to take 20 g or more of zolpidem per year. The epilepsy group had higher prevalence of comorbidities than the control group.
Table 1 shows the estimated risk of epilepsy associated with zolpidem exposure and comorbidities. Patients with epilepsy had an adjusted OR of 1.86 (95% CI, 1.70-2.03) and were, therefore, more strongly associated with zolpidem exposure than control patients were. The adjusted OR of epilepsy increased with the increase of mean zolpidem exposure (g/year). Compared with the OR of nonusers, the adjusted OR was 1.64 (95% CI, 1.44-1.86) for those who had taken < 1.0 g/y of zolpidem and 2.38 (95% CI, 2.06-2.74) for those who had taken ≥ 20.0 g/y of zolpidem. The comorbidities, excluding hyperlipidemia, were significantly associated with the risk of epilepsy. Stroke, head injury, and brain tumor were strongly associated with epilepsy.
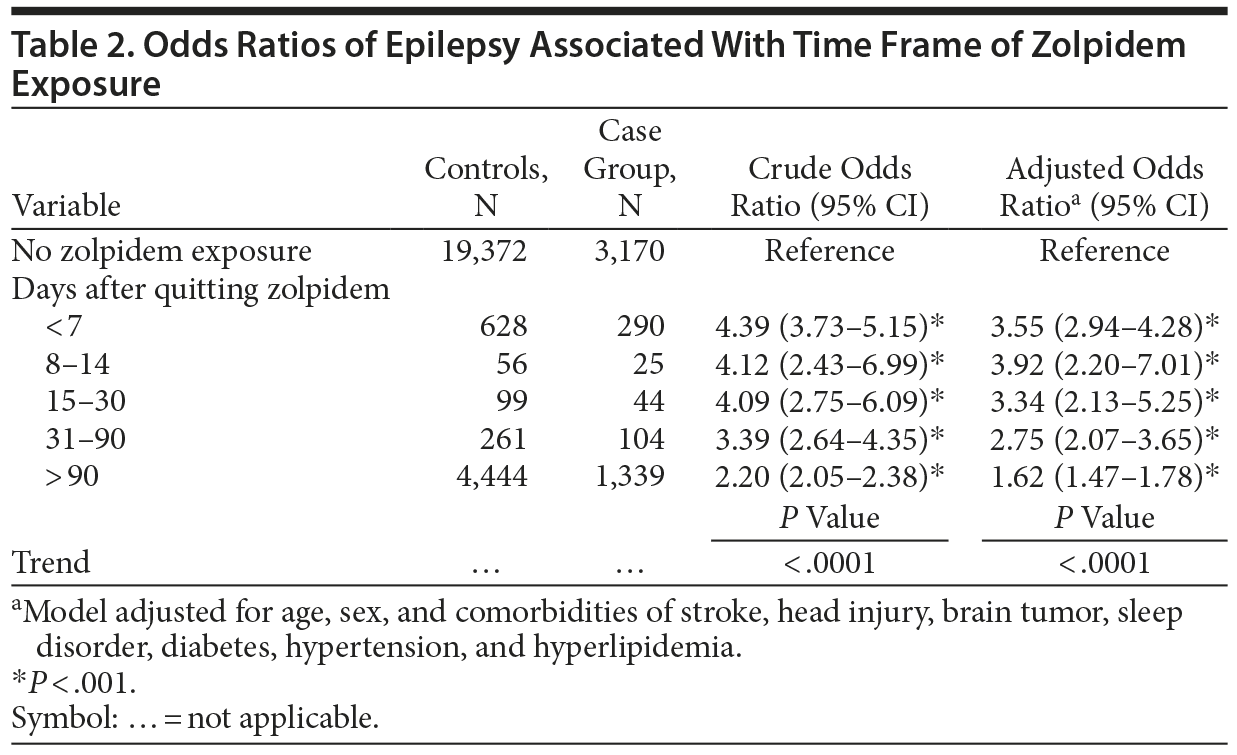
Table 2 shows that an adjusted OR of 3.55 (95% CI, 2.94-4.28) was associated with epilepsy when users stopped using zolpidem for less than 7 days. The estimated risk declined to an adjusted OR of 1.62 (95% CI, 1.47-1.78) when users stopped using the drug for more than 90 days.
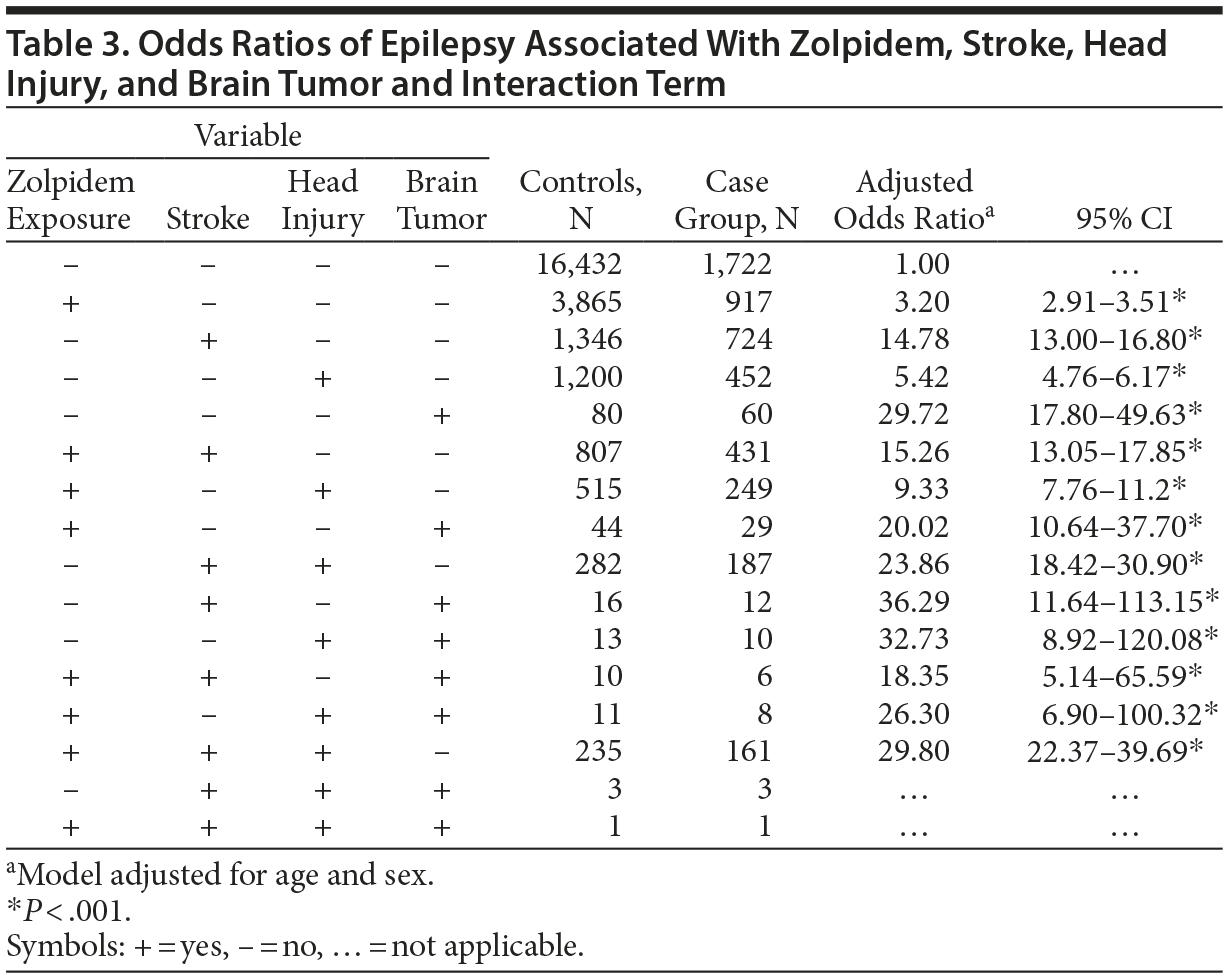
Table 3 shows the estimated risks of epilepsy in relation to zolpidem use, stroke, head injury, and brain tumor, and the interaction among these factors. Zolpidem use exerted little effect on the risk of epilepsy in interaction with stroke, head injury, and brain tumor. Among all factors, brain tumor exhibited the strongest association with epilepsy in the absence of zolpidem exposure (OR = 29.7, 95% CI, 17.8-49.6).
DISCUSSION
The current study is the first population-based study on the risk of developing epilepsy for zolpidem users in Taiwan. Contrary to our expectation, the results demonstrated a significant association between the use of zolpidem and an increased risk of developing epilepsy. Because zolpidem is a widely used hypnotic agent worldwide, the relationship between its long-term use and the risk of neurologic disorder development is crucial for clinicians. The results of this population-based study indicated that zolpidem use might be related to the increased risk of subsequent epilepsy. However, zolpidem exposure did not add risk of developing epilepsy in patients with stroke and reduced the risk of epilepsy in patients with brain tumors as well as in those with brain tumor and head injury (see Table 3). When each comorbidity (stroke, head injury, brain tumor) was examined with and without zolpidem exposure, there was generally no difference in odds ratios. These findings implied that the zolpidem itself, in fact, might not be driving the results alone. Brain tumor alone even had a much greater odds ratio than brain tumor plus zolpidem.
Zolpidem is an imidazopyridine short-acting hypnotic drug that binds at the specific benzodiazepine binding site of GABA-A receptors.16,17 By interacting with the GABA receptor-coupled chloride channel, it enhances inhibitory neurotransmission and inhibitory neurotransmitters. GABA is also considered to regulate various stages of cell proliferation and differentiation in the brain and periphery.18,19 Furthermore, zolpidem has been reported to promote viral infections, which might indicate suppression of the immune function of users.20
Previous studies have revealed that supratherapeutic doses of zolpidem (> 150 mg/d) and sudden withdrawal result in epileptic seizures.7,8,21,22 Typically, zolpidem is administered in a daily dose of 10 mg,9,23,24 and supratherapeutic or long-term use should be avoided to prevent drug abuse, dependence, and withdrawal symptoms. However, in this study, 52.1% of subjects in the epilepsy group and 42.5% of the control group were taking zolpidem at more than 4 g/year, which averaged to more than 10 mg/d and was higher than the approved dose for insomnia. This finding makes us realize that overuse, dependence, or abuse of zolpidem could happen in a medical care system if we do not regulate the prescription of zolpidem. In the group that discontinued use of zolpidem, higher risk of developing epilepsy remained when the quitting time was less than 30 days, following by quitting time of 31-90 days. Conversely, the group that had stopped taking zolpidem more than 90 days earlier exhibited the lowest risk of developing epilepsy. These findings imply that the withdrawal effects of zolpidem play a role in epileptogenesis and require considerable time to taper the dose when a patient quits the drug, especially after having taken more than 4 g of zolpidem per year. In the literature, certain benzodiazepines were also reported to be associated with hyperexcitability phenomena during treatment and following withdrawal. The suppression of the locus ceruleus-norepinephrine and hypothalamic-pituitary-adrenal axes could be followed, on an interdose basis, by a significant rebound and activation.25
Taking therapeutic doses of zolpidem was thought to be safer than taking benzodiazepines because zolpidem does not typically cause drug abuse, dependence, or withdrawal symptoms. We also observed a higher risk of developing epilepsy even in patients with smaller or moderate exposure to zolpidem (hazard ratio [HR]: 1.64 for < 1 g/y, HR: 1.70 for 1-4 g/y). Table 2 demonstrates that patients who had stopped taking zolpidem more than 90 days earlier still exhibited an OR of 1.62 for developing epilepsy. These findings convinced us that the cumulative effects of long-term, regular zolpidem treatment might play a role in epileptogenesis. A recent study in vitro suggested that continuous zolpidem exposure was able to induce adaptive changes in GABA-A receptors and to develop tolerance and dependence.26 A study in rats suggested that, upon repeated treatment, zolpidem produced tolerance to its anticonvulsive and sedative effects.27 Meanwhile, with the nighttime use of zolpidem for sleep disorders, the daytime interdose stage would be out of range of its anticonvulsive effects. According to a review of the relevant literature, the present study is the first to provide evidence that patients taking typical doses of zolpidem exhibit an increased risk of developing epilepsy in adulthood. However, another possibility is that the arousal parasomnias for which zolpidem use is indicated could be found in a person or family in relation to frontal lobe epilepsy even before the diagnosis of epilepsy is made,28 meaning that the factors which led to the use of zolpidem, rather than zolpidem itself, may be the factors contributing to the brain instability and epilepsy.29,30
One of the strengths of this study is the highly representative, nationwide, population-based design. However, the study also has limitations. First, detailed information such as smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of cancer is not available from the NHIRD, each of which is a major risk factor of epilepsy and could plausibly be associated with zolpidem. However, because the NHIRD covers nearly the entire population of Taiwan and the reimbursement policy is universal, it is unlikely that these factors affected the prescription of zolpidem. Second, the evidence derived from a case-control study is generally of lower quality than that from randomized controlled trials because a case-control study design is subject to many biases related to adjustment for confounding factors. Despite our meticulous study design and adequate control of confounding factors, a key limitation is that bias might remain if there are unmeasured or unknown confounders. Third, we could follow the codes for zolpidem prescription by administrative billing in the NHI claims, but we were unable to contact the patients directly regarding their exact doses of zolpidem or their seizure frequency because their identification numbers were anonymous. Therefore, the dose-response relationship could not be accounted for, ie, whether more severe insomnia would be accompanied by more severe epilepsy, thus requiring higher doses of zolpidem. Prescriptions for these drugs before 1996 were not included in the NHI system and thus were not in our analysis, either, which could have caused underestimation of the cumulative dosage and might have weakened the relevance of the observed association. However, the data on the prescription of zolpidem and epilepsy diagnoses were highly reliable in the NHIRD, and previous studies showed the accuracy and validity of the database.31,32
CONCLUSION
The results of this population-based case-control study demonstrated a possible association between the use of zolpidem and an increased risk of developing epilepsy, for both typical and supratherapeutic doses. These findings might stimulate public interest in safety issues regarding zolpidem use. Additional large, population-based, unbiased studies and randomized controlled trials are necessary before any confirmatory conclusion can be drawn.
Drug names: zolpidem (Ambien, Edluar, and others).
Author affiliations: Department of Neurosurgery, Buddhist Tzu Chi General Hospital and College of Medicine, Tzu Chi University, Hualien (Dr Harnod); Management Office for Health Data, China Medical University Hospital and College of Medicine, China Medical University, Taichung (Ms Wang); Graduate Institute of Clinical Medical Science and School of Medicine, College of Medicine, China Medical University, Taichung (Drs Sung and Kao); and Department of Nuclear Medicine and PET Center, China Medical University Hospital, Taichung (Dr Kao), Taiwan.
Author contributions: Conception/design: Drs Harnod and Kao; provision of study material or patients: Drs Sung and Kao; collection and/or assembly of data: Drs Harnod and Kao and Ms Wang; data analysis and interpretation: Drs Harnod and Kao and Ms Wang; manuscript writing: all authors; and final approval of manuscript: all authors.
Potential conflicts of interest: None reported.
Funding/support: This work was partially supported by research grants provided by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), Health and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan).
Role of the sponsors: The study sponsors collected data from the Taiwan National Health Insurance Research Database (NHIRD).
Additional information: The claims data of the Longitudinal Health Insurance Database (LHID), a data subset of the NHIRD, were also analyzed. The LHID consists of 1,000,000 people randomly selected from all insurants in the Taiwanese National Health Insurance program, with claims data abstracted from 1996 to 2010. The National Health Research Institutes reported no substantial differences in age and sex between the patients in the LHID and the NHIRD. The NHIRD, including the LHID subset, can be accessed at http://nhird.nhri.org.tw/en/index.htm.
REFERENCES
1. Petersen A. Dawn of a new sleep drug? Wall Street Journal. 2011:D1-D4. http://online.wsj.com/news/articles/SB10001424052702304567604576454102061138630. Updated July 19, 2011. Accessed July 16, 2014.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130. PubMed doi:10.1016/j.sleep.2005.08.008
3. Cho YW, Shin WC, Yun CH, et al. Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol. 2009;5(1):20-23. PubMed doi:10.3988/jcn.2009.5.1.20
4. Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia. 1991;32(4):429-445. PubMed doi:10.1111/j.1528-1157.1991.tb04675.x
5. Benbadis SR, Allen Hauser W. An estimate of the prevalence of psychogenic non-epileptic seizures. Seizure. 2000;9(4):280-281. PubMed doi:10.1053/seiz.2000.0409
6. Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: contributions of the Rochester epidemiology project. Mayo Clin Proc. 1996;71(6):570-575. PubMed doi:10.4065/71.6.570
7. Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol. 2000;23(5):281-283. PubMed doi:10.1097/00002826-200009000-00008
8. Barrero-Hernández FJ, Ruiz-Veguilla M, López-López MI, et al. Epileptic seizures as a sign of abstinence from chronic consumption of zolpidem [in Spanish]. Rev Neurol. 2002;34(3):253-256. PubMed
9. Schlich D, L’ Heritier C, Coquelin JP, et al. Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res. 1991;19(3):271-279. PubMed
10. Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. PubMed doi:10.1136/bmjopen-2012-000850
11. Toner LC, Tsambiras BM, Catalano G, et al. Central nervous system side effects associated with zolpidem treatment. Clin Neuropharmacol. 2000;23(1):54-58. PubMed doi:10.1097/00002826-200001000-00011
12. Inagaki T, Miyaoka T, Tsuji S, et al. Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6). PubMed
13. Vlainić J, Peričić D. Effect of acute and repeated zolpidem treatment on pentylenetetrazole-induced seizure threshold and on locomotor activity: comparison with diazepam. Neuropharmacology. 2009;56(8):1124-1130. doi:10.1016/j.neuropharm.2009.03.010
14. Lu JF, Hsiao WC. Does universal health insurance make health care unaffordable? lessons from Taiwan. Health Aff (Millwood). 2003;22(3):77-88. PubMed doi:10.1377/hlthaff.22.3.77
15. Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar. In: Okma KGH, Crivelli L, eds. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Hackensack, NJ: World Scientific; 2009:171-204. doi:10.1142/9789814261593_0007
16. Kovacic P, Somanathan R. Zolpidem, a clinical hypnotic that affects electronic transfer, alters synaptic activity through potential GABA receptors in the nervous system without significant free radical generation. Oxid Med Cell Longev. 2009;2(1):52-57. PubMed doi:10.4161/oxim.2.1.7859
17. Licata SC, Lowen SB, Trksak GH, et al. Zolpidem reduces the blood oxygen level-dependent signal during visual system stimulation. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1645-1652. PubMed doi:10.1016/j.pnpbp.2011.05.015
18. Enna SJ. Role of gamma-aminobutyric acid in anxiety. Psychopathology. 1984;17(suppl 1):15-24. PubMed doi:10.1159/000284073
19. Jezewska E, Scinska A, Kukwa W, et al. Gamma-aminobutyric acid concentrations in benign parotid tumours and unstimulated parotid saliva. J Laryngol Otol. 2011;125(5):492-496. PubMed doi:10.1017/S0022215110002574
20. Kripke DF. Evidence that new hypnotics cause cancer. http://escholarship.org/uc/item/12r2f32g. Accessed July 21, 2011.
21. Sethi PK, Khandelwal DC. Zolpidem at supratherapeutic doses can cause drug abuse, dependence and withdrawal seizure. J Assoc Physicians India. 2005;53:139-140. PubMed
22. Cubała WJ, Landowski J. Seizure following sudden zolpidem withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):539-540. PubMed doi:10.1016/j.pnpbp.2006.07.009
23. Hajak G, Müller WE, Wittchen HU, et al. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98(10):1371-1378. PubMed doi:10.1046/j.1360-0443.2003.00491.x
24. Licata SC, Jensen JE, Penetar DM, et al. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: a proton MRS study at 4 T. Psychopharmacology (Berl). 2009;203(4):819-829. PubMed doi:10.1007/s00213-008-1431-1
25. Vgontzas AN, Kales A, Bixler EO. Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology. 1995;51(4):205-223. PubMed doi:10.1159/000139363
26. Vlainić J, Jembrek MJ, Vlainić T, et al. Differential effects of short- and long-term zolpidem treatment on recombinant α1β2γ2s subtype of GABA(A) receptors in vitro. Acta Pharmacol Sin. 2012;33(12):1469-1476. PubMed doi:10.1038/aps.2012.89
27. Auta J, Impagnatiello F, Kadriu B, et al. Imidazenil: a low efficacy agonist at alpha1- but high efficacy at alpha5-GABA(A) receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidem. Neuropharmacology. 2008;55(2):148-153. doi:10.1016/j.neuropharm.2008.05.002
28. Manni R, Terzaghi M. Comorbidity between epilepsy and sleep disorders. Epilepsy Res. 2010;90(3):171-177. PubMed doi:10.1016/j.eplepsyres.2010.05.006
29. Vendrame M, Yang B, Jackson S, et al. Insomnia and epilepsy: a questionnaire-based study. J Clin Sleep Med. 2013;9(2):141-146. PubMed
30. Abad-Alegría F, López-Mallén ME, de Francisco-Maqueda P. Insomnia and somnolence in epilepsy[Spanish]. Rev Neurol. 1997;25(144):1171-1172. PubMed
31. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
32. Harnod T, Lin CL, Sung FC, et al. An association between benzodiazepine use and occurrence of benign brain tumors. J Neurol Sci. 2014;336(1-2):8-12. PubMed doi:10.1016/j.jns.2013.11.009