ABSTRACT
Importance: Most people with dementia will experience neuropsychiatric symptoms, including psychosis characterized by hallucinations and delusions. Across dementia subtypes, hallucinations and delusions are common, though their prevalence and presentation may vary. These symptoms have been associated with worse outcomes compared with dementia alone, including accelerated functional decline and mortality. Many people with dementia reside in long-term care facilities, and identification and management of hallucinations and delusions in this setting are critical.
Observations: For residents in long-term care facilities, the following factors can hinder management of hallucinations and delusions related to dementia: (1) delayed recognition of symptoms; (2) reluctance of staff and family members to acknowledge psychiatric issues; (3) lack of approved pharmacotherapies to treat hallucinations and delusions associated with dementia-related psychosis; and (4) regulatory and institutional guidelines, including the long-term care regulatory guidelines established by the Centers for Medicare and Medicaid Services and the 5-star rating system.
Conclusions and Relevance: Barriers to the treatment of hallucinations and delusions in patients with dementia in the long-term care setting are myriad and complex. Early diagnosis of dementia-related psychosis and new treatment options for managing hallucinations and delusions are needed to improve care of this patient population.
J Clin Psychiatry 2022;83(2):21m14050
To cite: Khanna R, White L, Bessey FY, et al. Barriers to treatment of hallucinations and delusions in people with dementia residing in long-term care. J Clin Psychiatry. 2022;83(2):21m14050.
To share: https://doi.org/10.4088/JCP.21m14050
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Creighton University School of Medicine, Phoenix, Arizona
bMedication Managers, LLC, Coppell, Texas
cMetro Rx Consulting, Milpitas, California
dTeamHealth, Asheville, North Carolina
eCentric Physicians Group, San Antonio, Texas
‡Current affiliation is Acadia Pharmaceuticals Inc., San Diego, California
*Corresponding author: Jason P. Caplan, Creighton University School of Medicine, 500 W Thomas Rd, Ste 230 Phoenix, AZ 85013 ([email protected]).
The cognitive decline exhibited by patients with dementia is often accompanied by neuropsychiatric symptoms, including psychosis, aggression, agitation, depression, and apathy.1–4 Patients may experience the hallucinations and delusions that characterize dementia-related psychosis regardless of the underlying disease causing dementia. These symptoms can be particularly distressing to both patients and caregivers and are associated with worse outcomes than dementia without psychosis.5–7
Many residents of long-term care (LTC) facilities have dementia, and neuropsychiatric symptoms including psychosis are extremely common in this population.8 Psychosis among patients in nursing homes has been associated with physical function decline, sleep disturbances, prevalence of pain, and medication use.9,10 Despite the impacts of dementia-related hallucinations and delusions for patients and the health care system, no therapies are currently approved in the United States for these symptoms. Antipsychotics are often used off-label, although they have been associated with serious safety concerns, including increased mortality.11 Careful consideration of the risks and benefits of antipsychotic use for each patient case is complex in the current landscape, particularly given narrow and (at times) conflicting guidance on the use of antipsychotics in the elderly.
The potential sequelae of dementia-related psychosis indicate a need for timely treatment. However, a number of challenges to treatment exist. First, accurate identification can be difficult for a myriad of reasons. Suboptimal training of staff in nursing homes and the reluctance of family members to accept psychiatric diagnoses present a complex set of challenges.12–14 Finally, regulatory considerations, cost, and coverage remain key barriers precluding proper treatment.1,11,15–17
In this review, we aim to address barriers to diagnosis of dementia-related psychosis and include an overview of prevalence and presentation by type of dementia to help with recognition of the disease. We also review current practices for treating patients with dementia-related psychosis in LTC settings and consider difficulties to initiating and sustaining effective treatment that are introduced by regulatory measures and social stigma.
HALLUCINATIONS AND DELUSIONS IN DEMENTIA
Alzheimer’s Disease
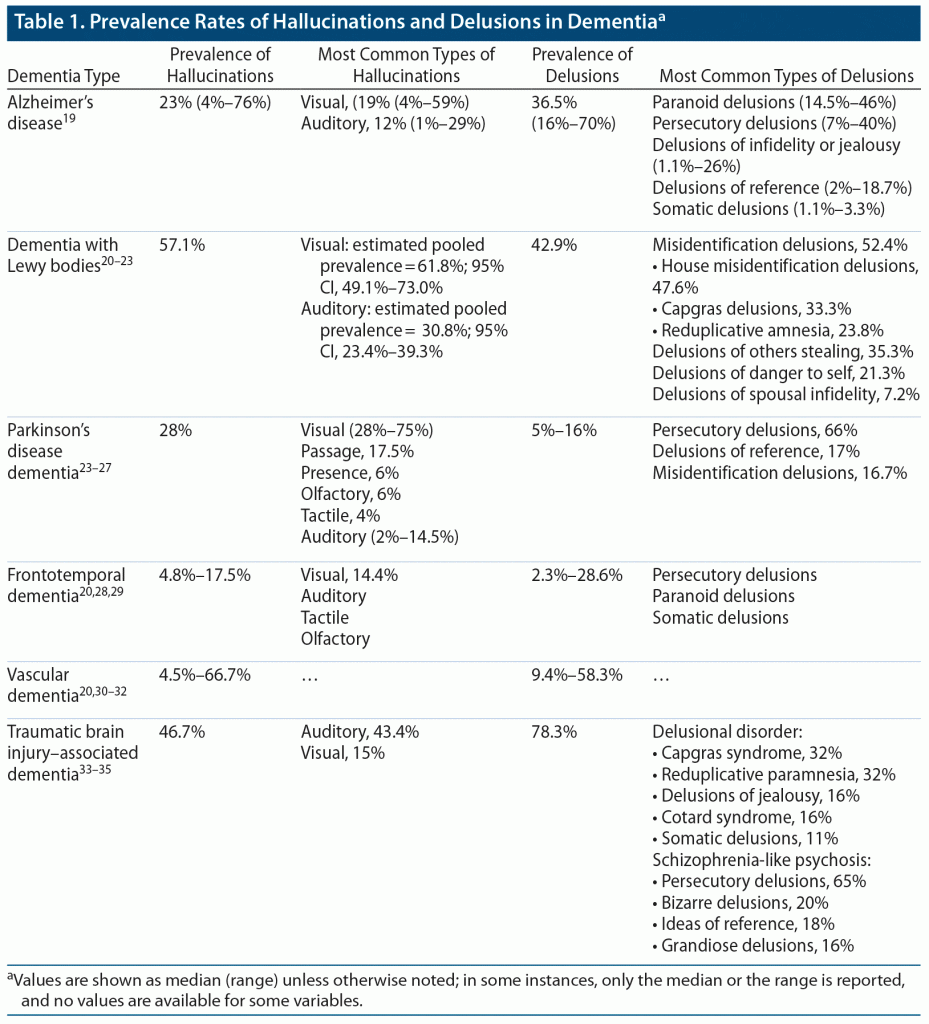
Alzheimer’s disease (AD) is the most common form of dementia and develops with accumulation of neuronal amyloid plaques and neurofibrillary tangles primarily in the hippocampus.18 As such, patients with AD suffer from progressive deterioration in memory alongside changes in behavior and cognitive abilities. The prevalence rates of delusions in individuals with AD have been reported to range from 16% to 70% (median = 36.5%; Table 1).19 Paranoid delusions are most common (noted in 14.5%–46% of patients), followed by persecutory delusions (7%–40%), delusions of infidelity or jealousy (1.1%–26%), delusions of reference (2%–18.7%), and somatic delusions (1.1%–3.3%).19 Reported prevalence rates of hallucinations range from 4% to 76% (median = 23%), with visual hallucinations most common (median = 19% [range, 4%–59%]) followed by auditory hallucinations (12% [1%–29%]).19 Hallucinations have been reported to manifest early in illness and are more commonly observed in individuals with a severe disease presentation.19 Delusions in patients with AD are commonly observed during the moderate stage of disease, yet the prevalence of both delusions and hallucinations in AD increases slowly with disease progression.19 One study36 found that delusions appeared at a mean (SD) of 2.23 (1.94) years after AD diagnosis, whereas hallucinations appeared at a mean of 2.50 (1.96) years after diagnosis. The preponderance of evidence therefore indicates that delusions occur more frequently than hallucinations in patients with AD, with paranoid delusions and visual hallucinations being the most common.37
Dementia With Lewy Bodies
Dementia with Lewy bodies (DLB) is classified among α-synucleinopathies as a type of dementia that shares symptoms with both AD and Parkinson’s disease (PD).38 Studies suggest that visual hallucinations are more prevalent (estimated pooled prevalence = 61.8%; 95% CI, 49.1%–73.0%) than auditory hallucinations (30.8%; 23.4%–39.3%) in patients with DLB,23 with hallucinations occurring more often than delusions (57.1% and 42.9%, respectively; Table 1).20 Common delusions observed in patients with DLB include misidentification delusions (52.4%), delusions of others stealing (35.3%), delusions of danger to self (21.3%), and delusions of spousal infidelity (7.2%).21,22 Of the misidentification delusion subtype, the most common delusions are house misidentification, Capgras delusions (belief that someone familiar has been replaced by an imposter), and reduplicative amnesia (belief that a place has been duplicated and is present in 2 locations simultaneously). Delusions and greater cognitive decline have been associated in DLB.21,27
Parkinson’s Disease
Parkinson’s disease dementia (PDD) is a complication of the disease marked by deficits in recognition memory, attention processes, and visual perceptions.39 Both neuropathological and genetic factors contribute to disease pathology, with Lewy bodies, neurofibrillary tangles, senile plaques, and microvascular disease all contributing factors.39 Visual hallucinations are more prevalent (28% [95% CI, 19.1%–39.5%]) than auditory hallucinations (8.9% [5.3%–14.5%]) in PDD,23 with some reports suggesting prevalence rates of visual hallucinations as high as 75%.25 Passage hallucinations (sightings of animals or objects passing in the peripheral visual field) are commonly observed in patients with PDD, along with extracampine hallucinations such as presence hallucinations (the sense of someone’s presence).27,40 After visual hallucinations, passage hallucinations are most common, followed by presence hallucinations, olfactory hallucinations (pungent odors such as garbage/rotten food, burning/gas), tactile hallucinations (insects biting or crawling on the skin), and auditory hallucinations (including incomprehensible voices, sound of steps, or music).25 Of note, extracampine hallucinations are often associated with visual hallucination onset, with both commonly presenting at the same time.40 Delusions, although less common, affect 5%–16% of patients with PDD; the most common subtypes are persecutory delusions, delusions of reference (interpreting an innocuous stimulus in the milieu as being specifically directed to them), and misidentification delusions.25–27 The prevalence of psychotic symptoms in PDD is time dependent and increases with duration of PDD.27
Frontotemporal Dementia
Frontotemporal dementia (FTD), a common cause of young-onset dementia, is characterized by intraneuronal tau protein deposition and atrophy of the frontal and anterior temporal lobes.41 As a progressive syndrome, FTD manifests as changes in personality and behavior with decline in language skills.41 Patients with FTD are less likely to present with delusions or hallucinations than are patients with other forms of dementia.37 The reported prevalence rates of delusions in FTD patients range from 2.3% to 28.6%, with prevalence rates of hallucinations ranging from 4.8% to 17.5%.20,28,29 Delusions are most commonly of the persecutory, paranoid, and somatic subtypes.28,29 Visual hallucinations are more commonly observed (14.4%) in FTD patients, with fewer reports of auditory, tactile, and olfactory hallucinations.28
Vascular Dementia
Vascular dementia (VaD) is a heterogeneous syndrome with a variety of pathological subtypes, including dementia associated with hemorrhagic strokes, cerebral hypoxic-ischemic events, and senile leukoencephalopathic lesions.42 Although cognitive changes in VaD are much more variable, predominant deficits in attention, executive function, and information processing are common.43 When compared with patients with other forms of dementia such as AD, patients with VaD are more likely to present with psychotic symptoms such as delusions.30 The prevalence rates for delusions and hallucinations in VaD range from 9.4% to 58.3% and from 4.5% to 66.7%, respectively.20,30–32 Historically, an association between severity of dementia and psychotic symptoms has not been reported; however, recent reports30,32 suggest an association between increased frequency of delusions in patients with VaD and more severe disease.
Traumatic Brain Injury
Traumatic brain injury (TBI)–associated dementia has been attributed to both genetic risk factors and white matter tract and neural network disruptions.44 Patient history of neurologic and neurodevelopmental conditions is often considered when assessing patient risk of dementia and psychotic disorder due to TBI (PDTBI).33 Patients with PDTBI are more likely to present with delusions (78.3%) than hallucinations (46.7%), with mean latency between injury and symptom onset ranging from 3 to 5 years.33–35 Auditory hallucinations (43.4%) are more common than visual hallucinations (15%) in patients with PDTBI, although visual hallucinations occur more often in earlier-onset TBI.34,35 Still, hallucinations are more commonly observed in patients with later-onset TBI than in patients with onset shortly after injury.34
PDTBI can be categorized into 2 subtypes: delusional disorder (DD) and schizophrenia-like psychosis (SLP). DD involves the occurrence of delusions alone, most commonly Capgras syndrome (32%) and reduplicative paramnesia (32%), followed by delusions of jealousy (16%), Cotard syndrome (delusion of being dead or dying; 16%), and somatic delusions (11%).33 Negative symptoms of psychosis are relatively uncommon, occurring in only 25% of cases.33 SLP involves the occurrence of both hallucinations and delusions, most commonly auditory hallucinations and persecutory delusions.33 Patients with SLP may also present with visual hallucinations, bizarre delusions (20%), ideas of reference (18%), and grandiose delusions (16%).33 Negative symptoms such as blunted affect, social withdrawal, and poor hygiene are more commonly observed in SLP than in DD. Results from mild, moderate, and severe injuries show no association between severity of TBI and onset of psychosis, but other risk factors such as family history of psychotic illness or substance use may predispose patients to psychosis.33
DIAGNOSIS OF DEMENTIA-RELATED PSYCHOSIS
Although understanding the potential for psychosis in patients with dementia may help with recognition of the condition, further challenges may hinder diagnosis and treatment. First, patients may lack insight into their condition, and those who are aware of symptoms may be hesitant to report them. Nonverbal or verbally challenged patients by definition cannot provide the necessary detail to define this issue. Family members may assume that some hallucinations or delusions are a normal process of aging and therefore not inquire about treatment options from health care providers. They may also resist reporting because they cannot imagine these sorts of symptoms or they want to avoid the social stigma of a “psychotic” diagnosis.
For patients in LTC facilities, the treating physician often relies on staff to report symptoms such as hallucinations and delusions. When these symptoms become the resident’s normal behavior pattern, staff members can overlook the frequency and severity of the hallucinations and delusions. The variability of symptom presentation in people with dementia-related psychosis can complicate reporting and diagnosis. Whereas some experience persistent symptoms, others may experience frequent, recurring symptoms.45 If symptoms are not troubling for the patient, they may not even recognize the actual frequency of symptom manifestation.
Additionally, caregivers providing frequent patient care within the LTC setting (eg, nursing staff, certified nursing assistants, restorative nurse assistants, dietary staff) may be concerned that reporting such symptoms will merely complicate care (ie, with additional documentation) and, in many cases, will not lead to any change in the care plan.
Over the past few years, with the focus on reducing unnecessary psychoactive medications across the country, fear around the use of psychoactive medications has also risen among LTC staff members. We must remember that the Centers for Medicare and Medicaid Services (CMS) regulations and guidance are to protect a resident from being prescribed unnecessary medications and to ensure that the lowest effective dose is used. Residents who experience hallucinations and delusions along with dementia or Parkinson’s disease often still need appropriate treatment. Working with an interdisciplinary team can help to ensure that all documentation is complete and includes evidence of the necessity and benefits and risks of any treatment considered.
CURRENT LANDSCAPE
Use of off-label antipsychotics in the elderly has been associated with an increased risk of mortality.46 In 2005, the US Food and Drug Administration (FDA) released a boxed warning of this risk associated with the use of atypical antipsychotics to treat dementia-related psychosis.47 Formal guidelines and recommendations for best practices have incorporated this warning into their recommendations, including those of the American Psychiatric Association (APA), the American Geriatrics Society (AGS), and the CMS.15,16 The degree of efficacy of available off-label antipsychotics has been reported elsewhere48 and is beyond the scope of the current review. However, hallucinations and delusions often warrant pharmacologic treatment to maintain safety of both the individual patient and the milieu as a whole, and there is sufficient efficacy to be considered in the risk-benefit analysis of each patient case. The AGS recommends that typical and atypical antipsychotics be avoided except when treating schizophrenia or bipolar disorder or for short-term management of chemotherapy-induced emesis.16 The APA cautions against the use of antipsychotics except when nonpharmacologic treatment approaches are ineffective and persisting symptoms are severe or dangerous or cause significant distress to the patient. The guidelines clarify that these recommendations apply to hallucinations and delusions caused by psychosis or agitation, rather than delirium, and that when used, antipsychotics should be tapered and withdrawn after 4 weeks if there is no response to the treatment or within 4 months if there is an adequate response to treatment. CMS guidelines, which set the regulatory standard of care for those living in nursing homes, also reflect the FDA boxed warning regarding the use of antipsychotics. Importantly, hallucinations and delusions can be dangerous and distressing to patients and caregivers, and while risk-benefit analyses of treatment options are critical, the use of antipsychotics remains the standard of care.
Several regulatory and organizational barriers impede the pharmacologic treatment of behavioral symptoms, including psychosis, in the LTC setting. The primary regulatory guidelines in the United States are the long-term care regulatory guidelines established by the CMS and the 5-star rating system.17,49 Both were amended in 2012 to specifically address the use of antipsychotics,50,51 and facilities are expected to adhere to both despite their differing requirements. Antipsychotic use has declined following these changes, demonstrating the power of these guidelines in shaping prescribing behavior.51–54
Care provided to residents living in nursing homes is governed by CMS rules and regulations as outlined in Appendix PP of the State Operations Manual (SOM).17 Each nursing home undergoes an annual survey during which it is evaluated for all aspects of care, including the general environment, staffing ratios, and use of certain medications. When facilities are found to be out of compliance, they can receive citations with monetary penalties or, in severe cases, closure to admissions. Areas of particular concern for surveyors are the evaluation, diagnosis, and treatment of psychiatric and behavioral conditions of LTC residents. The importance placed on behavioral health treatment is evident in the emphasis and detail given in the SOM guidelines. In fact, behavioral health services are the only areas of medical specialty that are specifically isolated and addressed in this document. Areas addressed include the definition of psychiatric illnesses, differential diagnoses to consider, goals for treatment, and guidelines for medication management, including a specific schedule by which clinicians must evaluate the ongoing use of all psychotropic medications. Although all psychotropic usage is addressed in the SOM, particular focus is given to the use of antipsychotics. Clinicians must follow a strict schedule of reducing doses of antipsychotics unless certain clinical contraindications are met. Acceptable contraindications for not initiating a dose reduction are as follows: (1) ongoing use is consistent with the accepted standard of care for a specific psychiatric diagnosis; (2) the patient has a documented significant failure of dose reduction in the recent past; or (3) the resident is highly likely to undergo a significant decompensation of mood or behavior with reduction in the antipsychotic (supported by specific clinical rationale). When treating residents with psychosis secondary to a nonprimary psychiatric illness, even more scrutiny is given to the use of antipsychotics to ensure they are not being used for chemical restraint. CMS encourages the use of nonpharmacologic measures for treatment of agitation related to psychosis secondary to dementia, but when these interventions fail, there is little by way of approved interventions.
The 5-star rating system was created by CMS to “help consumers, their families, and caregivers compare nursing homes more easily.”49 In theory, this system provides an objective way to evaluate and compare various nursing facilities by using a specific set of criteria associated with better patient outcomes. Families generally prefer to have their loved ones cared for at a highly rated facility, and because acute care settings are also incentivized to minimize rehospitalization, they are more inclined to discharge patients to highly rated facilities. Therefore, LTC facilities are motivated to earn the highest rating possible because it positively impacts their occupancy and finances.
The 5-star rating is divided into 3 areas: health inspection, staffing, and quality measures. Although health inspection and staffing are straightforward, the quality measure is divided into 15 different clinical measurements that are used to determine an overall score. One clinical measurement is the total number of residents within a nursing home who are receiving an antipsychotic for a diagnosis other than schizophrenia, Huntington’s disease, or Tourette’s syndrome, even if the use of the antipsychotic is in accordance with FDA-approved guidelines, is being used on-label, and is consistent with the patient’s overall clinical picture. The following hypothetical case can illustrate the potential problem with this guidance: A 66-year-old woman with longstanding bipolar disorder suffers a fall with resulting hip fracture. After surgical repair at her local hospital, she is admitted to a long-term care facility for rehabilitation. She has been psychiatrically stable on quetiapine 300 mg administered orally twice a day for 5 years. Even though she has had psychiatric stability and favorable treatment outcomes, the facility will now face a drop in their quality measures if they continue to treat this resident with her prior medications.
Although both are created by and enforced through CMS, the SOM and the 5-star rating system are incongruent. These complicated regulatory requirements also create a confusing and frustrating clinical landscape for LTC behavioral health clinicians, who are already few in number.
The decision to prescribe an antipsychotic for patients in LTC requires consideration of the associated risks and benefits specific to each case. Regulatory pressures imposed by the CMS and other guidelines have added additional variables to this equation. While the use of antipsychotics in these patients may have dropped substantially due to guidelines cautioning against their use and incentives for facilities to limit their use,51–54 this drop in use has not necessarily resulted in improved care for all patients. The 5-star rating has made it substantially more difficult for providers to treat patients with psychosis in accordance with guidelines, which do allow for the use of antipsychotics and acknowledge their value in terms of potential benefits in certain circumstances, though the amount of work required to meet the regulatory criteria for documentation (including initial justification for use and subsequent required dose reductions) adds additional stress to a system that is largely underserved. Untreated hallucinations and delusions in patients in LTC could also create risks for staff and impose additional burdens on the health care system, particularly related to costs of hospitalizations prompted by barriers to effective treatment in the LTC setting itself.
CONSEQUENCES OF ANTIPSYCHOTIC MEDICATION REDUCTIONS
Antipsychotic use in the LTC environment has decreased in recent years, partly because of the encouragement from regulators and greater understanding of the risks associated with current antipsychotics, particularly in patients with dementia.54 However, alternative treatment options for LTC residents with psychosis remain limited. Often, LTC facilities attempt nonpharmacologic approaches for the management of behaviors secondary to symptomatology, but if these methods are not effective, little can be done to help patients, especially in cases of dementia-related psychosis. Also, because of the decrease in LTC staffing, facilities do not have the necessary time or expertise to appropriately carry out these nonpharmacologic approaches, leading to suboptimal outcomes and the need for renewed or alternative medication management or adjustments.55 When an antipsychotic is prescribed and is effective at reducing a patient’s symptoms, tapering and withdrawing a medication can destabilize patients and cause symptoms to recur.56,57 In such cases, it is often more difficult to restabilize the patient even if the same medication is reinitiated. Another consequence of the shift away from antipsychotic use is that prescribers may substitute other medications that do not have proven effectiveness, including mood stabilizers (such as antiepileptic drugs) and sedatives.54,58 Substituting medications may result in new problems, because doing so can further destabilize the patient while not addressing their underlying psychiatric issue, or introduce new safety and tolerability issues. All of these in turn can lead to increased avoidable hospitalizations or movement from one LTC environment to another.59 Many gradual dose reductions and discontinuations within the LTC environment are being completed by nonpsychiatric providers who are not specialists in psychiatric medication management, further destabilizing patients’ psychiatric disorders.60
Encouragement (from a regulatory standpoint) to reduce antipsychotic use in LTC may be directly related to the increase in hospitalizations of patients suffering from mental illness.59 Some newly admitted LTC patients have suffered from mental illness before admission, but their antipsychotic medications are reduced or changed in accordance with the guidelines.60 Placement into LTC facilities also becomes difficult, with many elderly patients with psychosis “stuck in limbo” in the emergency department/hospital as LTC facilities reject them in favor of other referrals (since accepting patients that need antipsychotic treatment would lower their 5-star rating). Most LTC facilities strive to meet the guideline standards, often at the risk of destabilizing patients or increasing the potential for rehospitalization or movement to another facility, or both.59 Within the 5-star system that regulates LTC facilities, antipsychotic use was deemed appropriate for only 3 diagnoses, which do not include all psychiatric diagnoses having FDA approval for the use of antipsychotic medications.55 As previously noted, patients with bipolar disorder or treatment-resistant depression require long-term medication regimens to stabilize their disease and maintain optimal mental health, outcomes that are often difficult to achieve when medications are being regularly altered because of regulations. In addition, no antipsychotics are currently approved for treatment of psychosis related to dementia, severely limiting the treatment options for this large population of patients in LTC facilities.
CONCLUSIONS
Barriers to treatment of hallucinations and delusions in patients with dementia are myriad and complex, especially within the LTC setting. The statistics around prevalence of this symptomatology are alarming, but the challenges to effectively treating this vulnerable patient population are also daunting. Appropriately identifying these symptoms is difficult, but with or without symptom diagnosis, treatment may be impeded by psychosocial stigma, compliance with complex regulatory requirements around certain medications, and the increased difficulties manifested during acute and post-acute care of patients with psychosis. While this review reflects our opinion and our interpretation and recommendations may not be applicable to settings outside of LTC, a shift in the treatment paradigm for these troubling symptoms that addresses the aforementioned issues is worthy of further discussion.
Submitted: April 30, 2021; accepted September 23, 2021.
Published online: March 7, 2022.
Potential conflicts of interest: Drs White and Bessey became employees of Acadia Pharmaceuticals Inc. after completion of the majority of the work on this manuscript. Ms Hoberg: is a consultant and speaker for Acadia Pharmaceuticals Inc., Avanir Pharmaceuticals, Teva Pharmaceuticals, and Intracellular Therapies. Dr Caplan is consultant to and speaker for Acadia Pharmaceuticals Inc. and Avanir Pharmaceuticals and is a speaker for Neurocrine Biosciences and Myriad Genetics. Ms Khanna and Ms Borntrager have nothing to disclose.
Funding/support: Funding for medical writing support was provided by Acadia Pharmaceuticals Inc. (San Diego, California).
Role of the sponsor: Acadia reviewed the manuscript and provided feedback to the authors. The authors had full control over content and approved the final version for submission.
Acknowledgments: Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Alison Adams, PhD; Dena McWain, BA, edited the manuscript and styled for journal submission (both are with Ashfield MedComms, an Ashfield Health company). Dr Adams and Ms McWain have no conflicts of interest to declare.
Clinical Points
- Barriers to the treatment of hallucinations and delusions in patients with dementia in the long-term care setting are myriad and complex.
- Appropriately identifying hallucinations and delusions is difficult, but with or without symptom diagnosis, treatment may be impeded by psychosocial stigma, compliance with complex regulatory requirements around certain medications, and the increased difficulties manifested during acute and post-acute care of patients with psychosis.
- Early diagnosis of dementia-related psychosis and new treatment options for managing hallucinations and delusions are needed to improve care of this patient population.
References (60)

- Margallo-Lana M, Swann A, O’Brien J, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatry. 2001;16(1):39–44. PubMed CrossRef
- Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170–177. PubMed CrossRef
- van der Linde RM, Dening T, Stephan BC, et al. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry. 2016;209(5):366–377. PubMed CrossRef
- Helvik A-S, Selbæk G, Šaltytė Benth J, et al. The course of neuropsychiatric symptoms in nursing home residents from admission to 30-month follow-up. PLoS One. 2018;13(10):e0206147. PubMed CrossRef
- Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601–1608. PubMed CrossRef
- Emanuel JE, Lopez OL, Houck PR, et al. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am J Geriatr Psychiatry. 2011;19(2):160–168. PubMed CrossRef
- Fischer CE, Verhoeff NPLG, Churchill K, et al. Functional outcome in delusional Alzheimer disease patients: a systematic review. Dement Geriatr Cogn Disord. 2009;27(2):105–110. PubMed CrossRef
- Selbæk G, Engedal K, Bergh S. The prevalence and course of neuropsychiatric symptoms in nursing home patients with dementia: a systematic review. J Am Med Dir Assoc. 2013;14(3):161–169. PubMed CrossRef
- Habiger TF, Achterberg WP, Flo E, et al. Psychosis symptoms in nursing home residents with and without dementia-cross-sectional analyses from the COSMOS study. Int J Geriatr Psychiatry. 2019;34(5):683–691. PubMed CrossRef
- Sverdrup K, Bergh S, Selbæk G, et al. Trajectories of physical performance in nursing home residents with dementia. Aging Clin Exp Res. 2020;32(12):2603–2610. PubMed CrossRef
- Yunusa I, Alsumali A, Garba AE, et al. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological sym ptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828. PubMed CrossRef
- Chenoweth L, Jessop T, Harrison F, et al. Critical contextual elements in facilitating and achieving success with a person-centered care intervention to support antipsychotic deprescribing for older people in long-term care. BioMed Res Int. 2018;2018:7148515. PubMed CrossRef
- Aerts L, Cations M, Harrison F, et al. Why deprescribing antipsychotics in older people with dementia in long-term care is not always successful: insights from the HALT study. Int J Geriatr Psychiatry. 2019;34(11):1572–1581. PubMed CrossRef
- Jessop T, Harrison F, Cations M, et al. Halting antipsychotic use in long-term care: (HALT): a single-arm longitudinal study aiming to reduce inappropriate use in long-term care residents with behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2017;8:1391–1403. PubMed CrossRef
- Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543–546. PubMed CrossRef
- By the 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–694. PubMed CrossRef
- Centers for Medicare & Medicaid Services. State Operations Manual. Appendix PP—Guidance to Surveyors for Long Term Care Facilities. Centers for Medicare & Medicaid Services. CMS.gov website. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/som107ap_pp_guidelines_ltcf.pdf. 2017.
- Wanleenuwat P, Iwanowski P, Kozubski W. Alzheimer’s dementia: pathogenesis and impact of cardiovascular risk factors on cognitive decline. Postgrad Med. 2019;131(7):415–422. PubMed CrossRef
- Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: review of the brain decade. Psychosomatics. 2003;44(5):388–401. PubMed CrossRef
- Perri R, Monaco M, Fadda L, et al. Neuropsychological correlates of behavioral symptoms in Alzheimer’s disease, frontal variant of frontotemporal, subcortical vascular, and Lewy body dementias: a comparative study. J Alzheimers Dis. 2014;39(3):669–677. PubMed CrossRef
- Perini G, Carlini A, Pomati S, et al. Misidentification delusions: prevalence in different types of dementia and validation of a structured questionnaire. Alzheimer Dis Assoc Disord. 2016;30(4):331–337. PubMed CrossRef
- Tzeng R-C, Tsai C-F, Wang C-T, et al. Delusions in patients with dementia with Lewy bodies and the associated factors. Behav Neurol. 2018;2018:6707291. PubMed CrossRef
- Eversfield CL, Orton LD. Auditory and visual hallucination prevalence in Parkinson’s disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol Med. 2019;49(14):2342–2353. PubMed CrossRef
- Dudley R, Aynsworth C, Mosimann U, et al. A comparison of visual hallucinations across disorders. Psychiatry Res. 2019;272:86–92. PubMed CrossRef
- Kulick CV, Montgomery KM, Nirenberg MJ. Comprehensive identification of delusions and olfactory, tactile, gustatory, and minor hallucinations in Parkinson’s disease psychosis. Parkinsonism Relat Disord. 2018;54:40–45. PubMed CrossRef
- Lizarraga KJ, Fox SH, Strafella AP, et al. Hallucinations, delusions and impulse control disorders in Parkinson disease. Clin Geriatr Med. 2020;36(1):105–118. PubMed CrossRef
- Ffytche DH, Aarsland D. Psychosis in Parkinson’s disease. Int Rev Neurobiol. 2017;133:585–622. PubMed CrossRef
- Landqvist Waldö M, Gustafson L, Passant U, et al. Psychotic symptoms in frontotemporal dementia: a diagnostic dilemma? Int Psychogeriatr. 2015;27(4):531–539. PubMed CrossRef
- Shinagawa S, Nakajima S, Plitman E, et al. Psychosis in frontotemporal dementia. J Alzheimers Dis. 2014;42(2):485–499. PubMed CrossRef
- Östling S, Gustafson D, Blennow K, Börjesson-Hanson A, Waern M. Psychotic symptoms in a population-based sample of 85-year-old individuals with dementia. 2011;24(1):3–8.
- Anor CJ, O’Connor S, Saund A, et al. Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegener Dis. 2017;17(4-5):127–134. PubMed CrossRef
- Deardorff WJ, Grossberg GT. Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. In: Reus VI, Lindqvist D, eds. Psychopharmacology of Neurologic Disease. Elsevier; Handbook of Clinical Neurology. 2019;165:5–32.
- Fujii DE, Ahmed I. Psychotic disorder caused by traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):113–124. PubMed CrossRef
- Arciniegas DB, Harris SN, Brousseau KM. Psychosis following traumatic brain injury. Int Rev Psychiatry. 2003;15(4):328–340. PubMed CrossRef
- Guerreiro DF, Navarro R, Silva M, et al. Psychosis secondary to traumatic brain injury. Brain Inj. 2009;23(4):358–361. PubMed CrossRef
- Connors MH, Ames D, Woodward M, et al. Psychosis and clinical outcomes in Alzheimer disease: a longitudinal study. Am J Geriatr Psychiatry. 2018;26(3):304–313. PubMed CrossRef
- Engelborghs S, Maertens K, Nagels G, et al. Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry. 2005;20(11):1028–1037. PubMed CrossRef
- Mrak RE, Griffin WS. Dementia with Lewy bodies: definition, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3(5):619–625. PubMed
- Gratwicke J, Jahanshahi M, Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138(Pt 6):1454–1476. PubMed CrossRef
- Wood RA, Hopkins SA, Moodley KK, et al. Fifty percent prevalence of extracampine hallucinations in Parkinson’s disease patients. Front Neurol. 2015;6:263. PubMed CrossRef
- Devenney EM, Ahmed RM, Hodges JR. Frontotemporal dementia. In: Dekosky ST, Asthana S, eds. Geriatric Neurology. Elsevier; Handbook of Clinical Neurology. 2019:167:279–299.
- Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. PubMed CrossRef
- O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. PubMed CrossRef
- Mendez MF. What is the relationship of traumatic brain injury to dementia? J Alzheimers Dis. 2017;57(3):667–681. PubMed CrossRef
- Ballard CG, Saad K, Patel A, et al. The prevalence and phenomenology of psychotic symptoms in dementia sufferers. 1995;10(6):477–485.
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. PubMed CrossRef
- US Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. http://psychrights.org/drugs/FDAatypicalswarning4elderly.pdf. Accessed August 31, 2021.
- Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J Manag Care Pharm. 2012;18(suppl B):S1–S20. PubMed
- Center for Clinical Standards and Quality/Quality. Safety & Oversight Group. Five-Star Quality Rating System. Centers for Medicare & Medicaid Services. CMS.gov website. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/FSQRS. October 7, 2019. Accessed April 1, 2021.
- Stefanacci RG. New CMS rules on psychotropic medications in SNFs. Ann Longterm Care. 2017;25(6):19–20. CrossRef
- Bowblis JR, Lucas JA, Brunt CS. The effects of antipsychotic quality reporting on antipsychotic and psychoactive medication use. Health Serv Res. 2015;50(4):1069–1087. PubMed CrossRef
- Centers for Medicare & Medicaid Services. National Partnership to Improve Dementia Care in Nursing Homes: Antipsychotic Medication Use Data Report. CMS.gov website. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/National-Partnership-to-Improve-Dementia-Care-in-Nursing-Homes. April 2021. Accessed July 30, 2021.
- Ogarek J, Neylon K, Gadbois E, et al. Effect of CMS’s measure of antipsychotic prescribing practices for nursing facilities on utilization of antipsychotic medications and changes in diagnostic patterns. NASMHPD website. https://www.nasmhpd.org/sites/default/files/TAC_Paper_2_508C.pdf. August 2019. Accessed July 30, 2021.
- Maust DT, Kim HM, Chiang C, et al. Association of the Centers for Medicare & Medicaid Services’ national partnership to improve dementia care with the use of antipsychotics and other psychotropics in long-term care in the United States from 2009 to 2014. JAMA Intern Med. 2018;178(5):640–647. PubMed CrossRef
- Simmons SF, Bonnett KR, Hollingsworth E, et al. Reducing antipsychotic medication use in nursing homes: a qualitative study of nursing staff perceptions. Gerontologist. 2018;58(4):e239–e250. PubMed CrossRef
- Devanand DP, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med. 2012;367(16):1497–1507. PubMed CrossRef
- Tariot PN, Cummings JL, Soto-Martin ME, et al. Trial of pimavanserin in dementia-related psychosis. N Engl J Med. 2021;385(4):309–319. PubMed CrossRef
- Early N, Fairman K, Ma J, et al. Changes in psychotropic prescribing for patients with dementia, 2014–2016: potential implications for pharmacists. Sr Care Pharm. 2020;35(5):207–219. PubMed CrossRef
- Wang S, Temkin-Greener H, Conwell Y, et al. Policy to reduce antipsychotic use and hospitalization of nursing home residents with dementia. J Am Med Dir Assoc. 2020;21(11):1617–1622.e3. PubMed CrossRef
- Kirkham J, Susan B, Harris D, et al. Antipsychotic reduction in long-term care: unintended prescribing outcomes. Am J Geriatr Psychiatry. 2020;28(suppl 4):S141. CrossRef
This PDF is free for all visitors!
Save
Cite