Objective: To evaluate the epidemiology, pathobiology, and management of benign ethnic neutropenia and determine the extent to which these factors should influence measures designed to avoid clozapine-induced agranulocytosis.
Data Sources: A structured MEDLINE search with no language limitation was performed from database inception until March 31, 2015, using the terms clozapine and benign ethnic neutropenia. Retrieved articles were cross-checked for additional relevant studies.
Study Selection: Included in the study were articles that reported on the prevalence, etiology, and complications of benign ethnic neutropenia and the hematologic outcome of clozapine treatment in patients with this condition.
Data Extraction: Study results that documented the epidemiology, pathobiology, and clozapine utilization in persons of African, Arabian, and Mediterranean descent with a neutrophil count in the 1,000-1,800/mm3 range.
Results: The search identified 342 publications. Forty-two articles described the epidemiology, pathobiology, and management of benign ethnic neutropenia. Of these, 12 articles described patients with benign ethnic neutropenia whose neutrophil count decreased during treatment with clozapine. Persons with benign ethnic neutropenia do not have signs of impaired phagocytosis, and the frequency, severity, and outcome of their infections are similar to those observed in the general population. These features suggest that a neutrophil count > 1,000/mm3 is safe for initiating and/or resuming clozapine therapy.
Conclusions: The presence of benign ethnic neutropenia should not prevent treatment with clozapine. Patients with benign ethnic neutropenia who develop a clozapine-induced decrease in the neutrophil count, but have no evidence of infection or impaired phagocytosis, may resume clozapine as soon as they have > 1,000 neutrophils/mm3.
Benign Ethnic Neutropenia and Clozapine Use:
A Systematic Review of the Evidence and Treatment Recommendations

ABSTRACT
Objective: To evaluate the epidemiology, pathobiology, and management of benign ethnic neutropenia and determine the extent to which these factors should influence measures designed to avoid clozapine-induced agranulocytosis.
Data Sources: A structured MEDLINE search with no language limitation was performed from database inception until March 31, 2015, using the terms clozapine and benign ethnic neutropenia. Retrieved articles were cross-checked for additional relevant studies.
Study Selection: Included in the study were articles that reported on the prevalence, etiology, and complications of benign ethnic neutropenia and the hematologic outcome of clozapine treatment in patients with this condition.
Data Extraction: Study results that documented the epidemiology, pathobiology, and clozapine utilization in persons of African, Arabian, and Mediterranean descent with a neutrophil count in the 1,000-1,800/mm3 range.
Results: The search identified 342 publications. Forty-two articles described the epidemiology, pathobiology, and management of benign ethnic neutropenia. Of these, 12 articles described patients with benign ethnic neutropenia whose neutrophil count decreased during treatment with clozapine. Persons with benign ethnic neutropenia do not have signs of impaired phagocytosis, and the frequency, severity, and outcome of their infections are similar to those observed in the general population. These features suggest that a neutrophil count > 1,000/mm3 is safe for initiating and/or resuming clozapine therapy.
Conclusions: The presence of benign ethnic neutropenia should not prevent treatment with clozapine. Patients with benign ethnic neutropenia who develop a clozapine-induced decrease in the neutrophil count, but have no evidence of infection or impaired phagocytosis, may resume clozapine as soon as they have > 1,000 neutrophils/mm3.
J Clin Psychiatry 2016;77(7):e909-e916
dx.doi.org/10.4088/JCP.15r10085
© Copyright 2016 Physicians Postgraduate Press, Inc.
aMedical Services and bDepartment of Psychiatry, The Zucker Hillside Hospital, Glen Oaks, New York
cDepartment of Psychiatry, Hofstra North Shore-Long Island Jewish School of Medicine, Hempstead, New York
dAlbert Einstein College of Medicine, Bronx, New York
eDivision of Medical Ethics, Transilvania University, Brasov, Romania
fThe Feinstein Institute for Medical Research, Manhasset, New York
*Corresponding author: Peter Manu, MD, South Oaks Hospital, 400 Sunrise Highway, Amityville, NY 11701 ([email protected]).
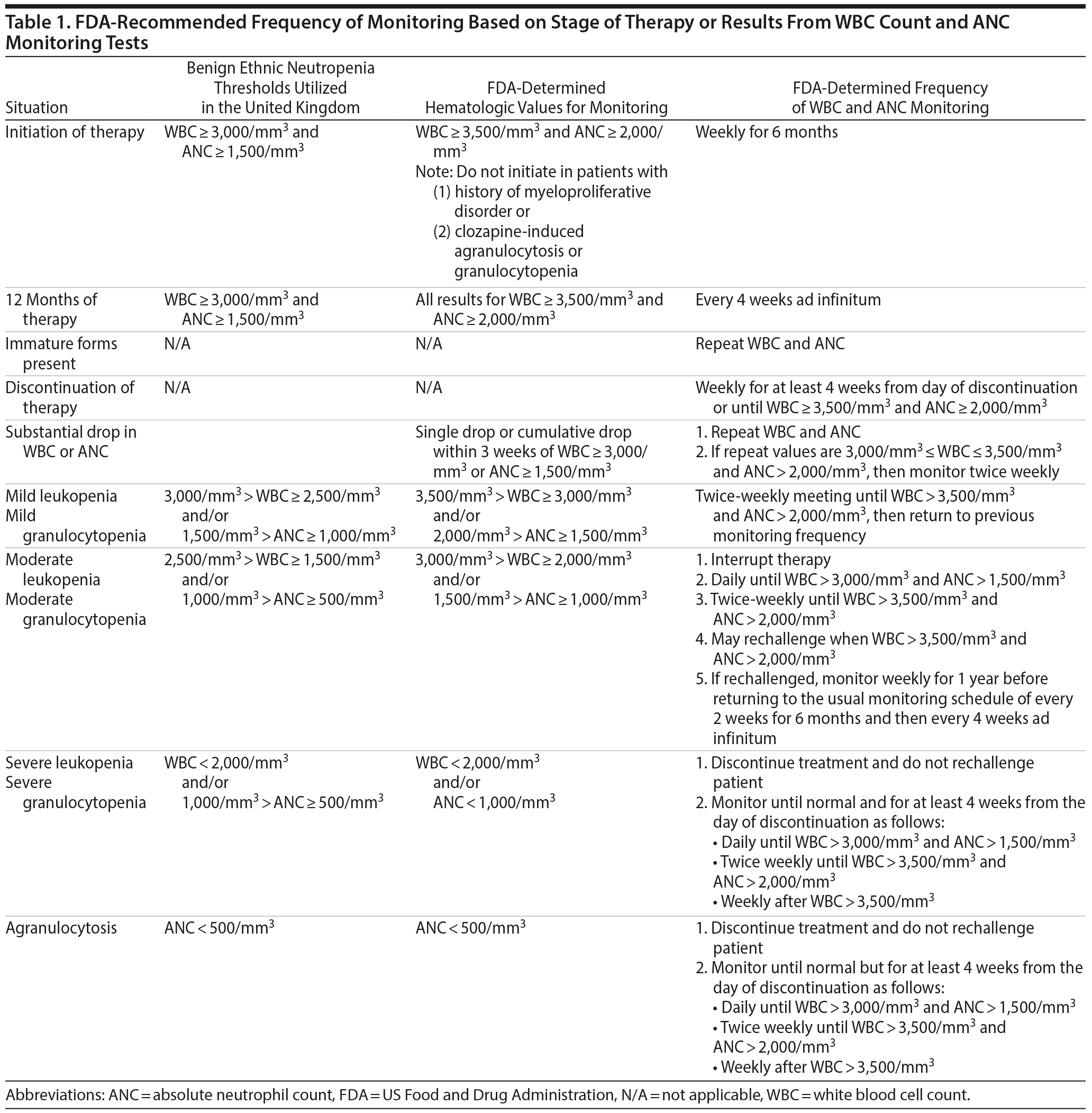
Clozapine is a tricyclic dibenzodiazepine atypical antipsychotic approved by the US Food and Drug Administration (FDA) for the management of treatment-resistant schizophrenia. In the United States, the drug is also indicated for patients with schizophrenia or schizoaffective disorder who experience recurrent suicidal behavior. Clozapine is the most effective antipsychotic for patients with schizophrenia spectrum disorders1-4 and bipolar disorders5 in whom first-line treatments fail. However, clozapine also has many adverse effects that complicate its use, the most potentially serious of which is agranulocytosis,6,7 defined as an absolute neutrophil count (ANC) of less than 500/mm3. Current US clinical standards indicate that in order to prevent clozapine-induced agranulocytosis, therapy should be initiated only in patients with a white blood cell count (WBC) of at least 3,500/mm3 and an ANC of at least 2,000/mm3. Hematologic monitoring should occur weekly for the first 6 months, every other week for the following 6 months, and every 4 weeks after the first year of treatment. If the WBC is between 2,000 and 3,000/mm3 or ANC is 1,000-1,500/mm3, clinicians should interrupt treatment, monitor daily, and rechallenge when WBC is > 3,500 and ANC is > 2,000/mm3. Clozapine treatment should be discontinued and rechallenge is not permitted in patients whose WBC is < 2,000/mm3 or ANC is < 1,000/mm3.7
These guidelines ignore the existence of benign ethnic neutropenia (BEN), a hereditary condition conservatively estimated to be present in 10% to 30% of healthy persons of African or Middle Eastern descent who have no history of repeated or severe infections.8 Benign ethnic neutropenia in African–Americans was discovered in 1939 in a group of 20 healthy Mississippi sharecroppers.9 Compared with native whites with the same diet and occupation, the African-Americans had significantly lower mean WBC (4,800/mm3 vs 7,600/mm3) and neutrophil (2,300/mm3 vs 4,600/mm3) counts. None of the subjects with neutropenia had any active disease at the time of the investigation. Their diet was adequate, and there was no evidence of gastrointestinal parasites. Evidence of “sickling” was reported in 2 blacks subjects (10%). The findings have been confirmed by large-scale epidemiologic studies10 and have been reported as a reason for clozapine’s underutilization and discontinuation in African-Americans11 and in the black and minority community in the United Kingdom.12 Furthermore, men have lower values than women, independently, across all ethnicities.13 Benign ethnic neutropenia is recognized as a modifier for the use of clozapine in the United Kingdom and Ireland, but details of the process and deliberations through which the restrictions have been relaxed in the presence of this condition are not available. Based on successful clozapine treatment in patients with BEN,14 adjusted thresholds for patients with habitually low WBCs have been proposed that are 500/mm3 lower than those in the general population for normal white cell/neutrophil count (≥ 3,000/≥ 1,500/mm3 instead of ≥ 3,500/≥ 2,000/mm3), mild leukopenia/granulocytopenia (≥ 2,500-3,000/≥ 1,000-1,500/mm3 instead of ≥ 3,000-3,500/≥ 1,500-2,000/mm3), and moderate leukopenia/granulocytopenia (< 2,500-> 2,000/< 1,000-> 500/mm3 instead of < 3,000-> 2,000/< 1,500-> 1,000/mm3)15 (Table 1). Of note, severe leukopenia/granulocytopenia and agranulocytosis thresholds are unaltered.
The risk factors for drug-induced agranulocytosis include the presence of autoimmune disease, a current or recent infection, chronic renal failure, and a history of allergic reaction to any antigen.16 The available data indicate that BEN has not been associated with increased risk of agranulocytosis in the general population.16 A MEDLINE search covering the period 1970-2015 did not reveal any cases of clozapine-induced agranulocytosis in patients with BEN. Therefore, clozapine therapy monitoring guidelines based on hematologic parameters of white populations are considered to restrict access to clozapine for individuals with BEN.12,17-19
In this review, we will present the main features of BEN and propose algorithms for clozapine utilization in patients with this condition.

- Many (10%-30%) persons of African, Arabian, and Mediterranean descent have benign ethnic neutropenia, ie, a neutrophil count in the 1,000-1,800/mm3 range. Current clinical guidelines prevent the use of clozapine in these individuals.
- Patients with benign ethnic neutropenia do not have signs of impaired phagocytosis, and the frequency, severity, and outcome of their infections are similar to those observed in the general population. These features suggest that a neutrophil count > 1,000/mm3 is safe for initiating and/or resuming clozapine therapy.
- The presence of benign ethnic neutropenia should not prevent treatment with clozapine. Patients with benign ethnic neutropenia who develop a clozapine-induced decrease in the neutrophil count, but have no evidence of infection, may be treated with clozapine as soon as they have at least 1,000 neutrophils/mm3.
METHODS
We conducted a systematic review in MEDLINE on March 31, 2015, dating back through inception, using the search terms benign ethnic neutropenia and clozapine to identify primary data articles and review articles on the phenomenon of BEN and the severity and management of hematologic complications in patients with BEN receiving clozapine.
RESULTS
The search identified a total of 342 publications. Forty-two articles described the epidemiology, pathobiology, and management of BEN and included 12 articles focusing on patients with BEN who developed decreased ANC during treatment with clozapine. All 42 articles were included in this review and used for the development of our recommendations.
Epidemiology
The prevalence of BEN in the US population has been most recently established in a representative sample of 25,222 participants in the 1999 to 2004 National Health and Nutrition Examination Survey.13 A neutrophil count of less than 1,500/mm3 was found in 4.5% (95% confidence interval, 3.9%-5.0%) of blacks but in only 0.79% (95% confidence interval, 0.57%-1.0%) of whites and in 0.38% (95% confidence interval, 0.24%-0.52%) of Mexican-Americans. Severe neutropenia (ANC < 1,000/mm3) was identified in 0.57% of black and 0.11% of white participants. Compared with whites, black participants had lower neutrophil counts (mean difference = 830/mm3). The lower number of neutrophils explained almost entirely the 890/mm3 difference in the WBC counts of the 2 groups. Black males had the higher prevalence of neutropenia (6.67%). Younger age increased the likelihood of neutropenia, while smoking decreased the likelihood of neutropenia.13 These data confirm the findings of previous investigations in smaller samples, which had identified a mean difference of 800 neutrophils/mm3 between black and white active-duty healthy service members20 and 960 neutrophils/mm3 in male veterans with these same ethnic backgrounds.21 Benign ethnic neutropenia has also been recognized at similar or higher rates in Afro-Caribbeans in the United Kingdom22 and the United States23; Arabs in Jordan, Kuwait, and the United Arab Emirates8,24; Yemenite and Ethiopian Jews and Bedouin Arabs living in Israel8,25; and blacks in Zimbabwe.26 Ancestry is more important than current geographical location, as demonstrated by the absence of neutropenia in members of a non-Arab tribe that relocated to the Arabian Peninsula a few centuries ago.24 A chronic neutropenia without bone marrow hypoplasia or increased risk of infections or leukemia has also been described in healthy white individuals,27 mostly female,8 and in patients with chronic hepatitis C.28
The health care implications of BEN have not been thoroughly investigated, but clinical experience suggests that individuals with this condition may be subject to extensive and costly hematologic evaluation, inappropriate antibiotic coverage for febrile viral illnesses, unnecessary and possibly harmful dose adjustments during chemotherapy, and discontinuation of treatment with clozapine8,24,29 as well as exclusion from clozapine treatment in the first place.
Pathobiology
The African and Arabian roots of individuals with BEN are considered to reflect acquired resistance to malaria, as the distribution of the ancestral homes matches the geographic distribution of Plasmodium falciparum.24 A similar superimposition has been found for the presence of hemoglobin S and thalassemia. In contrast, benign neutropenia is much more infrequent in persons tracing their ancestry in Mexico, because pre-Columbian inhabitants of that territory were never exposed to P falciparum.24 Pedigrees of Arab families indicate that as an “ancient” and widely spread inherited trait, the genetic transmission of BEN occurred before the tribal partitioning and is not the effect of increased homozygosity due to endogamy and inbreeding.24
The mechanism of the association has not been elucidated, but neutrophil activation in malaria produced by P falciparum may increase the risk of secondary (bacterial) infection and fatal outcomes.24,30 The genetic signature of BEN in Africans appears to be a polymorphism within the Duffy antigen receptor for chemokines (DARC),30 which influences the neutrophils’ chemotaxis, migration, and localization and leads to the inactivation of the proinflammatory cytokines. The Duffy antigen enhances transendothelial migration of neutrophils in response to chemotaxis.31 DARC-positive phenotypes in Africans are also associated with decreased susceptibility to tuberculosis and HIV infection.30 The null allele of the Duffy antigen is present in close to 80% of African–Americans and in 58% of Yemenite Jews, the 2 populations with a relative high incidence of BEN.31
Patients with BEN have normal circulating neutrophils, ie, normal proportions of mature and immature cells. There is no evidence of degenerated hypersegmented granulocytes8 or increased margination in the spleen, liver, bone marrow, or lungs.32,33 Bone marrow aspirates indicate normal cellularity, maturation of the white blood cell line,34 and granulocyte colony-forming ability.8 Physical activity, intravenous corticosteroid administration, and endotoxin challenges have demonstrated decreased release of mature granulocyte from the bone marrow into the peripheral blood.8,31,32,34
The incidence and outcome of bacterial infections are similar in persons with BEN and populations with higher baseline neutrophil counts.29,35 A study comparing patients with and without BEN admitted for the same acute bacterial infection found similar systemic abnormalities (fever, heart rate), response to antibiotics, and duration of hospitalization.35 Patients with BEN do not develop absolute leukocytosis during bacterial infections,29,35 but the average proportional increment in the WBC count is similar (39.0% vs 38.6%) to that observed in patients without leukopenia with the same ethnic background.35 The findings remind us that neutrophils are not “functioning” in the blood, but use the blood as a transport medium to a site to which they are attracted by an inflammatory stimulus.36 The excess of circulating uncommitted cells in persons with “normal” neutrophil counts confers no advantage in the fight to localize and limit an infection, which depends on the number of committed cells made available hic et nunc by the bone marrow. An adequate increment of at least 2,000/mm3 neutrophils has been identified in response to bacterial infection in youth and adults with BEN.8,35,37
Differential Diagnosis
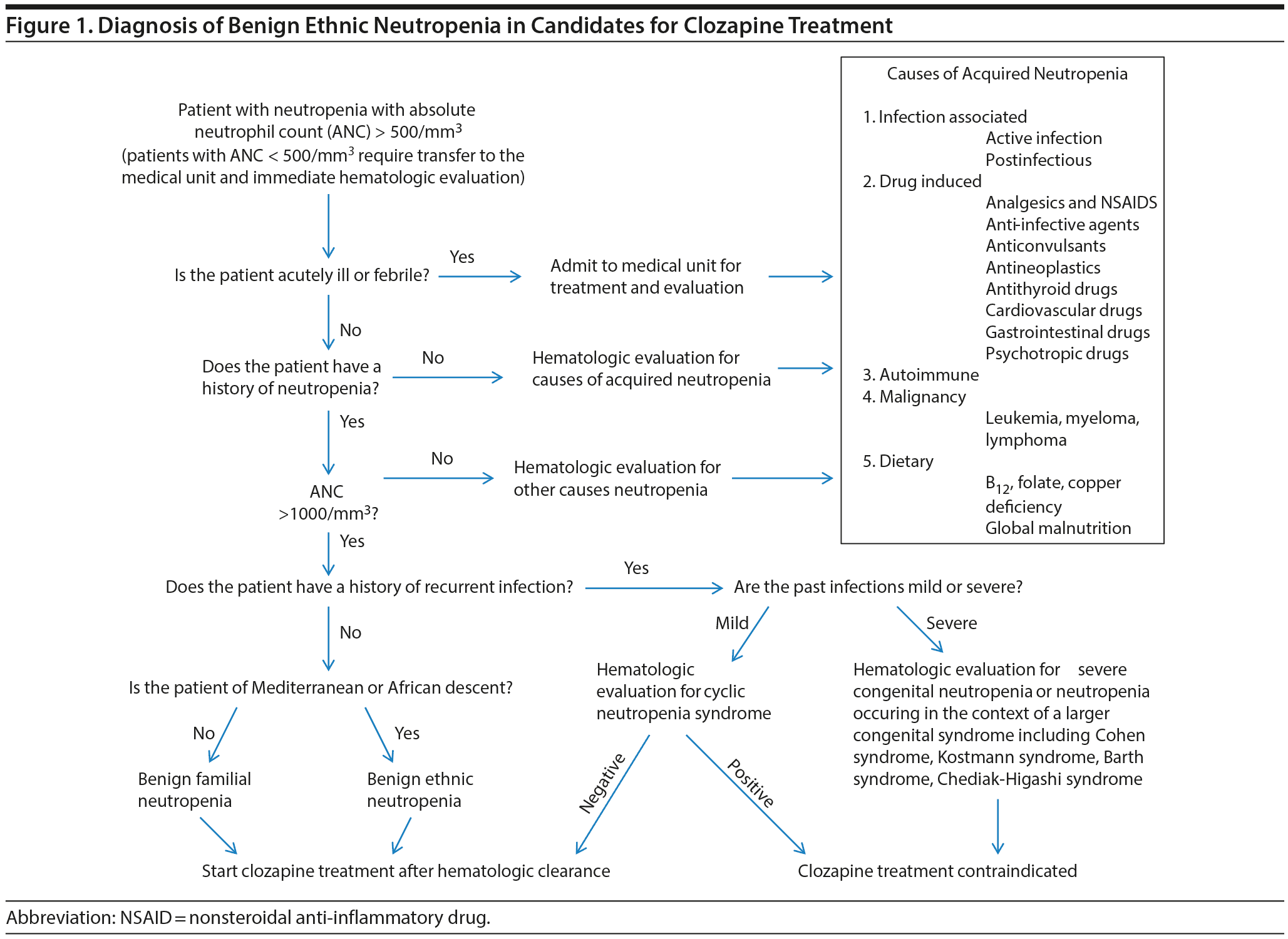
Benign ethnic neutropenia is a chronic congenital condition that should be suspected in persons of African, Arabian, and Mediterranean descent with an ANC in the 1,000-1,800/mm3 range without evidence of pancytopenia, current infection, or sepsis.38 It is a diagnosis of exclusion that should be established only after a careful evaluation conducted by an hematologist, who should first investigate the following causes of acquired neutropenia malignancy (leukemia, lymphoma, myeloma, myelodysplastic syndrome), primary or secondary autoimmune disorders, global or specific nutritional deficiency (vitamin B12, folate, copper), hypersplenism, storage diseases, radiotherapy and radioactive treatment, hypogammaglobulinemia, and exposure to drugs known to produce severe neutropenia or agranulocytosis.38-40 The last category is remarkably vast and in addition to antineoplastics includes psychotropic drugs other than clozapine; antithyroid drugs; anticonvulsants; analgesics and nonsteroidal anti-inflammatory drugs; cardiovascular drugs; diuretics; and antibiotics, antifungals, and antiretroviral agents16,38,39 (Table 2). The mechanisms of drug-induced neutropenia include bone marrow suppression and immune dysfunctions with and without antineutrophil antibodies.38,40 In patients with vitamin B12 deficiency, the neutropenia is associated with macrocytosis, while copper deficiency is seen almost exclusively in patients who have had gastric bypass surgery.38 The hematologic workup must be initiated after excluding pseudoneutropenia due to laboratory errors and diurnal variation in the neutrophil count. A general internal medicine specialist must be involved in the evaluation of neutropenia in situations in which a hematologist could not be available.
Once acquired neutropenia is ruled out, the workup should establish the type of inherited neutropenia. Cyclic neutropenia features episodes of severe neutropenia occurring every 2 to 5 weeks during which the patients may develop severe infections or evidence of phagocytic cell disease, such as oral ulcerations.38 A history of recurrent severe infections starting in infancy is the hallmark of severe congenital neutropenia, a syndrome characterized by ANC values being consistently less than 200/mm3 and due to a maturation arrest at the promyelocyte stage, requiring periodic administration of granulocyte colony-stimulating factor for survival.40,41 Severe congenital neutropenia and cyclic neutropenia are autosomal dominant syndromes and are produced by mutations in the granule protein neutrophil elastase. The 2 phenotypic expressions of these mutations may occur in the same kindred.42 A chronic idiopathic neutropenia with a relatively benign course despite ANC counts being as low as 200/mm3 has been identified in the Mediterranean area and is due to myeloid hypoplasia and a selective decrease of CD34+/CD33– progenitors.43 However, adults with BEN are not likely to have other congenital neutropenic conditions, such as dyskeratosis congenita, cartilage-hair hypoplasia, myelokathexis, or Fanconi anemia, as these syndromes manifest clinically in childhood.38
The absence of recurrent infections is crucial for the classification of isolated neutropenia as a benign condition. As a general rule, an ANC less than 200/mm3 will almost always lead to a serious localized infection, bacteremia, or meningitis.38,40 In patients with chronic neutropenia, recurrent infections are unusual if the ANC is greater than 500/mm3.38,40 Recurrent otitis media and oral ulcerations may be signs of neutropenia and should not be automatically attributed to an infectious process.40 A bone marrow aspiration and core biopsy is not considered mandatory in adults with chronic neutropenia whose ANC has never been lower than 500/mm3.40 In contrast, a bone marrow examination, including cytogenetics, is recommended if the ANC has been lower than 500/mm3 on 3 separate tests.38 Dysplastic features observed in the bone marrow and peripheral blood, such as promyelocytes with reticulated nuclei or hypogranular circulating neutrophils, help the hematology consultant establish the diagnosis of myelodysplastic syndrome.41
DISCUSSION
Legal and ethical considerations. Prescribing clozapine for patients with an ANC < 2,000/mm3 should be considered off-label, ie, a situation in which a drug is used for “a medical purpose not in accordance with the authorized product information.”44(p537) Off-label use is common in the United States and Europe (ie, 10%-29% of all prescriptions) and legal, because the regulatory agencies have not been granted constitutional authority to control or direct the practice of medicine.44-46 The medical practice reflects the challenges created by limited data regarding certain populations and treatment strategies, manufacturers’ inability or unwillingness to request revised labels for their products, and documented effectiveness in trials that do not fulfill all requirements indicated in the manufacturer’s prescribing information.7,46 Off-label or unlicensed prescribing might also be considered compassionate use, which, at least in the European Union, is allowed for “humanitarian reasons to a group of patients suffering from a debilitating and chronic or serious illness,”44(p540) but is unacceptable when a high probability of major adverse effects and only marginal clinical benefit exist.44-46
The off-label use of atypical antipsychotics in the United States was evaluated by a federal regulatory agency for health care quality in 2006.47 The agency found an increased risk of adverse events and questionable evidence of efficacy for the conditions most often treated off-label with antipsychotics, which were dementia with behavioral disturbance, autism, depression, obsessive-compulsive disorder, and posttraumatic stress syndrome.47 Clozapine was not included in the review because of its second-line status due to the potential for producing agranulocytosis.
With the exception of a few case reports,48,49 we could not find published reports on prospective studies of clozapine treatment in patients with BEN, although it is quite possible that clinicians have tried the off-label use of the drug in this population. However, clozapine underutilization in patients of African descent has been well-documented,11 even though the incidence of agranulocytosis is similar or lower than in white subjects.17,18 The lack of data stands in contrast to the ethical principle that all patient groups should be given access to medications that have been sufficiently tested44 to determine their risk and benefits.
Our review and the recommendations included in this text have limitations inherent to clinical domains that have not been rigorously assessed. The main reason for this shortcoming in our case is the fact that agranulocytosis had been identified before the clinical trials that led to the introduction of clozapine in the treatment of schizophrenia in the United States. These trials were designed with restrictions regarding cutoffs for leukocyte and neutrophil counts and excluded patients with BEN.
Clinical recommendations. The evaluation of patients who have ANC levels that raise suspicion of BEN and require treatment with clozapine should be informed by the severity of neutropenia, history of recurrent infections, and the need to rule out the aforementioned acquired or congenital causes of a decreased ANC. Patients with new-onset neutropenia and presence of fever and/or ANC < 1,000/mm3 must be referred immediately for extensive hematologic evaluation, preferably in an inpatient medical unit. Patients from appropriate ethnic groups with chronic neutropenia (ANC > 1,000/mm3) but no history of serious and/or recurrent infection should undergo an outpatient hematologic evaluation aiming to obtain clearance for clozapine treatment (Figure 1). The evaluation should start only after the exclusion of pseudoneutropenia due to laboratory error or diurnal variation in the neutrophil count.
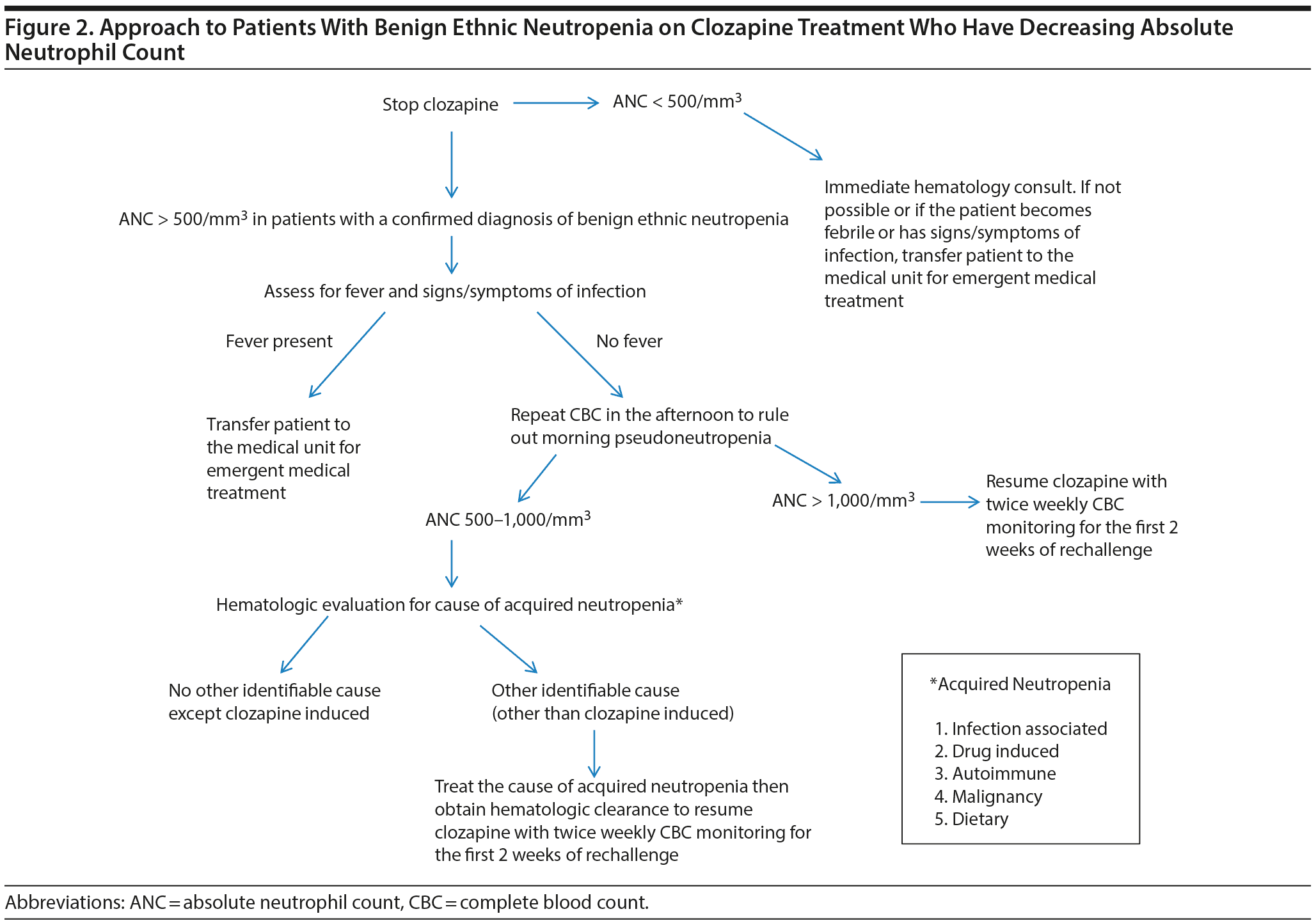
For patients with BEN treated with clozapine, a prudent approach is to stop the drug administration if ANC < 1,000/mm3, as no data are yet available to examine the safety below these levels. If a repeated WBC performed in the afternoon indicates an ANC > 1,000/mm3, which is indicative of morning pseudoneutropenia,50,51 clozapine treatment may be resumed with an increase from once- to twice-weekly WBC measurements. If a newly acquired cause of neutropenia is identified, clozapine therapy should be restarted only after the new issue has been successfully treated and the ANC has returned to baseline (Figure 2).
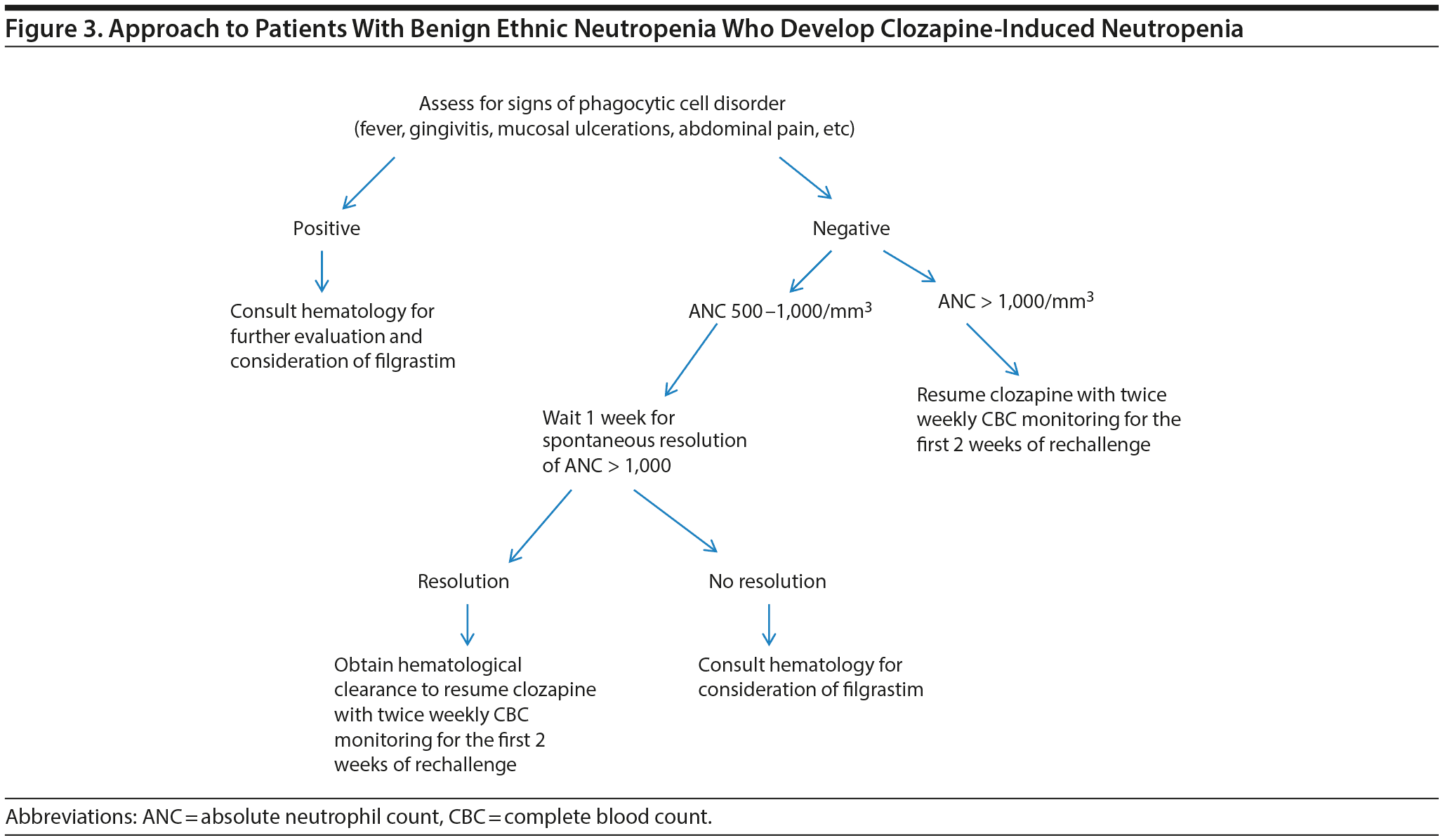
Patients with BEN who develop clozapine-induced decreases in ANC in the 500-1,500/mm3 range and who have no evidence of infection should be assessed for signs of phagocytic cell disorder (gingivitis, mucosal ulcerations, abdominal pain, diarrhea).22,40,41 When these abnormalities are present, a hematologist’s opinion must be sought regarding use of filgrastim to increase the neutrophil count. In patients without evidence of phagocytic cell disease, clozapine can be resumed if, or as soon as, the ANC is > 1,000/mm3 (Figure 3).
CONCLUSIONS
Benign ethnic neutropenia is a hereditary condition in which ANC levels are in the 1,000-1,800/mm3 range, but people have normal bone marrow cellularity, white blood cell line maturation, and granulocyte colony-forming ability. Therefore, the incidence and outcome of bacterial infections are similar in persons with BEN and populations with higher ANC levels. Benign ethnic neutropenia is present in 10%-30% of persons of African, Arabian, and Mediterranean descent and is due to a polymorphism within the Duffy antigen receptor for chemokines that has protective effects against malaria infections. Since guidelines regulate and restrict the use of clozapine in patients based on WBC and, especially, ANC levels, BEN is an important reason for clozapine underutilization and discontinuation.17
Based on the benign nature of habitual neutropenia and successful clozapine treatment in patients with BEN,14 it has been suggested that WBC and ANC thresholds be lowered by 500/mm3 compared to the general population, as done in the United Kingdom.15 We propose a complete assessment and treatment algorithm to identify patients with BEN and enable them to benefit from clozapine treatment by proposing assessment and treatment steps that both assure patient safety and appropriate utilization of clozapine in this important subgroup of patients. Our recommendations must be read within the limitations of the available publications, primarily the absence of any prospective study of the hematologic or infectious complications of patients with BEN treated with clozapine. With this important caveat, we hope that these recommendations can help clinicians use clozapine in patients who currently are denied this invaluable therapeutic intervention.
Submitted: April 27, 2015; accepted August 14, 2015.
Drug names: carbamazepine (Tegretol, Epitol, and others), clopidogrel (Plavix), clozapine (Clozaril, FazaClo, and others), filgrastim (Neupogen, Granix), fluoxetine (Prozac and others), furosemide (Lasix and others), lamotrigine (Lamictal and others), mirtazapine (Remeron and others), olanzapine (Zyprexa and others), phenytoin (Dilantin, Phenytek, and others), valproic acid (Depakene and others), ziprasidone (Geodon and others).
Potential conflicts of interest: Dr Kane has been a consultant and/or received honoraria from Amgen, Alkermes, Bristol-Myers Squibb, Eli Lilly, Forrest Laboratories, Genentech, IntraCellular Therapies, Janssen, Johnson & Johnson, Lundbeck, MedAvante, Novartis, Otsuka, Roche, Reviva, Sunovion, and Teva; and is a shareholder of MedAvante and Vanguard Research Group. Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Actavis, Alkermes, Bristol-Myers Squibb, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen, Johnson & Johnson, Lundbeck, MedAvante, Medscape, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Supernus, and Takeda; has received grant support from the American Academy of Child and Adolescent Psychiatry, Bristol-Myers Squibb, Janssen, Johnson & Johnson, National Institute of Mental Health (NIMH), Novo Nordisk A/S, Otsuka, Takeda, and the Thrasher Foundation; and has provided expert testimony for Janssen. Drs Manu, Sarvaiya, and Rogozea have nothing to disclose.
Funding/support: None.
Role of the sponsor: None.
REFERENCES
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796. PubMed doi:10.1001/archpsyc.1988.01800330013001
2. Meltzer HY. Treatment-resistant schizophrenia—the role of clozapine. Curr Med Res Opin. 1997;14(1):1-20. PubMed doi:10.1185/03007999709113338
3. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627. PubMed doi:10.1016/S0140-6736(09)60742-X
4. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
5. Nielsen J, Kane JM, Correll CU. Real-world effectiveness of clozapine in patients with bipolar disorder: results from a 2-year mirror-image study. Bipolar Disord. 2012;14(8):863-869. PubMed doi:10.1111/bdi.12018
6. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613. PubMed doi:10.4088/JCP.12r08064
7. Novartis Pharmaceutical Corporation. Clozaril (clozapine) tablets. Briefing Book for the Psychopharmacological Drugs Advisory Committee Meeting, May 19, 2003 Available at: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3959B1_01_E-Novartis-Appendix 4.pdf.
8. Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133(1):15-22. PubMed doi:10.1053/lc.1999.v133.a94931
9. Forbes WH, Johnson RE, Consolazio F. Leucopenia in Negro workmen. Am J Med Sci. 1941;201(3):407-412.
10. Fulwood R, Johnson CL, Bryner JD, et al; National Center for Health Statistics. Hematological and Nutritional Biochemistry Reference Data for Persons Six Months to 74 Years of Age: United States, 1976-1980. Vital and Health Statistics, Series II, No. 232, DHHS Pub. No. (PHS) 83-1682. Public Health Service. Washington, DC: US Government Printing Office; December 1982. Available at: http://www.cdc.gov/nchs/data/series/sr_11/sr11_232.pdf.
11. Kelly DL, Dixon LB, Kreyenbuhl JA, et al. Clozapine utilization and outcomes by race in a public mental health system: 1994-2000. J Clin Psychiatry. 2006;67(9):1404-1411. PubMed doi:10.4088/JCP.v67n0911
12. Whiskey E, Olofinjana O, Taylor D. The importance of the recognition of benign ethnic neutropenia in black patients during treatment with clozapine: case reports and database study. J Psychopharmacol. 2011;25(6):842-845. PubMed doi:10.1177/0269881110364267
13. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the US population: age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146(7):486-492. PubMed doi:10.7326/0003-4819-146-7-200704030-00004
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35. PubMed doi:10.2165/00023210-200721010-00003
15. Rajagopal S. Clozapine, agranulocytosis, and benign ethnic neutropenia. Postgrad Med J. 2005;81(959):545-546. PubMed doi:10.1136/pgmj.2004.031161
16. Andersohn F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med. 2007;146(9):657-665. PubMed doi:10.7326/0003-4819-146-9-200705010-00009
17. Kelly DL, Kreyenbuhl J, Dixon L, et al. Clozapine underutilization and discontinuation in African Americans due to leucopenia. Schizophr Bull. 2007;33(5):1221-1224. PubMed doi:10.1093/schbul/sbl068
18. Maher KN, Tan M, Tossell JW, et al. Risk factors for neutropenia in clozapine-treated children and adolescents with childhood-onset schizophrenia. J Child Adolesc Psychopharmacol. 2013;23(2):110-116. PubMed doi:10.1089/cap.2011.0136
19. Bray A. Ethnic neutropenia and clozapine. Aust N Z J Psychiatry. 2008;42(4):342-345. PubMed doi:10.1080/00048670701881595
20. Reed WW, Diehl LF. Leukopenia, neutropenia, and reduced hemoglobin levels in healthy American blacks. Arch Intern Med. 1991;151(3):501-505. PubMed doi:10.1001/archinte.1991.00400030063011
21. Freedman DS, Gates L, Flanders WD, et al. Black/white differences in leukocyte subpopulations in men. Int J Epidemiol. 1997;26(4):757-764. PubMed doi:10.1093/ije/26.4.757
22. Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664-666. PubMed doi:10.1136/jcp.49.8.664
23. Grann VR, Bowman N, Joseph C, et al. Neutropenia in 6 ethnic groups from the Caribbean and the US. Cancer. 2008;113(4):854-860. PubMed doi:10.1002/cncr.23614
24. Denic S, Showqi S, Klein C, et al. Prevalence, phenotype and inheritance of benign neutropenia in Arabs. BMC Blood Disord. 2009;9(1):3. PubMed doi:10.1186/1471-2326-9-3
25. Weingarten MA, Pottick-Schwartz EA, Brauner A. The epidemiology of benign leukopenia in Yemenite Jews. Isr J Med Sci. 1993;29(5):297-299. PubMed
26. Adewuyi J, Sigola LB, Keogh E. African neutropaenia in Zimbabwe. Cent Afr J Med. 1994;40(5):108-110. PubMed
27. Kyle RA, Linman JW. Chronic idiopathic neutropenia: a newly recognized entity? N Engl J Med. 1968;279(19):1015-1019. PubMed doi:10.1056/NEJM196811072791902
28. Sheehan V, Weir A, Waters B. Severe neutropenia in patients with chronic hepatitis C: a benign condition. Acta Haematol. 2013;129(2):96-100. PubMed doi:10.1159/000342964
29. Weingarten MA, Kahan E, Brauner A. Normal fluctuations of leucocyte counts and the response to infection in benign familial leucopenia. Acta Haematol. 1992;87(3):126-128. PubMed doi:10.1159/000204738
30. Thobakgale CF, Ndung’ u T. Neutrophil counts in persons of African origin. Curr Opin Hematol. 2014;21(1):50-57. PubMed doi:10.1097/MOH.0000000000000007
31. Paz Z, Nails M, Ziv E. The genetics of benign neutropenia. Isr Med Assoc J. 2011;13(10):625-629. PubMed
32. Shoenfeld Y, Modan M, Berliner S, et al. The mechanism of benign hereditary neutropenia. Arch Intern Med. 1982;142(4):797-799. PubMed doi:10.1001/archinte.1982.00340170157024
33. von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol. 2008;181(8):5183-5188. PubMed doi:10.4049/jimmunol.181.8.5183
34. Mason BA, Lessin L, Schechter GP. Marrow granulocyte reserves in black Americans: hydrocortisone-induced granulocytosis in the “benign” neutropenia of the black. Am J Med. 1979;67(2):201-205. PubMed doi:10.1016/0002-9343(79)90391-7
35. Shoenfeld Y, Ben-Tal O, Berliner S, et al. The outcome of bacterial infection in subjects with benign familial leukopenia (BFL). Biomed Pharmacother. 1985;39(1):23-26. PubMed
36. Crosby WH. How many “polys” are enough? Arch Intern Med. 1969;123(6):722-723. PubMed doi:10.1001/archinte.1969.00300160112019
37. Sadowitz PD, Amanullah S, Souid AK. Hematologic emergencies in the pediatric emergency room. Emerg Med Clin North Am. 2002;20(1):177-198. PubMed doi:10.1016/S0733-8627(03)00057-9
38. Gibson C, Berliner N. How we evaluate and treat neutropenia in adults. Blood. 2014;124(8):1251-1258. PubMed doi:10.1182/blood-2014-02-482612
39. James RM, Kinsey SE. The investigation and management of chronic neutropenia in children. Arch Dis Child. 2006;91(10):852-858. PubMed doi:10.1136/adc.2006.094706
40. Boxer LA. How to approach neutropenia. Hematology (Am Soc Hematol Educ Program). 2012;2012:174-182. PubMed
41. Palmblad J, Dufour C, Papadaki HA. How we diagnose neutropenia in the adult and elderly patient. Haematologica. 2014;99(7):1130-1133. PubMed doi:10.3324/haematol.2014.110288
42. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol. 2013;50(3):198-206. PubMed doi:10.1053/j.seminhematol.2013.06.010
43. Papadaki HA, Gibson FM, Psyllaki M, et al. Assessment of bone marrow stem cell reserve and function and stromal cell function in patients with severe congenital neutropenia. Eur J Haematol. 2001;67(4):245-251. PubMed doi:10.1034/j.1600-0609.2001.00495.x
44. Lenk C, Duttge G. Ethical and legal framework and regulation for off-label use: European perspective. Ther Clin Risk Manag. 2014;10:537-546. PubMed doi:10.2147/TCRM.S40232
45. Aronsohn A, Reau N, Jensen D. Preparing for the uncertain yet inevitable: off-label combinations of antiviral agents in hepatitis C virus. Hepatology. 2014;59(5):1688-1691. PubMed doi:10.1002/hep.26903
46. Fitzgerald AS, O’ Malley PG. Staying on track when prescribing off-label. Am Fam Physician. 2014;89(1):4-5. PubMed
47. Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. J Manag Care Pharm. 2012;18(5 suppl B):S1-S20. PubMed
48. Spencer BWJ, Williams HRJ, Gee SH, et al. Granulocyte colony stimulating factor (G-CSF) can allow treatment with clozapine in a patient with severe benign ethnic neutropaenia (BEN): a case report. J Psychopharmacol. 2012;26(9):1280-1282. PubMed doi:10.1177/0269881112450782
49. Nykiel S, Henderson D, Bhide G, et al. Lithium to allow clozapine prescribing in benign ethnic neutropenia. Clin Schizophr Relat Psychoses. 2010;4(2):138-140. PubMed doi:10.3371/CSRP.4.2.5
50. Esposito D, Chouinard G, Hardy P, et al. Successful initiation of clozapine treatment despite morning pseudoneutropenia. Int J Neuropsychopharmacol. 2006;9(4):489-491. PubMed doi:10.1017/S146114570500605X
51. McKee JR, Wall T, Owensby J. Impact of complete blood count sampling time change on white blood cell and absolute neutrophil count values in clozapine recipients. Clin Schizophr Relat Psychoses. 2011;5(1):26-32. PubMed doi:10.3371/CSRP.5.1.4
Please sign in or purchase this PDF for $40.00.
Save
Cite