Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Standard pharmacologic treatment options for depressive symptoms are generally unable to achieve remission quickly. This delay may account for the substantial morbidity and mortality associated with unremitted depression. The discovery of the rapid-acting antidepressant effects of ketamine has generated significant interest and led to ketamine being labeled "the most important [drug] discovery in half a century."
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
The Antidepressant Effect of Repeat Dose Intravenous Ketamine Is Delayed by Concurrent Benzodiazepine Use
To the Editor: Standard pharmacologic treatment options for depressive symptoms are generally unable to achieve remission quickly. This delay may account for the substantial morbidity and mortality associated with unremitted depression.1 The discovery of the rapid-acting antidepressant effects of ketamine has generated significant interest and led to ketamine being labeled "the most important [drug] discovery in half a century."2(p68) A growing literature attests to ketamine’s rapid but transient antidepressant actions in patients with treatment-resistant depression (TRD).3,4 To extend the clinical applicability of ketamine, several open-label trials4-6 have used ketamine as an augmenting agent, allowing participants to continue prestudy medications. Results from these studies enable characterization of potential drug interactions and shed light on the underlying mechanism of ketamine response.
Method. We present a post hoc analysis of an original study5 examining the effect of concurrent benzodiazepine treatment on antidepressant response to 6 ketamine infusions (0.5 mg/kg; 1 infusion every Monday, Wednesday, and Friday for 2 weeks) in individuals with TRD. The study was conducted at the Minneapolis Veterans Affairs Medical Center from October 2012 to August 2013 and approved by the institutional review board. All subjects provided written and informed consent prior to participation.
Inclusion criteria included men and women aged 18-70 years, with major depressive disorder (MDD) resistant to treatment (defined as failure to achieve remission from 2 adequately dosed antidepressants of different pharmacologic classes per the Antidepressant Treatment History Form7).
Exclusion criteria included any unstable medical condition, any nonpsychiatric central nervous system disorder, any Axis I disorder other than MDD judged to be the primary diagnosis, active substance use disorder within 6 months of study entry, lifetime history of psychosis, positive urine toxicology, or positive urine pregnancy screen for women. The Montgomery-Asberg Depression Rating Scale (MADRS)8 was administered by study clinicians immediately before and after each infusion, as well as 1 hour and 2 hours after cessation of each infusion.
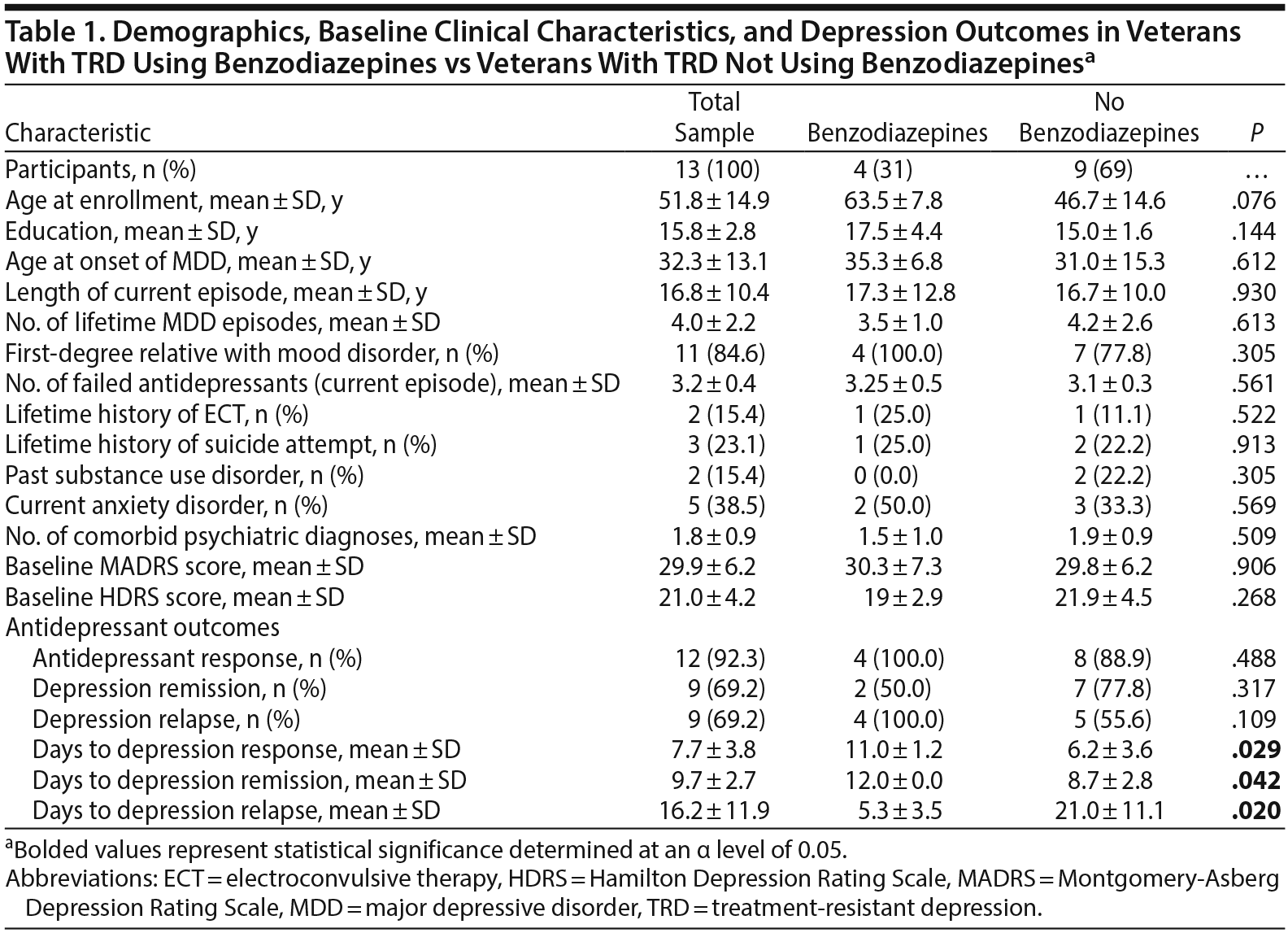
Of the 13 individuals who completed all 6 infusions, 4 were taking benzodiazepines (mean daily dose = 2.75 mg lorazepam equivalents).
Results. There was no statistically significant difference between benzodiazepine users and benzodiazepine nonusers in baseline demographic or clinical characteristics, depression response (MADRS score ≤ 50% of baseline) rate or remission (MADRS score < 10) rate after 6 infusions or in depression relapse (MADRS score ≥ 50% of baseline) rate during the 28-day follow-up period (Table 1). However, benzodiazepine users showed a significantly longer time to antidepressant response (P = .029), a significantly longer time to depression remission (P = .042), and a significantly shorter time to depression relapse (P = .020) (Table 1). In short, benzodiazepine users reached the same outcomes as benzodiazepine nonusers but took a longer time to reach those outcomes and also showed a significantly shorter time to loss of therapeutic effect.
The current findings add to a prior clinical report suggesting that benzodiazepines interfere with ketamine response.9 Interestingly, ketamine has been noted to be particularly effective for individuals with anxious depression and thus would be expected to be more effective in individuals requiring anxiolytic medications (eg, benzodiazepines).10 The paradoxical finding of decreased ketamine efficacy in benzodiazepine users supports the hypothesis that benzodiazepines themselves interfere with ketamine’s mechanism of action.
Ketamine is a noncompetitive N-methyl-d-aspartate receptor (NMDAR) antagonist. The precise mechanism of ketamine’s therapeutic effects continues to be a source of debate.11 One hypothesis suggests that ketamine modulates NMDARs on inhibitory γ-aminobutyric acid-ergic (GABAergic) interneurons that exert tonic suppression of excitatory glutamatergic networks.12 By blocking NMDARs on these interneurons, ketamine decreases inhibition, resulting in a burst of glutamate, signaling through the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and up-regulation of neuroplasticity-related transcription factors. Consistent with this model, agonism of the GABA-A receptor (as occurs with benzodiazepines) would increase inhibitory tone of these interneurons, thereby decreasing excitatory glutamatergic signal transduction and blocking the therapeutic effects of ketamine.
The current post hoc analysis provides support for the hypothesis that ketamine’s mechanism of action involves effects on GABAergic neurotransmission. This finding may bear on the variable response rates to ketamine, particularly in single-infusion studies, and suggests that coadministration of medications interfering with GABAergic neurotransmission may hamper optimal ketamine response. Though limited by small sample size, this report corroborates a previous report of attenuated response to multiple ketamine infusions via GABAergic mechanisms.9 As the likelihood of widespread use of ketamine for TRD nears and clinical trials examining the antidepressant efficacy of intranasal esketamine continue,13 consideration of concurrent benzodiazepine treatment is evidently necessary when designing clinical trials aimed at understanding the magnitude, timing, and durability of ketamine effects. Ultimately, however, the hypothesis that concomitant benzodiazepine exposure attenuates ketamine response would best be addressed in a randomized clinical trial.
References
1. Mauskopf JA, Simon GE, Kalsekar A, et al. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress Anxiety. 2009;26(1):83-97. PubMed doi:10.1002/da.20505
2. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72. PubMed doi:10.1126/science.1222939
3. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864. PubMed doi:10.1001/archpsyc.63.8.856
4. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250-256. PubMed doi:10.1016/j.biopsych.2012.06.022
5. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123-129. PubMed doi:10.1016/j.jad.2013.10.036
6. Rasmussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444-450. PubMed doi:10.1177/0269881113478283
7. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17. PubMed
8. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. PubMed doi:10.1192/bjp.134.4.382
9. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336. PubMed doi:10.1097/JCP.0000000000000316
10. Ionescu DF, Luckenbaugh DA, Niciu MJ, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry. 2014;75(9):e932-e938. PubMed doi:10.4088/JCP.14m09049
11. Lester HA, Lavis LD, Dougherty DA. Ketamine inside neurons? Am J Psychiatry. 2015;172(11):1064-1066. PubMed doi:10.1176/appi.ajp.2015.14121537
12. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697. PubMed doi:10.1002/da.22501
13. A study to evaluate the safety and efficacy of intranasal esketamine in treatment-resistant depression (SYNAPSE). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01998958. Updated June 6, 2016. Accessed October 3, 2016.
aDepartment of Psychiatry, University of Minnesota Medical School, Minneapolis
bMental Health Service Line, Minneapolis VA Medical Center, Minneapolis, Minnesota
cDepartment of Psychiatry, North Memorial Medical Center, Minneapolis, Minnesota
dDepartment of Anesthesiology, Minneapolis VA Medical Center, Minneapolis, Minnesota
eGeriatric Research Education and Clinical Center, Minneapolis VA Medical Center, Minneapolis, Minnesota
Potential conflicts of interest: The authors report no financial or other relationship relevant to the subject of this letter.
Funding/support: This research was supported by the Mental Health Service Line at the Minneapolis VA Medical Center and by a Career Development Award from the Center for Epidemiological and Clinical Research-VA Clinical Research Center of Excellence awarded to Dr Shiroma. Dr Albott’s work is supported by a National Institute on Drug Abuse training grant (T320A037183).
Role of the sponsor: The funding source had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit these data for publication.
Previous presentation: Poster presented at the 54th Annual Meeting of the American College of Neuropsychopharmacology (ACNP); Hollywood, Florida; December 9, 2015.
J Clin Psychiatry 2017;78(3):e308-e309
https://doi.org/10.4088/JCP.16l11277
© Copyright 2017 Physicians Postgraduate Press, Inc.
Save
Cite
Advertisement
GAM ID: sidebar-top