Objective: To summarize the effects of antenatal benzodiazepine exposure as monotherapy and in combination with antidepressants on the risk of congenital malformations.
Data Sources: MEDLINE, PsycINFO, CINAHL, Embase, and the Cochrane Library were searched from inception to June 30, 2018, using controlled vocabulary and keywords (eg, prenatal, benzodiazepines, malformation).
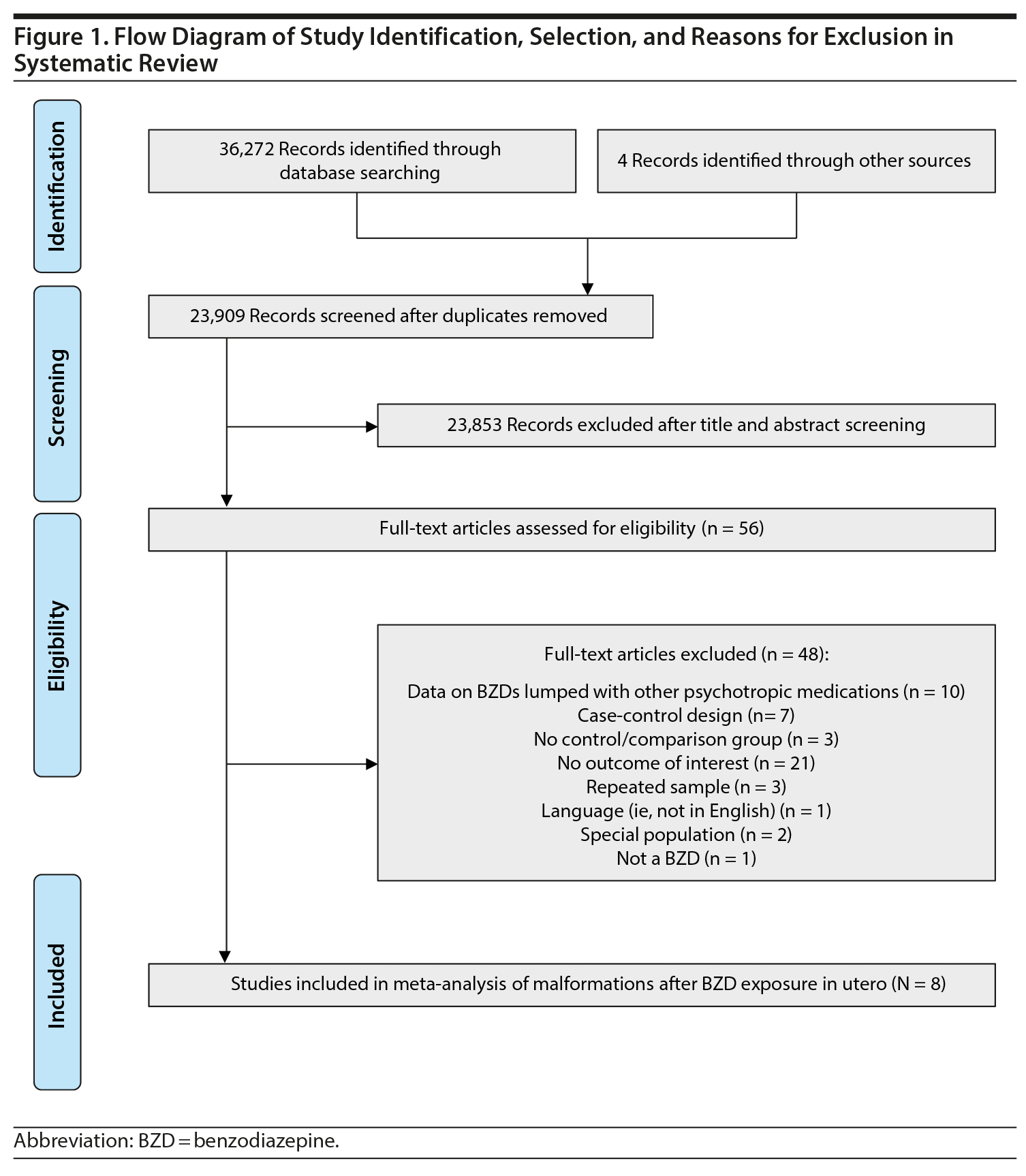
Study Selection: English-language cohort studies with prospectively collected data on the risk of malformations in benzodiazepine-exposed and -unexposed offspring were evaluated. 23,909 records were screened, 56 studies were assessed for eligibility, and 8 studies were included.
Data Extraction: Quality was assessed by 2 independent reviewers and data extracted. Random-effects models were used for outcomes (≥ 3 studies). Subanalyses examined effect of potential moderators including study quality and timing of exposure, among others.
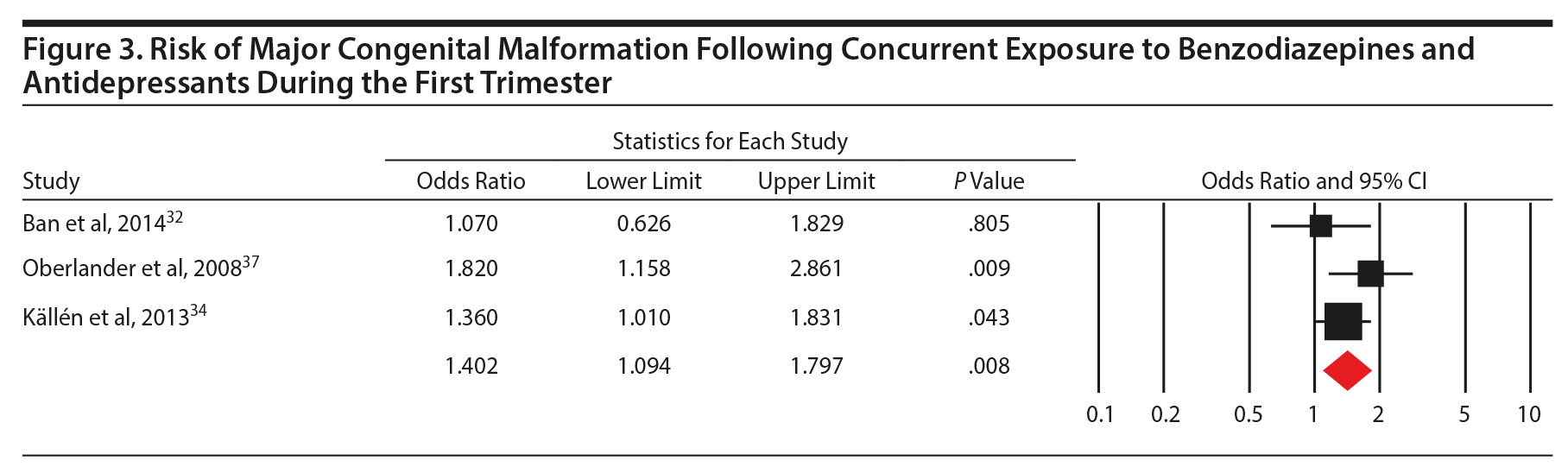
Results: Prenatal benzodiazepine use was not associated with an increased risk of congenital malformations (odds ratio [OR] = 1.13; 95% CI, 0.99 to 1.30, 8 studies, n = 222/5,195 exposed and 64,335/2,082,467 unexposed), including with first trimester exposure specifically (OR = 1.08; 95% CI, 0.93 to 1.25, P = .33; 5 studies, n = 181/4,331 exposed and 64,308/2,081,463 unexposed). There was no significant association with cardiac malformation following exposure (OR = 1.27; 95% CI, 0.98 to 1.65, P = .07; 4 studies, n = 61/4,414 exposed and 19,260/2,033,402 unexposed). However, concurrent use of benzodiazepine and antidepressants during pregnancy was associated with a significantly increased risk of congenital malformations (OR = 1.40; 95% CI, 1.09 to 1.80, P = .008; 3 studies).
Conclusions: Benzodiazepine exposure during pregnancy does not appear to be associated with congenital malformations or with cardiac malformations specifically. There may be an increased risk of congenital malformations when benzodiazepines are used in conjunction with antidepressants, suggesting that caution with this combination is warranted.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
‘ ¢Use caution when prescribing benzodiazepine-antidepressant combinations to pregnant women, as there may be an increased risk of congenital malformations
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in July 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2021. The latest review of this material was June 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Benzodiazepine Use During Pregnancy Alone or in Combination With an Antidepressant and Congenital Malformations:
Systematic Review and Meta-Analysis
ABSTRACT
Objective: To summarize the effects of antenatal benzodiazepine exposure as monotherapy and in combination with antidepressants on the risk of congenital malformations.
Data Sources: MEDLINE, PsycINFO, CINAHL, Embase, and the Cochrane Library were searched from inception to June 30, 2018, using controlled vocabulary and keywords (eg, prenatal, benzodiazepines, malformation).
Study Selection: English-language cohort studies with prospectively collected data on the risk of malformations in benzodiazepine-exposed and -unexposed offspring were evaluated. 23,909 records were screened, 56 studies were assessed for eligibility, and 8 studies were included.
Data Extraction: Quality was assessed by 2 independent reviewers and data extracted. Random-effects models were used for outcomes (≥ 3 studies). Subanalyses examined effect of potential moderators including study quality and timing of exposure, among others.
Results: Prenatal benzodiazepine use was not associated with an increased risk of congenital malformations (odds ratio [OR] = 1.13; 95% CI, 0.99 to 1.30, 8 studies, n = 222/5,195 exposed and 64,335/2,082,467 unexposed), including with first trimester exposure specifically (OR = 1.08; 95% CI, 0.93 to 1.25, P = .33; 5 studies, n = 181/4,331 exposed and 64,308/2,081,463 unexposed). There was no significant association with cardiac malformation following exposure (OR = 1.27; 95% CI, 0.98 to 1.65, P = .07; 4 studies, n = 61/4,414 exposed and 19,260/2,033,402 unexposed). However, concurrent use of benzodiazepine and antidepressants during pregnancy was associated with a significantly increased risk of congenital malformations (OR = 1.40; 95% CI, 1.09 to 1.80, P = .008; 3 studies).
Conclusions: Benzodiazepine exposure during pregnancy does not appear to be associated with congenital malformations or with cardiac malformations specifically. There may be an increased risk of congenital malformations when benzodiazepines are used in conjunction with antidepressants, suggesting that caution with this combination is warranted.
J Clin Psychiatry 2019;80(4):18r12412
To cite: Grigoriadis S, Graves L, Peer M, et al. Benzodiazepine use during pregnancy alone or in combination with an antidepressant and congenital malformations: systematic review and meta-analysis. J Clin Psychiatry. 2019;80(4):18r12412.
To share: https://doi.org/10.4088/JCP.18r12412
© Copyright 2019 Physicians Postgraduate Press, Inc.
aMood and Anxiety Disorders Clinic: Reproductive Transitions, Department of Psychiatry, Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, Ontario, Canada
bDepartment of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo, Michigan
cEvaluative Clinical Sciences, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
dLawrence S. Bloomberg Faculty of Nursing, University of Toronto, Toronto, Ontario, Canada
eDepartment of Psychiatry, Women’s College Hospital and the University of Toronto, Toronto, Ontario, Canada
fDepartment of Psychiatry and Behavioural Neurosciences, St Joseph’s Healthcare Hamilton and McMaster University, Hamilton, Ontario, Canada
gDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
hDepartment of Psychiatry, Sunnybrook Health Sciences Centre and the University of Toronto, Toronto, Ontario, Canada
iHealth Nexus, Toronto, Ontario, Canada
jDivision of Neurology, St Michael’s Hospital, Toronto, Ontario, Canada
*Corresponding author: Sophie Grigoriadis, MD, PhD, Women’s Mood and Anxiety Clinic: Reproductive Transitions, Department of Psychiatry, Sunnybrook Health Sciences Centre, FG 29, 2075 Bayview Ave, Toronto, Ontario, M4N 3M5, Canada ([email protected]).
While anxiety treatment recommendations and prescribing patterns have shifted in recent years away from benzodiazepine therapy to newer antidepressants,1,2 benzodiazepines continue to be used frequently for anxiety disorders.3 Importantly, benzodiazepines are often used concurrently with antidepressants at the beginning of treatment with an antidepressant for depression when anxiety is a prominent feature4 or as augmentation/combination with an antidepressant, for example, when depression is comorbid with anxiety disorders.5 Anxiety disorders are common in pregnancy, with rates up to 15% being reported,6 and they are often comorbid with depression, rendering them even more common.7,8 This leaves women with a treatment conundrum as to the choice of whether to use benzodiazepines during pregnancy, as monotherapy or in combination with an antidepressant. In addition, given the frequent use of medications in women compared to men9,10 and in reproductive-aged women,11 women may have exposure without awareness during the first trimester, when organogenesis is taking place. Benzodiazepines were found to be the third most frequently used psychotropic drug in pregnancy (following selective serotonin reuptake inhibitors and benzodiazepine-like medications),12 and a recent large cohort study reported a rate of prenatal benzodiazepine use of 2.5%, with 84% of use occurring in the first trimester.13 Due to conflicting results across studies, it is unknown if benzodiazepine exposure during pregnancy is associated with an increased risk of congenital malformations. While 2 meta-analyses (one with > 1,000,000 women14,15) suggested nonteratogenicity with benzodiazepine use in general, retrospective studies have indicated an increase in congenital malformations16,17 and in oral clefts specifically, suggesting an almost 2-fold risk (odds ratio [OR] = 1.79)14,18 in an examination of case-control studies. Moreover, although combination treatment may be used, subsequent risks are unknown. Newer data are now available, including data on the risk of cardiac anomaly and the combined use of benzodiazepines and antidepressants in pregnancy specifically. Up-to-date information about risks constitutes a critical component of the risk-benefit profile that clinicians and patients require to inform their treatment decisions.
The aim of this study was to reevaluate the risk of congenital malformations following exposure to benzodiazepines. To minimize bias,19 we assessed prospectively collected data from cohort studies comparing malformations in benzodiazepine-exposed and -unexposed pregnancies. We conducted a systematic review and meta-analysis where possible on the risk of antenatal benzodiazepine exposure and congenital malformations in the infant, including cardiac malformations. Potential effect modifiers as sources of heterogeneity were also assessed through subanalyses. The effect of benzodiazepines combined with an antidepressant, which to our knowledge has not been previously summarized, was also evaluated.

- Benzodiazepines have not been found to be teratogenic overall in previous work but have been associated with specific malformations in retrospective studies.
- Our systematic review and meta-analysis replicated previous work concluding that benzodiazepines are not teratogenic. Further, we found that benzodiazepines are not associated with cardiac malformations.
- Benzodiazepines in combination with antidepressants appear to convey an increased risk for major malformations, although the clinical significance appears low.
METHODS
Search Strategy
This work followed the guidelines of the Meta-Analyses and Systematic Reviews of Observational Studies,20 using methods previously described.21 Briefly, as part of a broader study of the impact of prenatal anxiety and prenatal use of anxiety-related medications, 2 psychopharmacology and psychiatry experts in library science independently conducted literature searches. One librarian searched Ovid MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, PsycINFO, CINAHL, Embase, and the Cochrane Library from their start date to June 30, 2018, using keywords that included antianxiety agents, anxiolytic, benzodiazepine, antenatal, congenital anomaly, congenital malformation, and malformation, among others (full search strategy available from the authors upon request). The second librarian used an iterative process to develop and test their search strategy in consultation with the review team. Strategies utilized a combination of keywords (eg, prenatal, anxiolytics) and controlled vocabulary (eg, “Pregnancy,” “Anti-Anxiety Agents”), and vocabulary and syntax were adjusted across databases as needed. Prior to execution, this strategy was reviewed by another senior information specialist using the Peer Review for Electronic Search Strategies checklist.22 Results were limited to the English language, human studies, and non-opinion pieces. We also searched reference lists from reviews, meta-analyses, and the final selection of included articles. The PRISMA checklist for reporting systematic reviews and meta-analyses was followed23 and reported in Figure 1.
Inclusion and Exclusion Criteria
Cohort studies that provided original, prospectively collected data on congenital malformations in a benzodiazepine-exposed and -unexposed comparison group were eligible. Acceptable assessment of benzodiazepine exposure included either a prescription for a benzodiazepine or notation of benzodiazepine use in maternal/clinical charts. For cases in which a sample was repeated in more than one publication, the article with the largest sample size was included. Studies presenting only data lumping benzodiazepines with other psychotropic medication(s) and case-control studies were excluded. Due to the volume of potentially eligible studies, unpublished data, abstracts, and conference proceedings were also excluded. Eligible studies that did not have outcome data comparable from at least 2 other studies were excluded (ie, minimum of 3 studies).
Quality Assessment and Data Extraction
Our team developed methods for quality assessment and data extraction, described in detail elsewhere.24 Briefly, independent quality assessments used the Systematic Assessment of Quality in Observational Research (SAQOR), which was adapted from the Downs and Black25 checklist and the Newcastle-Ottawa Scale.26 Quality categories included (1) sample, (2) control group, (3) quality of exposure/outcome measure, (4) follow-up, and (5) distorting influences/control for confounders. Combined with a modification of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system,27 SAQOR assessments provided each article with a quality rating of high, moderate, low, or very low quality. Ratings were then further classified as either “above quality threshold” (high, moderate, and low ratings) or “below quality threshold” (very low rating) for parsimony and to include enough data to permit meta-analysis.
Procedures for data extraction followed the Strengthening the Reporting of Observational Studies in Epidemiology criteria.28 Extracted data included authors, year of publication, source country, details of study design, participants (sample, control, demographics, and clinical characteristics), inclusion/exclusion criteria, details of benzodiazepine use (timing and indication of use in pregnancy, benzodiazepine type, and dose), use of other psychotropics in pregnancy, outcomes and their assessment methods and definitions, statistical adjustment for confounders, and loss to follow-up. For studies presenting outcome data following more than one benzodiazepine exposure time point in pregnancy, data from all time points were extracted and the largest sample size was used for the overall analysis, keeping in mind that time was assessed separately. When separate first trimester data were provided and included a smaller sample size, these data were used for the first trimester analysis. Adjusted estimates with their variances were extracted when available; when adjusted estimates were not provided in the published data, we calculated crude odds ratios or mean differences and sample variances. Before calculating the odds ratio for studies that included cells with a 0 count, we added 0.5 to these cells. Data were extracted by one reviewer and checked by another and the primary author (S.G.); disagreements were resolved by the primary author. Outcomes were as defined by the authors of the original publication. We contacted one author to request adjusted estimates and received a reply indicating that these data were not available (T. F. Oberlander, electronic communication; November 16, 2017). As such, raw data from this study were included.
Statistical Analyses
Pooled estimates of the odds ratio following random effects models were calculated using the DerSimonian and Laird method.29 Between-study heterogeneity was assessed with Cochrane Q and quantified with I2, which represents the percentage of the between-study variance in excess of what is expected due to chance.30
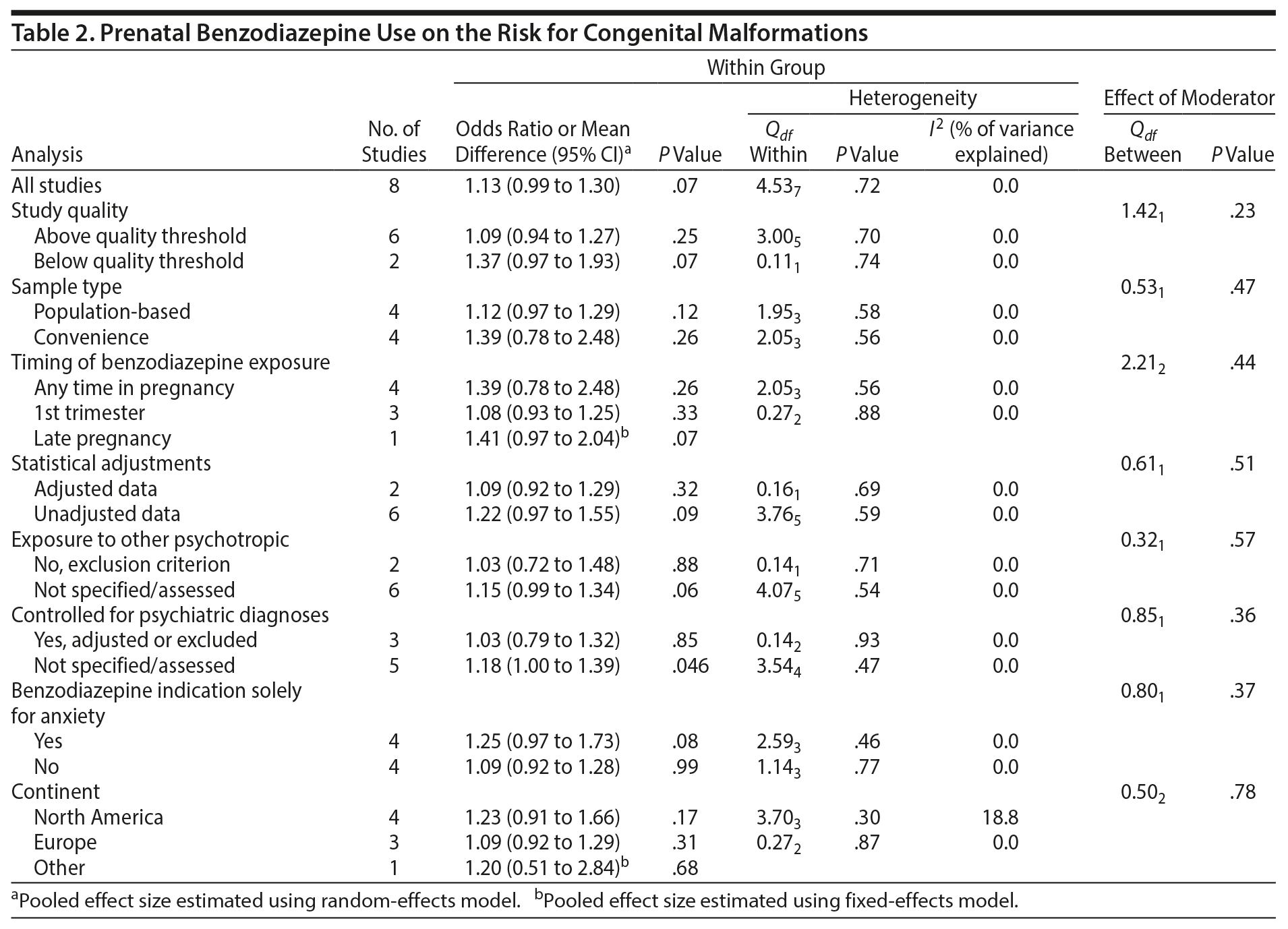
As part of the main analysis, we examined potential sources of heterogeneity through subgroup analyses, regardless of the Q statistic. These subgroup/moderator analyses identified between- and within-group differences in pooled effects based on study characteristics including (a) study quality (above or below quality threshold), (b) sample type (population-based or convenience), (c) timing of benzodiazepine exposure (any time, first trimester or late pregnancy), (d) statistical adjustments (adjusted or unadjusted estimates), (e) exposure to other psychotropic drugs (benzodiazepine monotherapy or use of any other psychotropic medications possible), (f) statistical adjustment/control for psychiatric diagnoses, (g) benzodiazepines used solely for anxiety, and (h) country. Separate analyses were conducted for the risk of malformations following benzodiazepine exposure in the first trimester, the risk of cardiac malformations following prenatal benzodiazepine exposure, and the risk of major congenital malformations following exposure to concurrent use of benzodiazepine and antidepressant in pregnancy.
Publication bias was assessed in analyses with a sufficient number of studies by visual inspection of funnel plots displaying individual study estimates (log odds ratio) by their standard error. The number of unpublished studies was estimated using Duval and Tweedie’s trim and fill method,31 and when one or more unpublished study was estimated, these were imputed around the summary estimate and a new (adjusted) summary OR was generated. All analyses were conducted using Comprehensive Meta-Analysis, Version 3 (Biostat; Englewood, New Jersey).
Patient Involvement
This study was conducted without patient involvement.
RESULTS
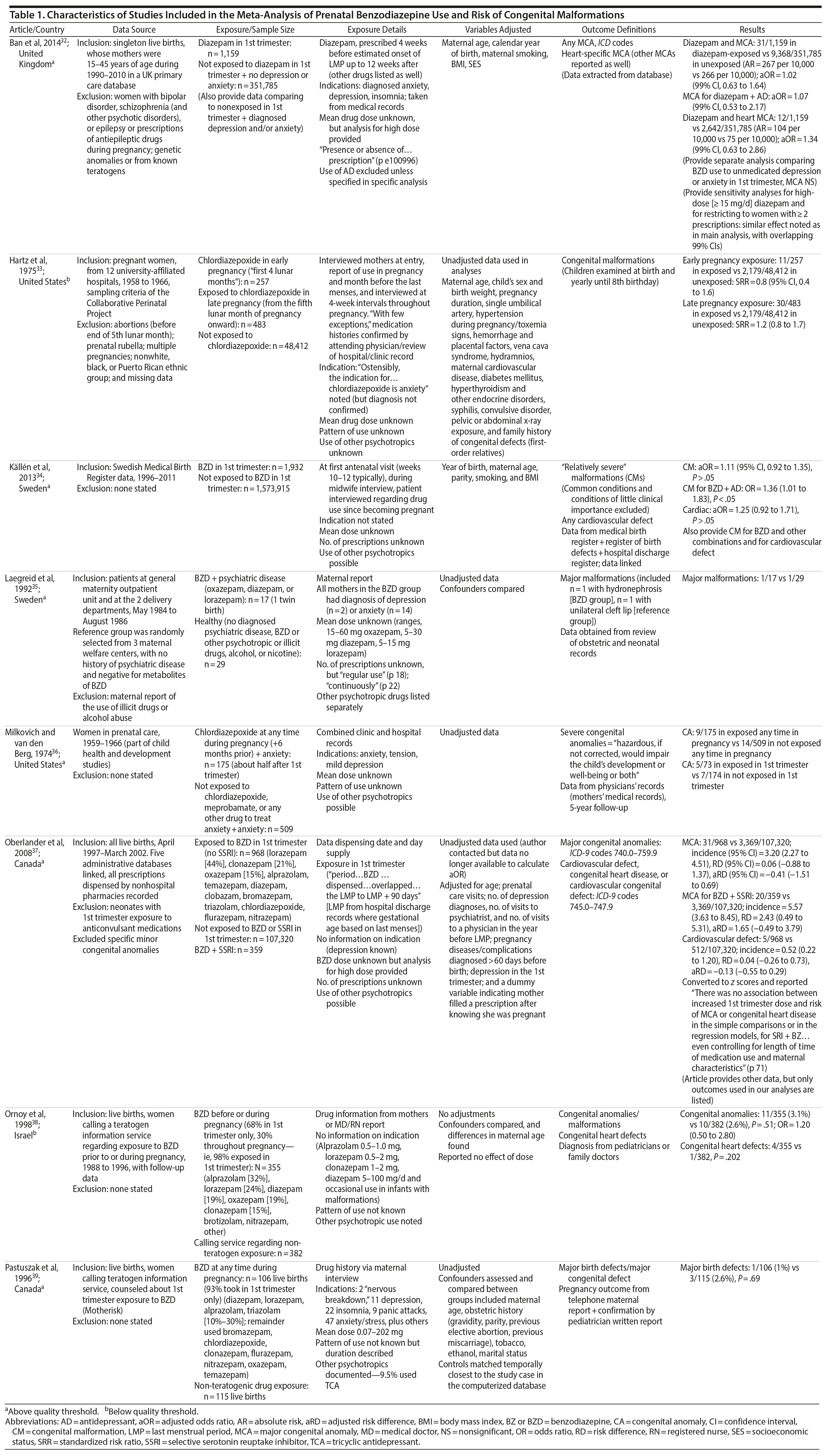
Of 23,909 unique records identified and screened, 56 full-text articles were retrieved and assessed for eligibility, and, of these, 8 studies were included in the present analyses (Figure 1).32-39 Characteristics of included studies are shown in Table 1, along with relevant descriptions. Six of the 8 studies that provided data on congenital malformations were above quality threshold, and 3 of the 4 studies with data on cardiac malformations were above quality threshold. Six of the 8 studies provided data on major malformations (Hartz et al33 [1975] and Ornoy et al38 [1998] did not specify).
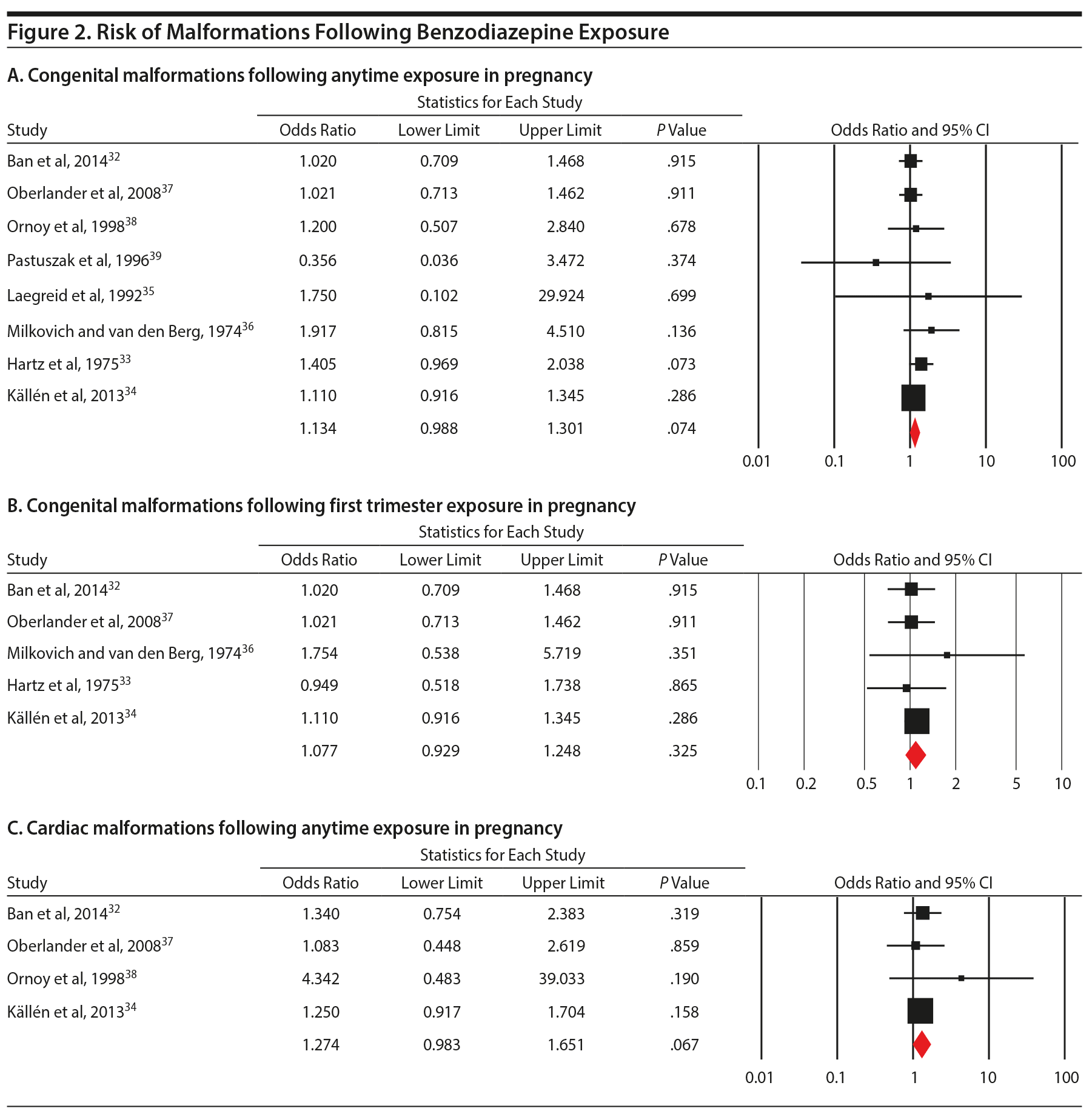
When pooling the 8 included studies, we found that benzodiazepine exposure in utero was not associated with the risk of congenital malformations (OR = 1.13; 95% CI, 0.99 to 1.30; P = .07, n = 222/5,195 exposed and 64,335/2,082,467 unexposed). The between-study heterogeneity for this analysis was not significant (Q7 = 4.53, P = .72, I2 = 0.0%) (Figure 2A). Not surprisingly, moderator analysis did not reveal significant moderators (all P values ≥ .10; Table 2). The subanalysis that included data that did not control for psychiatric diagnosis was significant (5 studies; OR = 1.18; 1.00 to 1.39, P = .046). Among 5 studies that specifically examined exposure to benzodiazepine in the first trimester, there again was no significant risk of congenital malformation (OR = 1.08; 0.93 to 1.25, P = .33, n = 181/4,331 exposed and 64,308/2,081,463 unexposed; Figure 2B), with no heterogeneity between studies (Q4 = 1.09, P = .90, I2 = 0.0%; Table 2).
Four studies investigating prenatal use of benzodiazepine (at any time during pregnancy) and cardiac malformations (OR = 1.27; 0.98 to 1.65, P = .07, n = 61/4,414 exposed and 19,260/2,033,402 unexposed, Figure 2C) were pooled, and a nonsignificant effect was shown. However, concurrent benzodiazepine and antidepressant use in the first trimester was significantly associated with the risk of major congenital malformations (OR = 1.40; 1.09 to 1.80, P = .008; 3 studies), with no significant between-study heterogeneity (Q2 = 2.30, P = .32; I2 = 12.9%; Figure 3). As there were only 3 studies, we did not run subgroup analyses.
Publication Bias
Assessment of publication bias was possible only for the main analysis and the analysis of malformations following first trimester benzodiazepine exposure. For the main analysis, publication bias was indicated by asymmetry of the funnel plot. The Duval and Tweedie trim and fill method imputed 1 missing study and provided a revised estimate similar to main results (OR = 1.12; 95% CI, 0.98 to 1.28), and this was still not significant. There was no evidence of publication bias for the analysis of the risk of malformations following first trimester benzodiazepine exposure.
DISCUSSION
In our meta-analysis that included 8 prospective cohort studies, we found no significant risk for congenital malformation following exposure at any time in pregnancy to benzodiazepine or during the first trimester exclusively. We also did not find an increased risk of cardiac malformations following benzodiazepine exposure. Interestingly, we found a significant pooled association between concurrent benzodiazepine and antidepressant use in pregnancy and the risk of major malformations, and to our knowledge this has not been previously reported. Despite the fact that an association would likely occur with a specific malformation if one exists, this study is the first step to help focus future research. Together, these results have significant implications for clinicians to streamline treatment regimen and for reproductive-age and pregnant women and their families. Of note, implications for clinicians extend beyond psychiatrists since the majority of benzodiazepine prescriptions are written by general practitioners9,40 and obstetricians.41
Strengths
This study has several strengths. We applied a rigorous process and conducted a comprehensive review. Our results update the previous meta-analysis by Enato et al (2011)14 and replicate the conclusion of the critical review by Bellantuono et al (2013)42 that there appears to be no association between benzodiazepine exposure in the first trimester and increased risk of congenital malformations. With the addition of new data, we were able to pool data on previously unsynthesized outcomes, namely, cardiac malformations and major congenital malformations specifically following concurrent prenatal use of benzodiazepine and antidepressant. By including only cohort studies with prospectively collected data, we have reduced the possibility of certain types of bias, keeping in mind that cohort studies inherently contain other types of design bias and between-study heterogeneity. Additionally, we explored potential sources of heterogeneity between pooled studies, and none of the moderators we identified a priori were significant. This is a very positive outcome as it suggests the data have little variability. The pooled association for studies that did not control for psychiatric diagnosis was significant but not the overall moderator analysis supporting the lack of heterogeneity overall. Interestingly, the “any time” in pregnancy exposure subanalysis was not significant, nor was the “first trimester” exposure. Organogenesis occurs during the first trimester primarily (note that the central nervous system continues to develop), and we would not expect significance in the “any time” exposure analysis. The fact that the “any time” analysis had a much wider confidence interval than the “first trimester” exposure analysis lends more confidence in the results.
Limitations
Results should nevertheless be interpreted with some caution as our analyses do have limitations. Namely, included studies reported on risks of malformations in populations/samples that included only live-born infants, potentially underestimating the risk of malformations following exposure to benzodiazepine antenatally,19 especially given that prenatal benzodiazepine may be associated with loss (ie, miscarriages and induced abortions), although this is not clear.43,44 The major malformations may have been selected out. We were also limited by the drug-specific data provided in the included studies that did not always specify indication and duration of use and, in all but 1 study (Ban et al32), did not examine variable effects for different doses of benzodiazepine. Different patterns of benzodiazepine use exist, with some women using these medications on occasion and others chronically; as a result, different patterns of use may have diverse implications for both mother and fetus. Further, large-scale studies that utilized data from prescription databases are limited by unclear “true” usage patterns. The poor adjustment for confounding cannot be totally overcome in the meta-analysis, and the original confounds can still influence the findings. For example, 6 of the studies (see Table 2) had unadjusted data, although it was reassuring that both the adjusted and unadjusted subanalyses were not significant. Finally, there were variable definitions of “major malformations” across studies, and we chose to group these, while providing original definitions (in Table 1). Some malformations therefore may be missed, but this is an issue with all teratogenic studies and points to the need for standardization for comparability across studies. Likewise, grouping all malformations together may mask associations between medication exposure and specific malformations (ie, cleft palate, which has been previously found in early case-control studies).14,18 We pooled data for specific malformation when available, and it was possible for only 1 outcome (cardiac malformations).
Implications
Benzodiazepines are commonly used in pregnancy, and we now have a sample size of 5,195 exposures compared to 2,082,467 not exposed, increasing our confidence in the absence or lack of an association with malformations when the drugs are used as monotherapy. Moreover, we are also reassured regarding cardiac malformations. Our analyses examined benzodiazepines as a class as there were insufficient data to analyze the pooled risk of malformations in association with specific drugs. As seen in research on use of other drugs in pregnancy, there may be differing risk profiles for adverse outcomes across different drugs within the same class. Of note, Källén et al (2013)34 reported that benzodiazepines, as a group, were not associated with a significant risk of severe congenital malformations or of congenital malformations, but significant risk of these outcomes was seen following alprazolam exposure from a small exposure group (total 444 events). The combination of benzodiazepines and antidepressants appears to be associated with major malformations, which is discouraging, as often these medications are used together, especially at the beginning of treatment. The mechanism needs to be determined if an association truly exists; it may be merely a function of increased surveillance for adverse effects given that more than one drug was used or that the women may have been more severely ill or perhaps the combination of medication may cause an interaction that results in increased blood drug levels, which may be associated with increased risk for anomaly.37 Given, however, that we are not provided with information on the pattern of use of the benzodiazepine with the antidepressant in the original studies, it makes intuitive sense to extrapolate that the occasional use would confer less risk, but this hypothesis also needs to be tested. Regardless, for now, our significant combined analysis of benzodiazepines and antidepressants argues against the use of more than one drug and supports the clinical recommendation of monotherapy that has often been made in past guidelines.45 Studies have been inconsistent, but some have implicated a potential signal for malformations following antidepressant exposure, although recent work has not found a signal overall.46,47 Further work is needed to determine if there is a particular combination of antidepressant with benzodiazepine that may be of concern versus the drugs in general. For example, a recent meta-analysis for citalopram specifically48 did not find an association, while for fluoxetine an effect was found.49 However, polypharmacy may still be the important factor as large, well-controlled studies of various medications did not find an effect.46 At least in one study, use of a benzodiazepine with antidepressant was found to aggravate adverse effects seen in the neonate.50 Fortunately, however, the OR in our analysis is small and, although statistically significant, clinically less likely to be so, especially since it is less than 2 (traditionally used a cutoff for clinical significance by clinical experts). Even the upper limit of the confidence interval was below the threshold of 2.47 As we are still hypotheses-driven in the area, this suggests that future research must look into the effects of polypharmacy and the compounding effects of severe mental illness.
Treatment of mood and anxiety disorders is paramount especially during pregnancy. This study provides more evidence that the risk-benefit ratio must be carefully considered and that it must be individual for every woman and her family. The weight load of the treatment can fluctuate and certainly may depend on symptom severity. Women with severe symptoms may not be able to participate in psychotherapy that has been recommended as first-line treatment, precisely because the symptoms render them unable. Monotherapy, although aspired to, may not be possible especially in the aforementioned cases. Treatment decisions are not easy to make and must take into account the effect of untreated illness on the mother, baby, and family as well as the potential adverse effects of the treatment itself. Although statistically significant, the clinical significance of the findings needs to be thought of carefully, especially in light of the small magnitude. Mothers are motivated to do what is best for their baby, and treatment of their illness must certainly be given heavy consideration. It is important not to lose sight of our role as health care providers, which is to work collaboratively with those we care for and do what is in their best interests within the context of their family.
Submitted: June 15, 2018; accepted March 1, 2019.
Published online: July 9, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, alprazolam is approved by the US Food and Drug Administration for the treatment of anxiety but should be discontinued during pregnancy and lactation; citalopram and fluoxetine are approved for the treatment of depression and should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Author contributions: Dr Grigoriadis conceptualized and designed the study and, with Dr Graves, obtained funding, directed data acquisition and analysis, and interpreted the data. Dr Grigoriadis is accountable for all aspects of the work and is the guarantor. Drs Grigoriadis and Peer drafted the manuscript. Dr Peer had substantial roles in data acquisition, data analysis, and drafting and revising the manuscript and with interpretation. Drs Vigod, Dennis, Steiner, Brown, Cheung, Rector, and Richter and Ms Dawson substantially contributed to study design and interpretation. Ms Mamisashvili had substantial role in data acquisition. Drs Grigoriadis, Graves, and Peer had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the manuscript critically and approved the final version.
Financial disclosures: Dr Grigoriadis has received personal fees from UpToDate, Eli Lilly, Psychotherapy Essentials to Go, and Compendium of Pharmaceuticals and Specialties over the last year, outside the submitted work, and Dr Vigod has received personal fees from UpToDate outside the submitted work; there are no other relationships or activities that could appear to have influenced the submitted work. Drs Graves, Peer, Dennis, Steiner, Brown, Cheung, Rector, and Richter and Mss Mamisashvili, Dawson, and Guenette have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This study was funded by the Canadian Institutes of Health Research, Ottawa, Ontario, Canada (FRN 141002).
Role of the sponsor: The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Previous presentation: A version of this paper was presented at the Canadian Psychiatric Association Conference, September 29, 2018, Toronto, Ontario, Canada.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1.Offidani E, Guidi J, Tomba E, et al. Efficacy and tolerability of benzodiazepines versus antidepressants in anxiety disorders: a systematic review and meta-analysis. Psychother Psychosom. 2013;82(6):355-362. PubMed CrossRef
2.Berney P, Halperin D, Tango R, et al. A major change of prescribing pattern in absence of adequate evidence: benzodiazepines versus newer antidepressants in anxiety disorders. Psychopharmacol Bull. 2008;41(3):39-47. PubMed
3.Starcevic V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert Rev Neurother. 2014;14(11):1275-1286. PubMed CrossRef
4.Bushnell GA, Stürmer T, Gaynes BN, et al. Simultaneous antidepressant and benzodiazepine new use and subsequent long-term benzodiazepine use in adults with depression, United States, 2001-2014. JAMA Psychiatry. 2017;74(7):747-755. PubMed CrossRef
5.Dold M, Bartova L, Souery D, et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders: results from a European multicenter study. J Psychiatr Res. 2017;91:1-13. PubMed CrossRef
6.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315-323. PubMed CrossRef
7.Falah-Hassani K, Shiri R, Dennis CL. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47(12):2041-2053. PubMed CrossRef
8.Dindo L, Elmore A, O’ Hara M, et al. The comorbidity of Axis I disorders in depressed pregnant women. Arch Women Ment Health. 2017;20(6):757-764. PubMed CrossRef
9.Alessi-Severini S, Bolton JM, Enns MW, et al. Use of benzodiazepines and related drugs in Manitoba: a population-based study. CMAJ Open. 2014;2(4):E208-E216. PubMed CrossRef
10.Petitjean S, Ladewig D, Meier CR, et al. Benzodiazepine prescribing to the Swiss adult population: results from a national survey of community pharmacies. Int Clin Psychopharmacol. 2007;22(5):292-298. PubMed CrossRef
11.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142. PubMed CrossRef
12.Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14(1):242. PubMed CrossRef
13.Yonkers KA, Gilstad-Hayden K, Forray A, et al. Association of panic disorder, generalized anxiety disorder, and benzodiazepine treatment during pregnancy with risk of adverse birth outcomes. JAMA Psychiatry. 2017;74(11):1145-1152. PubMed CrossRef
14.Enato E, Moretti M, Koren G. The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can. 2011;33(1):46-48. PubMed CrossRef
15.National Collaborating Centre for Mental Health, National Institute for Health and Clinical Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance, updated edition. NICE Clinical Guidelines No 192. Leicester, UK: The British Psychological Society & The Royal College of Psychiatrists. NICE website. https://www.ncbi.nlm.nih.gov/books/NBK305023/. 2014.
16.Altshuler LL, Cohen L, Szuba MP, et al. Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592-606. PubMed CrossRef
17.Iqbal MM, Sobhan T, Ryals T. Effects of commonly used benzodiazepines on the fetus, the neonate, and the nursing infant. Psychiatr Serv. 2002;53(1):39-49. PubMed CrossRef
18.Dolovich LR, Addis A, Vaillancourt JM, et al. Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and case-control studies. BMJ. 1998;317(7162):839-843. PubMed CrossRef
19.Grzeskowiak LE, Gilbert AL, Morrison JL. Investigating outcomes associated with medication use during pregnancy: a review of methodological challenges and observational study designs. Reprod Toxicol. 2012;33(3):280-289. PubMed CrossRef
20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. PubMed CrossRef
21.Grigoriadis S, Graves L, Peer M, et al. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J Clin Psychiatry. 2018;79(5):17r12011. PubMed CrossRef
22.McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the Peer Review of Electronic Search Strategies (PRESS EBC). Evid Based Libr Inf Pract. 2016;5(1):149-154. CrossRef
23.Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. PubMed CrossRef
24.Ross LE, Grigoriadis S, Mamisashvili L, et al. Quality assessment of observational studies in psychiatry: an example from perinatal psychiatric research. Int J Methods Psychiatr Res. 2011;20(4):224-234. PubMed CrossRef
25.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed CrossRef
26.Wells A, Shea B, O’ Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa Hopital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 2, 2016.
27.Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. PubMed CrossRef
28.von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. PubMed CrossRef
29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. PubMed CrossRef
30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. PubMed CrossRef
31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. PubMed CrossRef
32.Ban L, West J, Gibson JE, et al. First trimester exposure to anxiolytic and hypnotic drugs and the risks of major congenital anomalies: a United Kingdom population-based cohort study. PLoS One. 2014;9(6):e100996. PubMed CrossRef
33.Hartz SC, Heinonen OP, Shapiro S, et al. Antenatal exposure to meprobamate and chlordiazepoxide in relation to malformations, mental development, and childhood mortality. N Engl J Med. 1975;292(14):726-728. PubMed CrossRef
34.Källén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel). 2013;6(10):1221-1286. PubMed CrossRef
35.Laegreid L, Hagberg G, Lundberg A. The effect of benzodiazepines on the fetus and the newborn. Neuropediatrics. 1992;23(1):18-23. PubMed CrossRef
36.Milkovich L, van den Berg BJ. Effects of prenatal meprobamate and chlordiazepoxide hydrochloride on human embryonic and fetal development. N Engl J Med. 1974;291(24):1268-1271. PubMed CrossRef
37.Oberlander TF, Warburton W, Misri S, et al. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res B Dev Reprod Toxicol. 2008;83(1):68-76. PubMed CrossRef
38.Ornoy A, Arnon J, Shechtman S, et al. Is benzodiazepine use during pregnancy really teratogenic? Reprod Toxicol. 1998;12(5):511-515. PubMed CrossRef
39.Pastuszak AL, Milich V, Can S, et al. Prospective assessment of pregnancy outcome following first trimester exposure to benzodiazepines. Can J Clin Pharmacol. 1996;3(4):167-171.
40.Kjosavik SR, Ruths S, Hunskaar S. Psychotropic drug use in the Norwegian general population in 2005: data from the Norwegian Prescription Database. Pharmacoepidemiol Drug Saf. 2009;18(7):572-578. PubMed CrossRef
41.Mark TL, Levit KR, Buck JA. Datapoints: psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009;60(9):1167. PubMed CrossRef
42.Bellantuono C, Tofani S, Di Sciascio G, et al. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry. 2013;35(1):3-8. PubMed CrossRef
43.Bech BH, Kjaersgaard MI, Pedersen HS, et al. Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: population based cohort study. BMJ. 2014;349:g5159. PubMed CrossRef
44.Ban L, Tata LJ, West J, et al. Live and non-live pregnancy outcomes among women with depression and anxiety: a population-based study. PLoS One. 2012;7(8):e43462. PubMed CrossRef
45.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413. PubMed CrossRef
46.Huybrechts KF, Palmsten K, Avorn J, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370(25):2397-2407. PubMed CrossRef
47.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. Antidepressant exposure during pregnancy and congenital malformations: is there an association? a systematic review and meta-analysis of the best evidence. J Clin Psychiatry. 2013;74(4):e293-e308. PubMed CrossRef
48.Kang H-H, Ahn KH, Hong S-C, et al. Association of citalopram with congenital anomalies: a meta-analysis. Obstet Gynecol Sci. 2017;60(2):145-153. PubMed CrossRef
49.Gao SY, Wu QJ, Zhang TN, et al. Fluoxetine and congenital malformations: a systematic review and meta-analysis of cohort studies. Br J Clin Pharmacol. 2017;83(10):2134-2147. PubMed CrossRef
50.Salisbury AL, O’ Grady KE, Battle CL, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry. 2016;173(2):147-157. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite