Do Benzodiazepines Reduce the Effectiveness of Exposure Therapy
for Posttraumatic Stress Disorder?
ABSTRACT
Objective: Benzodiazepines, other anxiolytics, or sedative hypnotics are prescribed for 30%–50% of posttraumatic stress disorder (PTSD) patients. Prior data and theory suggest that these medications may inhibit response to exposure therapy, one of the most effective PTSD treatments. The present post hoc study reanalyzed results from a psychotherapy trial to assess whether benzodiazepine use was associated with reduced response to exposure therapy.
Method: Between August 2002 and October 2005, 283 female veterans
and soldiers meeting DSM-IV criteria for PTSD were randomly assigned to 10 weekly 90-minute sessions of either prolonged exposure (n = 140) or present-centered psychotherapy (n = 143). Benzodiazepine use (n = 57) or non-use (n = 226) at intake was not randomly assigned. Multilevel modeling was used to assess the effects of benzodiazepine status, psychotherapy condition, and their interaction on changes on the Clinician-Administered PTSD Scale and the PTSD Checklist during the treatment and 6-month follow-up periods.
Results: Consistent with prior reports from these data, prolonged exposure psychotherapy produced greater reductions per week in PTSD symptoms than did present-centered psychotherapy (b = −0.48, P = .02). Patients prescribed benzodiazepines did not have weaker response to prolonged exposure, but demonstrated poorer posttreatment maintenance of gains from present-centered psychotherapy (b = −0.78, P < .001).
Conclusions: Prolonged exposure is a sufficiently robust treatment that patients who are taking benzodiazepines can benefit from it. It is unclear whether benzodiazepine use or other patient factors accounted for benzodiazepine recipients’ poorer maintenance of gains in present-
centered psychotherapy.
Trial Registration: ClinicalTrials.gov identifier: NCT00032617
J Clin Psychiatry 2013;74(12):1241–1248
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: May 20, 2013; accepted October 24, 2013 (doi:10.4088/JCP.13m08592).
Corresponding author: Craig S. Rosen, PhD, VA Palo Alto Health Care System, 795 Willow Rd (334-PTSD), Menlo Park, CA 94025 ([email protected]).
About 8% of the US population and 13% of military personnel returning from deployment in Iraq or Afghanistan are affected by posttraumatic stress disorder (PTSD).1,2 In the US population 18 to 64 years of age, lifetime prevalence of PTSD is substantially higher among women (11.4%) than men (4.0%).1,3 PTSD often has a chronic course and is associated with greater risk for substance misuse, smoking, cardiovascular disease, violence, divorce, impaired vocational functioning, and suicide.4–6
The most efficacious treatments for PTSD are trauma-focused psychotherapies that involve reprocessing of trauma-related memories or cognitions.7,8 One of the most extensively studied trauma-focused psychotherapies, prolonged exposure psychotherapy,9 has consistently been shown to be more effective than wait list or non–trauma-focused treatments.10 However, not all patients who receive trauma-focused treatments experience full and sustained remission of PTSD symptoms.11–13
There has been relatively little research examining how commonly prescribed medications affect response to exposure therapy. Between 30% and 50% of Veteran and civilian patients diagnosed with PTSD are prescribed benzodiazepines, other anxiolytics, or sedative hypnotics.14–16 Although benzodiazepines are not effective for treating reexperiencing, avoidance, or most arousal symptoms of PTSD,17 they are often prescribed to PTSD patients with disturbed sleep or other anxiety disorders.18
Prior research suggests several ways in which benzodiazepines might interfere with exposure therapy.19,20 In humans, benzodiazepine use is associated with decreased attention and deficits in several memory domains.21,22 Animal research shows that extinction of fear responses is blocked by administration of GABAergic agents (including benzodiazepines) before or immediately after extinction training.20 These extinction trials in animal models are analogous to prolonged exposure psychotherapy. Benzodiazepines may also interfere with extinction through dose-response suppression of cortisol, which plays a critical role in habituation and extinction of fear.19,23 Finally, people using benzodiazepines during exposure may attribute their reduction in anxiety to the medication, which may reduce generalization of the effects of exposure.24
Little research has examined how benzodiazepines influence response to exposure therapy for PTSD. Research on combined cognitive-behavioral therapy and pharmacotherapy for PTSD has largely been limited to selective serotonin reuptake inhibitors.25 One study of people receiving exposure therapy for PTSD found that taking benzodiazepines at intake was associated with less improvement in symptoms at 1-month follow-up.26 Because that study did not include a comparison condition of people receiving non–trauma-focused psychotherapy, it is uncertain whether benzodiazepine use interfered with psychotherapy in general or specifically blunted the effects of exposure. A review27 of studies for panic disorder concluded that receiving cognitive-behavioral therapy plus benzodiazepines might produce better results than receiving cognitive-behavioral therapy alone immediately after treatment, yet contribute to deterioration in the 6 months after treatment, especially if benzodiazepines are not continued. It is unclear whether these findings would generalize to PTSD.
The present post hoc study reanalyzed data from a randomized controlled trial11 designed to estimate the effectiveness of prolonged exposure relative to present-centered therapy among female veterans and active duty soldiers diagnosed with PTSD. The study showed that PTSD symptoms improved more among women who received prolonged exposure than among women who received present-centered therapy. We focus here on whether benzodiazepine use at baseline moderated the effects of psychotherapy condition, especially prolonged exposure therapy, on patients’ PTSD symptoms during psychotherapy and in the 6 months after psychotherapy was completed.
Exposure therapy is posited to work through learning of new responses to fear-invoking cues. On the basis of previous research findings on the effects of benzodiazepines on memory and extinction, we hypothesized that the effects of prolonged exposure therapy would be blunted by benzodiazepine use. We hypothesized that present-centered therapy, which is not based on an exposure/extinction paradigm, would be less affected by benzodiazepine use. This difference in effect between treatments would be reflected in a significant interaction of psychotherapy condition by benzodiazepine use on both clinician-assessed and self-reported PTSD symptoms. Although we expected prolonged exposure therapy to produce more improvement in PTSD symptoms than would present-centered therapy, we also anticipated that the effect of psychotherapy condition on outcomes would be smaller among people taking benzodiazepines than among those not taking benzodiazepines.
METHOD
Participants
A total of 284 female veterans and active duty soldiers meeting DSM-IV criteria for PTSD were recruited between August 2002 and October 2005, from 9 Veterans Affairs (VA) Medical Centers, 2 VA Readjustment Counseling Centers, and 1 military hospital. Eligibility criteria included being female, having a current diagnosis of PTSD based on the Clinician-Administered PTSD Scale (CAPS)28 and a CAPS score of 45 or higher, receiving no other concurrent PTSD psychotherapy other than brief visits with an existing therapist or participation in self-help groups, and having no change in psychoactive medications for at least 2 months prior to study recruitment. Exclusion criteria were substance dependence not in remission for at least 3 months; current psychotic symptoms, mania, or bipolar disorder; prominent current suicidal or homicidal ideation; cognitive impairment indicated by chart diagnosis or observable cognitive difficulties; current involvement in a violent relationship; or self-mutilation within the past 6 months. The study is registered at ClinicalTrials.gov (identifier: NCT00032617).
Medication use at intake was decided by patients and their prescribers and was not randomly assigned. One participant who did not report medication status at intake was excluded from the present analyses, leaving 283 participants. Of those, 213 participants (75%) were receiving psychoactive medications at the start of the trial, including 57 (20%) who were prescribed benzodiazepines. Patients using benzodiazepines were evenly split between the prolonged exposure (n = 28) and present-centered (n = 29) psychotherapy conditions. Forty-eight (84%) of the participants who were prescribed benzodiazepines reported their medication use at the end of the study. Of those, 40 participants (83%) maintained the same dose, 2 (4%) increased their dose, 1 (2%) decreased her dose, and 5 (10%) discontinued benzodiazepines.
Measures
PTSD symptom severity was assessed with the CAPS (by clinicians who were blind to participants’ psychotherapy condition) and by self-report using the PTSD Checklist (PCL).29 Both measures were administered at baseline, posttreatment, and 3 and 6 months posttreatment. The PCL was also completed every other session during the treatment phase.
Several possible confounders in the relationships between benzodiazepine use and PTSD symptoms were also assessed. Depressive symptoms were measured with the Beck Depression Inventory,30 and overall mental and health functioning was assessed with the Medical Outcomes Study 36-item Short Form Health Survey (SF-36).31 Trauma exposure was assessed with the Life Events Checklist.32 Current psychoactive prescriptions, prior engagement in psychotherapy, and PTSD disability pension status were assessed by self-report. We classified participants’ benzodiazepine use on the basis of their self-reports (0 = no, 1 = yes) of medication use at baseline assessment and follow-up. Additional details of the study design have been published previously.11,33
Procedure
Study procedures were overseen by institutional review boards at each of the study sites. Participants who gave consent were randomly assigned to receive either prolonged exposure psychotherapy or present-centered psychotherapy. Both psychotherapies were delivered in 10 weekly 90-minute sessions using manualized protocols. Prolonged exposure includes psychoeducation about common reactions to trauma, breathing retraining, repeated recounting of trauma memories during sessions, and exposure homework (for example, listening to a recording of the recounting during the therapy session and in vivo exposure to safe situations that the person has been avoiding because of the trauma).9,11 The study protocol did not provide specific instructions regarding benzodiazepine use during exposure exercises. Rather than focusing on past traumatic events, present-centered therapy focuses on current life problems as manifestations of PTSD. Therapists helped patients identify and review daily stressors and discussed them in a nondirective mode, but did not teach cognitive restructuring or assign exposure exercises.
As has been previously reported,11 dropout from psychotherapy was significantly higher among participants in the prolonged exposure condition (n = 53, 38%) than among those in the present-centered therapy condition (n = 30, 21%; odds ratio = 2.3; 95% CI, 1.2–4.5; P < .05). Dropout rates did not vary by either benzodiazepine status or the interaction of benzodiazepine status and psychotherapy condition.
Analysis Plan
Baseline characteristics of participants in the 4 treatment groups (2 psychotherapy conditions × benzodiazepine use or non-use) were compared using analysis of variance and logistic regression with 3 planned contrasts (benzodiazepine status, psychotherapy condition, benzodiazepine status × psychotherapy condition). Race/ethnicity, previous psychotherapy, psychoactive medication use, PTSD symptoms, and SF-36 mental health component score varied by treatment group (Table 1).
Outcome analyses were performed on an intent-to-treat basis. As in the original trial, we estimated participants’ missing outcome values with SAS PROC MI and SAS PROC MI ANALYZE (SAS Institute Inc; Cary, North Carolina) multiple imputation procedures using Markov chain Monte Carlo methods initiated using estimates from an expectation-maximization algorithm.34,35 Due to the large number of covariates in the multiple-imputation model, the quantity of initial burn-in iterations (ie, NBITER) and the number of iterations between imputations (ie, NITER) were each set at 5,000. We used 30 imputations for the CAPS and 40 for the PCL to minimize between-imputation variance and maximize relative efficiency. Trace plots of coefficient means and autocorrelation plots were consistent with good mixing and convergence at the target distribution. Multilevel regression analyses (SAS 9.0, Proc GLIMMIX) were conducted to assess the effects of psychotherapy condition (coded +0.5 for prolonged exposure psychotherapy, –0.5 for present-centered psychotherapy), benzodiazepine status (coded +0.5 for benzodiazepines, –0.5 for none), and their interaction on initial status and rate of change in PTSD symptoms.36 One analysis predicted changes in clinician-assessed PTSD symptoms using the CAPS; a second predicted changes in the self-report PCL. Both analyses covaried clinical factors associated with benzodiazepine use: prior receipt of psychotherapy, use of psychoactive medications, PTSD symptoms, depression symptoms, and SF-36 mental health scores, as well the interaction of initial severity × time (the latter controls for initial severity influencing the slope of treatment response). As in the original trial,11 baseline PTSD symptom severity, psychotherapy condition, and site were controlled as fixed effects, and random intercepts were employed for participant and for therapist to account for possible clustering of repeated measures within participants and of participants within therapists, respectively.
In addition to controlling for additional covariates, we modified the analysis plan from the original study11 in 3 ways. First, we added benzodiazepine status and a benzodiazepine × psychotherapy interaction term as fixed effects. Second, in the analysis with PCL as the outcome, we included PCL scores obtained during treatment, as well as those obtained at baseline, posttreatment, and the 2 follow-up assessments. Third, parameter estimates of interest were rates of change in PTSD symptoms, rather than mean outcome symptom scores at individual time points. This allowed us to maximize statistical power by using all available symptom assessments rather than fitting a separate model for each assessment. Because some prior research suggests that the moderating effects of benzodiazepines might be different during and after completion of psychotherapy,27 we fit our data using piecewise-linear regression, which allowed rate of change over time in the average participant’s PTSD symptoms to differ between psychotherapy (weeks 0–16) and the 6-month posttreatment period (weeks 16–42).
RESULTS
Clinician-Assessed PTSD Symptoms
Changes in clinician-assessed PTSD symptoms are shown in Figure 1. In Table 2, b weights indicate mean change in symptoms per week associated with each increment of the predictors in the model. Clinician-assessed PTSD symptoms declined over the course of psychotherapy (b = −1.20, P < .001) and declined more rapidly among participants who received prolonged exposure rather than present-centered psychotherapy (b = –0.48, P = .02). More severe symptoms at intake were associated with less improvement, regardless of therapy type (b = 0.24, P = .03). Baseline use of benzodiazepines, the interaction of psychotherapy type with benzodiazepines, and the interaction of symptom severity at intake and therapy type had no significant effect on rate of change in clinician-assessed PTSD symptoms during the course of treatment (see weeks 0–16 in Figure 1).
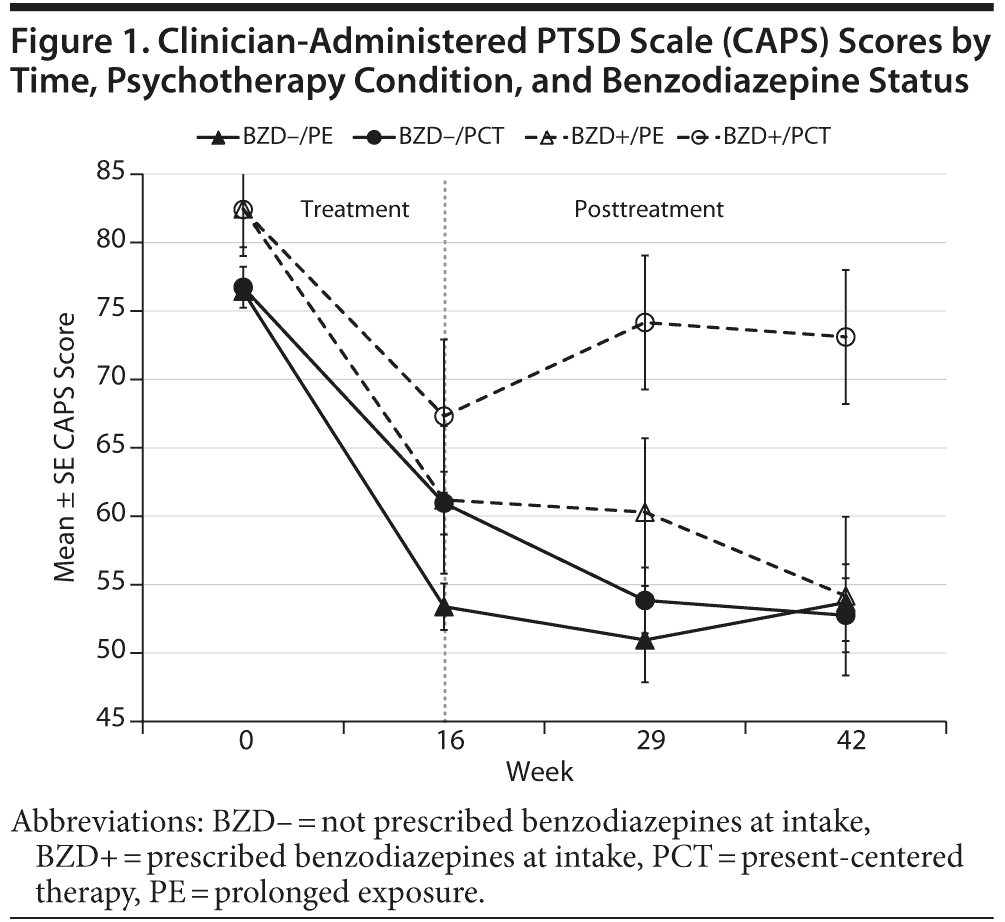
After psychotherapy was completed, type of psychotherapy, benzodiazepine use, and initial symptom severity did not have a statistically significant main effect on change in clinician-assessed symptoms during the 6-month follow-up period (see Table 2). However, benzodiazepine use interacted with type of psychotherapy to predict the rate of change in symptoms—but in the opposite direction than predicted (b = –0.78, P < .001). Benzodiazepine use was not associated with change in symptoms after the end of prolonged exposure therapy, but it was associated with worsening PTSD symptoms after the end of present-centered therapy (see weeks 16–42 in Figure 1). The mean improvement in CAPS scores from pretreatment to 6-month follow-up among benzodiazepine users who received present-centered therapy was less than half as large as the improvement among veterans in the other 3 groups (see Figure 1).
Self-Reported PTSD Symptoms
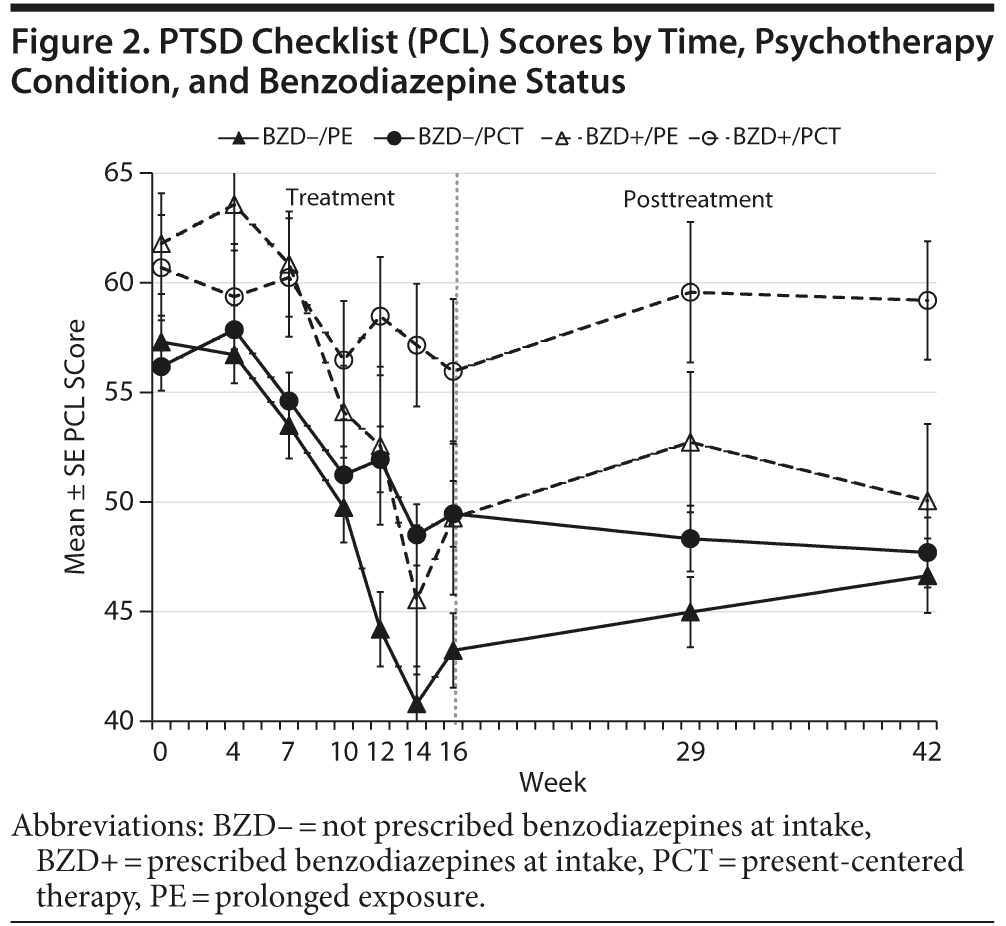
As shown in Figure 2 and Table 2, participants’ self-reported PTSD symptoms improved over the course of treatment (b = –0.72, P < .001) and improved more rapidly among those who received prolonged exposure than among those who obtained present-centered therapy (b = –0.60, P < .001). Benzodiazepine use was associated with less improvement (b = 0.23, P = .03), whereas greater initial symptom severity (controlling for benzodiazepine use) was associated with slightly more improvement (b = –0.11, P = .03). However, the interaction of benzodiazepine use and psychotherapy condition had no effect on symptom course during treatment (see Table 2 and weeks 0–16 in Figure 2). Participants’ self-reported symptoms tended to increase slightly (b = 0.10, P < .01) during the posttreatment follow-up period. However, neither the main effects of psychotherapy condition, benzodiazepine, and initial symptom severity nor their interactions predicted symptom course after the end of treatment (see Table 2 and Figure 2).
DISCUSSION
The present study reanalyzed data from a randomized clinical trial to determine whether benzodiazepine use might impede response to exposure therapy. Contrary to our hypotheses, benzodiazepine use was not associated with weaker response to exposure therapy during either treatment or the follow-up period. This was particularly striking because patients who were prescribed benzodiazepines tended to have other clinical characteristics (more severe PTSD symptoms, worse SF-36 mental health functioning scores, more prior sessions of psychotherapy, and more use of psychotropic medications) that could indicate failure to respond to prior treatments. Yet, prolonged exposure was a sufficiently robust treatment that these patients were able to benefit.
Unexpectedly, benzodiazepine use was associated with poorer maintenance of clinician-rated PTSD symptom improvements by participants who received present-centered psychotherapy. Our findings are not accounted for by discontinuation of benzodiazepines, because most participants using benzodiazepines at the beginning of the study also reported using them at the end of the study.
Benzodiazepines may have made it more difficult to attend to and retain information from present-centered psychotherapy. Beyond their effects on fear extinction, benzodiazepines have broader effects on memory and attention that may have interfered with retention of psychotherapy content.21,22
It is also possible that present-centered therapy may not be sufficiently powerful to produce lasting changes in patients with a history of poor response to other mental health treatments. As noted above, variables associated with benzodiazepine use suggest that these patients may have been hard to treat. An American Psychological Association workgroup concluded that trials in which present-centered therapy was used as a comparator condition suggest that present-centered therapy is efficacious,37 but the efficacy of present-centered therapy has not been tested relative to placebo.
Our findings contrast with those of van Minnen and colleagues,26 who found that benzodiazepine use was associated with poorer outcomes 1 month after completion of prolonged exposure therapy for PTSD. The measure of benzodiazepine use differed in the 2 studies: our sample of benzodiazepine users included anyone with a benzodiazepine prescription, whereas the van Minnen et al study defined benzodiazepine use as daily use. Van Minnen et al also analyzed outcomes among treatment completers only; this may have biased their findings toward poorer outcomes for the benzodiazepine group because dropout was much higher among benzodiazepine non-users than among benzodiazepine users in their sample.
Neither our study nor van Minnen and colleagues’ study controlled dosage or timing of benzodiazepine use. Because the effects of benzodiazepines on learning are dose dependent, effects might be different for people taking different doses.19 Although dosage was not assessed in this study, the average dose of benzodiazepines prescribed for VA PTSD patients at the time of the study was 1.9 standard daily dosage units, comparable to 1.9 mg/d of clonazepam or 19.0 mg/d of diazepam.14 Timing may also be a factor. Benzodiazepines have been shown to interfere with extinction of conditioned fear responses if they are used during or shortly after the time of exposure to fear-inducing conditions.19,20,24 If many veterans were primarily taking benzodiazepines at bedtime to help them sleep,18 this would be unlikely to interfere with exposure exercises conducted during waking hours.
Aside from the issue of whether benzodiazepines can interfere with psychotherapy, many veterans with PTSD have comorbid conditions for which benzodiazepines are contraindicated. Substance misuse38 and prescribing of opiates for pain39 are both common in the PTSD patient population. For patients with these comorbidities, there are efficacious and safer alternatives to benzodiazepines for managing anxiety or insomnia.17,40
The present study has several limitations. Our sample was limited to treatment-seeking female veterans and military personnel. Benzodiazepine use was not randomly assigned, and we did not control for benzodiazepine half-life, dosage, or timing. Moreover, benzodiazepine users were a relatively small subsample of our patients. Further research using larger samples of benzodiazepine users might provide more definitive information about the influence of benzodiazepines on psychotherapies to alleviate PTSD symptoms. Further information is also needed about specific patient characteristics associated with benzodiazepine use that account for its influence on psychotherapy outcomes.
Notwithstanding these limitations, findings from this investigation add to previous research by demonstrating that veterans who are prescribed benzodiazepines can benefit from prolonged exposure therapy and thus do not necessarily need to discontinue benzodiazepines before undergoing this efficacious psychotherapy. More research is needed on the efficacy of present-centered therapy for PTSD. Further investigation is also needed on whether cognitive effects of benzodiazepines may impede retention of material taught in psychotherapy.
Drug names: clonazepam (Klonopin and others), diazepam (Diastat, Valium, and others), trazodone (Oleptro and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, clonazepam and diazepam are not approved by the US Food and Drug Administration for the treatment of sleep or PTSD symptoms.
Author affiliations: National Center for PTSD Dissemination & Training Division, VA Palo Alto Health Care System, Menlo Park, California, and Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, California (Drs Rosen and Holmes); VA Sierra-Pacific Mental Illness Research, Education, and Clinical Center (Mr Greenbaum and Dr Rosen) and HSR&D Center for Innovation to Implementation (Drs Brennan and Rosen), VA Palo Alto Health Care System, Palo Alto, California; National Center for PTSD Executive Division, White River Junction VA Medical Center, White River Junction, Vermont (Drs Schnurr and Friedman); and Departments of Psychiatry (Drs Schnurr and Friedman) and Pharmacology & Toxicology (Dr Friedman), Geisel School of Medicine at Dartmouth, Hanover, New Hampshire.
Financial disclosure: Drs Rosen, Schnurr, Holmes, Brennan, and
Friedman and Mr Greenbaum have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This study was conducted with support from grant CSP #494 from the VA Cooperative Studies Program, support from the Department of Defense for CSP #494, and support from the VA Sierra-Pacific Mental Illness, Research, Education and Clinical Center (MIRECC) and the VA Palo Alto Health Care System.
Role of the sponsors: The sponsors had no role in the conduct or publication
of the study.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the Department of Defense, or any US government agency.
REFERENCES
1. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21(3):169–184. doi:10.1002/mpr.1359 PubMed
2. Schell TL. Survey of individuals previously deployed for OEF/OIF. In: Tanielian TL, ed. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: Rand; 2008:
87–115.
3. Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132(6):959–992. doi:10.1037/0033-2909.132.6.959 PubMed
4. Schnurr PP, Lunney CA, Bovin MJ, et al. Posttraumatic stress disorder and quality of life: extension of findings to veterans of the wars in Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):727–735. doi:10.1016/j.cpr.2009.08.006 PubMed
5. Dedert EA, Calhoun PS, Watkins LL, et al. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med. 2010;39(1):61–78. doi:10.1007/s12160-010-9165-9 PubMed
6. Del Gaizo AL, Elhai JD, Weaver TL. Posttraumatic stress disorder, poor physical health and substance use behaviors in a national trauma-exposed sample. Psychiatry Res. 2011;188(3):390–395. doi:10.1016/j.psychres.2011.03.016 PubMed
7. Forbes D, Creamer M, Bisson JI, et al. A guide to guidelines for the treatment
of PTSD and related conditions. J Trauma Stress. 2010;23(5):537–552. doi:10.1002/jts.20565 PubMed
8. Bradley R, Greene J, Russ E, et al. A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 2005;162(2):214–227. doi:10.1176/appi.ajp.162.2.214 PubMed
9. Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. Oxford, UK: Oxford University Press; 2007.
10. Powers MB, Halpern JM, Ferenschak MP, et al. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;
30(6):635–641. doi:10.1016/j.cpr.2010.04.007 PubMed
11. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297(8):820–830. doi:10.1001/jama.297.8.820 PubMed
12. de Kleine RA, Hendriks GJ, Kusters WJ, et al. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71(11):962–968. doi:10.1016/j.biopsych.2012.02.033 PubMed
13. Eftekhari A, Ruzek JI, Crowley JJ, et al. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care.
JAMA Psychiatry. 2013;70(9):949–955. doi:10.1001/jamapsychiatry.2013.36 PubMed
14. Lund BC, Bernardy NC, Alexander B, et al. Declining benzodiazepine use
in veterans with posttraumatic stress disorder. J Clin Psychiatry. 2012;73(3):
292–296. doi:10.4088/JCP.10m06775 PubMed
15. Harpaz-Rotem I, Rosenheck RA, Mohamed S, et al. Pharmacologic treatment of posttraumatic stress disorder among privately insured Americans.
Psychiatr Serv. 2008;59(10):1184–1190. doi:10.1176/appi.ps.59.10.1184 PubMed
16. Mellman TA, Clark RE, Peacock WJ. Prescribing patterns for patients with posttraumatic stress disorder. Psychiatr Serv. 2003;54(12):1618–1621. doi:10.1176/appi.ps.54.12.1618 PubMed
17. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Version 2.0 2010. US Department of Veterans Affairs Website. Washington, DC. http://www.healthquality.va.gov/Post_Traumatic_Stress_Disorder_PTSD.asp. Updated May 29, 2013. Accessed December 12, 2012.
18. Jain S, Greenbaum MA, Rosen CS. Concordance between psychotropic prescribing for veterans with PTSD and clinical practice guidelines.
Psychiatr Serv. 2012;63(2):154–160. doi:10.1176/appi.ps.201100199 PubMed
19. Otto MW, McHugh K, Kantak KM. Combined pharmacotherapy and cognitive-behavioral therapy for anxiety disorders: medication effects, glucocorticoids, and attenuated treatment outcomes. Clin Psychol Sci Pract. 2010;17(2):91–103. doi:10.1111/j.1468-2850.2010.01198.x
20. Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–1652. PubMed
21. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45–58. doi:10.2174/1381612023396654 PubMed
22. Tannenbaum C, Paquette A, Hilmer S, et al. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):
639–658. PubMed
23. Yang YL, Chao PK, Ro LS, et al. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharmacology. 2007;32(5):1042–1051. doi:10.1038/sj.npp.1301215 PubMed
24. Başoğlu M, Marks IM, Kiliç C, et al. Alprazolam and exposure for panic disorder with agoraphobia: attribution of improvement to medication
predicts subsequent relapse. Br J Psychiatry. 1994;164(5):652–659. doi:10.1192/bjp.164.5.652 PubMed
25. Hetrick SE, Purcell R, Garner B, et al. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2010;7(7):CD007316. PubMed
26. van Minnen A, Arntz A, Keijsers GPJ. Prolonged exposure in patients with chronic PTSD: predictors of treatment outcome and dropout. Behav Res Ther. 2002;40(4):439–457. doi:10.1016/S0005-7967(01)00024-9 PubMed
27. Spiegel DA, Bruce TJ. Benzodiazepines and exposure-based cognitive behavior therapies for panic disorder: conclusions from combined treatment trials. Am J Psychiatry. 1997;154(6):773–781. PubMed
28. Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD
Scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):
132–156. doi:10.1002/da.1029 PubMed
29. Blanchard EB, Jones-Alexander J, Buckley TC, et al. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669–673. doi:10.1016/0005-7967(96)00033-2 PubMed
30. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi:10.1001/archpsyc.1961.01710120031004 PubMed
31. Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36), 1: conceptual framework and item selection. Med Care. 1992;
30(6):473–483. doi:10.1097/00005650-199206000-00002 PubMed
32. Gray MJ, Litz BT, Hsu JL, et al. Psychometric properties of the Life Events Checklist. Assessment. 2004;11(4):330–341. doi:10.1177/1073191104269954 PubMed
33. Schnurr PP, Friedman MJ, Engel CC, et al. Issues in the design of multisite clinical trials of psychotherapy: VA Cooperative Study No. 494 as an example. Contemp Clin Trials. 2005;26(6):626–636. doi:10.1016/j.cct.2005.09.001 PubMed
34. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. doi:10.1093/biomet/63.3.581
35. Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman & Hall; 1997. doi:10.1201/9781439821862
36. Wolfinger R, O’Connell M. Generalized linear mixed models: a pseudo-likelihood approach. J Statist Comput Simulation. 1993;48(3–4):233–243. doi:10.1080/00949659308811554
37. Research-supported psychological treatments: posttraumatic stress disorder. Society of Clinical Psychology, American Psychological Association Division 12 Website. http://www.div12.org/PsychologicalTreatments/disorders/ptsd_main.php. Updated 2008. Accessed October 11, 2013.
38. Pietrzak RH, Goldstein RB, Southwick SM, et al. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord. 2011;25(3):456–465. doi:10.1016/j.janxdis.2010.11.010 PubMed
39. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–947. doi:10.1001/jama.2012.234 PubMed
40. Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567–590. doi:10.2165/00023210-200620070-00003 PubMed



