J Clin Psychiatry 2022;83(4):22ac14500
To cite: Schoretsanitis G, de Leon J. Best practices for starting clozapine in patients with schizophrenia: how to switch from the prior antipsychotic(s). J Clin Psychiatry. 2022;83(4):22ac14500.
To share: https://doi.org/10.4088/JCP.22ac14500
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Psychotherapy and Psychosomatics, Psychiatric Hospital, University of Zürich, Zürich, Switzerland
bThe Zucker Hillside Hospital, Psychiatry Research, Northwell Health, Glen Oaks, New York
cDepartment of Psychiatry and Molecular Medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
dMental Health Research Center, Eastern State Hospital, Lexington, Kentucky
eBiomedical Research Centre in Mental Health Net (CIBERSAM), Santiago Apóstol Hospital, University of the Basque Country, Vitoria, Spain
*Corresponding author: Jose de Leon, MD, Mental Health Research Center at Eastern State Hospital, 1350 Bull Lea Rd, Lexington, KY 40511 ([email protected]).
Clozapine is a highly efficacious antipsychotic medication and is the standard of care for treatment-resistant schizophrenia (TRS)1 as well as for reducing the risk of suicidal behaviors2 and aggression3 in schizophrenia and schizoaffective disorder. A major obstacle to its use has been tolerability, and efforts have been undertaken to provide advice as to its optimal and safe titration.4 A prior ASCP Corner column discussed ways of offering clozapine.5 This article provides advice on how to switch from the prior antipsychotic(s).
To reduce the risk of psychotic relapse, we prefer to add clozapine to a prior antipsychotic or antipsychotics and not to decrease the other antipsychotic dosage until the fourth week or even later, when the clozapine dosage has reached a therapeutic level by providing a plasma concentration of at least 350 ng/mL.
We think that adding clozapine to most individual antipsychotics or antipsychotic combinations is safe, with the exception of the following 4 antipsychotics, which may require slower titrations. These are discussed in turn.
Olanzapine
Olanzapine by itself may not present the risk of myocarditis,6 but in > 3,000 myocarditis reports worldwide, olanzapine increased the risk of myocarditis seriousness by an odds ratio (OR) of 1.90 (95% confidence interval [CI], 1.35 to 2.68).7 Olanzapine is mainly metabolized by cytochrome P450 (CYP)1A2, but usually it does not behave as a CYP1A2 inhibitor. In circumstances of saturation of clozapine metabolism, which may occur during myocarditis,8 olanzapine may behave as a competitive inhibitor of clozapine metabolism. Other adverse drug reactions (ADRs) to consider during the clozapine titration, before olanzapine is discontinued, are sedation and constipation. Both olanzapine and clozapine are antagonists of histaminic (H1) and muscarinic receptors, and their effects may be additive for, respectively, sedation and constipation. Bowel movements should be monitored, and the need for laxatives should be considered.
Quetiapine
Rarely, quetiapine may be associated with myocarditis during overdose or rapid titration.6 More importantly, in the large sample of clozapine myocarditis reports,7 the quetiapine OR was 2.83 (95% CI, 1.82–4.40) for seriousness and 2.12 (95% CI, 1.03–4.35) for lethality. These results suggest that quetiapine may have some pharmacodynamic synergies at the immunologic level that contribute to the risk of myocarditis.6 Other ADRs to consider during clozapine titration, before quetiapine is discontinued, are sedation and orthostatic hypotension. Both quetiapine and clozapine are antagonists of histaminic (H1) and α1 receptors, and their effects may be additive for, respectively, sedation and orthostatic hypotension. The clozapine titration guideline4 recommends monitoring orthostatic changes in blood pressure and pulse.
Perphenazine
An in vitro study9 suggests that perphenazine can be an inhibitor of CYP1A2 activity. Thus, it is not surprising that perphenazine can make some clozapine patients behave as poor metabolizers (PMs). Our limited experience in the US10 and China11 suggests that perphenazine in therapeutic doses may cause a clinically relevant inhibition of clozapine metabolism in some patients. Clozapine-induced myocarditis was first described in Denmark, and one of the first cases was a patient in whom clozapine was added to perphenazine.12
Flupenthixol
Flupenthixol (or “flupentixol”) is an antipsychotic used in some European countries and has lesser-known pharmacokinetic properties; according to a case report, it can inhibit clozapine metabolism.13 Until better studies are available, it appears safer to consider any patient treated with flupenthixol as a potential clozapine PM.
Update to Titration Guideline
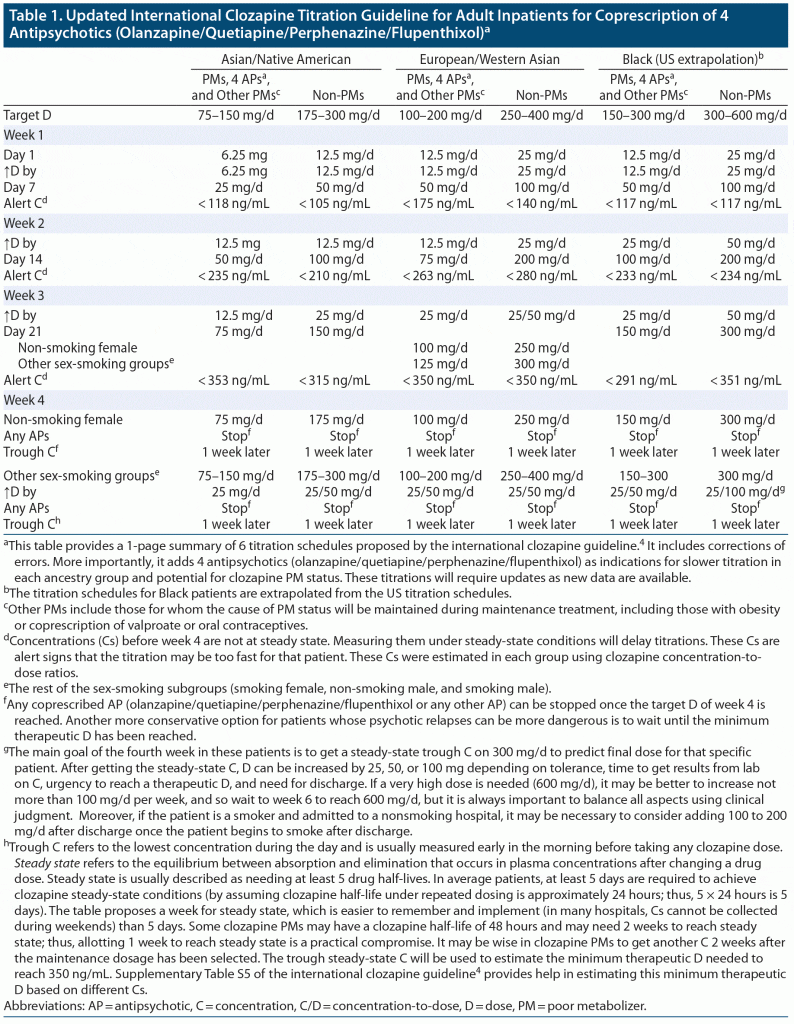
Table 1 presents a summary of the 6 clozapine titrations proposed by the international clozapine guideline4 and modifies the 3 titrations for clozapine PMs, which should also be used for patients prescribed olanzapine, quetiapine, perphenazine, and flupenthixol. These modifications may help further increase safety during titration and reduce the risk of clozapine-induced myocarditis; they serve to update the clozapine titration guideline.4 The table recommends stopping any coprescribed antipsychotic, including any long-acting antipsychotic, in week 4, but another option is to wait until the clozapine dosage has provided the minimum therapeutic concentration of 350 ng/mL. The table provides alert numbers for clozapine concentrations during the first 3 weeks, in non-steady conditions, that may indicate that the patient is a clozapine PM (eg, through inhibitory effects of perphenazine or flupenthixol). In the absence of rapid access to clozapine concentrations, the guideline4 recommends measuring C-reactive protein (CRP; measured at baseline and weekly at the same time as the white blood cell count) to detect CRP elevations during titration, which could indicate that the titration used may be too rapid for that specific patient.
Published online: July 4, 2022.
Relevant financial relationships: In the last 3 years, Dr Schoretsanitis has served as a consultant for HLS Therapeutics. Dr de Leon reports no conflicts of interest.
Funding/support: This article received no support from any funding agency, commercial business, or not-for-profit institution.
Acknowledgment: The authors thank Lorraine Maw, MA (Mental Health Research Center at Eastern State Hospital), for editorial assistance. Ms Maw declares no competing interest during the last 36 months.
ORCID: Georgios Schoretsanitis: https://orcid.org/0000-0002-3851-4117; Jose de Leon: https://orcid.org/0000-0002-7756-2314
The ASCP Corner, edited by Leslie L. Citrome, MD, MPH, is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s).

References (13)

- Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–392. PubMed CrossRef
- Taipale H, Lähteenvuo M, Tanskanen A, et al. Comparative effectiveness of antipsychotics for risk of attempted or completed suicide among persons with schizophrenia. Schizophr Bull. 2021;47(1):23–30. PubMed CrossRef
- Citrome L, Volavka J. Specific anti-hostility effects of atypical antipsychotics in persons with schizophrenia: from clozapine to cariprazine. Harv Rev Psychiatry. 2021;29(1):20–34. PubMed CrossRef
- de Leon J, Schoretsanitis G, Smith RL, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. 2022;55(2):73–86. PubMed CrossRef
- Rubio JM, Kane JM. How to make an effective offer of clozapine. J Clin Psychiatry. 2021;83(1):21ac14000. PubMed CrossRef
- De Las Cuevas C, Sanz EJ, Rohde C, et al. Association between myocarditis and antipsychotics other than clozapine: a systematic literature review and a pharmacovigilance study using VigiBase. Expert Rev Clin Pharmacol. 2022;15(1):65–78. PubMed CrossRef
- De Las Cuevas C, Sanz EJ, Ruan CJ, et al. Clozapine-associated myocarditis in the World Health Organization’s pharmacovigilance database: focus on reports from various countries [published online July 21, 2021]. Rev Psiquiatr Salud Ment (Engl Ed). 2021;21:S1888-9891(21)00070-7.
- Koenig M, McCollum B, Spivey JK, et al. Four cases of myocarditis in US hospitals possibly associated with clozapine poor metabolism and a comparison with prior published cases. Neuropsychopharmacol Hung. 2022;24:29–41.
- Shin JG, Soukhova N, Flockhart DA. Effect of antipsychotic drugs on human liver cytochrome P-450 (CYP) isoforms in vitro: preferential inhibition of CYP2D6. Drug Metab Dispos. 1999;27(9):1078–1084. PubMed
- Cooke C, de Leon J. Adding other antipsychotics to clozapine. J Clin Psychiatry. 1999;60(10):710. PubMed CrossRef
- Ruan CJ, Zang YN, Cheng YH, et al. Around 3% of 1,300 levels were elevated during infections in a retrospective review of 131 Beijing Hospital in-patients with more than 24,000 days of clozapine treatment. Psychother Psychosom. 2020;89(4):255–257. PubMed CrossRef
- Jensen VE, Gøtzsche O. Allergisk myocarditis ved klozapinbehandling [Allergic myocarditis in clozapine treatment]. Ugeskr Laeger. 1994;156(28):4151–4152. PubMed
- Wagner X, Kluge M. Abrupt decrease of clozapine plasma concentration after discontinuation of flupentixol comedication. J Clin Psychopharmacol. 2019;39(2):168–169. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite