Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Few studies have evaluated optimal dosing of intravenous (IV) ketamine for treatment-resistant depression (TRD). Landmark studies infused ketamine at 0.5 mg/kg actual body weight. However, a consensus statement by Sanacora et al1 suggested dosing based on calculated ideal body weight, especially in patients with body mass index (BMI) ≥ 30 given concerns about greater hemodynamic instability.
The Association Between Body Mass Index and Remission Rates in Patients With Treatment-Resistant Depression Who Received Intravenous Ketamine
To the Editor: Few studies have evaluated optimal dosing of intravenous (IV) ketamine for treatment-resistant depression (TRD).1,2 Landmark studies3-5 infused ketamine at 0.5 mg/kg actual body weight. However, a consensus statement by Sanacora et al1 suggested dosing based on calculated ideal body weight, especially in patients with body mass index (BMI) ≥ 30 given concerns about greater hemodynamic instability. Preliminary studies6 identified an association between BMI and ketamine response with 0.5 mg/kg infused over 40 minutes. The association was postulated to be due to clinically effective dose, as patients with the highest dose had more improvement.6 We completed 2 open-label trials7,8 of racemic ketamine infused intravenously, 0.5 mg/kg actual body weight, over 100 minutes in TRD patients. We conducted a secondary analysis from these studies to assess association between BMI and ketamine response at a slower infusion rate.
Methods. We combined subject-level data from 2 open-label, adjunct IV ketamine trials7,8 that enrolled adults with treatment-resistant DSM-IV unipolar or bipolar major depressive disorder (defined as failure to respond to 2 adequate trials of antidepressive treatments, including pharmacotherapy with antidepressants, mood stabilizers, or atypical antipsychotic drugs with bipolar antidepressive effects;, electroconvulsive therapy; or transcranial magnetic stimulation). Multiple infusions were given weekly until remission was achieved or maximum number of infusions administered (twice weekly up to 4 infusions8 or thrice weekly up to 6 infusions7). Remission was defined as Montgomery-Asberg Depression Rating Scale (MADRS) score ≤ 9 after the 4 infusions.9 The quantitative outcome percentage change in MADRS score was also analyzed (see Supplementary Appendix 1 for details). We analyzed BMI as a continuous measure and categorized BMI per World Health Organization guidelines (normal, overweight, obesity classes I and II).
Continuous variables are reported as mean ± SD and categorical variables as counts and percentages. The Wilcoxon rank sum test was used to compare continuous variables. Chi-square and Fisher exact tests were used to compare categorical variables. Logistic regression was used to evaluate the association between BMI and remission. Because the sample size was small, the Cochran-Armitage trend test was utilized to test for association between BMI obesity categories (stepwise increases from normal BMI through class II obesity) and remission. JMP Pro 13.0.0 statistical software (SAS Institute, Cary, North Carolina) and R version 3.4.210 were used for the analysis.
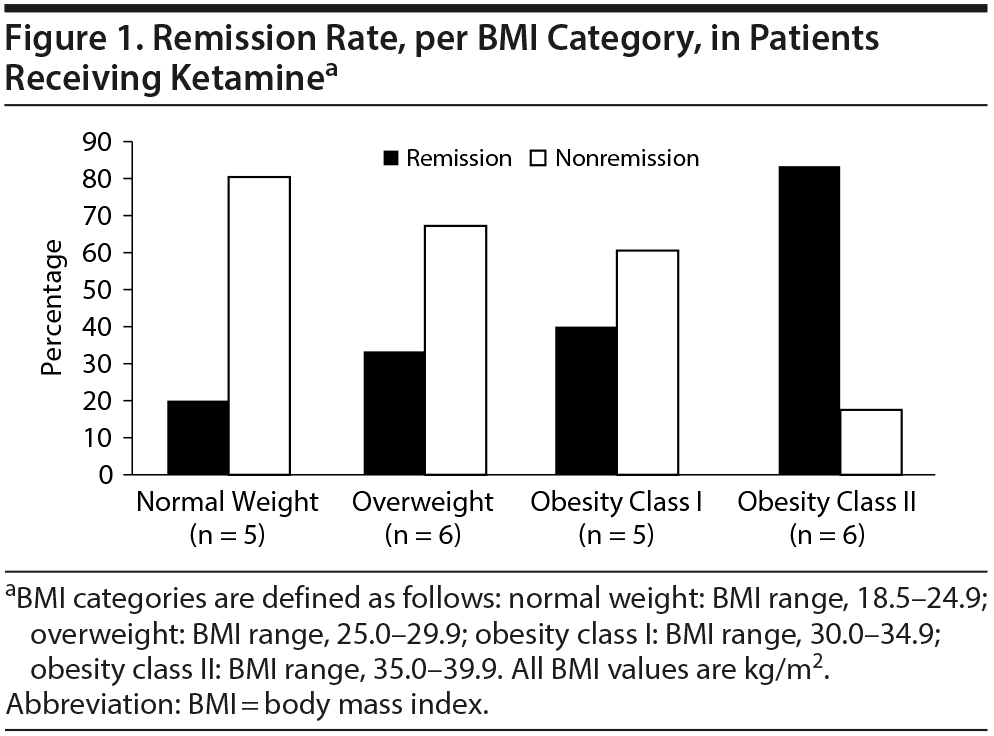
Results. The study sample consisted of 22 subjects with depression who were middle-aged (mean ± SD age = 46.4 ± 11.5 years), had a mean ± SD BMI of 30.7 ± 6.6, and were predominantly female (77%) (Supplementary Tables S1 and 2). Mean (SE) change in MADRS score was −16.18 ± 2.84 (P < .0001), a significant reduction (improvement) from baseline (mean ± SD = 31.18 ± 1.53). There was a trend association of BMI and remission (OR = 1.17; 95% CI, 0.98-1.39; P = .07). When data were categorized into 4-level ordinal variables (BMI categories), there was a significant association between higher BMI category and remission (P = .03, Figure 1).
We found an association between baseline BMI and odds of remission and an effect modification of the relationship between ketamine and remission by BMI category. Our major limitation is very small sample size, especially in the class II obesity category, which drove the BMI association with remission and percentage change in MADRS score; thus, replication of these findings in a larger sample is needed. Nevertheless, these data, alongside those of Niciu et al,6 suggest that higher BMI may be associated with higher rates of remission regardless of infusion rate. Ketamine is highly lipid soluble and is rapidly transferred across the blood-brain barrier, directly impacting brain lipids. One proposed hypothesis is that patients with higher BMI may very likely have received a clinically effective dose of ketamine, which may have led to greater improvement in depression scores.6 These data have implications when evaluating merits of actual versus ideal body weight in ketamine dosing and raise an important clinical question in need of an adequately powered randomized trial (probably with restriction as to the upper allowable BMI limit for enrollees for safety purposes). This issue is important as US obesity rates steadily increase.11 Interestingly, a recent study (Cullen et al12) dosed ketamine in TRD adolescents on ideal body weight. However, after observation that most subjects were nonresponders, the study design was switched to dosing on actual body weight, which resulted in significantly increased response rates.
Further consideration is needed before dosing patients based on ideal body weight, as the risk for underdosing this population may exist. Although higher BMI favors efficacy, safety considerations of lipophilic drug use in obese patients should be considered and need further investigation.
REFERENCES
1.Sanacora G, Frye MA, McDonald W, et al; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405. PubMed CrossRef
2.Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD) [published online ahead of print October 3, 2018]. Mol Psychiatry. 2018. PubMed CrossRef
3.Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864. PubMed CrossRef
4.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802. PubMed CrossRef
5.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142. PubMed CrossRef
6.Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry. 2014;75(5):e417-e423. PubMed CrossRef
7.Vande Voort JL, Morgan RJ, Kung S, et al. Continuation phase intravenous ketamine in adults with treatment-resistant depression. J Affect Disord. 2016;206:300-304. PubMed CrossRef
8.Rasmussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444-450. PubMed CrossRef
9.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed CrossRef
10.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. R Foundation for Statistical Computing website. https://www.R-project.org/.
11.Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief. 2017;(288):1-8. PubMed
12.Cullen KR, Amatya P, Roback MG, et al. Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol. 2018;28(7):437-444. PubMed CrossRef
aDepartment of Psychiatry and Psychology, Mayo Clinic Depression Center, Mayo Clinic College of Medicine, Rochester, Minnesota
bDepartment of Psychiatry and Psychology, Mayo Clinic College of Medicine, Jacksonville, Florida
cDepartment of Health Sciences Research, Mayo Clinic, Rochester, Minnesota
Potential conflicts of interest: Dr Singh received research time support from Medibio. It is unrelated to the current study. Dr Frye received grant support from Assurex Health, Mayo Foundation, and Medibio; consultancy from Actify Neurotherapies, Allergan, Intra-Cellular Therapies, Janssen, Myriad, Neuralstem,Takeda, and Teva; and continuing medical education (CME)/travel/honoraria from American Physician Institute, CME Outfitters, and Global Academy for Medical Education, none of which are related to the current study. Dr Vande Voort is co-Principal Investigator on an investigator-initiated study that has a grant-in-kind for supplies and genotyping only through Assurex. It is unrelated to the current study. Drs Bobo, Rasmussen, Schak, and Biernacka; Ms Stoppel; and Mr Rico report no potential conflicts of interest relative to the subject of this letter.
Funding/support: Department of Psychiatry and Psychology, Mayo Clinic, Rochester, Minnesota.
Role of the sponsor: The funder of the study had no role in study design, data analysis, data interpretation, and writing of the report.
Previous presentation: The abstract was presented as an oral presentation at the Annual American Society of Clinical Psychopharmacology Meeting; May 28, 2019; Scottsdale, Arizona.
Supplementary material: See accompanying pages.
Published online: November 12, 2019.
J Clin Psychiatry 2019;80(6):19l12852
To cite: Singh B, Bobo WV, Rasmussen KG, et al. The association between body mass index and remission rates in patients with treatment-resistant depression who received intravenous ketamine. J Clin Psychiatry 2019;80(6):19l12852
To share: https://doi.org/10.4088/JCP.19l12852
© Copyright 2019 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!
Save
Cite