ABSTRACT
Objective: To evaluate the short- and long-term effects of brexpiprazole on patient functioning in schizophrenia.
Methods: Data were included from three 6-week, randomized, double-blind, placebo-controlled studies (hospitalized patients); a 52-week, randomized, double-blind, placebo-controlled maintenance treatment study (terminated early by the study sponsor based on the positive result of an interim analysis); and two 52-week, open-label extension studies—all in patients with schizophrenia (DSM-IV-TR criteria) and conducted from July 2011–February 2016. Patients allocated to oral brexpiprazole received 2–4 mg/d (short-term studies) or 1–4 mg/d (long-term studies). Functioning was measured using the Personal and Social Performance (PSP) and Global Assessment of Functioning (GAF) scales, with response defined as a PSP/GAF increase of ≥ 10 points and remission as PSP score ≥ 71 or GAF score ≥ 61.
Results: Patients receiving brexpiprazole (n = 831) showed greater improvement than those receiving placebo (n = 490) from baseline to week 6 in PSP score (least squares mean difference, 3.20; 95% confidence interval, 1.82–4.58; P < .0001; Cohen d = 0.31) and in all 4 PSP domains. At week 52 of the maintenance study (which had a low completion rate primarily due to the early termination), GAF functional remission was achieved by 65.3% (62/95) of stabilized patients randomized to brexpiprazole and 47.1% (48/102) of stabilized patients randomized to placebo, with a number needed to treat of 6 (95% confidence interval, 4–22; P = .0076). At week 52 of the open-label studies (n = 177), PSP functional response and remission were achieved by 84.2% and 41.8% of patients receiving brexpiprazole, respectively.
Conclusions: Although limited by the lack of an active comparator, analyses of this large dataset demonstrate that brexpiprazole treatment is associated with clinically relevant improvement in functioning among patients with schizophrenia, in the short term and long term.
Trial Registration: Data used in this post hoc analysis were from studies with ClinicalTrials.gov identifiers: NCT01396421, NCT01393613, NCT01810380, NCT01668797, NCT01397786, and NCT01810783.
J Clin Psychiatry 2022;83(2):20m13793
To cite: Correll CU, He Y, Therrien F, et al. Effects of brexpiprazole on functioning in patients with schizophrenia: post hoc analysis of short- and long-term studies. J Clin Psychiatry. 2022;83(2):20m13793.
To share: https://doi.org/10.4088/JCP.20m13793
© Copyright 2022 Physicians Postgraduate Press, Inc.
aThe Zucker Hillside Hospital, Department of Psychiatry, Glen Oaks, New York
bThe Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Department of Psychiatry and Molecular Medicine, Hempstead, New York
cCharité-Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department of Child and Adolescent Psychiatry, Berlin, Germany
dOtsuka Pharmaceutical Development & Commercialization Inc, Princeton, New Jersey
eOtsuka Canada Pharmaceutical Inc, Saint-Laurent, Quebec, Canada
fLundbeck Canada Inc, Saint-Laurent, Quebec, Canada
gH. Lundbeck A/S, Valby, Copenhagen, Denmark
*Corresponding author: Christoph U. Correll, MD, The Zucker Hillside Hospital, Psychiatry Research, 75–59 263rd St, Glen Oaks, NY 11004 ([email protected]).
See free commentary by Schooler
The progressive nature of schizophrenia during the early stages of the disease allows residual and persistent symptoms to accumulate, such that patients experience a continuing loss of functionality.1 Antipsychotics are efficacious for treating the positive symptoms of acute schizophrenia2,3 and for reducing the risk of relapse in the long term, with modest differences between drugs.4–6 However, patients may remain functionally impaired because of persisting negative, cognitive, and other residual symptoms.7–10 Antipsychotic side effects can also contribute to impaired functioning.11,12
Treatment guidelines emphasize that improving a patient’s level of functioning in the acute phase of treatment, and maintaining or improving functioning in the maintenance phase, should be major goals of schizophrenia management.7,13,14 Moreover, functionality is a key component of recovery,15 achieved by relatively few individuals with schizophrenia.16
Brexpiprazole acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors and as an antagonist at serotonin 5-HT2A and norepinephrine α1B/α2C receptors, all with subnanomolar affinity.17 The efficacy and safety of brexpiprazole for the treatment of adults with acute schizophrenia have been demonstrated in 2 pivotal randomized controlled studies,18,19 supported by a third study with flexible dosing.20,21 In the long term, a randomized controlled study demonstrated the efficacy and safety of brexpiprazole as maintenance treatment in stabilized patients,22 and 2 open-label extension (OLEx) studies showed that brexpiprazole was generally well tolerated for up to 52 weeks in patients with schizophrenia.23,24
The aim of this post hoc analysis was to comprehensively evaluate the short- and long-term effects of brexpiprazole on functioning in adult patients with schizophrenia, based on data from all phase 3 studies of brexpiprazole in schizophrenia (randomized, double-blind, placebo-controlled studies and long-term studies) that included a functioning outcome measure. Phase 2 studies were not included in the present analysis as they studied brexpiprazole doses outside the approved dosing range, had no titration schedule, and/or showed insufficient assay sensitivity.
METHODS
Study Design and Patients
The studies were conducted in compliance with the International Conference on Harmonisation Good Clinical Practice Consolidated Guideline and the World Medical Association Declaration of Helsinki. The protocols were approved by independent ethics committees or institutional review boards at each site/country, and all patients provided written informed consent to participate after procedures and possible side effects were explained to them.
Short-term Studies
The short-term analysis included data from 3 randomized, double-blind, placebo-controlled studies of oral brexpiprazole in patients with acute schizophrenia: VECTOR (ClinicalTrials.gov identifier: NCT01396421),18 BEACON (NCT01393613),19 and LIGHTHOUSE (NCT01810380),20,21 conducted from July 2011–December 2014.
The methodology of these studies has been published.18–21 In brief, the studies enrolled patients aged 18–65 years experiencing an acute exacerbation of schizophrenia (DSM-IV-TR criteria). The studies had similar designs, comprising a ≤ 14-day screening phase, a 6-week double-blind treatment phase during which patients were hospitalized, and a safety follow-up phase. Depending on the study, eligible patients were randomized to placebo, brexpiprazole (fixed-dose 0.25, 1, 2, or 4 mg/d or flexible-dose 2–4 mg/d), or active reference (quetiapine extended-release [XR] flexible-dose 400–800 mg/d, included in a single study for assay sensitivity).
Long-term Maintenance Study
EQUATOR (NCT01668797)22 was a 52-week, randomized, double-blind, placebo-controlled maintenance treatment study conducted from October 2012–February 2015.
The methodology has been published.22 In brief, the study enrolled inpatients and outpatients aged 18–65 years experiencing an acute exacerbation of schizophrenia (DSM-IV-TR criteria) and with a history of relapse and/or symptom exacerbation in the absence of antipsychotic treatment. The study comprised an as-needed conversion/washout phase, a 12–36 week single-blind stabilization phase in which all patients received oral brexpiprazole 1–4 mg/d, a 52-week double-blind maintenance phase (for only those patients who met stabilization criteria, including outpatient status), and a safety follow-up phase. In the maintenance phase, patients were randomized to continue receiving brexpiprazole 1–4 mg/d or switch to placebo.
Long-term OLEx Studies
The OLEx long-term analysis included data from 2 OLEx studies in schizophrenia: ZENITH (NCT01397786)23 and Study 14644B (NCT01810783),24 conducted from September 2011–February 2016.
Methodology has been published for ZENITH23 and is summarized online for Study 14644B.24 In brief, outpatients (and inpatients in Study 14644B) who completed the short-term studies were eligible to roll over (also, de novo and maintenance study patients were eligible; not included in the present post hoc analyses). Patients received flexibly dosed oral brexpiprazole 1–4 mg/d for up to 52 weeks (ZENITH was amended to 26 weeks toward the end; this amendment only applied to 11.2% of patients).
Assessments
The primary outcomes were change in schizophrenia symptoms (short-term studies), time to relapse (maintenance study), or safety and tolerability (OLEx studies); these outcomes have been previously reported for the individual studies and the pooled short-term sample.18–24 The present analysis addresses functioning outcomes, measured using the Personal and Social Performance (PSP) and Global Assessment of Functioning (GAF) scales.
The PSP25 is a brief, clinician-rated measure of 4 domains of patient functioning: (1) socially useful activities; (2) personal and social relationships; (3) self-care; and (4) disturbing and aggressive behaviors. Each domain is rated on a 6-degree severity scale from absent to very severe. By cross-referencing the domain ratings with a descriptive table, an overall PSP score is determined from 1 (worst) to 100 (best), where a score of ≥ 71 represents, at worst, mild functional difficulties; 31–70 represents manifest disabilities of various degrees; and 1–30 represents functioning sufficiently poor that intensive support or supervision is needed.
The PSP was administered at baseline and weeks 3 and 6 of the short-term studies and at baseline and weeks 2, 8, 26, and 52 of the OLEx studies. In the maintenance study, although PSP was measured at baseline of the conversion phase, it was not measured at randomization; thus, it is not possible to separate the effects of placebo and brexpiprazole, and so the data were not included in the present analysis.
The GAF26 is a brief, clinician-rated measure of psychological, social, and occupational/school functioning, which also takes symptoms into account. The patient’s level of functioning is scored on a hypothetical continuum of mental health illness from 1 (worst) to 100 (best), with descriptors at 10-point intervals.
In the maintenance study, GAF was administered at baseline of the conversion phase (or baseline of the stabilization phase if not converted); weeks 12, 24, and 36 of the stabilization phase; and weeks 12, 24, 36, and 52 of the maintenance phase. GAF was not used in the short-term or OLEx studies.
Statistical Analysis
Criteria for functional response and remission. PSP functional response was defined as an increase of ≥ 10 points from baseline, corresponding to improvement of at least one 10-point category.25 This is a conservative approach based on clinically meaningful improvement estimates of 9 points in acute schizophrenia27 and 4–7 points in stable schizophrenia.28 PSP functional remission was defined as a score of ≥ 71, which can be interpreted as no dysfunction or mild difficulties known only to someone who is very familiar with the person.25
GAF functional response was defined as an increase of ≥ 10 points from baseline, corresponding to improvement of at least one 10-point category.26 GAF functional remission was defined as a score of ≥ 61, as is standard in schizophrenia studies.29–32
Short-term studies. Data from the short-term studies were pooled for all patients allocated to placebo, and for all patients allocated to a brexpiprazole dose in the recommended dose range of 2–4 mg (ie, 2 mg, 4 mg, and 2–4 mg).33 The brexpiprazole 0.25 mg and 1 mg groups, intended to evaluate the lower dose range, were not included in the post hoc analyses. The quetiapine XR group was not included because the data came from a small arm in a single study that was intended for assay sensitivity rather than head-to-head comparison. Furthermore, it has been suggested that the known side effect profile of quetiapine XR (primarily sedation) led to functional unblinding in this dataset, thereby introducing a source of bias toward symptom improvement among patients receiving quetiapine XR.21
Baseline was defined as the randomization visit (prior to the first dose of study drug).
Changes in overall PSP score and PSP domain scores were analyzed using a mixed model for repeated measures (MMRM), with fixed effect of protocol, trial center within protocol, treatment, visit, treatment–visit interaction, baseline value, and baseline–visit interaction as covariates, and with an unstructured variance–covariance matrix. Cohen d effect size (ES) was calculated as the treatment–placebo difference divided by the pooled standard deviation, with ES = 0.2 as “small,” ES = 0.5 as “medium,” and ES = 0.8 as “large.”34 Functional response and remission were analyzed using a Cochran–Mantel–Haenszel (CMH) general association test with last observations carried forward (LOCF). The number needed to treat (NNT) to achieve functional response/remission was calculated, with 95% confidence limits (CLs).
Analyses were repeated, stratifying by age (18–35 years, 36–65 years) and baseline PSP score (≥ median = “better baseline functioning,” < median = “worse baseline functioning”).
Maintenance study. Analyses were performed in the sample of patients who entered the randomized phase of the study. Baseline was defined as the first visit of the conversion/stabilization phase; the term randomization was used to describe the first visit of the maintenance phase.
Mean GAF score was calculated at baseline and last visit in the stabilization phase, and mean change in GAF score was calculated relative to randomization in the maintenance phase (LOCF; analysis of covariance model with treatment and trial center as factors and baseline value as covariate), with Cohen d. Functional response and remission were analyzed using a CMH general association test with LOCF and observed cases (OC); NNTs were calculated. MMRM analyses were not performed for the maintenance study as they were underpowered at week 52.
OLEx studies. Data were combined from the 6-week short-term studies (for those patients who ultimately rolled over) and the 52-week OLEx studies, in order to investigate the longest possible continuous brexpiprazole treatment duration (a total of up to 58 weeks). With this treatment duration in mind, the analyses included only those patients in the long-term studies who had previously received brexpiprazole 2–4 mg in the short-term studies.
Baseline was defined as the randomization visit of the short-term studies. Mean changes from baseline in PSP and PSP domain scores, and the proportions with functional response and remission, were summarized using descriptive statistics with OC data. Within-group Cohen d ES was calculated for the change from baseline to open-label week 52.
Analyses were performed using SAS Enterprise Guide 7.1 software (SAS Institute Inc; Cary, NC).
RESULTS
Patients
Short-term studies. In the short-term studies pooled sample, 1,414 patients were randomized to placebo (n = 531) or brexpiprazole 2–4 mg (n = 883), of whom 1,411 received at least 1 dose of study drug (n = 529; n = 882). The studies were completed by 335 patients (63.1%) in the placebo group and 617 patients (69.9%) in the brexpiprazole group; the most common reasons for discontinuation were adverse events (placebo: 65 patients, 12.2%; brexpiprazole: 70 patients, 7.9%), lack of efficacy (63, 11.9%; 70, 7.9%), and patient withdrew consent (50, 9.4%; 103, 11.7%).
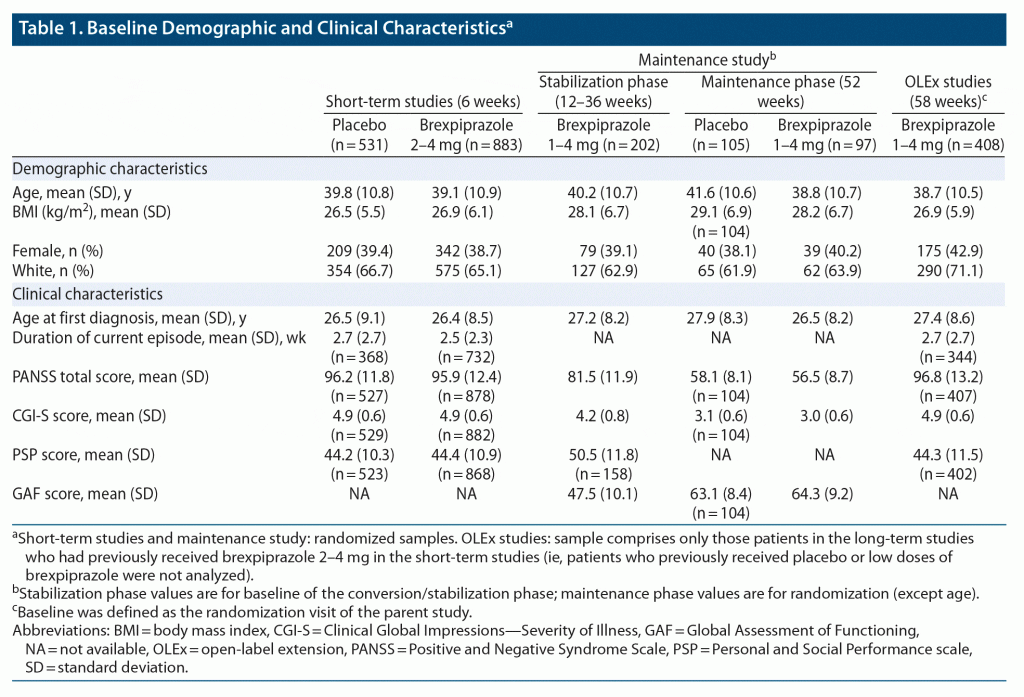
Baseline demographic and clinical characteristics were similar between treatment groups (Table 1). The median PSP score at baseline was 44, indicating marked/severe difficulties in functioning.25 The majority of patients had a baseline PSP score in the range 31–50 (placebo, 65.1%; brexpiprazole, 63.3%). Of the remaining patients, 24.3% (placebo) and 25.3% (brexpiprazole) had a baseline PSP score in the range 51–70, 10.0% and 10.8% in the range 1–30, and 0.6% and 0.6% in the range 71–100.
Baseline characteristics were also similar between treatment groups when stratified by age (Supplementary Table 1) and baseline functioning severity (Supplementary Table 2).
Maintenance study. In the maintenance study, 202 patients met stability criteria in the stabilization phase and were randomized to placebo (n = 105) or brexpiprazole (n = 97), of whom 201 received at least 1 dose of randomized study drug (n = 104; n = 97). The study was terminated early by the sponsor based on the positive result of an interim analysis. Only 9 patients (8.6%) in the placebo group and 14 patients (14.4%) in the brexpiprazole group completed the study; the most common reasons for discontinuation were early termination of the study (placebo: 38 patients, 36.2%; brexpiprazole: 49 patients, 50.5%), and lack of efficacy (impending relapse) (40, 38.1%; 13, 13.4%).
At randomization, demographic and clinical characteristics were similar between treatment groups and reflected that patients were stabilized and generally functioning well (mean GAF score = 64) (Table 1).
The mean daily dose of brexpiprazole was 3.4 mg during the stabilization phase (n = 202) and 3.6 mg at patients’ last visit in the maintenance phase (n = 97).
OLEx studies. The OLEx long-term sample comprised 408 patients who rolled over from the short-term studies, all of whom received at least 1 dose of study drug. The studies were completed by 177 patients (43.4%); the most common reasons for discontinuation were adverse events (83 patients, 20.3%) and patient withdrew consent (77, 18.9%).
Baseline demographic and clinical characteristics are presented in Table 1. The median PSP score at baseline was 43.
Across the duration of the studies, the mean brexpiprazole dose was 3.0 mg/d.
Efficacy
Short-term studies.
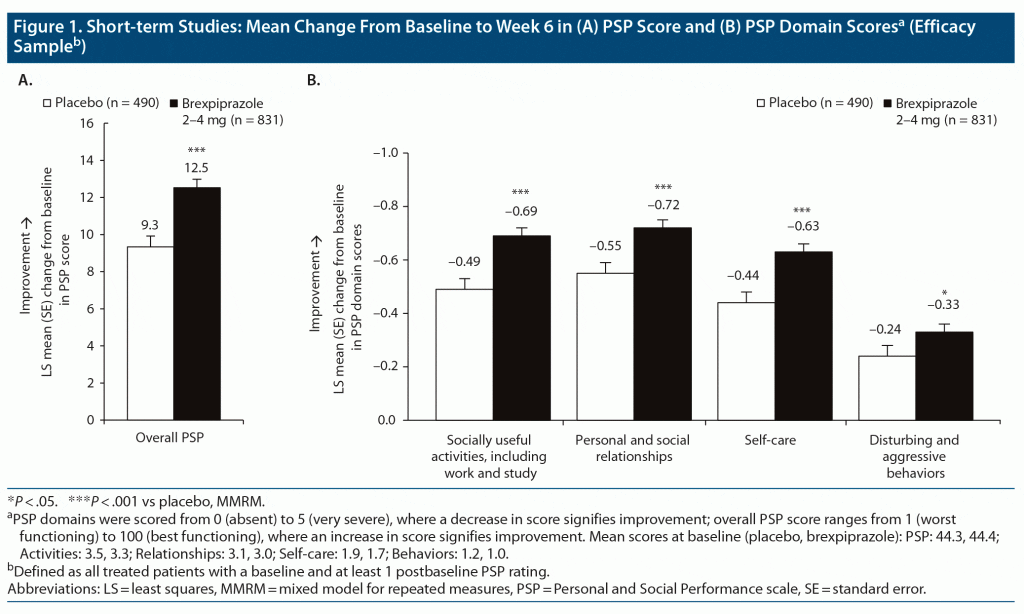
Change in PSP score. Brexpiprazole was associated with greater improvement than placebo from baseline to week 6 in overall PSP score and all 4 PSP domain scores (Figure 1). Least squares mean differences (95% CLs) versus placebo at week 6 were PSP: 3.20 (1.82, 4.58), P < .0001, ES = 0.31; Activities: −0.20 (−0.29, −0.10), P < .0001, ES = 0.28; Relationships: −0.16 (−0.26, −0.07), P = .0007, ES = 0.23; Self-care: −0.19 (−0.28, −0.10), P < .0001, ES = 0.27; and Behaviors: −0.09 (−0.18, 0.00), P = .042, ES = 0.14.
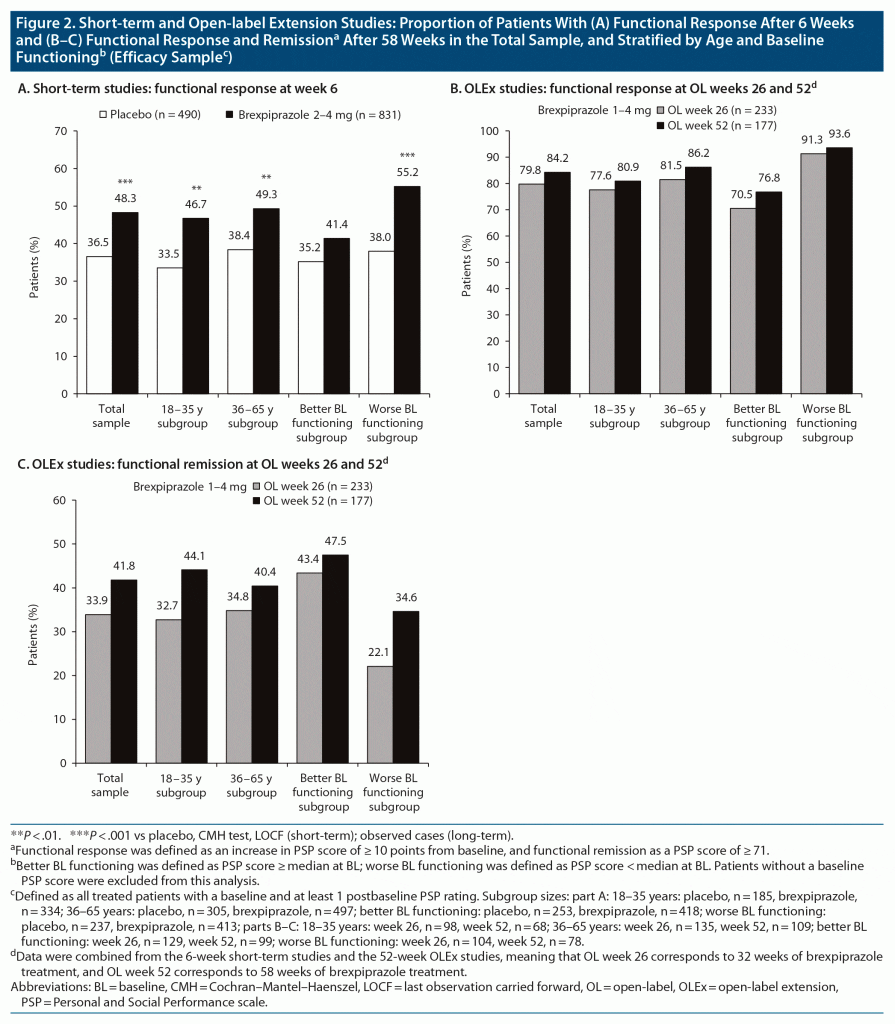
Functional response and remission. A greater proportion of patients receiving brexpiprazole versus placebo showed functional response at week 6 in the total sample, in both age subgroups, and for patients with worse baseline functioning (Figure 2A). NNTs for functional response (95% CLs) were 9 (6, 16) (P < .0001) in the total sample, 8 (5, 23) (P = .0012) in the 18–35 years subgroup, 10 (6, 26) (P = .0032) in the 36–65 years subgroup, 17 (8, −75) (P = .086) in the better baseline functioning subgroup, and 6 (4, 11) (P < .0001) in the worse baseline functioning subgroup.
At week 6, functional remission was achieved by 11.0% (91/831) of patients receiving brexpiprazole and 6.7% (33/490) receiving placebo, with an NNT of 24 (14, 88) (P = .0071).
Maintenance study.
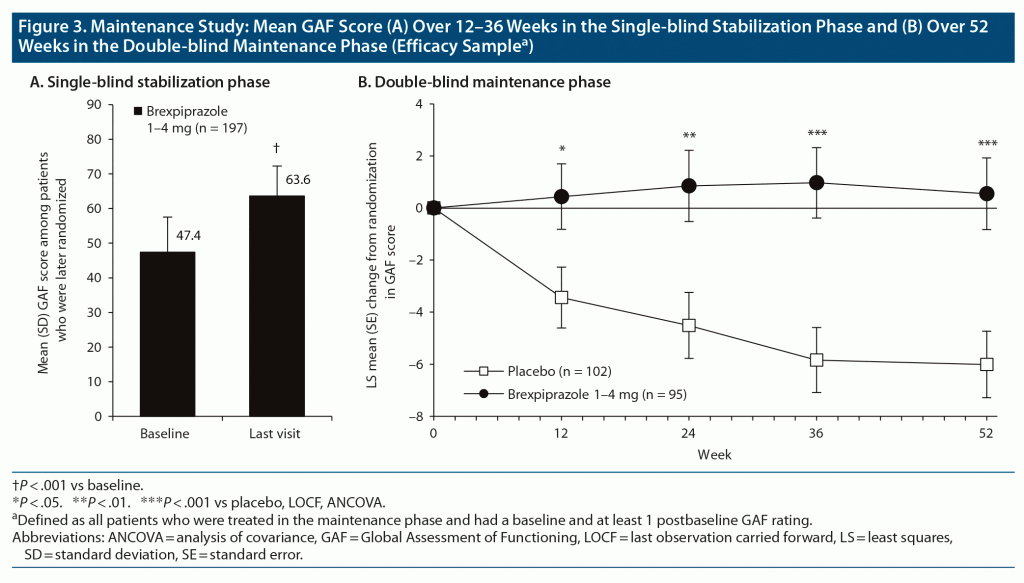
Change in GAF score. GAF score improved in the stabilization phase and this improvement was maintained with brexpiprazole treatment during the maintenance phase, whereas the placebo group worsened (Figure 3). The least squares mean difference (95% CLs) versus placebo at week 52 of the maintenance phase was 6.55 (3.28, 9.83), P = .0001, ES = 0.56.
Functional response and remission. The proportions of patients achieving functional response at week 52 of the maintenance phase (LOCF) were 70.5% (67/95) for brexpiprazole and 49.0% (50/102) for placebo, with an NNT (95% CLs) of 5 (3, 13) (P = .0011). Considering OC data, functional response rates for brexpiprazole and placebo at week 24 were 78.0% (39/50) and 63.9% (23/36), respectively (P = .15); and at week 52 were 93.3% (14/15) and 55.6% (5/9), respectively, with an NNT of 3 (2, 34) (P = .031).
The proportions of patients with functional remission at week 52 of the maintenance phase (LOCF) were 65.3% (62/95) for brexpiprazole and 47.1% (48/102) for placebo, with an NNT (95% CLs) of 6 (4, 22) (P = .0076). The OC data at week 24 were 84.0% (42/50) for brexpiprazole and 72.2% (26/36) for placebo (P = .19); and at week 52 were 80.0% (12/15) and 55.6% (5/9), respectively, with an NNT of 5 (2, −8) (P = .21).
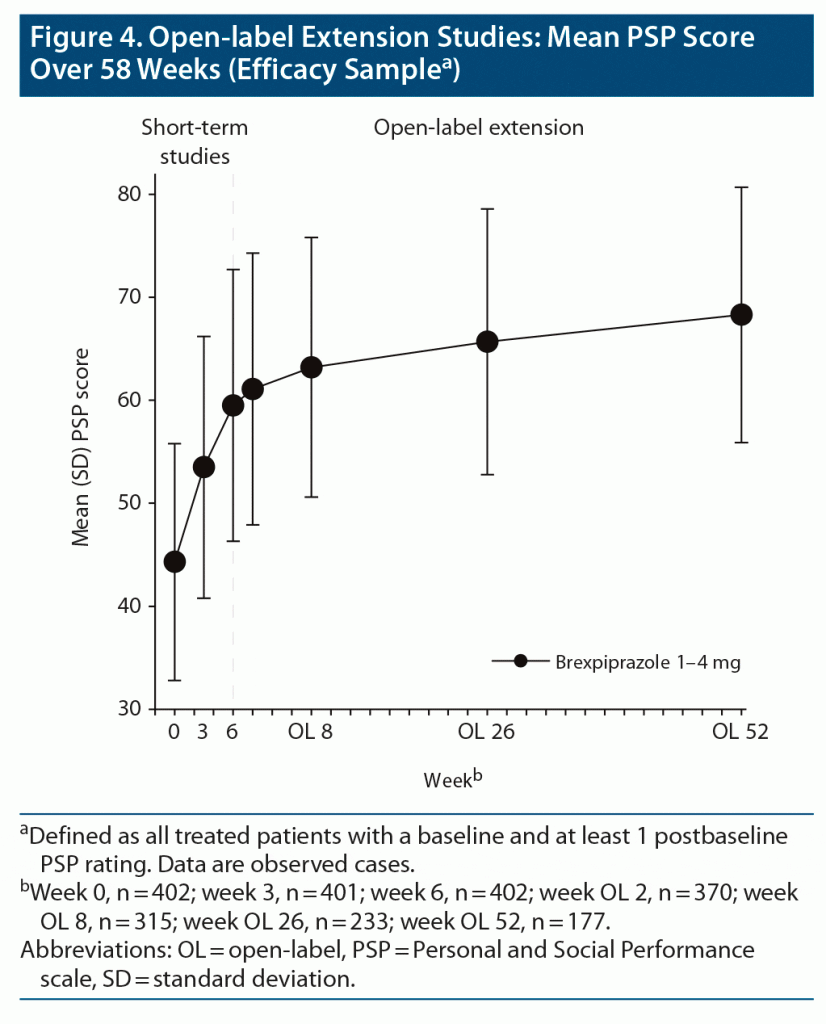
OLEx studies.
Change in PSP score. Change in PSP score over 58 weeks of brexpiprazole treatment is shown in Figure 4. The mean (standard deviation) changes from baseline to open-label week 52 were PSP: 23.6 (12.9), within-group ES = 1.82; Activities: −1.5 (1.0), ES = 1.53; Relationships: −1.4 (1.0), ES = 1.46; Self-care: −1.1 (0.9), ES = 1.20; Behaviors: −0.6 (1.0), ES = 0.64. (Baseline values were PSP: 44.3 [11.5]; Activities: 3.4 [0.8]; Relationships: 3.0 [0.8]; Self-care: 1.7 [1.1]; Behaviors: 1.0 [1.0].)
Functional response and remission. At open-label week 52, functional response was achieved by 80.9%–86.2% of patients, and functional remission by 40.4%–44.1% of patients, depending on age (Figure 2B and 2C).
Split by median PSP at baseline, the functional response rate at open-label week 52 was higher among patients with worse baseline functioning, and the functional remission rate was higher among patients with better baseline functioning (Figure 2B and 2C).
DISCUSSION
Deficits in psychosocial functioning, attributable to persisting symptoms and/or antipsychotic side effects, are a core feature of schizophrenia and an important treatment target.9,11,35,36 The present analyses demonstrate that brexpiprazole treatment is associated with clinically relevant improvement in patient functioning among adults with schizophrenia, in the short term and the long term.
Across 3 short-term studies in acute schizophrenia, brexpiprazole improved levels of functioning versus placebo in all 4 PSP domains—socially useful activities, personal and social relationships, self-care, and disturbing and aggressive behaviors—with small-to-medium benefits over placebo (between-group ES = 0.23–0.31, except behaviors, which was limited by low baseline values). These data are supported by a previous 6-week, open-label, exploratory study in patients with acute schizophrenia, in which flexibly dosed brexpiprazole (mean dose during week 6, 3.6 mg/d) was associated with improvements in all 4 domains of everyday functioning (as measured by the Specific Levels of Functioning Scale), whereas aripiprazole was associated with improvements in 2 domains.37 Additionally, in the present short-term analysis, a significantly greater proportion of patients receiving brexpiprazole versus placebo demonstrated functional response—in younger and older adults, and among patients with worse baseline functioning. In the subgroup with better baseline functioning, the numerical advantage for brexpiprazole on functional response did not reach statistical significance, attributed to a relatively high baseline score, which allowed less opportunity for improvement.
In the maintenance study, acutely ill patients treated with brexpiprazole improved in functioning during the stabilization phase, and stabilized patients receiving brexpiprazole maintained this improved level of functioning, whereas placebo was associated with a decline. The GAF score difference versus placebo at endpoint (week 52 of the maintenance phase) was 6.6 points, which is above the suggested minimum clinically important difference in schizophrenia (4 points),38 with a medium-to-large between-group ES. Functional response rates at week 52 also favored brexpiprazole, although the nature of this maintenance study (in which patients with impending relapse were withdrawn) and the low completion rate (primarily due to early termination by the study sponsor) limit the interpretation of the data. Furthermore, since the GAF is not a pure measure of functioning (it also includes diverse psychiatric symptoms, with the final score being determined by the dimension with greater severity/impairment),26 the benefit of brexpiprazole treatment over placebo may reflect other factors besides functioning, such as worsening of symptoms in the placebo group.
Finally, across 2 OLEx studies, brexpiprazole showed improvement of PSP functioning and maintenance of effect for up to 58 weeks, with large within-group effect sizes (all > 1, except behaviors, 0.64). Of the 177 patients who received brexpiprazole for 58 weeks, more than 4 in 5 achieved functional response, and more than 2 in 5 achieved functional remission, using established, clinically meaningful criteria for response and remission.25,27,28 This finding suggests that continued treatment with brexpiprazole results in improved and sustained functioning over time, and benefits are not limited to an initial/acute improvement.
In general, patients with schizophrenia taking atypical antipsychotics can experience a variety of side effects, including activating and sedating side effects, sexual side effects, and weight gain.12,36,39 If unaddressed, antipsychotic side effects can cause long-term distress and functional impairment, can contribute to chronic health complications, and can have a negative impact on treatment adherence.11,40 Side effects have a negative impact on multiple domains of functioning (physical, social, vocational, and emotional) and reduce quality of life.36 Although other atypical antipsychotics (olanzapine, paliperidone, quetiapine, and lurasidone) have shown improvement in social functioning versus placebo in short-term studies,3 this must be considered in the context of their side effects. For example, some patients taking sedating antipsychotics, such as olanzapine and quetiapine, experience persistent sedation or somnolence that can have a marked impact on multiple domains of functioning and quality of life.41,42 At the other end of the spectrum, anxiety symptoms in schizophrenia are also associated with poorer functioning.43 Treatment guidelines recommend that special attention is given to potential side effects when selecting an antipsychotic.7,13,14 Brexpiprazole is well tolerated in the short and long term, being neither activating nor sedating,41 and with low rates of important adverse events that are associated with other antipsychotics (akathisia, sedation, weight gain, or QTc prolongation).44
Strengths of this analysis include the large dataset, that patients were followed for up to 58 weeks, the subanalyses according to age and baseline functional status, and the application of consistent and validated criteria for functional response and remission. The analysis is limited by its post hoc nature, the patient selection criteria (which may limit generalizability), the lack of an active comparator, the lack of PSP measurement at randomization in the maintenance study, the small number of patients who completed the maintenance study, and the lack of correction for multiple comparisons.
In conclusion, treatment with brexpiprazole was associated with clinically relevant improvement in functioning among adults with acute schizophrenia, and maintenance of this improvement over the long term, with high rates of functional response and remission.
Submitted: November 18, 2020; accepted October 20, 2021.
Published online: March 1, 2022.
Potential conflicts of interest: Dr Correll has been a consultant and/or advisor to or has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva; has provided expert testimony for Janssen and Otsuka; has served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; and is a stock option holder of LB Pharma. Drs He, Weiss, and Hobart are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Dr Therrien is a full-time employee of Otsuka Canada Pharmaceutical Inc. Dr MacKenzie is a full-time employee of Lundbeck Canada Inc. Dr Meehan and Mr Hefting are full-time employees of H. Lundbeck A/S.
Funding/support: This work was supported by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ) and H. Lundbeck A/S (Valby, Denmark).
Role of the sponsor: Authors affiliated with the sponsors were involved in the design of the study, the analysis and interpretation of data, and the writing and reviewing of this article.
Previous presentations: Parts of this study were presented at the Royal Australian and New Zealand College of Psychiatrists’ Annual Congress, Adelaide, Australia, April 30–May 4, 2017; American College of Neuropsychopharmacology Annual Meeting, Palm Springs, California, December 3–7, 2017; and 6th Biennial Meeting of the Schizophrenia International Research Society Conference, Florence, Italy, April 4–8, 2018.
Acknowledgments: Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge—a Prime Global Agency (Cambridge, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Patients with schizophrenia often remain functionally impaired, making improvement of functioning an important area of need.
- Brexpiprazole can improve functioning in patients with schizophrenia, measured using the Personal and Social Performance and Global Assessment of Functioning scales.
- Improvement of functioning with brexpiprazole begins during treatment of acute schizophrenia, and benefits are generally maintained over a 1-year treatment period.
References (44)

- Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. PubMed CrossRef
- Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97. PubMed CrossRef
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. PubMed CrossRef
- Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. PubMed CrossRef
- Kishimoto T, Agarwal V, Kishi T, et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry. 2013;18(1):53–66. PubMed CrossRef
- Kishimoto T, Hagi K, Nitta M, et al. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208–224. PubMed CrossRef
- Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association. Steering Committee on Practice Guidelines. Practice Guideline for the Treatment of Patients With Schizophrenia. Second Edition. PsychiatryOnline.org website. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Accessed November 13, 2020.
- Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19(suppl 1):38–52, quiz 35–37, 53. PubMed CrossRef
- Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107–116. PubMed CrossRef
- Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–534. PubMed CrossRef
- Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567–620. PubMed CrossRef
- Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757–777. PubMed CrossRef
- Hasan A, Falkai P, Wobrock T, et al; World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–378. PubMed CrossRef
- Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44. PubMed CrossRef
- Correll CU, Kishimoto T, Nielsen J, et al. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. 2011;33(12):B16–B39. PubMed CrossRef
- Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–1306. PubMed CrossRef
- Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604. PubMed CrossRef
- Correll CU, Skuban A, Ouyang J, et al. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(9):870–880. PubMed CrossRef
- Kane JM, Skuban A, Ouyang J, et al. A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res. 2015;164(1–3):127–135. PubMed CrossRef
- Marder SR, Hakala MJ, Josiassen MK, et al. Brexpiprazole in patients with schizophrenia: overview of short- and long-term phase 3 controlled studies. Acta Neuropsychiatr. 2017;29(5):278–290. PubMed CrossRef
- Marder SR, Eriksson H, Zhao Y, et al. Post hoc analysis of a randomised, placebo-controlled, active-reference 6-week study of brexpiprazole in acute schizophrenia. Acta Neuropsychiatr. 2020;32(3):153–158. PubMed CrossRef
- Fleischhacker WW, Hobart M, Ouyang J, et al. Efficacy and safety of brexpiprazole (OPC-34712) as maintenance treatment in adults with schizophrenia: a randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2017;20(1):11–21. PubMed
- Forbes A, Hobart M, Ouyang J, et al. A long-term, open-label study to evaluate the safety and tolerability of brexpiprazole as maintenance treatment in adults with schizophrenia. Int J Neuropsychopharmacol. 2018;21(5):433–441. PubMed CrossRef
- ClinicalTrials.gov. Brexpiprazole in patients with schizophrenia (Study 14644B). Identifier: NCT01810783. 2017. ClinicalTrials.gov website. https://clinicaltrials.gov/show/NCT01810783. Accessed November 13, 2020.
- Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated Personal and Social Performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25(2):325–338. PubMed CrossRef
- Nasrallah H, Morosini P, Gagnon DD. Reliability, validity and ability to detect change of the Personal and Social Performance scale in patients with stable schizophrenia. Psychiatry Res. 2008;161(2):213–224. PubMed CrossRef
- Bachmann S, Bottmer C, Schröder J. One-year outcome and its prediction in first-episode schizophrenia: a naturalistic study. Psychopathology. 2008;41(2):115–123. PubMed CrossRef
- Lambert M, De Marinis T, Pfeil J, et al. Establishing remission and good clinical functioning in schizophrenia: predictors of best outcome with long-term risperidone long-acting injectable treatment. Eur Psychiatry. 2010;25(4):220–229. PubMed CrossRef
- Verma S, Subramaniam M, Abdin E, et al. Symptomatic and functional remission in patients with first-episode psychosis. Acta Psychiatr Scand. 2012;126(4):282–289. PubMed CrossRef
- Boyer L, Richieri R, Guedj E, et al. Validation of a functional remission threshold for the Functional Remission of General Schizophrenia (FROGS) scale. Compr Psychiatry. 2013;54(7):1016–1022. PubMed CrossRef
- Rexulti (brexpiprazole) tablets, for oral use. Prescribing information. https://www.otsuka-us.com/media/static/Rexulti-PI.pdf. 2020. Accessed November 13, 2020.
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. PubMed CrossRef
- Burns T, Patrick D. Social functioning as an outcome measure in schizophrenia studies. Acta Psychiatr Scand. 2007;116(6):403–418. PubMed CrossRef
- Tandon R, Lenderking WR, Weiss C, et al. The impact on functioning of second-generation antipsychotic medication side effects for patients with schizophrenia: a worldwide, cross-sectional, web-based survey. Ann Gen Psychiatry. 2020;19(1):42. PubMed CrossRef
- Citrome L, Ota A, Nagamizu K, et al. The effect of brexpiprazole (OPC-34712) and aripiprazole in adult patients with acute schizophrenia: results from a randomized, exploratory study. Int Clin Psychopharmacol. 2016;31(4):192–201. PubMed CrossRef
- Amri I, Millier A, Toumi M. Minimum clinically important difference in the Global Assessment Functioning in patients with schizophrenia. Value Health. 2014;17(7):A765–A766. PubMed CrossRef
- DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20. PubMed CrossRef
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. PubMed CrossRef
- Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138–147. PubMed CrossRef
- Kane JM, Sharif ZA. Atypical antipsychotics: sedation versus efficacy. J Clin Psychiatry. 2008;69(suppl 1):18–31. PubMed
- Lysaker PH, Salyers MP. Anxiety symptoms in schizophrenia spectrum disorders: associations with social function, positive and negative symptoms, hope and trauma history. Acta Psychiatr Scand. 2007;116(4):290–298. PubMed CrossRef
- Kane JM, Skuban A, Hobart M, et al. Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res. 2016;174(1–3):93–98. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite