Objective: To systematically review studies examining the longitudinal associations between cannabis use and symptomatic outcomes among individuals with an anxiety or mood disorder at baseline.
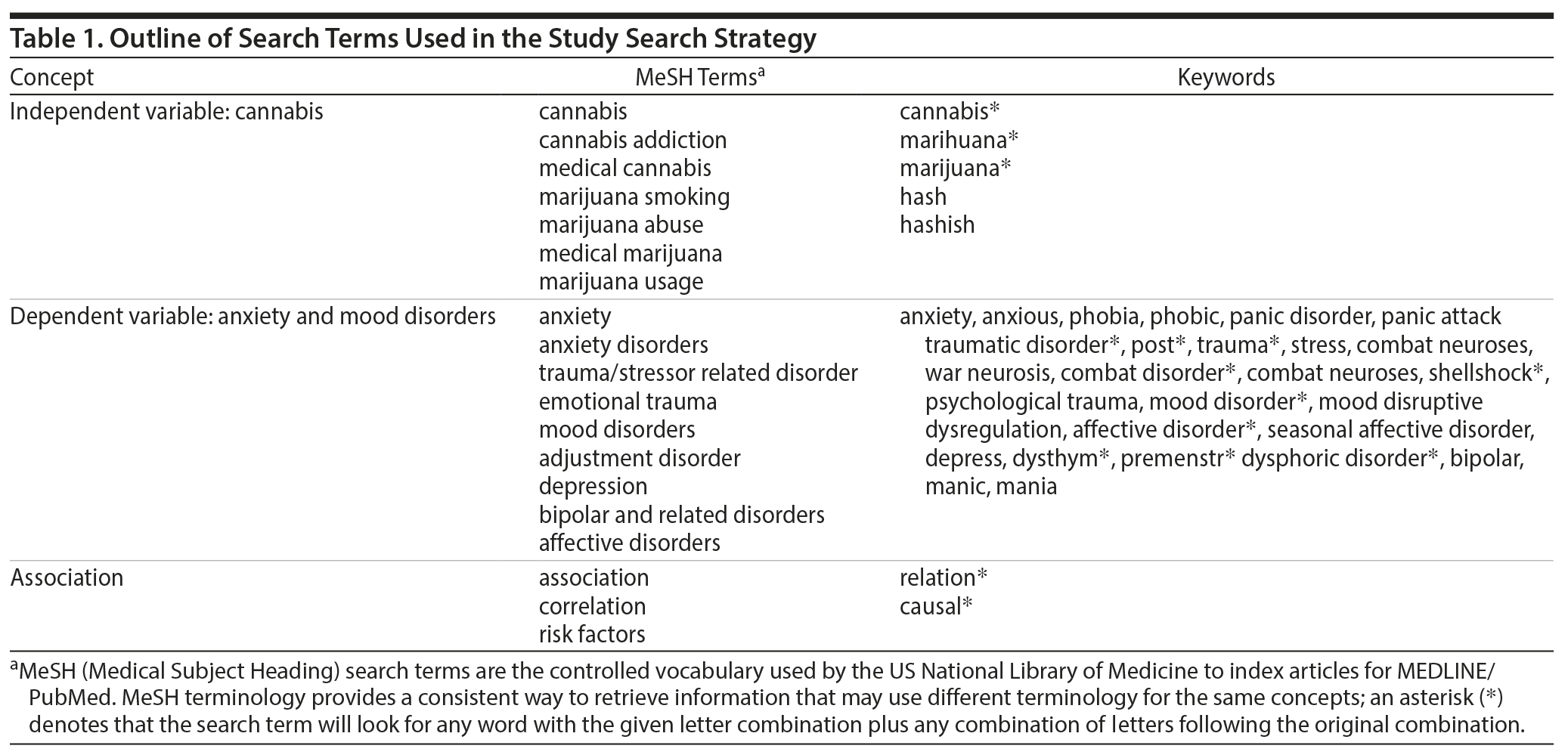
Data Sources: A search of the literature up to May 2017 was conducted using several databases. Search terms related to the exposure (ie, cannabis) and outcome (ie, symptoms) variables of interest. There were no search restrictions.
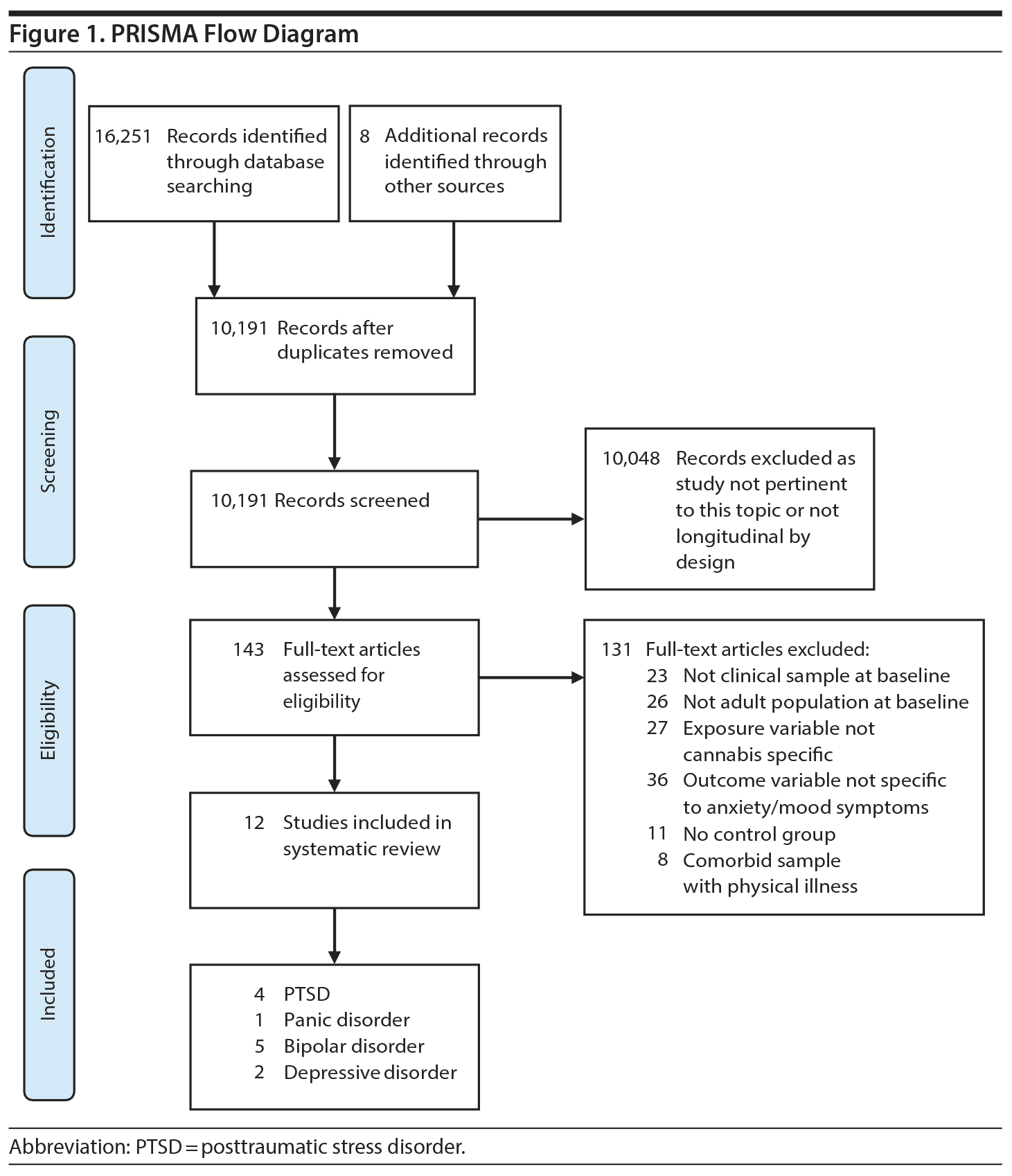
Study Selection: In total, 10,191 citations were screened. Key inclusion criteria related to (1) cohort-based longitudinal study design using adults who met criteria for a mood or anxiety disorder at baseline, (2) an independent variable focusing on at least baseline cannabis use, and (3) a dependent variable focusing on the symptomatic course and/or outcomes in anxiety and mood disorders (AMD).
Data Extraction: We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Methodological characteristics and key findings were extracted from each study, and quality assessments were conducted for each study.
Results: Twelve studies (with a total of 11,959 individuals) met inclusion criteria related to posttraumatic stress disorder (n = 4), panic disorder (n = 1), bipolar disorder (n = 5), and depressive disorder (n = 2). Across 11 studies, “recent” cannabis use (ie, any/greater frequency of use during the last 6 months) was associated with higher symptomatic levels over time relative to comparison groups (ie, no/lesser frequency of use). Ten of these studies further suggested that cannabis use was associated with less symptomatic improvement from treatment (eg, medication, psychotherapy for AMD).
Conclusions: Recent cannabis use was associated with negative long-term symptomatic and treatment outcomes across AMD. The findings should be interpreted with caution, considering the observational designs across studies and the biases associated with the samples (eg, inpatients) and sources of cannabis consumed (ie, unregulated sources). Nonetheless, clinicians can use the insight gained to inform their own and their patients’ knowledge concerning potential risks of cannabis with regard to symptoms of AMD.
Association of Cannabis With Long-Term Clinical Symptoms in Anxiety and Mood Disorders:
A Systematic Review of Prospective Studies

ABSTRACT
Objective: To systematically review studies examining the longitudinal associations between cannabis use and symptomatic outcomes among individuals with an anxiety or mood disorder at baseline.
Data Sources: A search of the literature up to May 2017 was conducted using several databases. Search terms related to the exposure (ie, cannabis) and outcome (ie, symptoms) variables of interest. There were no search restrictions.
Study Selection: In total, 10,191 citations were screened. Key inclusion criteria related to (1) cohort-based longitudinal study design using adults who met criteria for a mood or anxiety disorder at baseline, (2) an independent variable focusing on at least baseline cannabis use, and (3) a dependent variable focusing on the symptomatic course and/or outcomes in anxiety and mood disorders (AMD).
Data Extraction: We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Methodological characteristics and key findings were extracted from each study, and quality assessments were conducted for each study.
Results: Twelve studies (with a total of 11,959 individuals) met inclusion criteria related to posttraumatic stress disorder (n = 4), panic disorder (n = 1), bipolar disorder (n = 5), and depressive disorder (n = 2). Across 11 studies, “recent” cannabis use (ie, any/greater frequency of use during the last 6 months) was associated with higher symptomatic levels over time relative to comparison groups (ie, no/lesser frequency of use). Ten of these studies further suggested that cannabis use was associated with less symptomatic improvement from treatment (eg, medication, psychotherapy for AMD).
Conclusions: Recent cannabis use was associated with negative long-term symptomatic and treatment outcomes across AMD. The findings should be interpreted with caution, considering the observational designs across studies and the biases associated with the samples (eg, inpatients) and sources of cannabis consumed (ie, unregulated sources). Nonetheless, clinicians can use the insight gained to inform their own and their patients’ knowledge concerning potential risks of cannabis with regard to symptoms of AMD.
J Clin Psychiatry 2018;79(4):17r11839
To cite: Mammen G, Rueda S, Roerecke M, et al. Association of cannabis with long-term clinical symptoms in anxiety and mood disorders: a systematic review of prospective studies. J Clin Psychiatry. 2018;79(4):17r11839.
To share: https://doi.org/10.4088/JCP.17r11839
© Copyright 2018 Physicians Postgraduate Press, Inc.
aInstitute for Mental Health Policy Research, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
bLibrary Services, Centre for Addiction and Mental Health, Toronto, Ontario, Canada
cAddiction Medicine and Dual Disorders Clinic, Lev Hasharon Medical Center, Pardesya, Israel
dSackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
*Corresponding author: George Mammen, PhD, Institute for Mental Health Policy Research, Centre for Addiction and Mental Health, 33 Russell St, Toronto, ON, Canada, M5S 2W6 ([email protected]).
Cannabis is commonly used among individuals living with anxiety and mood disorders (AMD), which are the most prevalent mental health conditions globally.1 For instance, those diagnosed with generalized anxiety disorder, posttraumatic stress disorder (PTSD), bipolar disorder, and depressive disorders have higher rates of lifetime and recent use (eg, past year and month) than individuals without such psychiatric conditions,2-4 with around 20%-30% of users consuming cannabis daily.5-9 This frequent use has been discussed in the context of self-medication. In line with this, AMD lead mental health conditions in which cannabis is used for therapeutic purposes,10-12 as users report that cannabis relieves acute symptoms in PTSD (eg, reduces nightmares), bipolar disorder (eg, stabilizes mood), and depressive disorders (eg, increases motivation).13-18
However, whether cannabis positively or negatively influences long-term symptoms in AMD is highly debated and is an understudied area of research.19-22 The majority of longitudinal studies examining relationships between cannabis and psychiatric disorders have focused on the general population and the incidence of developing mental health conditions as predicted by cannabis use. Systematic reviews show that higher frequencies of use may increase risk in the onset of anxiety, depressive, and bipolar disorders,23-26 in addition to schizophrenia and psychoses.27 On the basis of this literature, mental health experts theorize that cannabis use most likely does not benefit long-term symptoms but rather exacerbates the course of illness.19,28
To the authors’ knowledge, no systematic review has focused on the longitudinal associations between cannabis use and AMD in a clinical population. Wilkinson and colleagues,29 in the Journal of Clinical Psychiatry, published a systematic review to determine the efficacy of cannabis in psychiatric indications. However, their review did not focus on AMD, as they examined PTSD, in addition to Alzheimer’s disease and Tourette’s syndrome. Narrowing the focus to AMD is warranted, particularly given the high prevalence of cannabis consumption in PTSD and other anxiety (eg, social anxiety disorder) and mood disorders (eg, major depressive disorder).2-9 Pursuing this aim can help address if cannabis is associated with negative or positive symptomatic outcomes, which clinicians can use as evidence to inform patients regarding potential long-term influence (eg, benefits, risks) of cannabis on AMD.
METHODS
To conduct this systematic review, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol was registered with PROSPERO (registration number: CRD42017037733).
Databases and Search Terms
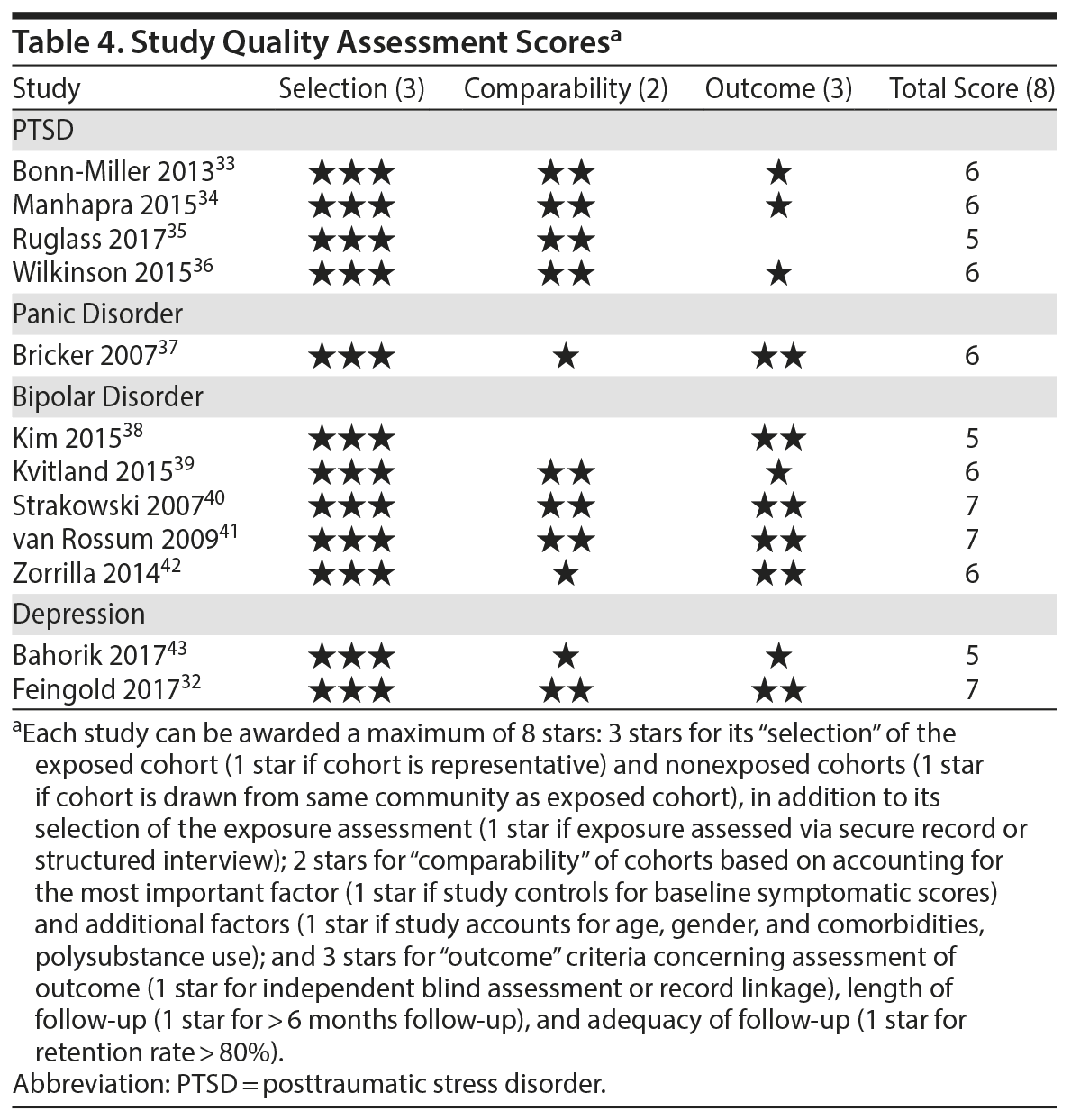
The following electronic databases were used for the literature search: Embase, MEDLINE, MEDLINE in Process and Other Non-Indexed Citations, MEDLINE Epub Ahead of Print, and PsycInfo. Databases including gray literature (ie, conference papers) were searched up until May 2017. There were no year, language, or study type restrictions. Search terms related to the exposure (ie, regulated and unregulated cannabis) and outcome (ie, AMD) variables of interest (Table 1). The search process was led by a professional health science librarian (S.B.).
Screening Process and Study Eligibility Criteria
The study screening and retrieval process was conducted in duplicate by 4 trained reviewers and documented by DistillerSR.30 Reference lists of relevant studies and literature reviews, in addition to “related articles” in electronic databases, were further examined. Meeting the inclusion criteria meant that the study (1) employed a cohort-based longitudinal design; (2) focused on adults (ie, 18+ years of age) meeting criteria for a mood or anxiety disorder at baseline (without comorbidities related to physical illness, schizophrenia, or psychoses), as determined by either clinician interviews or screening instruments with established cutoff thresholds; (3) assessed symptomatic course (operationalized as using multiple follow-up assessments in analysis) and/or symptomatic outcome (operationalized as using only 1 follow-up measure) as the dependent variable; (4) assessed at least baseline cannabis use as the independent variable (isolated cannabis without polysubstance use); and (5) included at least 1 comparison/control group.
Data Extraction and Quality Assessments
Methodological characteristics and key findings were extracted from each study. Authors of eligible studies were contacted to provide missing or additional data. To assess study quality, we used the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies,31 which examines quality via indicators related to sample recruitment, group comparability, and ascertainment of the exposure and outcome variables of interest. Two appraisers performed these assessments independently. Interrater agreement was assessed using the κ statistic (κ = 0.79), and any discrepancies were resolved with the coauthors of this review.
RESULTS
Retrieved Results
The search process yielded 16,251 citations. After deduplication, 10,191 titles/abstracts were screened, resulting in 12 included studies. Figure 1 displays the flow diagram and reasoning for study exclusions. Five anxiety disorder-based studies (PTSD, n = 4, panic disorder, n = 1) and 7 mood disorder-based studies (bipolar disorder, n = 5; depressive disorder, n = 2) were used in the synthesis, using data from a total sample of 11,959 individuals (2,588 individuals “more exposed” to cannabis, operationalized as any recent use or greater use; 9,371 individuals “less exposed” to cannabis, operationalized as no recent use or lesser use).
Study Characteristics and Quality
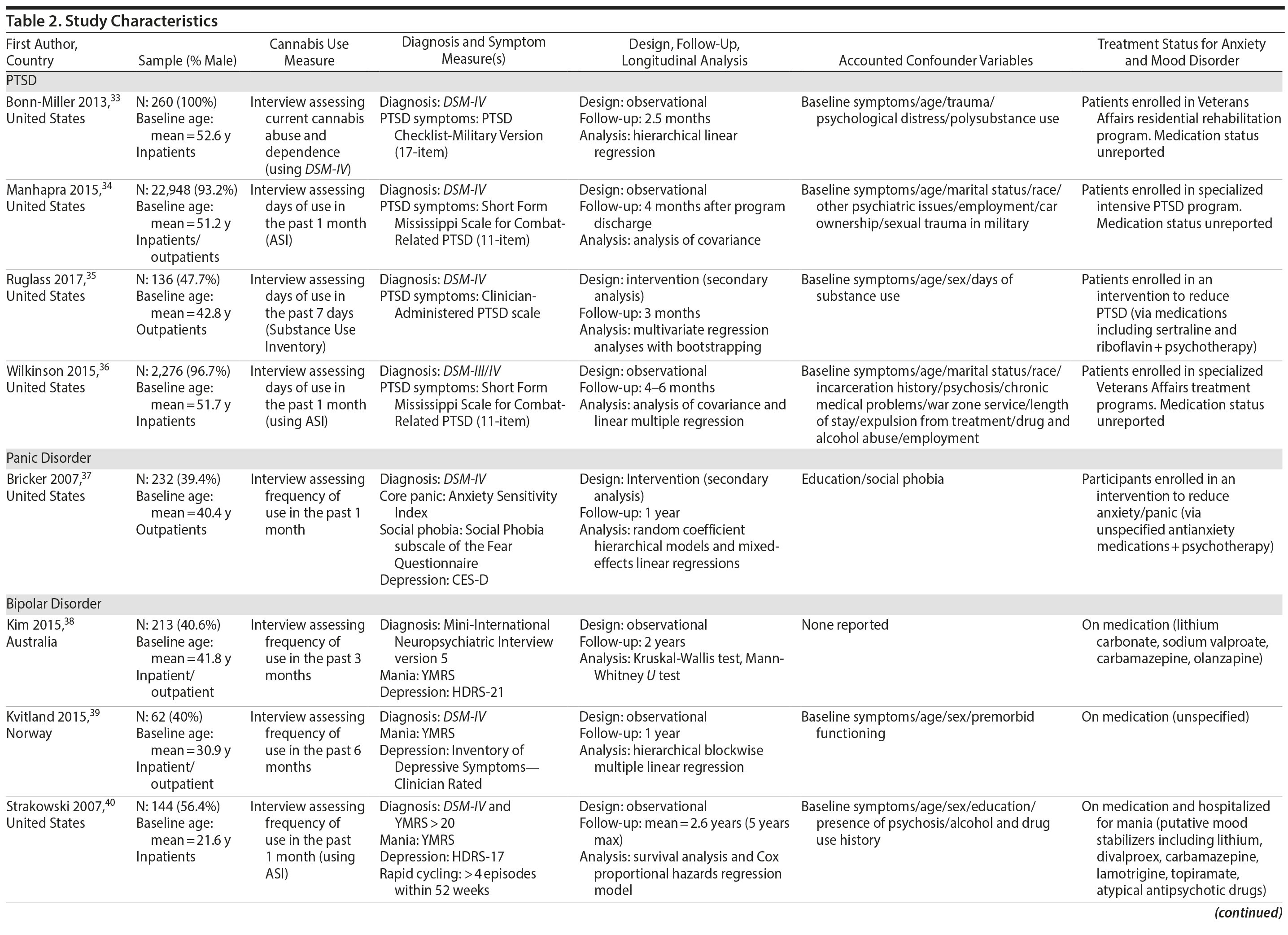
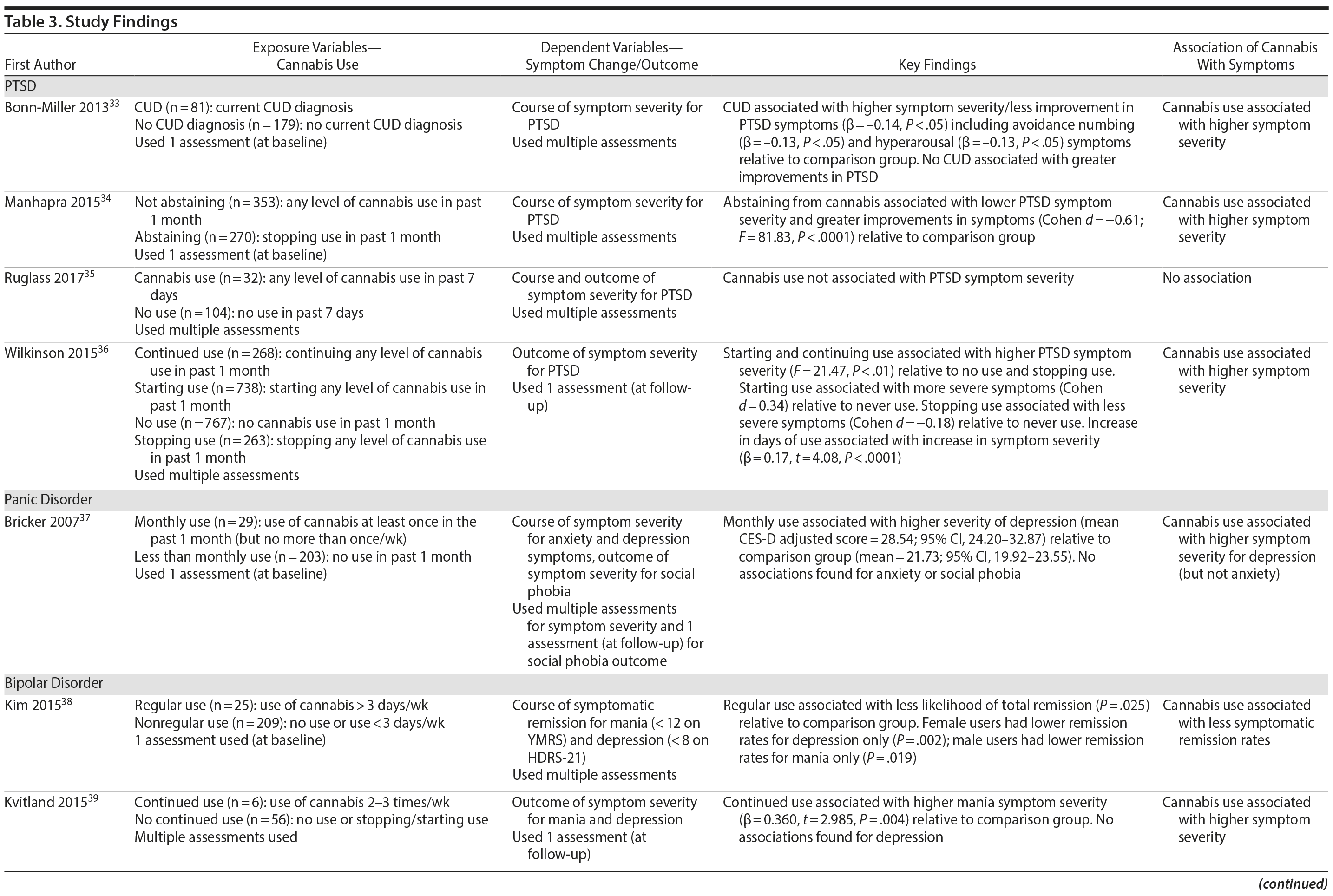
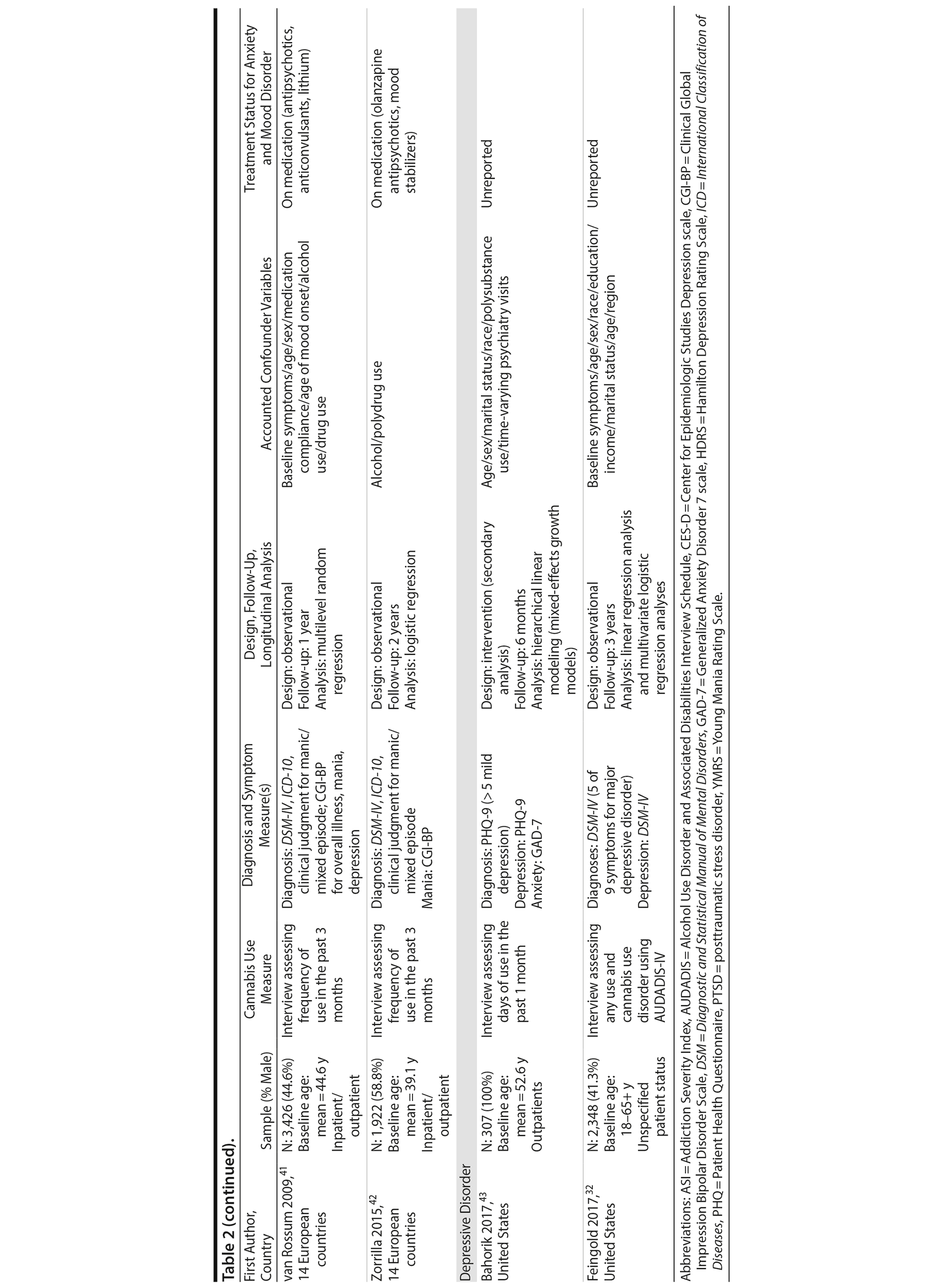
Tables 2 and 3 highlight methodological characteristics and findings, respectively, of each study. The majority of studies were based in the United States (n = 8), followed by Europe (n = 3) and Australia (n = 1). Study samples ranged from 62 to 22,948 individuals who qualified for an anxiety or mood disorder at baseline and were psychiatric-based patients (majority inpatients) receiving symptomatic treatment. The baseline age ranged from 18 to 65+ years, with the baseline mean age across the cohorts being close to 43 years. There was fairly even representation of genders across the bipolar, panic, and depressive disorder studies. Among the 4 PTSD studies, 3 were heavily focused on males (93%-100%). In terms of study design, 9 were observational cohort studies, and 3 were based from secondary analyses of interventions aimed at treating the anxiety or mood disorder. Follow-up periods ranged from 2.5 months to 5 years.
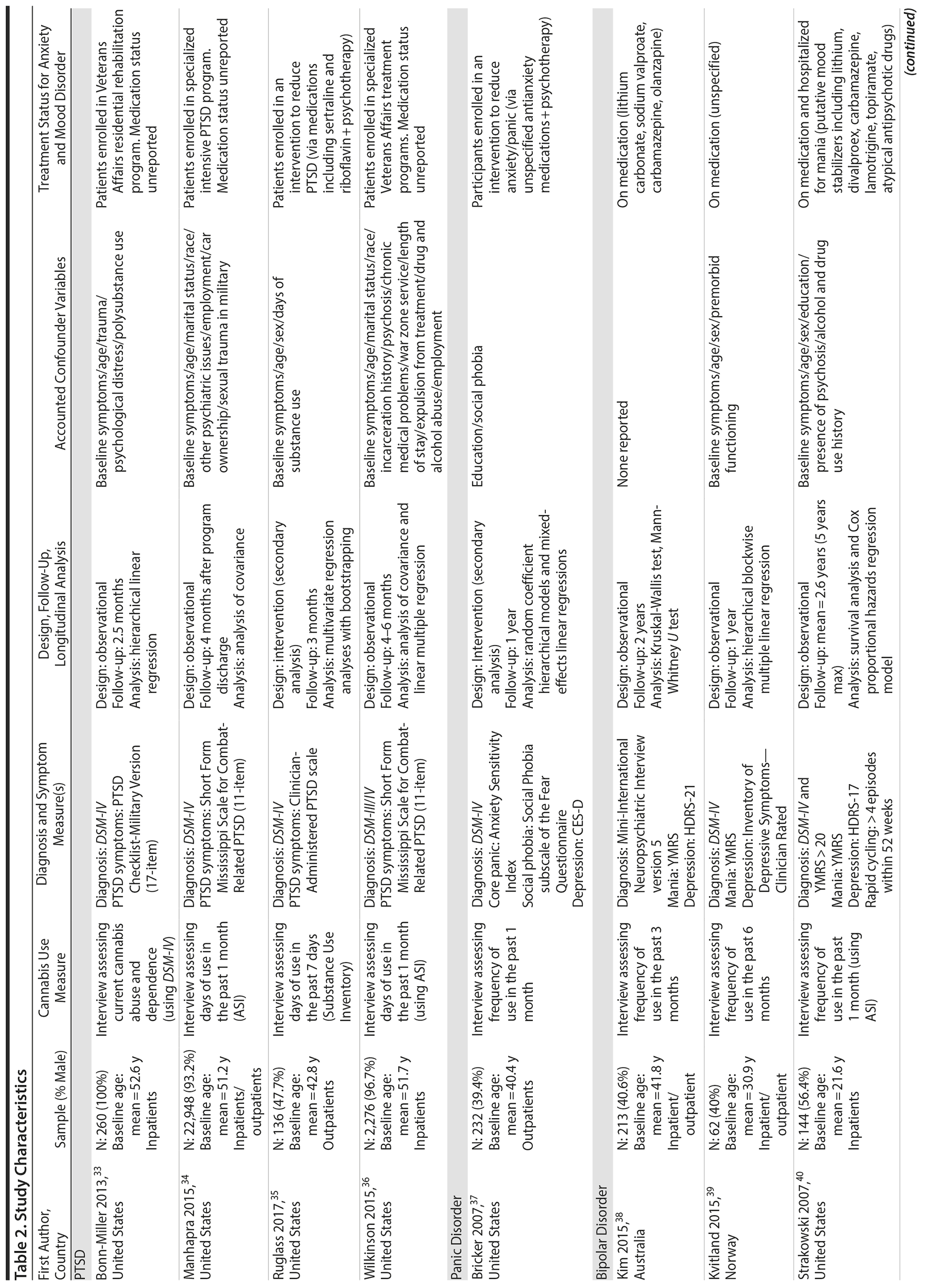
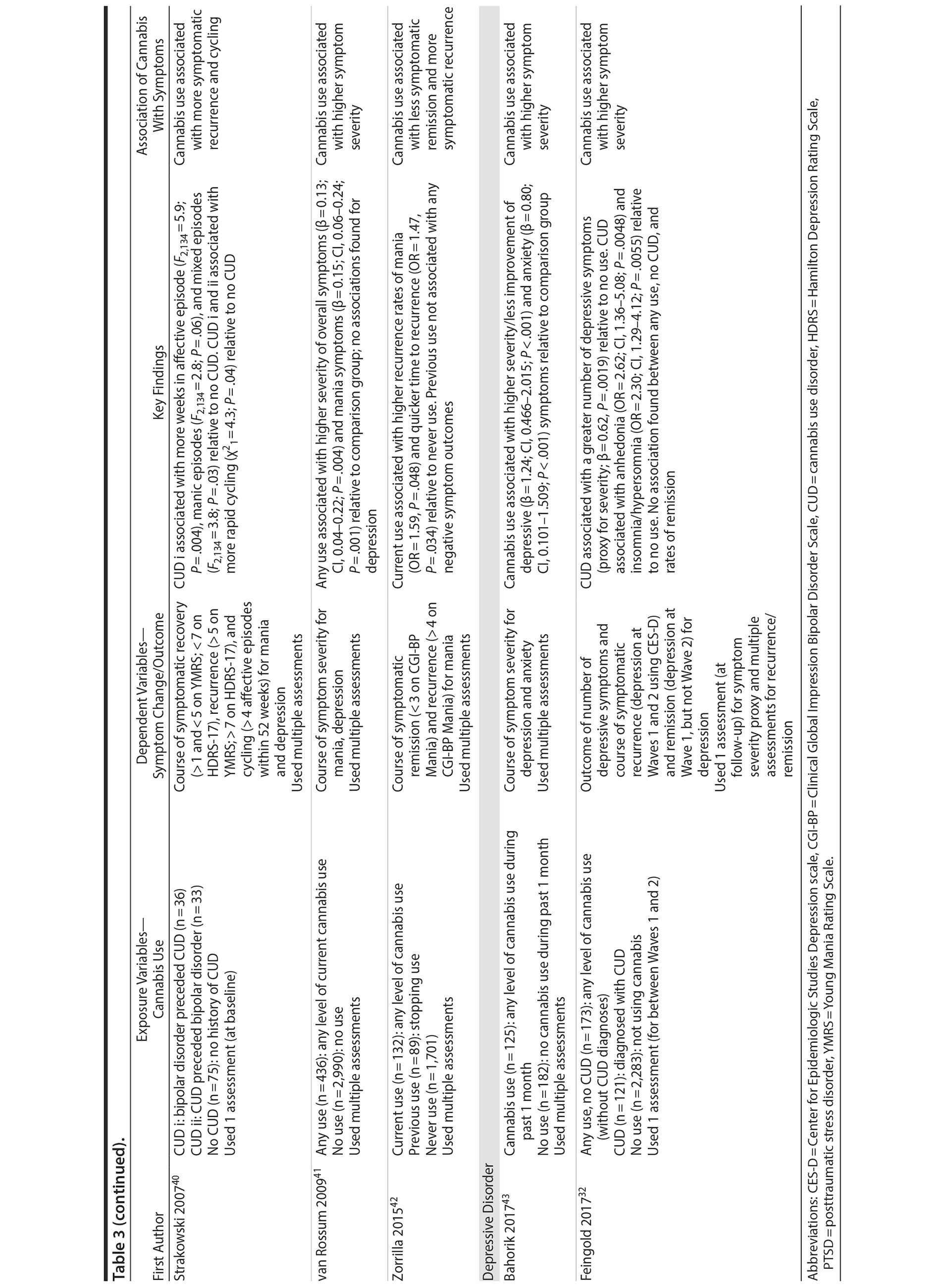
Cannabis use was assessed subjectively across studies, primarily using self-report measures via clinical interviews. All studies focused on frequency of recent use during the past 7 days, 1 month, 3 months, or 6 months. Six studies used only baseline assessments of cannabis use, and 6 studies used multiple assessments to help determine how changes in use (eg, reducing it) affect symptomatic outcomes. In terms of source of cannabis, all studies examined use of illicit “street cannabis” (ie, unregulated cannabis) in relation to outcomes of AMD.
The majority of studies (n = 10) used a version of the DSM (eg, –III, –IV) for diagnosing AMD. In terms of the dependent variable, various measures of symptoms were used across the AMD (see Table 2), though all but 1 study (ie, number of symptoms)32 used a scale of symptom severity. Two studies used only 1 follow-up outcome of symptoms, 7 studies used multiple follow-up measures of symptoms to help determine how cannabis affects the course of symptoms, and 3 studies measured both course and outcomes of symptoms.
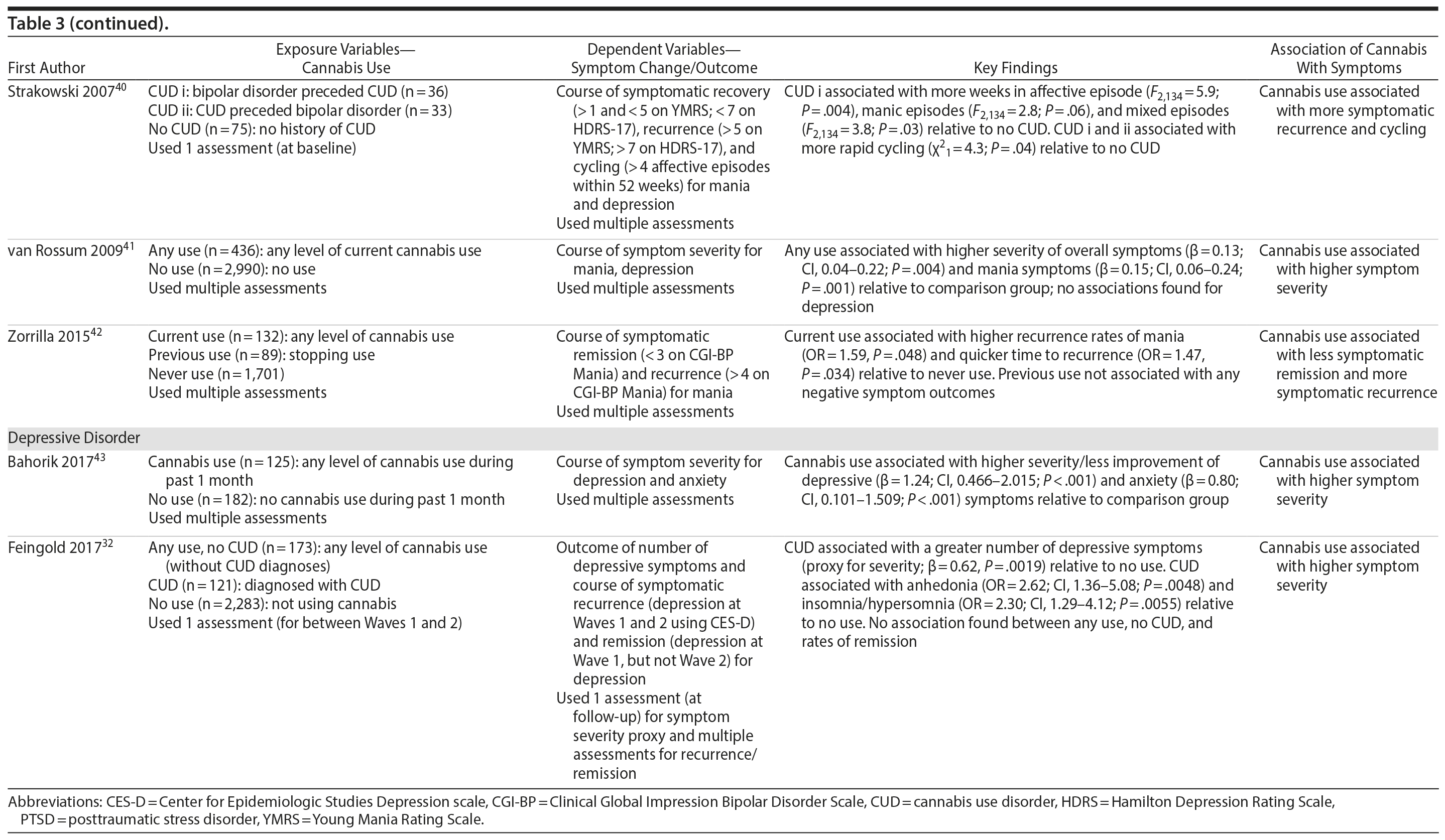
In terms of study quality (Table 4), out of a possible 8 stars, 3 studies were rated 7 stars, 6 studies were rated 6 stars, and 3 studies were rated 5 stars, suggesting that the majority of studies can be considered of good overall methodological rigor. Specifically, all studies received maximum stars for cohort representativeness and assessment of exposure, and most studies received maximum stars for the following: controlling for baseline symptoms (n = 9) and multiple additional confounding variables in final statistical models (n = 10) (eg, age, sex, comorbidities, polysubstance use); conducting follow-up assessments 1 year after baseline (n = 7); and having high retention rates (> 80%; n = 10). Detailed scoring of each study is available upon request.
Cannabis and PTSD or Panic Disorder
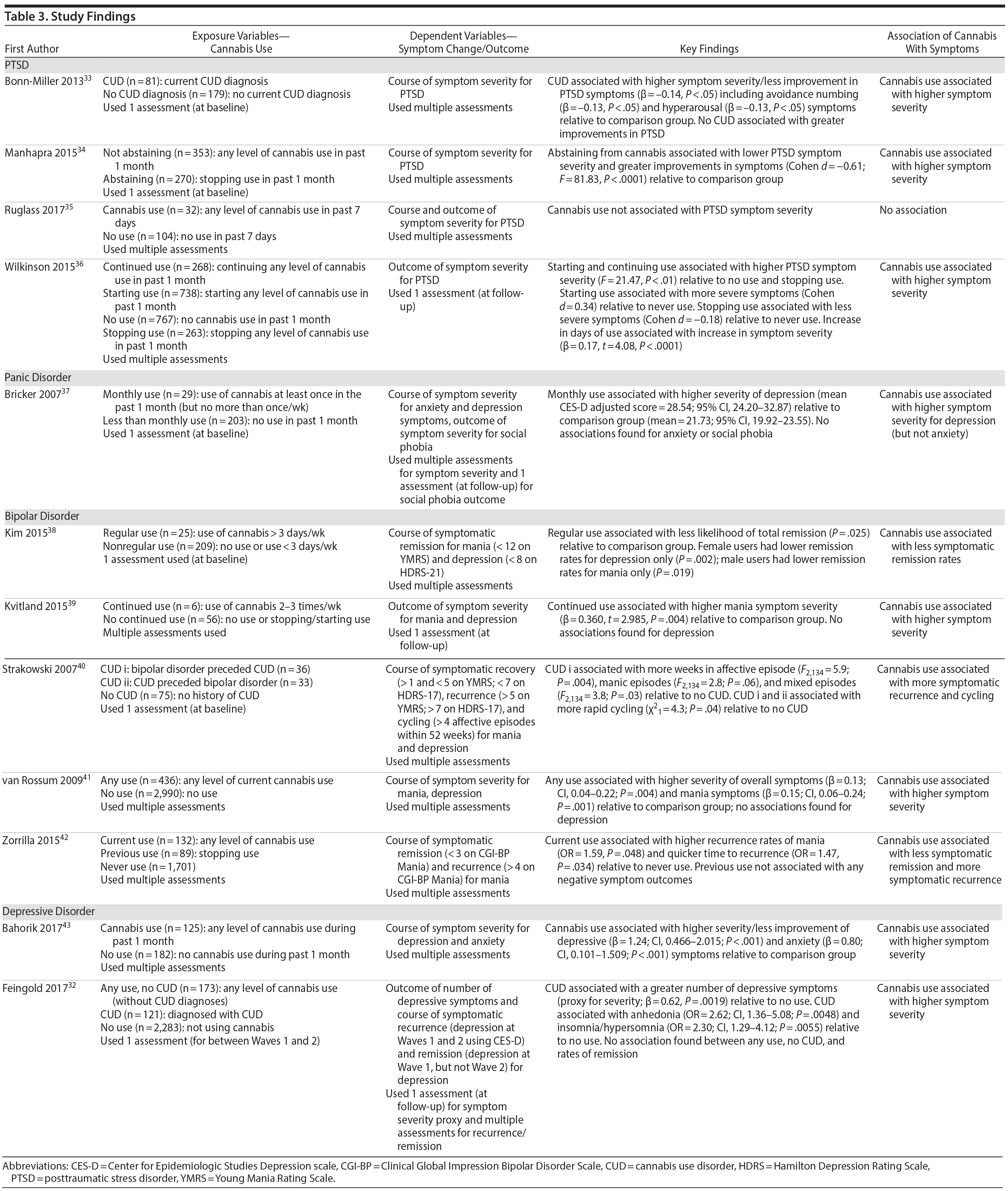
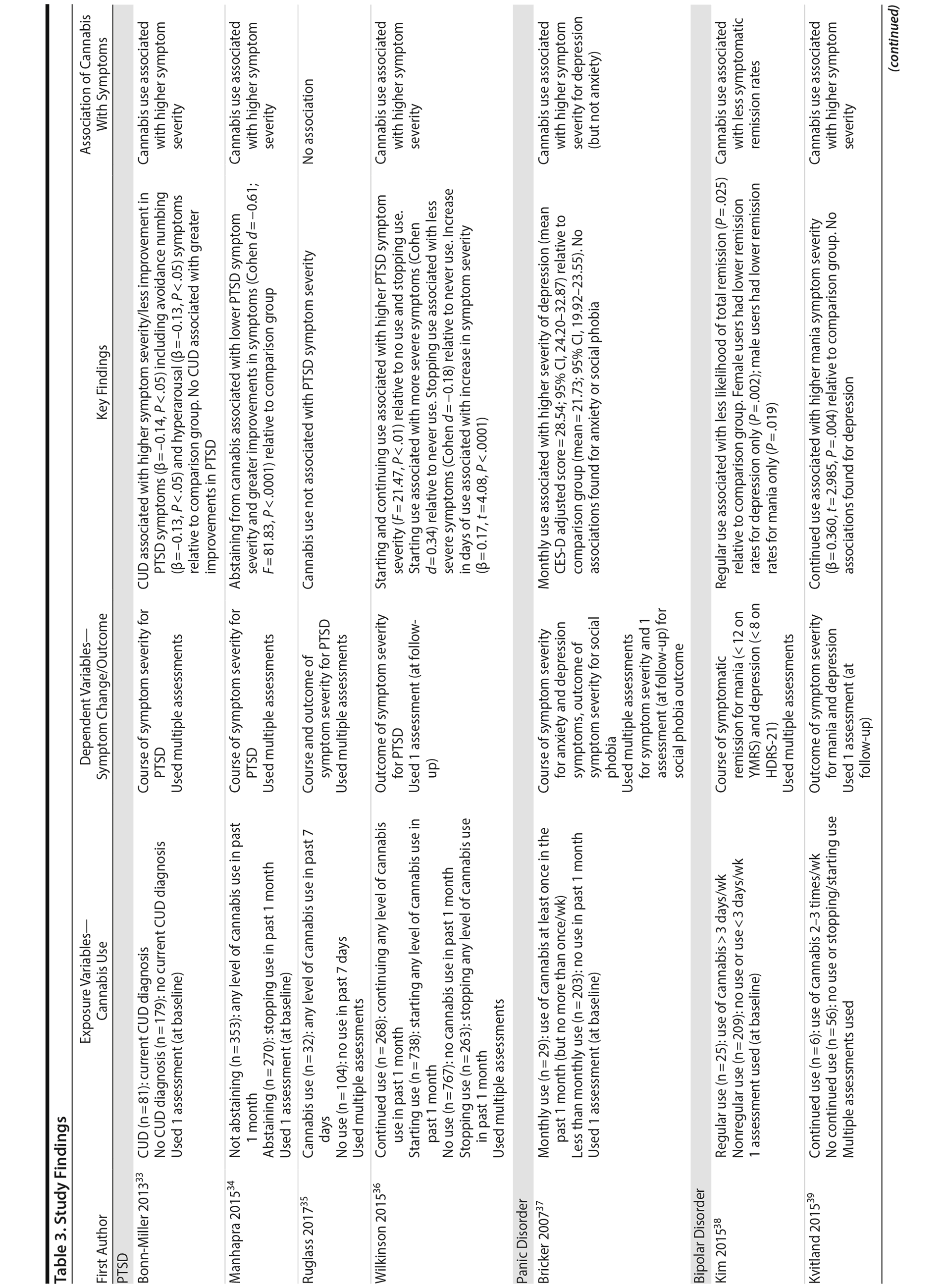
Four studies examined the association of cannabis with long-term symptoms in PTSD.33-36 Three studies collectively showed that recent cannabis use (eg, past month) was associated with a negative course33,34 and negative outcomes36 of PTSD symptom severity, while 1 study found no significant relationships (note that this study included only 32 individuals in the “exposure” group).35 Among the significant findings, 2 studies found that “any” level of baseline cannabis use (F = 81.83, P < .0001)34 or sustained use over time (F = 21.47, P < .01)36 was associated with greater PTSD symptom severity 4 months following baseline assessments, compared to abstinence. These studies also supported that stopping use is associated with less severe symptoms (Cohen d = −0.18)36 and greater improvements in symptoms from treatment (Cohen d = −0.61)34 compared to continuing or starting cannabis use. Bonn-Miller and colleagues33 revealed that a baseline current cannabis use disorder (CUD) diagnosis was associated with less improvement from treatment regarding PTSD symptoms (β = -0.14, P < .05) over the course of 2.5 months, relative to the comparison group (ie, no CUD), specifically concerning avoidance numbing (β = -0.13, P < .05) and hyperarousal (β = -0.13, P < .05) symptoms. In these 3 studies finding significance, it is worth noting that all participants were predominantly men who were enrolled in a Veterans Affairs residential rehabilitation program to help treat their combat-related PTSD. Hence, the findings may not be generalizable to females experiencing non-combat-related PTSD, for instance.
One study examined the association of cannabis with symptoms in panic disorder, using secondary data from a trial aimed to reduce symptoms via medication and psychotherapy. Bricker et al37 showed that, in the intervention group, there were no differences between monthly users and less than monthly users in terms of symptomatic outcomes over 1 year, suggesting that cannabis use did not impede recovery. Within the control group not receiving the intervention, however, monthly cannabis use (Center for Epidemiologic Studies Depression Scale [CES-D] adjusted mean = 28.54, P < .01) was associated with higher levels of depressive symptoms over 1 year compared to less than monthly use (CES-D adjusted mean = 21.73, P < .01). No associations were found for core panic symptoms or social phobia symptoms. The limited findings in this study could be due to the small sample of monthly users (ie, 29 individuals) and the focus on lower frequencies of use (ie, using cannabis at least once in the past month but no more than once per week was operationalized as monthly use).
Cannabis and Bipolar Disorder
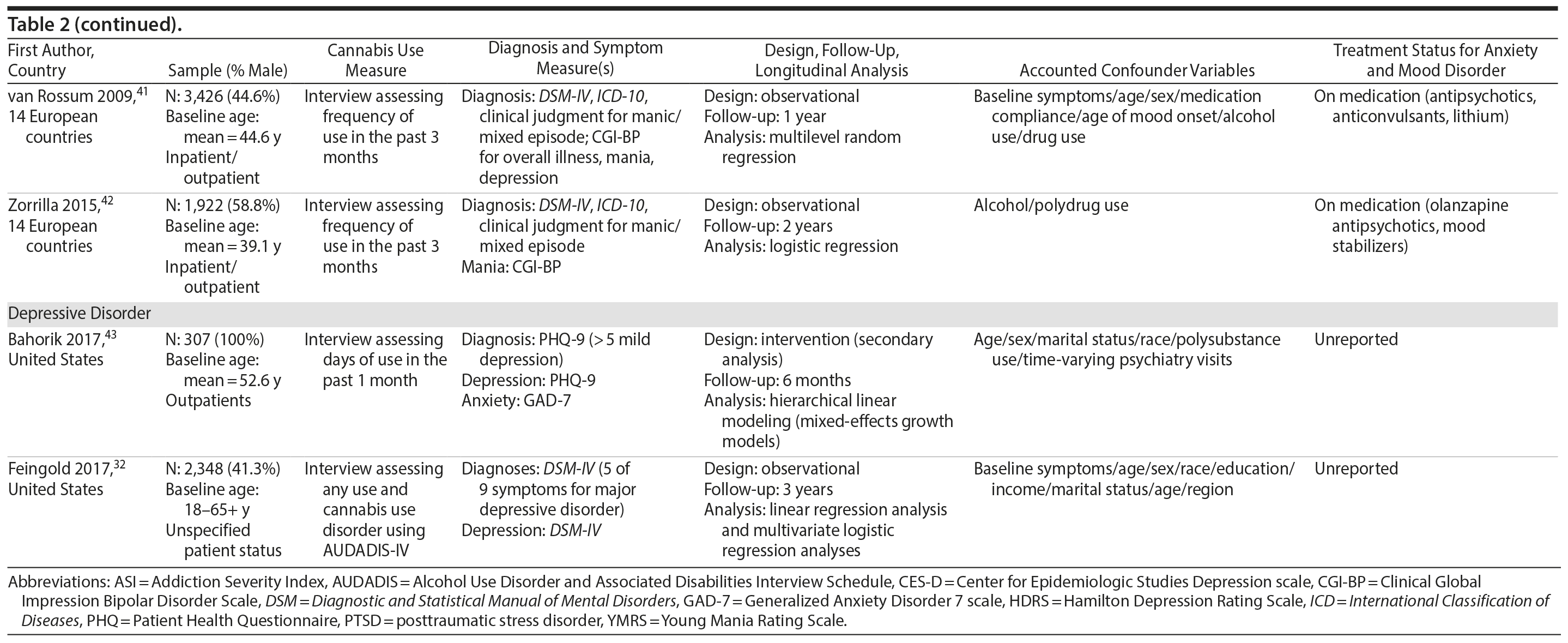
Five studies examined the association of cannabis with long-term symptoms in bipolar disorder.38-42 Each study provided indication that recent cannabis use (eg, past month) was associated with negative symptomatic outcomes. For instance, 2 studies revealed that “any recent use” of cannabis (eg, 2-3 times per week) over 1 year was associated with greater symptom severity for mania throughout the course of 1 year (β = 0.15, P = .001)41 and at 1-year follow-up39 compared to no use or stopping use. Both studies found no significant associations related to depressive symptoms, likely attributed, as the authors note, to the focus on patients with more severe baseline mania than depression.
The remaining 3 studies38,40,42 collectively showed that cannabis use was associated with greater symptom severity, as measured through thresholds concerning symptomatic remission, recovery, and recurrence for mania and depressive symptoms. Specifically, Kim and colleagues38 showed that baseline “regular” use (ie, > 3 days/week) was associated with less occurrence of remission for mania and depressive symptoms throughout the course of 2 years compared to nonregular use (ie, < 3 days/week). Aligned with this finding, Zorrilla et al42 showed that continued use over 1 year (ie, any use) was associated with higher recurrence rates of mania (OR = 1.59, P = .048) and quicker time to recurrence (OR = 1.47, P = .034) over the course of 2 years, relative to never use. Zorrilla and colleagues’ study further suggested that stopping cannabis use was associated with more favorable symptomatic outcomes over time. Lastly, Strakowski and colleagues40 found that a baseline CUD diagnosis was associated with more weeks spent in an affective episode (F2,134 = 5.9; P = .004), more time in mixed episodes (F2,134 = 3.8; P = .03), and more rapid cycling between episodes over the course of up to 5 years compared to having no CUD diagnosis. All bipolar disorder studies were conducted in the context of symptomatic treatment including medication and inpatient care, which revealed that cannabis use was associated with less improvement from treatment.
Cannabis and Depressive Disorder
Two studies examined the association of cannabis with long-term symptoms in depressive disorders. Both studies revealed that cannabis use was associated with higher symptom severity43 and the number of symptoms.32 Specifically, Feingold and colleagues32 showed that a CUD diagnosis between baseline and follow-up was associated with a greater number of depressive symptoms (β = 0.62, P = .0019), specifically related to anhedonia (OR = 2.62; P = .0048) and insomnia/hypersomnia (OR = 2.30 P = .0055) at 3-year follow-up relative to no use. Bahorik and colleagues43 showed that continued cannabis use was associated with greater symptom severity and less symptomatic improvement for depressive (β = 1.24, P < .001) and anxiety symptoms (β = 0.80, P < .001) over the course of 6 months relative to no use. This study was in the context of cognitive behavior therapy, suggesting that cannabis use may potentially interfere with psychotherapy effectiveness.
DISCUSSION
This review provides consistent evidence that—among individuals living with a baseline PTSD, panic disorder, bipolar disorder, or depressive disorder—recent cannabis use was associated with negative symptomatic outcomes (including course of symptoms) over time. Specifically, the collective findings suggest that individuals using cannabis (ie, any/greater frequency of use in the last 6 months) experienced greater symptom severity and number of symptoms and less occurrence of symptomatic remission and recovery up to 5 years following baseline assessment relative to the comparison groups (ie, no/lesser frequency of use). All but 1 study32 across the review was in the context of treatment for an anxiety or mood disorder (eg, medication, psychotherapy), implying that cannabis use may potentially interfere with recovery efforts and contribute to long-term persistent symptoms.
These results are supported by the broader substance use literature inferring a detrimental effect of various substances (eg, alcohol, tobacco) on the clinical course and treatment outcomes in anxiety, depressive, and bipolar disorders.44-46 Although cannabis is considered less harmful than most psychoactive substances,47,48 the results nonetheless support its link to negative symptomatic outcomes in AMD. Specific to the cannabis literature, the results support studies (ineligible for inclusion in the current review; eg, no comparison groups, not longitudinal) showing that cannabis users with AMD experience “negative” symptoms,49,50 psychological distress,51 and a low quality of life52 and that reducing use may benefit symptoms.53 The evidence from the general population, which shows cannabis use to increase the risk in developing AMD over time23-26 and other adverse mental health effects,54 further supports association of cannabis with negative symptomatic outcomes in a clinical population.
Our review provides no indication that cannabis benefits AMD over time. This finding opposes other studies (ineligible for inclusion) suggesting that cannabis can play a role in alleviating symptoms in PTSD (eg, reduces nightmares), bipolar disorder (eg, stabilizes mood), and depressive disorders (eg, increases motivation).13-18,55,56 However, these studies are primarily based on acute therapeutic effects of cannabis. Mechanistically, acute effects of cannabis are mediated by the human endocannabinoid system.57 This homeostatic system serves to regulate mood, cognition, appetite, and sleep, among other functions, by the interactions between endogenous cannabinoids (anandamide and 2-arachidonoylglycerol) and G-protein cannabinoid receptors (CB1, CB2).58 When cannabis is consumed, its constituents such as tetrahydrocannabinol (THC) and cannabidiol (CBD), which structurally resemble the mentioned endogenous cannabinoids, activate the endocannabinoid system by reacting with the brain’s CB1 receptors. This mechanism is the basis by which cannabis facilitates a “high” effect, emulating antianxiety and antidepressive states in some individuals.
Particularly among individuals living with AMD, research shows there are deficiencies in cannabinoid production and signaling dysfunctions within the endocannabinoid system that may contribute to the disorder.59-63 Targeting this system has therefore been recommended to help treat AMD62,63 by helping to synthesize endocannabinoids, regulate signaling, and overall facilitate the endocannabinoid system. However, whether exogenous cannabinoids, such as THC, can intervene in the endocannabinoid system to sustainably improve related symptoms is controversial and understudied. Based on the literature and mechanisms explaining acute effects of cannabis, in conjunction with the review’s longitudinal-associative findings, it can be speculated that cannabis may serve as a “Band-Aid” strategy to relieve acute symptoms, but over time the drug may contribute to persistent symptoms and the prevention of symptomatic recovery.
Study Limitations and Future Research
Despite the consistent results of cannabis’s association with negative long-term symptomatic and treatment outcomes, the review’s findings need to be interpreted with caution when considering the individual studies’ methodological limitations in conjunction with the broader limitations of the systematic review. First, the observational designs across studies disallow causal inferences to be made between cannabis use and persisting symptoms of AMD. Though the review found a consistent longitudinal association between cannabis use and symptoms in AMD, the conclusion that cannabis can negatively influence the course and outcomes of symptoms and treatment efforts over time can only be speculative. Second, the review’s findings may be biased toward a sample with a higher severity of symptoms, as the large majority of the total sample (11,959 individuals) included psychiatric inpatients and outpatients. Including individuals with varying degrees of symptom severity can provide further knowledge on the influence of cannabis in AMD, particularly among those not needing psychiatric intervention (ie, lower severity).
Third, unaccounted confounding variables around cannabis consumption in each study may have further biased the results. For example, the earlier age at onset for cannabis use significantly increases risk in developing AMD in addition to cannabis dependence.64 Hence, the age at onset could have mediated the association between cannabis use and negative symptomatic outcomes.
Further, none of the studies captured the dose of cannabis consumed, but rather the broader, subjective frequency of recent use, which is subject to recall and social desirability biases. Within frequency of use, there was heterogeneity in the definitions and operationalizations of individuals “exposed” and “nonexposed” to cannabis. For example, the exposed groups in multiple studies may have included those who use cannabis daily, in addition to those who used cannabis “at least once in the past month,” whereas the nonexposed groups in multiple studies may have included those who have never used cannabis with those who may use cannabis at lower frequencies. Distinguishing these users in analysis, among low, moderate, and heavy/daily consumption, for example, is important since the adverse psychological effects of cannabis are considered to be frequency and dose dependent.57,65
Regarding frequency and dose, it is imperative to also collect information on the concentrations of cannabinoids consumed (eg, THC). A proxy for this in the review was based on the source of cannabis used, which were unregulated-illicit sources. Over the last several decades, “street cannabis” has contained increasingly potent levels of THC and decreases in CBD production.66 Researchers have noted that the drug’s effects and risks can depend upon the potency and dose of THC and its ratio with CBD.57,65 At higher levels, THC—the primary and psychoactive constituent of cannabis responsible for the “high”—may overstimulate CB1 receptors and contribute to adverse effects including increased acute anxiety, paranoia, memory impairment, and sedation and subsequent addiction issues related to withdrawal and tolerance.58,67 Thus, the frequency of cannabis use among a clinical population (eg, daily)68,69 and the increasingly potent THC levels in street cannabis may help explain the review’s findings of cannabis’s association with negative symptomatic and treatment outcomes.
Interestingly, emerging evidence shows that THC’s adverse effects can be limited by CBD, which is cannabis’s secondary and nonpsychoactive constituent that has been bred out of street cannabis. Unlike THC, CBD does not yield a “high” experience, is perceived as having limited side effects, and is generally well tolerated across doses.70 One role of CBD is to mitigate THC’s effects, mechanistically explained by its indirect antagonist actions71,72 and low affinity for CB1 receptors.73 This action helps prevent THC from acting at full strength.74,75 Independent of THC, CBD further contains its own antianxiety properties involving mechanisms with other mood regulatory receptors (eg, GPR55, 5-HT1A).76 Hence, due to its nonpsychoactive property, safety and tolerability, and encouraging evidence as an antianxiety drug, “CBD is possibly the cannabinoid more likely to have initial findings translated into clinical practice.”70(p1224)
Fourth, there was further heterogeneity regarding the outcome assessments. For instance, the symptoms across AMD were measured and operationalized by severity, remission, recurrence, recovery, relapse, cycling, or number of symptoms. Compounding the heterogeneity was the review’s inclusion of individual AMD in PTSD, panic disorder, bipolar disorder, and depressive disorder. This decision was based on the limited number of studies available for each disorder, and it limited the ability to conduct a meaningful meta-analysis. Of notice was the lack of cohort studies on generalized anxiety disorder (n = 0), social anxiety disorder (n = 0), and depressive disorders (n = 2). Considering that AMD are the most common mental health conditions,1 with strong links to frequent cannabis use,2-9 more monitoring of consumption and symptoms within each disorder is warranted.
Overall, future research in this area needs to address the limitations of the current literature by increasing methodological rigor to better ascertain the influence of cannabis on the course and outcomes of symptoms in AMD. In addition to accounting for the potential confounding variables noted above (eg, age at onset of use, dose of cannabinoid concentration), greater focus is needed in examining changes in severity scale scores between baseline and follow-up assessments. These data would help determine if cannabis “worsens” symptoms over time within subjects, as opposed to the current review, which moreover indicates that cannabis users are more likely to experience greater symptom severity over time compared to those abstaining from use. Additionally, clinical trials are needed to rigorously examine cannabis’s short- and long-term medical application for AMD, with specifics on cannabinoid concentrations (eg, THC, CBD), route of administration (eg, pill form, vaporization), dosage (eg, low vs moderate), different forms of cannabis (eg, oils, dried cannabis), interactions with medications (eg, antianxiety drugs), and mechanisms (eg, endocannabinoid system functional magnetic resonance imaging studies).
Clinical Implications
Though the findings of the review have clear limitations, this systematic review is the first to provide unique insight into the longitudinal associations between cannabis use and symptomatic outcomes among those living with a baseline anxiety or mood disorder. Clinicians can use this “best available” evidence to inform their own and their patients’ knowledge concerning potential long-term risks of cannabis on symptoms and recovery. The results can be useful for health care professionals (eg, psychiatrists, family doctors, nurse practitioners) who are asked to prescribe medical cannabis from patients living with AMD. With increasing legalization of recreational cannabis in North America (eg, Canada in 2018), communicating this evidence to patients requesting medical cannabis is timely and important when one considers they will arguably have easier and quicker access to regulated recreational cannabis than regulated medical cannabis.
CONCLUSION
Across AMD, recent cannabis use was associated with negative symptomatic and treatment outcomes over time. The findings should be interpreted with caution when considering the observational designs across studies, biases linked with the samples (eg, inpatients) and sources of cannabis consumed (ie, unregulated sources), and limitations surrounding the heterogeneity in exposure and outcome measurements. This review can inform future research to provide more rigorous data to better ascertain cannabis’s influence on the course and outcomes of symptoms in AMD. Clinicians can use the insight gained from the review to help inform their own and their patients’ knowledge concerning potential risks of cannabis on long-term symptoms and recovery.
Submitted: August 1, 2017; accepted November 3, 2017.
Published online: June 5, 2018.
Potential conflicts of interest: None.
Funding/support: This review was conducted when Dr Mammen was a postdoctoral fellow funded by the Centre for Addiction and Mental Health (CAMH) Fellowship Program.
Role of the sponsor: CAMH had no role in the conduct or publication of the study.
Acknowledgments: The authors acknowledge and thank Eva Pila, PhD (University of Toronto, Toronto, Ontario, Canada); Rose Wang, MSc (Lakehead University, Thunder Bay, Ontario, Canada); and Tereza Florica, BSc (McMaster University, Hamilton, Ontario, Canada), all of whom reported no conflict of interests, for their valuable contributions toward the study screening and retrieval processes.
REFERENCES
1. Whiteford HA, Ferrari AJ, Degenhardt L, et al. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820. PubMed CrossRef
2. Blanco C, Hasin DS, Wall MM, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry. 2016;73(4):388-395. PubMed CrossRef
3. Lev-Ran S, Le Foll B, McKenzie K, et al. Cannabis use and cannabis use disorders among individuals with mental illness. Compr Psychiatry. 2013;54(6):589-598. PubMed CrossRef
4. Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the US population. Drug Alcohol Depend. 2011;119(1-2):28-36. PubMed CrossRef
5. Bonn-Miller MO, Harris AH, Trafton JA. Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol Serv. 2012;9(4):404-416. PubMed CrossRef
6. Cheung JT, Mann RE, Ialomiteanu A, et al. Anxiety and mood disorders and cannabis use. Am J Drug Alcohol Abuse. 2010;36(2):118-122. PubMed CrossRef
7. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25(3):554-558. PubMed CrossRef
8. Gentes EL, Schry AR, Hicks TA, et al. Prevalence and correlates of cannabis use in an outpatient VA posttraumatic stress disorder clinic. Psychol Addict Behav. 2016;30(3):415-421. PubMed CrossRef
9. Hunt GE, Malhi GS, Cleary M, et al. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349. PubMed CrossRef
10. Hazekamp A, Ware MA, Muller-Vahl KR, et al. The medicinal use of cannabis and cannabinoids: an international cross-sectional survey on administration forms. J Psychoactive Drugs. 2013;45(3):199-210. PubMed CrossRef
11. Ogborne AC, Smart RG, Weber T, et al. Who is using cannabis as a medicine and why: an exploratory study. J Psychoactive Drugs. 2000;32(4):435-443. PubMed CrossRef
12. Walsh Z, Callaway R, Belle-Isle L, et al. Cannabis for therapeutic purposes: patient characteristics, access, and reasons for use. Int J Drug Policy. 2013;24(6):511-516. PubMed CrossRef
13. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162-165. PubMed CrossRef
14. Denson TF, Earleywine M. Decreased depression in marijuana users. Addict Behav. 2006;31(4):738-742. PubMed CrossRef
15. Elliott L, Golub A, Bennett A, et al. PTSD and cannabis-related coping among recent veterans in New York City. Contemp Drug Probl. 2015;42(1):60-76. PubMed CrossRef
16. Gruber AJ, Pope HG Jr, Brown ME. Do patients use marijuana as an antidepressant? Depression. 1996;4(2):77-80. PubMed CrossRef
17. Healey C, Peters S, Kinderman P, et al. Reasons for substance use in dual diagnosis bipolar disorder and substance use disorders: a qualitative study. J Affect Disord. 2009;113(1-2):118-126. PubMed CrossRef
18. Osborn LA, Lauritsen KJ, Cross N, et al. Self-medication of somatic and psychiatric conditions using botanical marijuana. J Psychoactive Drugs. 2015;47(5):345-350. PubMed CrossRef
19. Haney M, Evins AE. Does cannabis cause, exacerbate or ameliorate psychiatric disorders? an oversimplified debate discussed. Neuropsychopharmacology. 2016;41(2):393-401. PubMed CrossRef
20. Khoury JM, Neves MCLD, Roque MAV, et al. Is there a role for cannabidiol in psychiatry? World J Biol Psychiatry. 2017;1-16. PubMed
21. Turna J, Patterson B, Van Ameringen M. Is cannabis treatment for anxiety, mood, and related disorders ready for prime time? Depress Anxiety. 2017;34(11):1006-1017. PubMed CrossRef
22. Walsh Z, Gonzalez R, Crosby K, et al. Medical cannabis and mental health: A guided systematic review. Clin Psychol Rev. 2017;51:15-29. PubMed CrossRef
23. Gibbs M, Winsper C, Marwaha S, et al. Cannabis use and mania symptoms: a systematic review and meta-analysis. J Affect Disord. 2015;171:39-47. PubMed CrossRef
24. Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population—a meta-analysis of 31 studies. BMC Psychiatry. 2014;14(1):136. PubMed CrossRef
25. Lev-Ran S, Roerecke M, Le Foll B, et al. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44(4):797-810. PubMed CrossRef
26. Twomey CD. Association of cannabis use with the development of elevated anxiety symptoms in the general population: a meta-analysis. J Epidemiol Community Health. 2017;71(8):811-816. PubMed CrossRef
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319-328. PubMed CrossRef
28. Johns A. Psychiatric effects of cannabis. Br J Psychiatry. 2001;178(2):116-122. PubMed CrossRef
29. Wilkinson ST, Radhakrishnan R, D’ Souza DC. A systematic review of the evidence for medical marijuana in psychiatric indications. J Clin Psychiatry. 2016;77(8):1050-1064. PubMed CrossRef
30. DistillerSR [computer program]. Ottawa, Ontario, Canada: Evidence Partners; 2011.
31. Wells G, Shea B, O’ Connell D, et al. Newcastle-Ottawa Quality Assessment Scale, Cohort Studies. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 2014 .
32. Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: a population based longitudinal study. Psychiatry Res. 2017;251:225-234. PubMed CrossRef
33. Bonn-Miller MO, Boden MT, Vujanovic AA, et al. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychol Trauma. 2013;5(2):193-200. CrossRef
34. Manhapra A, Stefanovics E, Rosenheck R. Treatment outcomes for veterans with PTSD and substance use: impact of specific substances and achievement of abstinence. Drug Alcohol Depend. 2015;156:70-77. PubMed CrossRef
35. Ruglass LM, Shevorykin A, Radoncic V, et al. Impact of cannabis use on treatment outcomes among adults receiving cognitive-behavioral treatment for PTSD and substance use disorders. J Clin Med. 2017;6(2):14. PubMed CrossRef
36. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(9):1174-1180. PubMed CrossRef
37. Bricker JB, Russo J, Stein MB, et al. Does occasional cannabis use impact anxiety and depression treatment outcomes? results from a randomized effectiveness trial. Depress Anxiety. 2007;24(6):392-398. PubMed CrossRef
38. Kim S-W, Dodd S, Berk L, et al. Impact of cannabis use on long-term remission in bipolar I and schizoaffective disorder. Psychiatry Investig. 2015;12(3):349-355. PubMed CrossRef
39. Kvitland LR, Melle I, Aminoff SR, et al. Continued cannabis use at one year follow up is associated with elevated mood and lower global functioning in bipolar I disorder. BMC Psychiatry. 2015;15(1):11. PubMed CrossRef
40. Strakowski SM, DelBello MP, Fleck DE, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry. 2007;64(1):57-64. PubMed CrossRef
41. van Rossum I, Boomsma M, Tenback D, et al; EMBLEM Advisory Board. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis. 2009;197(1):35-40. PubMed CrossRef
42. Zorrilla I, Aguado J, Haro JM, et al. Cannabis and bipolar disorder: does quitting cannabis use during manic/mixed episode improve clinical/functional outcomes? Acta Psychiatr Scand. 2015;131(2):100-110. PubMed CrossRef
43. Bahorik AL, Leibowitz A, Sterling SA, et al. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord. 2017;213:168-171. PubMed CrossRef
44. Bahorik AL, Leibowitz A, Sterling SA, et al. The role of hazardous drinking reductions in predicting depression and anxiety symptom improvement among psychiatry patients: a longitudinal study. J Affect Disord. 2016;206:169-173. PubMed CrossRef
45. Fontana A, Rosenheck R, Desai R. Comparison of treatment outcomes for veterans with posttraumatic stress disorder with and without comorbid substance use/dependence. J Psychiatr Res. 2012;46(8):1008-1014. PubMed CrossRef
46. Strakowski SM, DelBello MP, Fleck DE, et al. The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry. 2000;48(6):477-485. PubMed CrossRef
47. Lachenmeier DW, Rehm J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci Rep. 2015;5(1):8126. PubMed CrossRef
48. Nutt DJ, King LA, Phillips LD; Independent Scientific Committee on Drugs. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376(9752):1558-1565. PubMed CrossRef
49. Bersani G, Bersani FS, Caroti E, et al. Negative symptoms as key features of depression among cannabis users: a preliminary report. Eur Rev Med Pharmacol Sci. 2016;20(3):547-552. PubMed
50. Galynker II, Cohen LJ, Cai J. Negative symptoms in patients with major depressive disorder: a preliminary report. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13(3):171-176. PubMed
51. Aspis I, Feingold D, Weiser M, et al. Cannabis use and mental health-related quality of life among individuals with depressive disorders. Psychiatry Res. 2015;230(2):341-349. PubMed CrossRef
52. Lev-Ran S, Le Foll B, McKenzie K, et al. Cannabis use and mental health-related quality of life among individuals with anxiety disorders. J Anxiety Disord. 2012;26(8):799-810. PubMed CrossRef
53. Moitra E, Anderson BJ, Stein MD. Reductions in cannabis use are associated with mood improvement in female emerging adults. Depress Anxiety. 2016;33(4):332-338. PubMed CrossRef
54. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227. PubMed CrossRef
55. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559-564. PubMed CrossRef
56. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84-88. PubMed CrossRef
57. Volkow ND, Hampson AJ, Baler RD. Don’ t worry, be happy: endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol. 2017;57(1):285-308. PubMed CrossRef
58. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64(1):21-47. PubMed CrossRef
59. Ashton CH, Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr Scand. 2011;124(4):250-261. PubMed CrossRef
60.Micale V, Tabiova K, Kucerova J, et al. Role of the endocannabinoid system in depression: from preclinical to clinical evidence. In: Campolongo T, Fattore L, eds. Cannabinoid Modulation of Emotion, Memory, and Motivation. New York, NY: Springer; 2015:97-129.
61.Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. In: Kendall D, Alexander S, eds. Behavioral Neurobiology of the Endocannabinoid System. Berlin, Germany: Srpinger-Verlag; 2009:347-371.
62. Saito VM, Wotjak CT, Moreira FA. Pharmacological exploitation of the endocannabinoid system: new perspectives for the treatment of depression and anxiety disorders?. Rev Bras Psiquiatr. 2010;32(suppl 1):S7-S14. PubMed
63. Serra G, Fratta W. A possible role for the endocannabinoid system in the neurobiology of depression. Clin Pract Epidemol Ment Health. 2007;3(1):25. PubMed CrossRef
64. Paruk S, Burns JK. Cannabis and mental illness in adolescents: a review. S Afr Fam Pract. 2015;58(suppl 1):S18-S21.
65. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383-1391. PubMed CrossRef
66. Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev. 2012;5(1):32-40. PubMed CrossRef
67. Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10(9):2825-2830. PubMed CrossRef
68. Sexton M, Cuttler C, Finnell JS, et al. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1(1):131-138. PubMed CrossRef
69. Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59(3):291-295. PubMed CrossRef
70. Bergamaschi MM, Queiroz RH, Zuardi AW, et al. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf. 2011;6(4):237-249. PubMed CrossRef
71. Hayakawa K, Mishima K, Hazekawa M, et al. Cannabidiol potentiates pharmacological effects of Δ(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157-164. PubMed CrossRef
72. Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16(5-6):315-331. PubMed CrossRef
73. Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. 2006;30(suppl 1):S13-S18. PubMed CrossRef
74. McPartland JM, Duncan M, Di Marzo V, et al. Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? a systematic review. Br J Pharmacol. 2015;172(3):737-753. PubMed CrossRef
75. Niesink RJ, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130. PubMed CrossRef
76. Blessing EM, Steenkamp MM, Manzanares J, et al. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825-836. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite