Objective: This phase 3 study evaluated the efficacy, safety, and tolerability of cariprazine in patients with acute exacerbation of schizophrenia.
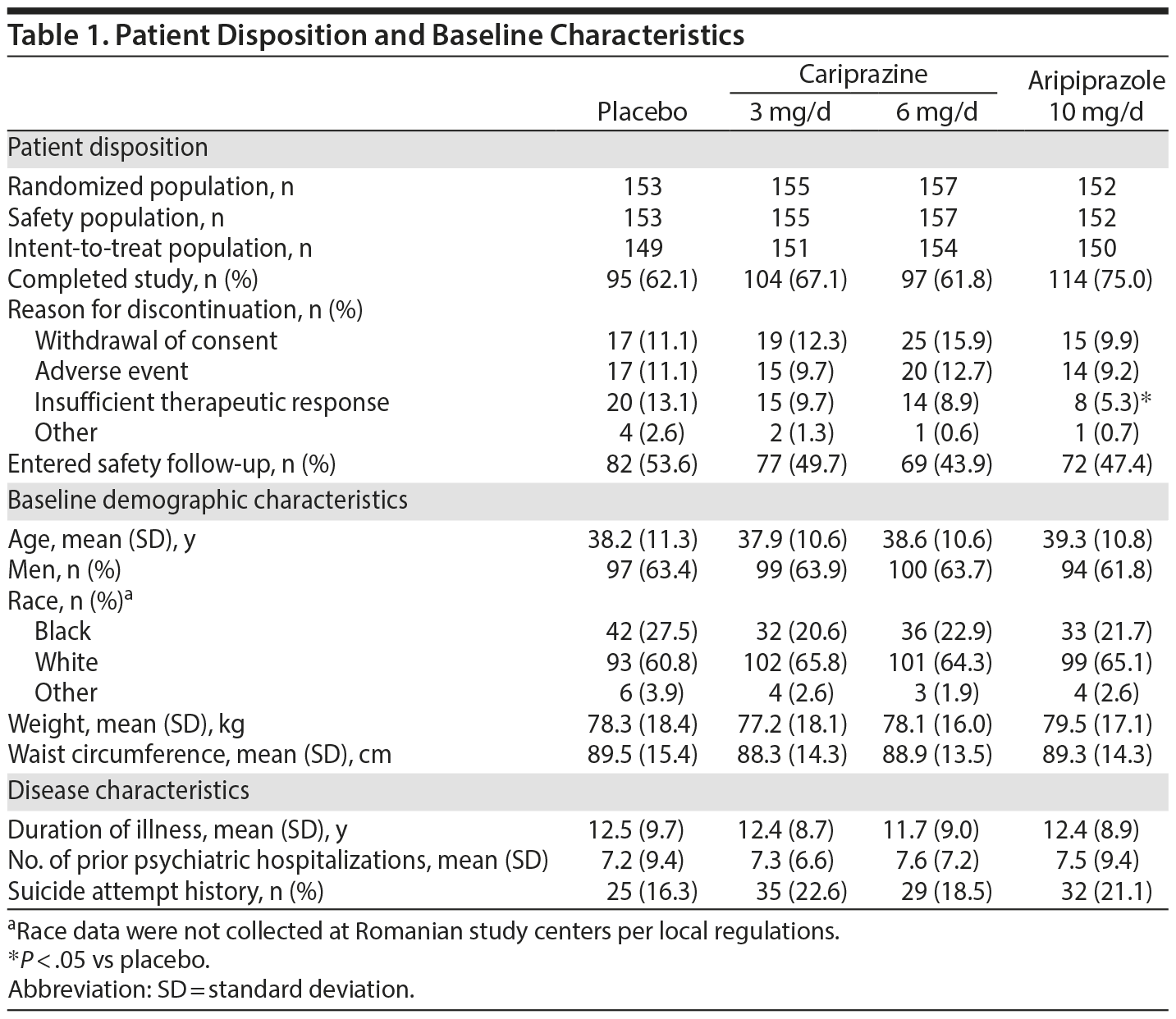
Method: This multinational, randomized, double-blind, placebo- and active-controlled study was conducted from April 2010 to December 2011. Patients who met DSM-IV-TR criteria for schizophrenia were randomized to placebo (n = 153), cariprazine 3 mg/d (n = 155), cariprazine 6 mg/d (n = 157), or aripiprazole 10 mg/d (n = 152) for 6 weeks of double-blind treatment. The primary and secondary efficacy parameters were mean change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score and Clinical Global Impressions-Severity of Illness (CGI-S) score, respectively.
Results: Least squares mean differences (LSMDs) in PANSS total score change at week 6 significantly favored cariprazine 3 and 6 mg/d versus placebo (LSMD [95% CI]: 3 mg/d, −6.0 [−10.1 to −1.9], adjusted P = .0044; 6 mg/d, −8.8 [−12.9 to −4.7], adjusted P < .0001). Cariprazine 3 and 6 mg/d were also associated with significant improvements relative to placebo in CGI-S scores (LSMD [95% CI]: 3 mg/d, −0.4 [−0.6 to −0.2], adjusted P = .0044; 6 mg/d, −0.5 [−0.7 to −0.3], adjusted P < .0001). Significant differences from placebo were also observed with aripiprazole on the PANSS (LSMD [95% CI]: −7.0 [−11.0 to −2.9], P = .0008) and CGI-S (LSMD [95% CI]: −0.4 [−0.6 to −0.2], P = .0001). Common treatment-emergent adverse events (≥ 10%) were insomnia (all groups), akathisia (cariprazine 6 mg/d), and headache (placebo, cariprazine 6 mg/d).
Conclusions: This study supports the efficacy, safety, and tolerability of cariprazine 3 and 6 mg/d in the treatment of patients with acute exacerbation of schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT01104766
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
ABSTRACT
Objective: This phase 3 study evaluated the efficacy, safety, and tolerability of cariprazine in patients with acute exacerbation of schizophrenia.
Method: This multinational, randomized, double-blind, placebo- and active-controlled study was conducted from April 2010 to December 2011. Patients who met DSM-IV-TR criteria for schizophrenia were randomized to placebo (n = 153), cariprazine 3 mg/d (n = 155), cariprazine 6 mg/d (n = 157), or aripiprazole 10 mg/d (n = 152) for 6 weeks of double-blind treatment. The primary and secondary efficacy parameters were mean change from baseline to week 6 in Positive and Negative Syndrome Scale (PANSS) total score and Clinical Global Impressions-Severity of Illness (CGI-S) score, respectively.
Results: Least squares mean differences (LSMDs) in PANSS total score change at week 6 significantly favored cariprazine 3 and 6 mg/d versus placebo (LSMD [95% CI]: 3 mg/d, −6.0 [−10.1 to −1.9], adjusted P = .0044; 6 mg/d, −8.8 [−12.9 to −4.7], adjusted P < .0001). Cariprazine 3 and 6 mg/d were also associated with significant improvements relative to placebo in CGI-S scores (LSMD [95% CI]: 3 mg/d, −0.4 [−0.6 to −0.2], adjusted P = .0044; 6 mg/d, −0.5 [−0.7 to −0.3], adjusted P < .0001). Significant differences from placebo were also observed with aripiprazole on the PANSS (LSMD [95% CI]: −7.0 [−11.0 to −2.9], P = .0008) and CGI-S (LSMD [95% CI]: −0.4 [−0.6 to −0.2], P = .0001). Common treatment-emergent adverse events (≥ 10%) were insomnia (all groups), akathisia (cariprazine 6 mg/d), and headache (placebo, cariprazine 6 mg/d).
Conclusions: This study supports the efficacy, safety, and tolerability of cariprazine 3 and 6 mg/d in the treatment of patients with acute exacerbation of schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT01104766
J Clin Psychiatry 2015;76(12):e1574-e1582
dx.doi.org/10.4088/JCP.15m09997
© Copyright 2015 Physicians Postgraduate Press, Inc.
aForest Research Institute, Jersey City, New Jersey, an Allergan affiliate
bFlorida Clinical Research Center, LLC, Bradenton, Florida
cPrescott Medical Communications Group, Chicago, Illinois
dGedeon Richter Plc, Budapest, Hungary
eNorthwestern Feinberg School of Medicine, Chicago, Illinois
*Corresponding author: Suresh Durgam, MD, Clinical Development, Forest Research Institute, an Allergan affiliate, Harborside Financial Center, Plaza V, Jersey City, NJ 07311 ([email protected]).
Schizophrenia is a complex disorder characterized by positive and negative symptoms and cognitive impairment.1 Like the first generation of antipsychotics used to treat schizophrenia, atypical (or second-generation) antipsychotics act primarily through blockade of dopamine D2 receptors, some degree of which is thought to be necessary for antipsychotic activity.2 Compared with their predecessors, atypical antipsychotics have similar efficacy in treating the positive symptoms of schizophrenia, and they are generally thought to have a potentially lower risk of extrapyramidal symptoms (EPS) and tardive dyskinesia, although they are associated with a number of adverse effects, including metabolic issues.3,4 While first-generation agents have limited efficacy on negative and cognitive symptoms,4 the effect of atypical antipsychotics on these symptom domains is controversial.5-9 Some atypical antipsychotics produce marked improvements in negative and cognitive domains in substantial numbers of patients,8 but efficacy differs among agents and patients. Reasons for these individual differences have not been fully elucidated, and the need for new pharmacologic treatments with broader efficacy and improved tolerability remains.
Cariprazine, a dopamine D3 and D2 receptor partial agonist with preferential binding to D3 receptors, is approved by the US Food and Drug Administration for the treatment of schizophrenia and the acute treatment of manic and mixed episodes associated with bipolar I disorder; it is also in development for the treatment of bipolar depression and the adjunctive treatment of major depressive disorder. Cariprazine differs from available atypical antipsychotics in that it has almost 10-fold greater affinity for D3 than D2 receptors in vitro10 and high and balanced in vivo occupancy of both D2 and D3 receptors in rats11 and humans.12 The dopamine D3 receptor is thought to be important in modulating mood and cognition13-17; therefore, a compound with high affinity and occupancy of both D2 and D3 receptors not only may be effective in treating the positive symptoms, but also may provide beneficial effects on negative symptoms, mood, and cognitive impairment associated with schizophrenia.15,18-21
In previous phase 2 (cariprazine 1.5, 3.0, or 4.5 mg/d22) and phase 3 (3-6 and 6-9 mg/d23) randomized, placebo-controlled studies in patients with acute exacerbation of schizophrenia, cariprazine was effective and generally well tolerated. This phase 3 study (ClinicalTrials.gov identifier: NCT01104766) evaluated the efficacy, safety, and tolerability of cariprazine in patients with schizophrenia. Aripiprazole was used as an active control for assay sensitivity; therefore, no inferential statistical testing was done to compare active treatment groups.
METHOD
Study Design
This was a double-blind, parallel-group, placebo- and active-controlled study, supported by funding from Forest Laboratories, LLC, an Allergan affiliate, and Gedeon Richter Plc. It was conducted at 57 centers in the United States, Romania, Russia, and Ukraine from April 23, 2010, to December 20, 2011. The protocol was approved by an institutional review board (US sites) or ethics committee (non-US sites). ICH-E6 Good Clinical Practice guidelines were followed, and written informed consent was obtained from all participants.
This 9-week study comprised a washout period of up to 7 days followed by 6 weeks of double-blind treatment and 2 weeks of safety follow-up. Patients were randomized (1:1:1:1) to receive placebo, cariprazine 3 mg/d, cariprazine 6 mg/d, or aripiprazole 10 mg/d (recommended dose). Patients randomly assigned to cariprazine initiated treatment at 1.5 mg/d; dosage was increased by 1.5 mg/d until the target dose was achieved (day 2 and day 4 for cariprazine 3 and 6 mg/d, respectively). Aripiprazole was initiated and maintained at 10 mg/d. Patients were hospitalized during washout/screening and for at least 4 weeks of treatment. Starting on day 28, patients with a Clinical Global Impressions-Severity of Illness (CGI-S)24 score ≤ 3 and no significant risk of suicide or violent behavior were eligible for discharge.

- In this 6-week trial of adults with schizophrenia, cariprazine at doses of 3 and 6 mg/d was generally well tolerated and was associated with significantly greater improvement compared with placebo across the range of schizophrenia symptoms.
- With its distinct pharmacology and preferential dopamine D3 receptor binding affinity, cariprazine may be an effective new treatment option for schizophrenia.
Inclusion Criteria
Patients aged 18-60 years with a current diagnosis of schizophrenia (paranoid, disorganized, catatonic, and/or undifferentiated types) per Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)25 criteria were included. Participants had to have been diagnosed for ≥ 1 year and have had ≥ 1 psychotic episode that required hospitalization or change in antipsychotic medication during the past year. In addition, to ensure that participants’ current psychotic episode was acute, duration of the current episode must be < 2 weeks, as captured by the Structured Clinical Interview for DSM-IV Disorders26 and by interviews with patients, caregiver, and other informants. A CGI-S score ≥ 4, Positive and Negative Syndrome Scale (PANSS)27 total score ≥ 80 and ≤ 120, and a score ≥ 4 on at least 2 of the PANSS positive symptoms of delusions, hallucinatory behavior, conceptual disorganization, or suspiciousness/persecution were also required.
Exclusion Criteria
Patients were excluded from the study for diagnosis of schizoaffective or schizophreniform disorder, bipolar disorder, or other DSM-IV-TR disorders of sufficient severity to interfere with study participation. Additional exclusionary conditions included first psychotic episode, substance abuse/dependence (past 3 months), suicide attempt (past 2 years), suicide risk (as judged by the investigator on the basis of the psychiatric interview or information collected in the Columbia-Suicide Severity Rating Scale [C-SSRS]),28 treatment resistance (ie, poor response to ≥ 2 antipsychotics at adequate dose/duration during the past 2 years), body mass index < 18 or > 40, pregnancy/breastfeeding, or medical conditions that might interfere with study participation. Psychotropic medications were prohibited except for lorazepam as needed for agitation, restlessness, irritability, or hostility; zolpidem, zaleplon, chloral hydrate, or eszopiclone for insomnia; diphenhydramine or benztropine for EPS; and propranolol for akathisia.
Outcome Measures
PANSS and CGI-S were administered at screening (week −1), baseline (week 0), and each double-blind treatment visit (weeks 1-6). Additional assessments included the 16-item Negative Symptom Assessment (NSA-16)29 (weeks 0, 2, 4, 6), Clinical Global Impressions-Improvement (CGI-I)24 (weeks 1-6), Schizophrenia Quality of Life Scale-Revision 4 (SQLS-R4)30 (weeks 0, 6), and the Cognitive Drug Research (CDR) Attention Battery31 and Color Trails Test (CTT)32 (weeks −1, 0, 6).
Safety assessments included treatment-emergent adverse events (TEAEs), clinical laboratory tests, vital signs, C-SSRS, physical examinations, ophthalmologic examinations (including Lens Opacities Classification System III scores for nuclear opalescence, color of lens nucleus, subcapsular opacity, visual acuity, and intraocular pressure), 12-lead electrocardiograms (ECGs), and EPS measures (Abnormal Involuntary Movement Scale,33 Barnes Akathisia Rating Scale [BARS],34 and Simpson-Angus Scale [SAS]35).
Statistical Analysis
Safety evaluations were based on the safety population (all randomized patients who received ≥ 1 dose of study medication); efficacy evaluations were based on the intent-to-treat (ITT) population (patients in the safety population who had ≥ 1 postbaseline PANSS assessment).
Change from baseline to week 6 in PANSS total score (primary efficacy) and CGI-S score (secondary efficacy) were assessed using a mixed-effects model for repeated measures (MMRM) with treatment group, study center, visit, and treatment group-by-visit interaction as fixed effects and baseline value and baseline value-by-visit interaction as covariates. An unstructured covariance matrix was used to model the covariance of within-patient scores. A matched parallel gatekeeping procedure was used to control for multiple comparisons across primary and secondary hypotheses for comparisons of the 2 cariprazine dose groups to placebo36; significance on the secondary endpoint for a dose level was not claimed unless its corresponding primary hypothesis was significant. Sensitivity analyses for the primary efficacy parameter included an analysis of covariance (ANCOVA) using a last-observation-carried-forward (LOCF) approach, with treatment group and study center as factors and baseline score as covariate, and a pattern-mixture model (PMM) based on non-future dependent missing value restrictions.37
Additional efficacy evaluations included change from baseline to week 6 in NSA-16 score and PANSS positive and negative subscales (MMRM); SQLS-R4 (ANCOVA, LOCF); CTT and CDR Attention Battery scores (Wilcoxon rank sum test, LOCF); week 6 CGI-I scores (MMRM using CGI-S baseline score); and PANSS response (≥ 30% improvement from baseline; logistic regression, LOCF). Change from baseline to week 6 in PANSS general psychopathology subscale, PANSS cognitive (items P2, N5, N7, G10, and G11)38,39 subscale, and the PANSS depression cluster (items G1, G2, G3, and G6)40 was evaluated post hoc.
Premature discontinuation in the active treatment groups versus placebo was compared using the Fisher exact test.41 Between-group differences for demographic and baseline characteristics were analyzed using 2-way analysis of variance for continuous variables and Cochran-Mantel-Haenszel tests42 for categorical variables, controlling for study center.
Safety parameters were analyzed using descriptive statistics. Post hoc statistical testing was performed for between-group differences in TEAEs (Fisher exact test) and changes in clinical and laboratory parameters (2-sample t test). Treatment-emergent parkinsonism was defined as SAS score ≤ 3 at baseline and > 3 at any postbaseline assessment; treatment-emergent akathisia was defined as BARS score ≤ 2 at baseline and > 2 at any postbaseline assessment.
Aripiprazole was compared to placebo for assay sensitivity; no testing was done to compare active treatment groups. Statistical tests were 2-sided tests performed at the 5% level of significance; confidence intervals (CIs) were 2-sided 95% CIs. For efficacy measures, statistical significance was defined as P values < .05. Primary and secondary efficacy measures were controlled for multiple comparisons; additional and by-visit efficacy analyses were not controlled for multiple comparisons.
RESULTS
Approximately 67% of patients completed the study. Discontinuation rates were similar for placebo and cariprazine and slightly lower for aripiprazole (Table 1). There were no statistically significant differences between placebo and either cariprazine group for overall discontinuations or individual reasons for discontinuation. Demographics, baseline characteristics, and disease history were similar among groups. Mean baseline PANSS and CGI-S scores indicated that most patients were markedly ill (Table 2).43
Efficacy
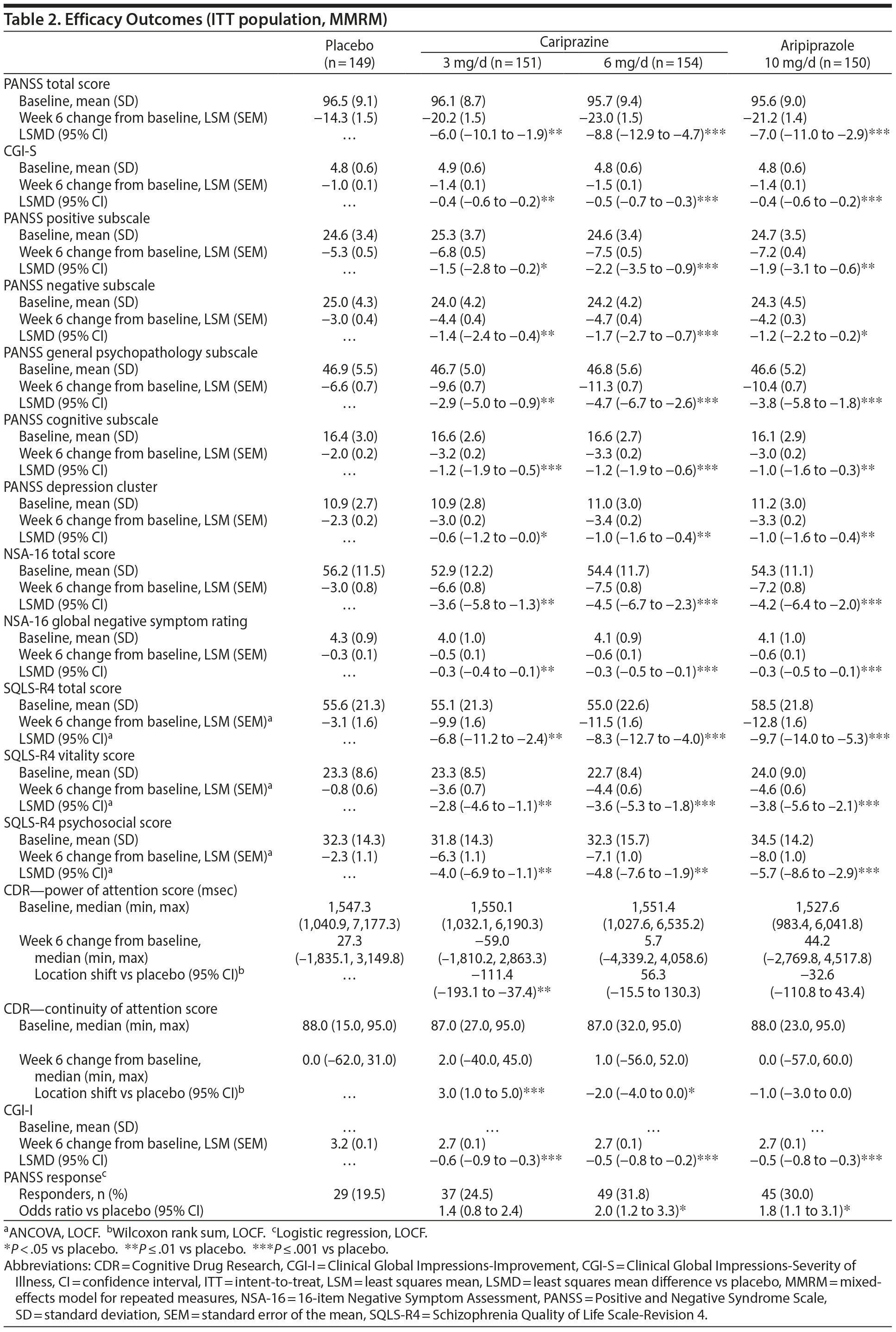
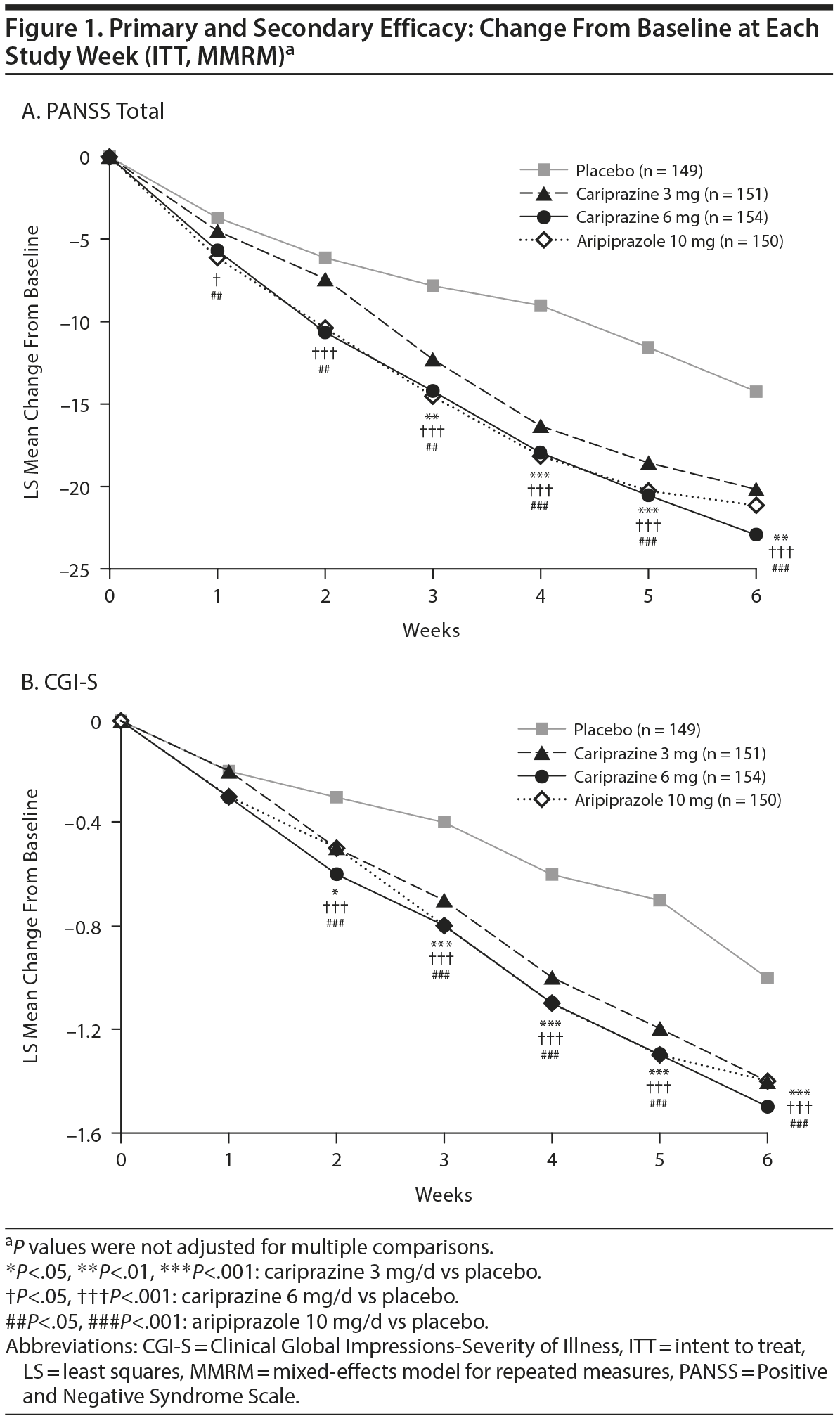
The primary efficacy parameter, mean change from baseline to week 6 in PANSS total score, was significantly greater for cariprazine 3 mg/d and 6 mg/d versus placebo (Table 2). The primary results were supported by LOCF (least squares mean difference [LSMD] [95% CI] for cariprazine versus placebo: 3 mg/d = −5.4 [−9.3 to −1.4], P = .0078; 6 mg/d = −7.9 [−11.8 to −4.0], P < .0001) and PMM sensitivity analyses (data not shown). Statistically significant separation from placebo was achieved at week 1 and week 3 in the cariprazine 6 and 3 mg/d groups, respectively, and was maintained through the end of the study (Figure 1A). PANSS total score change at week 6 was also significantly greater for aripiprazole than placebo (MMRM, PMM, and LOCF), indicating sufficient assay sensitivity.
Mean change in CGI-S score (secondary efficacy) was significantly greater at week 6 for cariprazine 3 mg/d and 6 mg/d versus placebo using MMRM (Table 2) and LOCF (LSMD [95% CI]: 3 mg/d = −0.4 [−0.6 to −0.2], P = .0005; 6 mg/d = −0.5 [−0.7 to −0.2], P < .0001) approaches. Improvement in CGI-S scores was significantly greater for both cariprazine groups versus placebo beginning at week 2 (Figure 1B).
Both cariprazine doses were statistically superior to placebo on positive, negative, cognitive, and mood symptoms, as measured by PANSS subscales, PANSS depression cluster, and NSA-16 scores (Table 2). Cariprazine was associated with early improvement on all 4 PANSS subscales (see Supplementary eFigure 1 at Psychiatrist.com). Cariprazine-treated patients also had significantly greater improvement versus placebo on the CGI-I and quality of life measures (Table 2). PANSS response rate (≥ 30% improvement from baseline) at week 6 was significantly higher for cariprazine 6 mg/d than placebo (Table 2). Statistically significant differences in favor of aripiprazole over placebo were seen on all the secondary and additional efficacy measures discussed above (Table 2).
On the CDR Attention Battery, significant improvement was seen for cariprazine 3 mg/d versus placebo on both power of attention (P = .0036) and continuity of attention (P = .0005) measures (Table 2). There were no significant differences between aripiprazole and placebo on the power of attention or continuity of attention measures. There were no significant differences between any treatment group and placebo on CDR cognitive reaction time and reaction time variability or on the CTT (data not shown).
Safety and Tolerability
Extent of exposure. Mean treatment duration was 33 days for placebo; 34 and 33 days for cariprazine 3 mg/d and cariprazine 6 mg/d, respectively; and 36 days for aripiprazole.
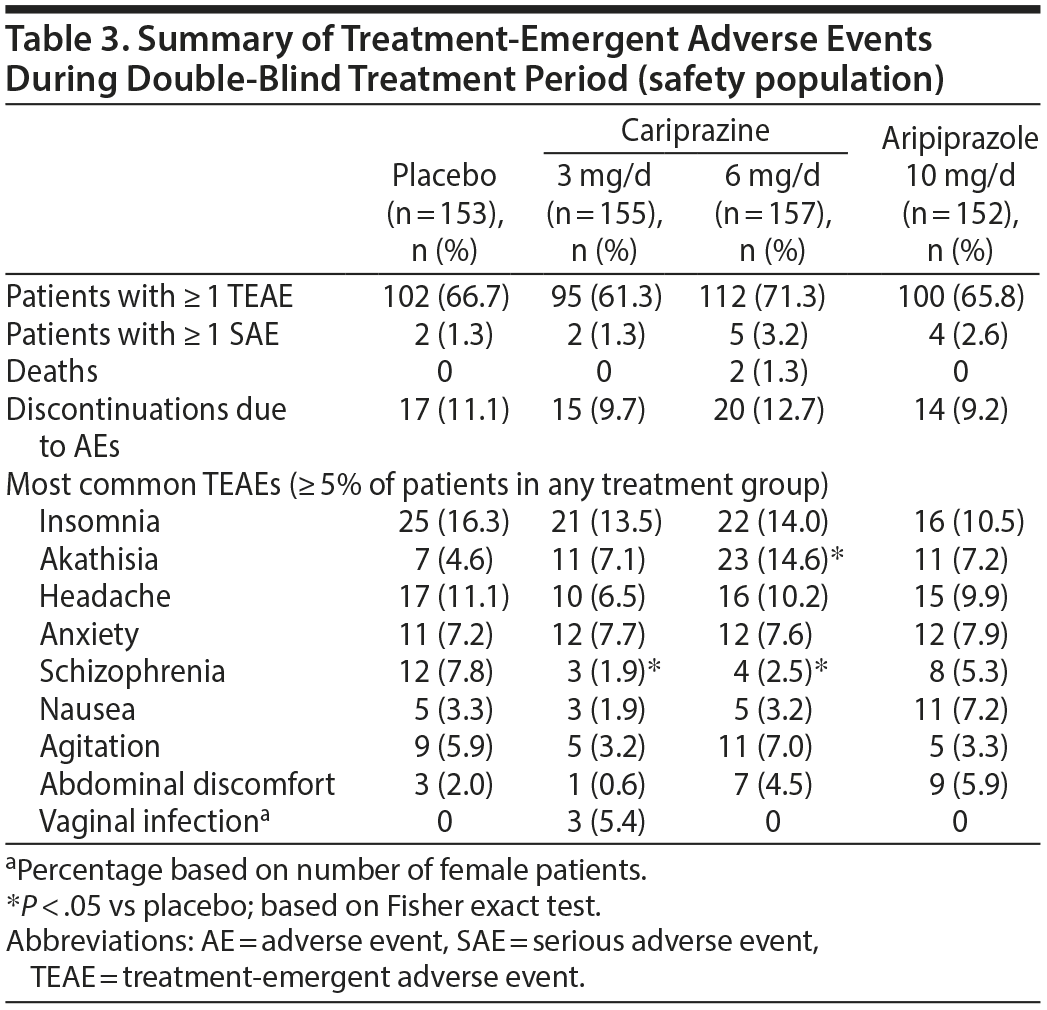
Adverse events. More than half of the patients in each treatment group reported a TEAE (Table 3); most TEAEs were considered to be mild or moderate in intensity. Discontinuations due to AEs were reported for 66 patients; the most frequent reasons were schizophrenia (placebo = 8, cariprazine 3 mg/d = 3, cariprazine 6 mg/d = 4, aripiprazole = 7) and psychotic disorder (placebo = 1, cariprazine 3 mg/d = 3, cariprazine 6 mg/d = 4, aripiprazole = 1). The only TEAE reported in ≥ 5% of cariprazine patients, twice the rate of placebo, and possibly related to treatment was akathisia in the 6 mg/d group (vaginal infection in the 3 mg/d group was unrelated to treatment). The incidence of akathisia was significantly greater for cariprazine 6 mg/d (14.6%) versus placebo (4.6%; P = .0034). Worsening of schizophrenia was significantly more frequent in the placebo group (7.8%) than in the cariprazine groups (3 mg/d: 1.9%, P = .0178; 6 mg/d: 2.5%, P = .0412). There were 17 serious AEs (SAEs) during double-blind treatment or safety follow-up (placebo = 2 [1.3%], cariprazine 3 mg/d = 4 [2.6%], cariprazine 6 mg/d = 7 [4.5%], and aripiprazole = 4 [2.6%]). The only SAEs reported in > 1 patient were schizophrenia (cariprazine 3 mg/d = 2, cariprazine 6 mg/d = 1), social stay hospitalization (cariprazine 6 mg/d and aripiprazole = 1 each), and psychotic disorder (cariprazine 3 mg/d = 2). The only SAE considered possibly related to study drug was supraventricular tachycardia, which was observed in 1 patient receiving cariprazine 3 mg/d; the patient was discontinued due to psychotic disorder (considered unrelated to treatment) on the same day, and the supraventricular tachycardia resolved within 1 week of study discontinuation. There were 2 deaths during the study; both occurred in the cariprazine 6 mg/d group (suicide and ischemic stroke/myocardial infarction) and were considered unrelated to treatment.
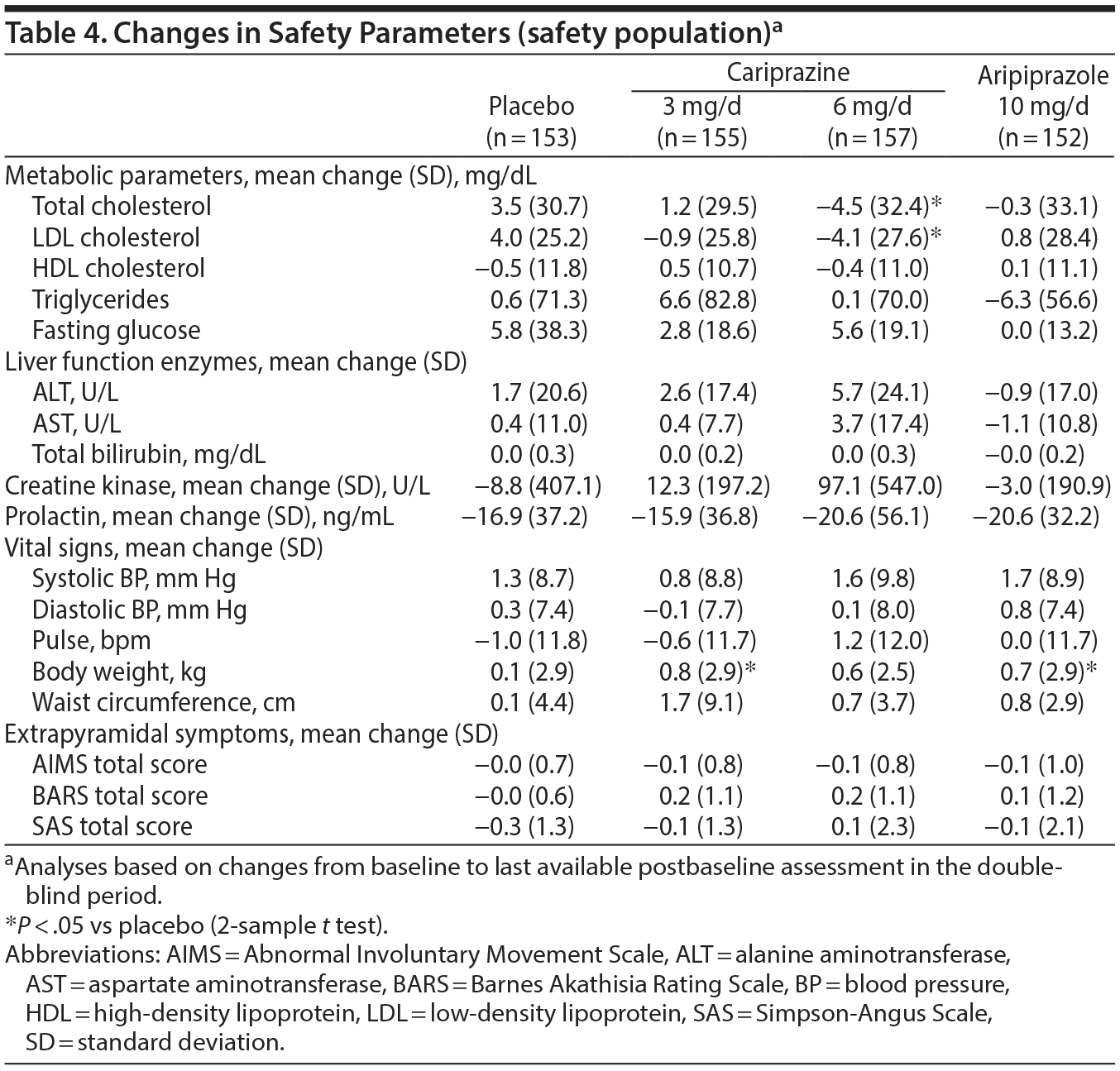
Laboratory parameters, vital signs, electrocardiogram, physical examination, and ophthalmologic assessments. Total and low-density lipoprotein cholesterol levels were significantly decreased in the cariprazine 6 mg/d group versus placebo; there were no other significant differences between groups for changes in clinical laboratory parameters (Table 4).
Mean changes in blood pressure and pulse were not clinically significant (Table 4). Body weight increased by 0.8 kg for cariprazine 3 mg/d, 0.6 kg for cariprazine 6 mg/d, 0.1 kg for placebo, and 0.7 kg for aripiprazole. Incidences of body weight increase ≥ 7% were 6% for cariprazine 3 mg/d, 5% for cariprazine 6 mg/d, 3% for placebo, and 6% for aripiprazole. The incidence of orthostatic hypotension was 18% for cariprazine 3 mg/d, 12% for cariprazine 6 mg/d, 12% for placebo, and 11% for aripiprazole. Mean ECG changes were small and similar across groups; slight increases in ventricular heart rate were noted with cariprazine 3 mg/d (2.8 bpm) and cariprazine 6 mg/d (3.5 bpm) versus placebo (−0.8 bpm) and with aripiprazole (2.8 bpm) versus placebo (−0.8 bpm). Small decreases in QT interval occurred across active treatment groups. One aripiprazole patient had a QTc (Bazett correction) interval > 500 msec; no patient had a QTc (Fridericia correction) interval > 500 msec. Mean changes in ophthalmologic parameters were similar among groups.
Extrapyramidal symptoms. Cariprazine patients had higher rates than placebo patients of treatment-emergent parkinsonism (SAS baseline ≤ 3 and postbaseline > 3; placebo = 3%, cariprazine 3 mg/d = 6%, cariprazine 6 mg/d = 11%) and akathisia (BARS baseline ≤ 2 and postbaseline > 2; placebo = 5%, cariprazine 3 mg/d = 14%, cariprazine 6 mg/d = 16%); aripiprazole patients also had higher rates than placebo of treatment-emergent parkinsonism (5%) and akathisia (11%). All but 2 cariprazine-related reports of akathisia were considered mild or moderate in intensity. Excluding akathisia and restlessness, EPS-related TEAEs resulted in the discontinuation of only 1 patient (cariprazine 6 mg/d: musculoskeletal stiffness). Discontinuation due to akathisia/restlessness occurred in 3 cariprazine 3 mg/d patients and 1 cariprazine 6 mg/d patient. There were no discontinuations due to EPS-related TEAEs in the aripiprazole group. Mean changes on movement disorder scales were generally similar among groups (Table 4).
Suicidality. C-SSRS-reported suicidal ideation occurred in 7 (5%) placebo, 3 (2%) cariprazine 3 mg/d, 4 (3%) cariprazine 6 mg/d, and 4 (3%) aripiprazole patients; most incidences were recorded in the least severe category (“wish to be dead,” no intent to act). No suicidal behavior was recorded on the C-SSRS. One completed suicide was reported during the study; the patient’s C-SSRS scores had not indicated suicidal ideation or behavior.
DISCUSSION
In this phase 3 clinical trial in adult patients with schizophrenia, significantly greater improvement for cariprazine 3 mg/d and 6 mg/d versus placebo was demonstrated on the primary efficacy parameter, change from baseline to week 6 in PANSS total score; persistent improvement over placebo was apparent by week 1 for cariprazine 6 mg/d and by week 3 for cariprazine 3 mg/d. Significant improvement versus placebo in the active-control group (aripiprazole 10 mg/d) supported the validity of the primary analysis. Significant improvement of cariprazine versus placebo on secondary (CGI-S) and additional efficacy measures (eg, SQLS-R4 total score, PANSS positive subscale, SQLS-R4 psychosocial and vitality scores) suggests improvement in overall disease severity, symptom intensity, and quality of life for cariprazine patients.
Blockade of the D2 receptor is thought to be necessary for efficacy on the positive symptoms of schizophrenia,2 and cariprazine, like aripiprazole, is a potent D2 receptor partial agonist. In addition, cariprazine also shows high D3 receptor affinity and selectivity.10-12 Research has supported a role for D3 receptors in modulating mood and cognition,13-15,21,44 suggesting that potent activity at D3 receptors may confer pharmacologic benefit on negative symptoms, cognitive deficits, and mood symptoms. Because these symptoms may be more strongly correlated with functional impairment and decreased quality of life than positive symptoms in schizophrenia,45-48 medications with efficacy in these symptom domains are important for disease management.
In this study, patients in the cariprazine and aripiprazole groups showed significantly greater improvement versus placebo in negative symptoms and depressed mood, as measured by the PANSS negative subscale, NSA-16 total score, NSA-16 global negative symptom rating, and PANSS depression cluster scores. Evaluation of PANSS negative subscale scores over time revealed significant improvement with cariprazine 6 mg/d compared with placebo after 1 week of double-blind treatment. On cognitive measures, cariprazine- versus placebo-treated patients had significant improvement on the CDR power of attention (3 mg/d), CDR continuity of attention (3 mg/d), and PANSS cognitive subscale scores (both doses), but not on the CDR cognitive reaction time or reaction time variability score or on the CTT.
The results corresponding to negative or cognitive symptom domains must be interpreted with caution since many antipsychotic treatments have shown improved negative and cognitive symptoms in clinical trials of patients with acutely exacerbated schizophrenia. In this patient population, improvement in these domains may only reflect pseudospecific effects resulting from concurrent improvements in positive symptoms, affective symptoms, or overall clinical status.49,50 To better parcellate the effects of cariprazine in improving negative, cognitive, or affective symptoms, prospectively defined studies designed to assess these specific symptom domains in patients with a stabilized disease state are warranted.
Although this study was not designed to compare cariprazine dose levels, inclusion of 2 dose groups permits observation of potential dosing effects. Changes of greater magnitude on most efficacy parameters and earlier differentiation from placebo on the primary efficacy analysis were seen in the cariprazine 6 mg/d group relative to the 3 mg/d group. PANSS response rates were statistically higher for cariprazine 6 mg/d versus placebo, but only numerically higher for cariprazine 3 mg/d. Conversely, the 3-mg/d dose appeared to have slightly better tolerability.
Cariprazine was generally well tolerated; most TEAEs were considered mild or moderate in severity, and discontinuations due to AEs were comparable between the cariprazine and placebo groups, although the rate was highest for cariprazine 6 mg/d. Similar to other atypical antipsychotics, akathisia was more frequent with active treatment than placebo; most incidences were mild to moderate in severity and resulted in few discontinuations. Mean changes from baseline in EPS scales were similar in all treatment groups.
Some atypical antipsychotics are associated with significant weight gain, metabolic issues, cardiovascular AEs, and type 2 diabetes,51 which is especially challenging given the high rate of comorbid medical conditions associated with schizophrenia.52 Consistent with previous studies in schizophrenia22 and bipolar disorder,53 cariprazine was not associated with clinically relevant changes in metabolic parameters, body weight, or waist circumference. Further, cariprazine showed no clinically relevant effects on sedation, QT prolongation, or prolactin elevation.
Interpretation of study results is limited by the relatively short duration of this trial. In addition, while aripiprazole was included as an active control to assess assay sensitivity, the study was not designed to allow for comparisons between the active treatments. Future cariprazine studies designed for head-to-head comparisons with other antipsychotics are warranted. Lastly, while the study included efficacy scales that allowed for measurement of changes in negative and cognitive symptoms, the study was not powered to detect between-group differences or designed to properly evaluate the effects of cariprazine on these domains.
This study supports the efficacy and tolerability of cariprazine 3 mg/d and 6 mg/d in the treatment of adult patients with acute exacerbation of schizophrenia. With its distinct pharmacology and preferential dopamine D3 receptor binding affinity, cariprazine may be an effective new treatment option for schizophrenia.
Submitted: March 27, 2015; accepted September 14, 2015.
Drug names: aripiprazole (Abilify), cariprazine (Vraylar), eszopiclone (Lunesta), lorazepam (Ativan and others), propranolol (Inderal and others), zaleplon (Sonata and others), zolpidem (Ambien, Edluar, and others).
Potential conflicts of interest: Drs Durgam and Lu and Mr Migliore acknowledge a potential conflict of interest as employees of Forest Research Institute, an Allergan affiliate; they are also stock shareholders of Actavis, PLC. Dr Cutler has received research support from AbbVie, Alkermes, AstraZeneca, Bristol-Meyers Squibb, Forest Laboratories, LLC, an Allergan affiliate, Forum, Janssen (Johnson & Johnson), Eli Lilly, Lundbeck, Novartis, Otsuka, Pfizer, Reckitt Benckiser, Sunovion, Takeda, and Vanda; served as a consultant for AbbVie, Alkermes, AstraZeneca, Bristol-Myers Squibb, Forest Laboratories, LLC, an Allergan affiliate, Forum, Janssen (Johnson & Johnson), Jazz, Eli Lilly, Lundbeck, Novartis, Otsuka, Pfizer, Sunovion, Takeda, Teva, and Vanda; and served as a speaker for Alkermes, AstraZeneca, Bristol-Myers Squibb, Forest Laboratories, LLC, an Allergan affiliate. Janssen (Johnson & Johnson), Jazz, Eli Lilly, Lundbeck, Novartis, Otsuka, Pfizer, Sunovion, Takeda, Teva, and Vanda. Dr Ruth acknowledges a potential conflict of interest as an employee of Prescott Medical Communications Group, a contractor of Forest Research Institute, an Allergan affiliate. Drs Laszlovszky and Németh acknowledge a potential conflict of interest as employees of Gedeon Richter Plc and as patent owners of cariprazine. Dr Meltzer has received research support from SureGene LLC, Forest, Boehringer Ingelheim, FORUM Pharmaceuticals, Sunovion, Dainippon Sumitomo, Janssen, Teva, Novartis, Reviva Pharmaceuticals, Astellas, Alkermes, Lundbeck, Eli Lilly, Takeda, Neurotherapeutics, Naurex, Auspex, Magceutics, and American Society for Suicide Prevention; served as a consultant for SureGene LLC, Forest, Boehringer Ingelheim, FORUM Pharmaceuticals, Sunovion, Dainippon Sumitomo, Janssen, Teva, Novartis, Reviva Pharmaceuticals, Astellas, Alkermes, Lundbeck, Eli Lilly, Guidepoint Global Advisors, Gerson Lehrman Group, and Clearview Health Partners; receives honoraria from International Society for Serotonin Research, University of Minnesota, University of Texas Medical Branch, Reviva Pharmaceuticals, and Georgia Psychiatric Physicians Association; serves on speakers or advisory boards for FORUM Pharmaceuticals, Sunovion, Dainippon Sumitomo, Novartis, and ProPhase LLC, is a stockholder in SureGene LLC, Acadia, and GlaxoSmithKline; and receives other financial or material support from McGraw Hill Publishing.
Funding/support: Supported by funding from Forest Laboratories, LLC, an Allergan affiliate (Jersey City, New Jersey), and Gedeon Richter Plc (Budapest, Hungary).
Role of the sponsors: Forest Laboratories, LLC and Gedeon Richter Plc. were involved in the study design, collection (via contracted clinical investigator sites), analysis and interpretation of data, and decision to present these results.
Previous presentations: Presented at the 166th American Psychiatric Association Annual Meeting, May 18-22, 2013, San Francisco, California; the 53rd New Clinical Drug Evaluation Unit (NCDEU) Annual Meeting, May 28-31, 2013, Hollywood, Florida; the 26th Annual US Psychiatric and Mental Health Congress, September 30-October 3, 2013 Las Vegas, Nevada; the 26th European College of Neuropsychopharmacology Congress, October 5-9, 2013, Barcelona, Spain; and the 2013 NEI Psychopharmacology Congress, November 14-17, 2013, Colorado Springs, Colorado.
Acknowledgments: The authors acknowledge the following individuals for their contributions to the study and/or development of the manuscript. Writing assistance and editorial support for the preparation of this manuscript were provided by Paul Ferguson, MS, and Jennifer Kaiser, PhD, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute, an Allergan affiliate. The investigators at each study center were as follows: United States: Jim G. Aukstuolis, MD, Woodland International Research Group, Inc.; Prakash Bhatia, MD, PhD, Synergy Clinical Research of Escondido; Steven J. Glass, MD, CRI Worldwide, LLC; Armen Goenjian, MD, CNS Network, LLC; Jelena Kunovac, MD, MS, Excell Research; Joseph Kwentus, MD, Precise Research Centers; Mark Lerman, MD, Alexian Brothers Center for Psychiatric Research; Michael Lesem, MD, Claghorn-Lesem Research Clinic, Ltd.; Adam F. Lowy, MD, Comprehensive Clinical Development, Inc.; Denis Mee-Lee, MD, Hawaii Clinical Research Center; Franco Sicuro, MD, Millenium Psychiatric Associates, LLC; and John G. Sonnenberg, PhD, Uptown Research Institute, LLC. Romania: Drs George Badescu, MD, PhD, Spitalul Clinic de Neuropsihiatrie Craiova; Doina Cosman, MD, PhD, Spitalul Clinic Judetean de Urgenta Cluj; Irina Ana Dan, MD, PhD, Spitalul Clinic de Psihiatrie “Prof Dr Alexandru Obregia”; Iosif Gabos, MD, PhD, Spitalul Clinic Judetean Mures; Gabriela Marian, MD, PhD, Spitalul Clinic de Psihiatrie “Prof Dr Alexandru Obregia”; Bogdan Pacala, MD, PhD, Spitalul de Psihiatrie Dr Gheorghe Preda” Sibiu; and Simona Tamasan, MD, PhD, Spitalul Clinic Judetean de Urgenta Timisoara. Russia: Drs Alexey Agarkov, MD, Regional State Healthcare Institution; Anatoly Bogdanov, MD, PhD, State Budget Healthcare Institution of the Arkhangelsk Region “Arkhangelsk Clinical Mental Hospital”; Olga Golubchikova, MD, PhD, State Healthcare Institution “Regional Clinical Specialized Psychoneurological Hospital #1”; Nikolay Govorin, MD, PhD, State Educational Institution of High Professional Education “Chita State Medical Academy of Roszdrav”; Mikhail Popov, MD, PhD, St. Petersburg State Healthcare Institution “City Mental Hospital # 3 named after I. I. Skvortsov-Stepanov”; and Olga Reshetko, MD, PhD, Municipal Healthcare Institution “City Clinical Hospital #2 named after V. I. Razumovsky.” Ukraine: Oleksandr Filts, MD, PhD, Lviv Regional Clinical Psychiatric Hospital; Pavlo Palamarchuk, MD, Kherson Regional Psychiatric Hospital; Vladlena Semenikhina, MD, I. I. Mecnikov Regional Clinical Hospital; and Viktoria Verbenko, MD, PhD, ScD, Crimean Republican Institution: Clinical Psychiatric Hospital #1.
Supplementary material: Available at Psychiatrist.com.
REFERENCES
1. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4: clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23. PubMed doi:10.1016/j.schres.2009.03.005
2. Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1081-1090. PubMed doi:10.1016/j.pnpbp.2003.09.004
3. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439. PubMed doi:10.1192/bjp.bp.109.066217
4. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5: treatment and prevention. past, present, and future. Schizophr Res. 2010;122(1-3):1-23. PubMed doi:10.1016/j.schres.2010.05.025
5. Keefe RS, Bilder RM, Davis SM, et al; Neurocognitive Working Group. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647. PubMed doi:10.1001/archpsyc.64.6.633
6. Leucht S, Arbter D, Engel RR, et al. How effective are second-generation antipsychotic drugs? a meta-analysis of placebo-controlled trials. Mol Psychiatry. 2009;14(4):429-447. PubMed doi:10.1038/sj.mp.4002136
7. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223. PubMed doi:10.1056/NEJMoa051688
8. Meltzer HY. Update on typical and atypical antipsychotic drugs. Annu Rev Med. 2013;64(1):393-406. PubMed doi:10.1146/annurev-med-050911-161504
9. Woodward ND, Purdon SE, Meltzer HY, et al. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8(3):457-472. PubMed doi:10.1017/S146114570500516X
10. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340. PubMed doi:10.1124/jpet.109.160432
11. Kiss B, Horti F, Bobok A. Cariprazine, a D3/D2 dopamine receptor partial agonist antipsychotic, displays greater D3 receptor occupancy in vivo compared with other antipsychotics. Schizophr Res. 2012;136(suppl 1):S190. doi:10.1016/S0920-9964(12)70588-1
12. Slifstein M, Abi-Dargham A, D’ Souza DC, et al. Cariprazine demonstrates high dopamine D3 and D2 receptor occupancy in patients with schizophrenia: a clinical PET study with [11C]- (+) -PHNO. Neuropsychopharmacology. 2013;38(S2):S520.
13. Cho DI, Zheng M, Kim KM. Current perspectives on the selective regulation of dopamine D₂ and D₃ receptors. Arch Pharm Res. 2010;33(10):1521-1538. PubMed doi:10.1007/s12272-010-1005-8
14. Gross G, Wicke K, Drescher KU. Dopamine D₃ receptor antagonism—still a therapeutic option for the treatment of schizophrenia. Naunyn Schmiedebergs Arch Pharmacol. 2013;386(2):155-166. PubMed doi:10.1007/s00210-012-0806-3
15. Laszy J, Laszlovszky I, Gyertyán I. Dopamine D3 receptor antagonists improve the learning performance in memory-impaired rats. Psychopharmacology (Berl). 2005;179(3):567-575. PubMed doi:10.1007/s00213-004-2096-z
16. Millan MJ, Buccafusco JJ, Loiseau F, et al. The dopamine D3 receptor antagonist, S33138, counters cognitive impairment in a range of rodent and primate procedures. Int J Neuropsychopharmacol. 2010;13(8):1035-1051. PubMed doi:10.1017/S1461145710000775
17. Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5(1):25-43. PubMed doi:10.2174/187152706784111551
18. Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10(13):917-925. PubMed doi:10.1016/S1359-6446(05)03491-4
19. Kiss B, Laszlovszky I, Horváth A, et al. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: I. neurochemical characterisation of RG-15. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):515-528. PubMed doi:10.1007/s00210-008-0308-5
20. Schwartz JC, Diaz J, Pilon C, et al. Possible implications of the dopamine D(3) receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31(2-3):277-287. PubMed doi:10.1016/S0165-0173(99)00043-0
21. Gyertyán I, Sághy K, Laszy J, et al. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: II: behavioural characterisation of RG-15. Naunyn Schmiedebergs Arch Pharmacol. 2008;378(5):529-539. PubMed doi:10.1007/s00210-008-0311-x
22. Durgam S, Starace A, Li D, et al. An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res. 2014;152(2-3):450-457. PubMed doi:10.1016/j.schres.2013.11.041
23. Kane JM, Zukin S, Wang Y, et al. Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol. 2015;35(4):367-373. PubMed
24. Guy W, ed. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Rockville, MD: National Institute of Mental Health, Psychopharmacology Research Branch; 1976:217-222.
25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
26. First MB, Williams JBW, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Clinical Trials Version (SCID-CT). New York, NY: New York State Psychiatric Institute, Biometrics Research; 2007.
27. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
28. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed doi:10.1176/appi.ajp.2011.10111704
29. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item Negative Symptom Assessment. J Psychiatr Res. 1993;27(3):253-258. PubMed doi:10.1016/0022-3956(93)90036-2
30. Martin CR, Allan R. Factor structure of the Schizophrenia Quality of Life Scale Revision 4 (SQLS-R4). Psychol Health Med. 2007;12(2):126-134. PubMed doi:10.1080/13548500500407383
31. Simpson PM, Surmon DJ, Wesnes KA, et al. The Cognitive Drug Research computerized Assessment System for demented patients: a validation study. Int J Geriatr Psychiatry. 1991;6(2):95-102. doi:10.1002/gps.930060208
32. D’ Elia LF, Satz P, Uchiyama CL, et al. Color Trails Test: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1996.
33. Guy W. The Abnormal Movement Scale. ECDEU Assessment Manual for Psychopharmacology. DHEW Publication No. 76-338. Rockville, MD: National Institute of Mental Health; 1976; 218-222.
34. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(5):672-676. PubMed doi:10.1192/bjp.154.5.672
35. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand suppl. 1970;212:11-19. PubMed
36. Chen X, Luo X, Capizzi T. The application of enhanced parallel gatekeeping strategies. Stat Med. 2005;24(9):1385-1397. PubMed doi:10.1002/sim.2005
37. Kenward MG, Molenberghs G, Thijs H. Pattern-mixture models with proper time dependence. Biometrika. 2003;90(1):53-71. doi:10.1093/biomet/90.1.53
38. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967. PubMed doi:10.1176/appi.ajp.2011.10060907
39. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829-836. PubMed doi:10.4088/JCP.08m04905
40. Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophr Bull. 1990;16(3):537-545. PubMed doi:10.1093/schbul/16.3.537
41. Mehta CR, Patel NR, Tsiatis AA. Exact significance testing to establish treatment equivalence with ordered categorical data. Biometrics. 1984;40(3):819-825. PubMed doi:10.2307/2530927
42. Johnson WD, May WL. Combining 2 x 2 tables that contain structural zeros. Stat Med. 1995;14(17):1901-1911. PubMed doi:10.1002/sim.4780141706
43. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2-3):231-238. PubMed doi:10.1016/j.schres.2005.04.008
44. Gross G, Drescher K. The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handbook Exp Pharmacol. 2012;213(213):167-210. PubMed doi:10.1007/978-3-642-25758-2_7
45. Conley RR, Ascher-Svanum H, Zhu B, et al. The burden of depressive symptoms in the long-term treatment of patients with schizophrenia. Schizophr Res. 2007;90(1-3):186-197. PubMed doi:10.1016/j.schres.2006.09.027
46. Ho BC, Nopoulos P, Flaum M, et al. Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am J Psychiatry. 1998;155(9):1196-1201. PubMed doi:10.1176/ajp.155.9.1196
47. Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162(3):495-506. PubMed doi:10.1176/appi.ajp.162.3.495
48. Savilla K, Kettler L, Galletly C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust N Z J Psychiatry. 2008;42(6):496-504. PubMed doi:10.1080/00048670802050512
49. Buchanan RW, Davis M, Goff D, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31(1):5-19. PubMed doi:10.1093/schbul/sbi020
50. Kirkpatrick B, Fenton WS, Carpenter WT Jr, et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214-219. PubMed doi:10.1093/schbul/sbj053
51. Newcomer JW, Haupt DW. The metabolic effects of antipsychotic medications. Can J Psychiatry. 2006;51(8):480-491. PubMed
52. Weber NS, Cowan DN, Millikan AM, et al. Psychiatric and general medical conditions comorbid with schizophrenia in the National Hospital Discharge Survey. Psychiatr Serv. 2009;60(8):1059-1067. PubMed doi:10.1176/ps.2009.60.8.1059
53. Durgam S, Starace A, Li D, et al. The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord. 2015;17(1):63-75. PubMed doi:10.1111/bdi.12238
This PDF is free for all visitors!
Save
Cite