ABSTRACT
Objective: Certain atypical antipsychotics, while efficacious as adjunctive treatments in major depressive disorder (MDD), are associated with metabolic adverse effects and weight gain. This analysis determined the effect of adjunctive brexpiprazole on metabolic parameters and body weight in adults with MDD and prediabetes (ie, at risk of developing diabetes) based on pooled data from 3 short-term studies and 1 long-term study.
Methods: The short-term studies were 6-week, randomized, double-blind, placebo-controlled studies of adjunctive oral brexpiprazole 1–3 mg/d in outpatients with MDD (DSM-IV-TR criteria) and inadequate response to antidepressant treatment, conducted between June 2011 and May 2016. The long-term study was a 26- to 52-week, open-label extension study conducted between October 2011 and May 2017. Prediabetes was defined based on fasting serum glucose and glycated hemoglobin (HbA1c) levels. Shifts in diabetes status and shifts/changes in fasting metabolic parameters and body weight were determined.
Results: Most patients receiving adjunctive brexpiprazole maintained their baseline diabetes status in the short term (568/751; 75.6%) and long term (1,919/2,746; 69.9%). The incidence of categorical shifts in fasting metabolic parameters generally did not differ between treatment groups or between prediabetes and non-diabetes subgroups. Mean changes from baseline in metabolic parameters were small in the short term (all < 5 mg/dL) and long term (all < 6 mg/dL, except < 20 mg/dL for triglycerides). Moderate weight gain was observed in the short term (1.5 kg) and long term (3.4–4.1 kg).
Conclusions: Adjunctive brexpiprazole had a limited impact on the metabolic profile of patients with MDD, regardless of diabetes status (prediabetes/non-diabetes).
Trial Registration: Data used in this post hoc analysis came from studies with ClinicalTrials.gov identifiers NCT01360645, NCT01360632, NCT02196506, and NCT01360866.
J Clin Psychiatry 2023;84(5):23m14786
Author affiliations are listed at the end of this article.
Patients with major depressive disorder (MDD) have an increased risk of developing metabolic syndrome (a combination of metabolic factors that increases the risk of developing type 2 diabetes mellitus and cardiovascular disease) and type 2 diabetes mellitus.1–5 One suggested contributor to this increased risk is that patients with depression may have unhealthy lifestyles and poor diet.3,6,7 It has also been hypothesized that depression and diabetes share common pathophysiologic alterations in the stress response system.3,4 In addition, the use of antidepressant treatments (ADTs) may be an independent risk factor for type 2 diabetes, potentially due to drug-related weight gain or via a direct effect on glucose metabolism by altering insulin resistance or secretion.8,9
Up to half of patients with MDD do not adequately respond to ADTs,10,11 and randomized trials show that the adjunctive administration of certain atypical antipsychotics can improve depressive symptoms in some of these patients.12 However, many antipsychotics are associated with metabolic adverse effects and weight gain that further increase the risk of developing diabetes.12–16 Consequently, in the population of patients with MDD for whom adjunctive antipsychotic treatment is deemed appropriate, it is vital that prescribing physicians select an agent that has minimal effect on metabolic parameters and weight—particularly among patients who may be clinically vulnerable to diabetes, such as those with prediabetes.
Brexpiprazole has a therapeutic action attributed to high-affinity partial agonism at serotonin 5-HT1A (Ki = 0.12 nM) and dopamine D2 (Ki = 0.30 nM) receptors and high-affinity antagonism at serotonin 5-HT2A (Ki = 0.47 nM) and norepinephrine α1B/α2C (Ki = 0.17/0.59 nM) receptors.17,18 Relative to its 5-HT1A/D2 receptor affinity, brexpiprazole has moderate-to-low affinity antagonism at histamine H1 receptors (Ki = 19 nM), limiting the risk for weight gain and diabetes.14,17 In clinical studies in patients with MDD and inadequate response to ADTs, the addition of brexpiprazole to ADT was efficacious and well-tolerated and was associated with only small changes in metabolic parameters (all < 2 mg/dL in the short term, and < 4 mg/dL in the long term, except triglycerides, 15.8 mg/dL) and moderate weight gain (approximately 1.5 kg in the short term and 3.8 kg in the long term).19,20 Although brexpiprazole has demonstrated a favorable metabolic profile in MDD, its metabolic profile in patients with MDD and prediabetes has not specifically been investigated.
The aim of this post hoc analysis was to determine the effect of brexpiprazole as an adjunctive treatment to ADT on metabolic parameters and body weight in adults with MDD and prediabetes compared with those who do not have prediabetes/diabetes (termed non-diabetes), based on pooled data from 3 short-term studies and 1 long-term extension study. It was hypothesized that adjunctive brexpiprazole would have limited impact on the metabolic profile of such patients.
METHODS
Study Design and Patients
The clinical studies included in this analysis were conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guideline and local regulatory requirements. The study protocols were approved by independent ethics committees or institutional review boards at each site/country. All patients provided written informed consent to participate after procedures and possible side effects were explained to them.
The 3 short-term studies included in the present analysis were conducted in North America and Europe between June 2011 and May 2016 (ClinicalTrials.gov identifiers: NCT01360645, NCT01360632, and NCT02196506). The methods have been published in detail for each study21–23 and are described in brief in this section.
These were randomized, double-blind, placebo-controlled studies with similar designs. The studies enrolled adult outpatients with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) diagnosis of single or recurrent nonpsychotic MDD, a current depressive episode of ≥ 8 weeks in duration, and inadequate response to 1–3 prior ADTs during the current episode, for which inadequate response was defined as < 50% improved according to the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire.24 Exclusion criteria included treatment with an adjunctive antipsychotic medication during the current episode, suicidal ideation or behavior, substance abuse or dependence, hallucinations or delusions during the current episode, another specified DSM-IV-TR disorder, or a clinically significant medical condition. Patients with insulin-dependent diabetes mellitus (IDDM; ie, any patients using insulin) were excluded, as were patients with non-IDDM who had glycated hemoglobin (HbA1c) ≥ 7.0% or screening glucose > 125 mg/dL (fasting) or ≥ 200 mg/dL (non-fasting). Minor or well-controlled medical conditions were permitted if not expected to interfere with safety or efficacy assessments. The following medications were prohibited during the studies: psychotropic agents (including but not limited to antipsychotics other than the study drug, antidepressants other than those prescribed as part of the study, mood stabilizers, and anticonvulsants), investigational agents, cytochrome P450 2D6 (CYP2D6) inhibitors, CYP3A4 inhibitors and inducers, and barbiturates.
Eligible patients received 1 of 6 investigator-determined, open-label ADTs (escitalopram, fluoxetine, paroxetine controlled-release, sertraline, duloxetine, or venlafaxine extended-release) + single-blind placebo for 8 weeks, during which their response was prospectively assessed. Patients who responded to ADT + placebo based on rating scale score thresholds (clinician-rated depression and global improvement) continued on the same ADT at the same dose for another 6 weeks, whereas patients with inadequate response to ADT + placebo entered a randomized treatment phase. In the randomized phase, patients continued the same ADT at the same dose and were randomized to double-blind treatment with adjunctive brexpiprazole (fixed doses in the range of 1–3 mg/d) or placebo for 6 weeks.
The long-term safety study was conducted in North America and Europe between October 2011 and May 2017 (ClinicalTrials.gov identifier: NCT01360866). The methods have been published in detail.25 In brief, this was an open-label extension study that enrolled patients who had completed the last scheduled visit of 1 of 3 short-term studies,21,22,26 regardless of whether or not they had been randomized in the short-term study. Eligible patients continued the same ADT they received in the parent study (dose adjustments were permitted) and were administered adjunctive brexpiprazole 0.5–3 mg/d (flexible dose). Prohibited medications were the same as for the short-term studies. The study duration was originally 52 weeks, but was amended to 26 weeks midway through because the safety profile of brexpiprazole was considered to be well established.
Assessments
The focus of this analysis was fasting metabolic parameters, especially those relating to prediabetes: serum HbA1c and glucose (diagnostic for diabetes/prediabetes), as well as triglycerides and high-density lipoprotein (HDL) cholesterol (which are characteristically altered in metabolic syndrome and diabetes27,28). For completeness, fasting serum total and low-density lipoprotein (LDL) cholesterol were analyzed, as well as fasting insulin to assess insulin resistance. In the short-term studies, a fasting blood draw was collected for clinical laboratory tests including metabolic parameters at randomization and at week 6 of the randomized phase, as well as at weeks 2 and 4 in two of the three studies. Body weight was measured at randomization and at weekly visits. In the long-term study, metabolic parameters and body weight were measured at multiple visits from week 1 to week 26 (post-amendment) or week 52 (pre-amendment).
The present analysis also investigated whether adjunctive brexpiprazole remains an efficacious treatment option for patients with MDD and inadequate response to ADTs, regardless of prediabetes status. Efficacy was measured using the Montgomery-Åsberg Depression Rating Scale (MADRS), a 10-item, clinician-rated measure of depressive symptom severity in MDD, with total scores ranging from 0 (least severe) to 60 (most severe).29 The MADRS was completed at randomization and at weekly intervals during the randomized phase.
Data Analysis
In this post hoc analysis, patients were split into subgroups according to diabetes status at baseline based on definitions adapted from the American Diabetes Association (plasma definitions were applied to serum data).30 Non-diabetes was defined as fasting serum glucose < 100 mg/dL and HbA1c < 5.7%. Prediabetes was defined as fasting serum glucose ≥ 100 mg/dL and < 125 mg/dL or HbA1c ≥ 5.7% and < 6.5%. Diabetes was defined as fasting serum glucose ≥ 125 mg/dL or HbA1c ≥ 6.5%. However, since patients with fasting serum glucose > 125 mg/dL or HbA1c ≥ 7.0% were excluded from the studies, only those with HbA1c ≥ 6.5% and < 7.0% met the criteria for diabetes (approximately 4% of patients); these patients were included in analyses of shift in diabetes status, but were not analyzed for changes in metabolic parameters due to the low number of patients and because this was beyond the scope of the study. Patients without a baseline fasting serum glucose or HbA1c measurement were also excluded from the present analyses.
Shifts in diabetes status and HbA1c status were determined in the short- and long-term samples from baseline to last visit using the American Diabetes Association definitions.30 Categorical shifts in fasting metabolic parameters (glucose, triglycerides, cholesterol) were determined for the following time periods: from baseline to any post-baseline visit during the first 6 weeks (short-term studies), from baseline to any post-baseline visit up to week 26 (ie, the first 6 months of treatment), and from week 26 to any post-week 26 visit (ie, the last 6 months of treatment). Glucose category definitions were those of the American Diabetes Association30; triglyceride and cholesterol category definitions were adapted from guidelines of the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III.31 Mean changes in metabolic parameters from baseline were also determined. To assess insulin resistance, mean homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as: (insulin [µU/mL] × glucose [mmol/L])/22.5.32 For body weight, mean changes from baseline and the proportions with clinically relevant change from baseline to any post-baseline visit were calculated. All analyses used observed-cases data.
In the short-term analysis, data were pooled for all patients randomized to ADT + brexpiprazole (any dose) and similarly for all patients randomized to ADT + placebo. The baseline value was defined as the last value prior to randomization. The long-term analysis included a greater number of patients than the short-term analysis since the extension study enrolled patients regardless of whether or not they were randomized in the short-term studies. In the long-term analysis, the baseline value was defined as the last value obtained prior to the start of adjunctive brexpiprazole treatment, whether at the start of the extension study (for those patients who had rolled over from adjunctive placebo) or at the start of the parent study (for those patients who had rolled over from adjunctive brexpiprazole). Thus, long-term data covered up to 58 weeks of brexpiprazole exposure (ie, 0 or 6 weeks from the parent study, plus up to 52 weeks from the extension study). Metabolic and weight analyses were performed on the safety sample, defined per final protocol in the short-term studies as all patients who received at least 1 dose of randomized treatment (brexpiprazole or placebo) and in the long-term analysis as all patients who received at least 1 dose of brexpiprazole.
In the short-term efficacy analysis, mean change in MADRS total score was calculated using a mixed model for repeated measures (MMRM) with terms of treatment, site nested within trial, visit, treatment by visit, and baseline by visit interaction for ADT + brexpiprazole 2–3 mg/d (the recommended-to-maximum dose for MDD in some countries33) and ADT + placebo. Efficacy analyses were performed on the efficacy sample per final protocol, defined as all patients in the safety sample with a baseline and post-baseline MADRS evaluation.
All analyses were performed using SAS Enterprise 8.1 software (SAS Institute Inc; Cary, NC).
RESULTS
Patients
In the short-term analysis, 743 of 797 patients (93.2%) randomized to brexpiprazole 1–3 mg and 557 of 586 patients (95.1%) randomized to placebo completed 6 weeks of treatment. In the long-term study (which was amended to a 26-week duration midway through), 2,132 of 2,938 patients (72.6%) in the safety sample received ADT + brexpiprazole for at least 6 months and 781 of 2,938 (26.6%) for at least 12 months.
During the short-term studies, lipid-modifying agents (eg, statins) were used by 124 of 1,383 randomized patients (9.0%), and drugs for diabetes (eg, metformin) were used by 41 of 1,383 randomized patients (3.0%). During the long-term study, lipid-modifying agents were used by 263 of 2,938 patients (9.0%), and drugs for diabetes were used by 97 of 2,938 patients (3.3%).
At baseline in the short-term analysis, across treatment groups, 831 patients (62.0%) did not have diabetes, 456 (34.0%) had prediabetes, and 54 (4.0%) had diabetes. At baseline in the long-term analysis, 1,712 patients (62.3%) did not have diabetes, 932 (33.9%) had prediabetes, and 102 (3.7%) had diabetes.
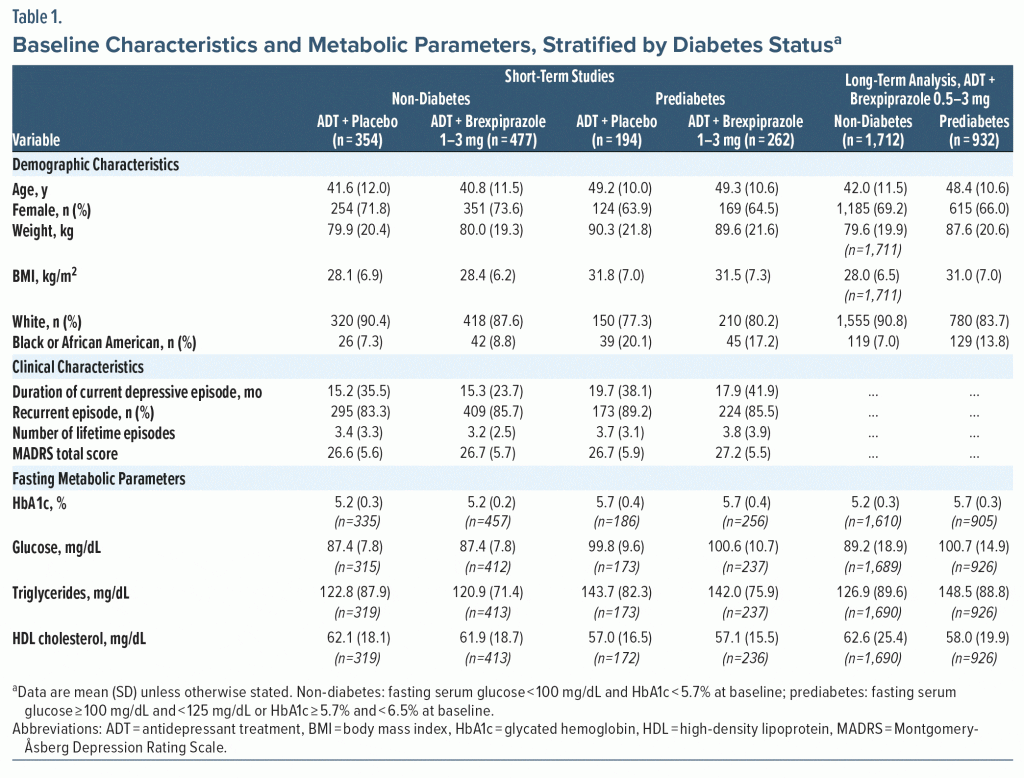
At baseline, on average, the prediabetes subgroups were older, had higher body weight and body mass index (BMI), and had a greater proportion of Black/African American patients than the non-diabetes subgroups (Table 1). Baseline clinical (ie, depression) characteristics were similar between the non-diabetes and prediabetes subgroups (Table 1).
Baseline mean fasting glucose (by definition) and triglycerides were higher in the prediabetes subgroups than in the non-diabetes subgroups, and mean fasting HDL was lower in the prediabetes subgroups than the non-diabetes subgroups (Table 1).
There were no notable differences in baseline characteristics between the ADT + brexpiprazole and ADT + placebo groups (Table 1).
Diabetes Status Changes
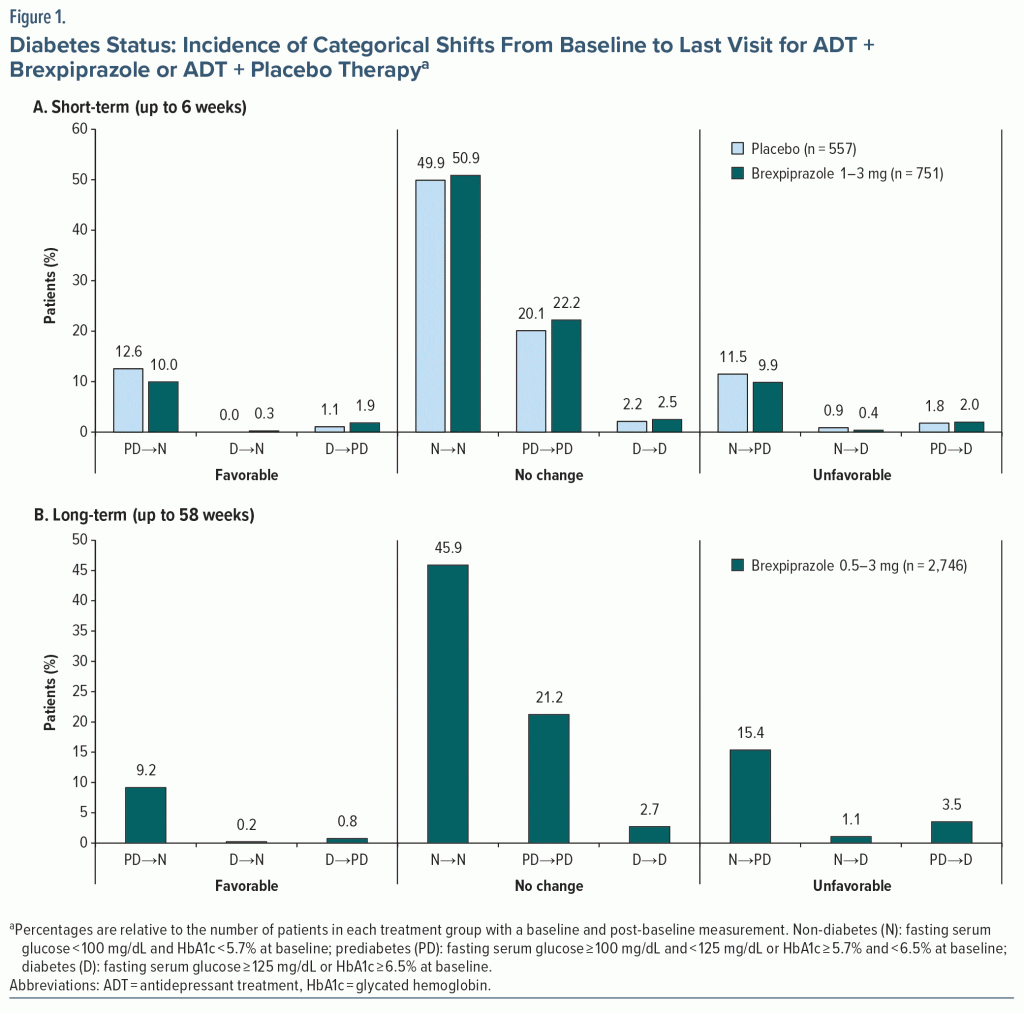
Diabetes categorical shifts. At last visit in the short-term studies (up to 6 weeks), the majority of patients maintained their baseline diabetes status (74.2%; 970/1,308), with no notable differences between the ADT + brexpiprazole group (75.6%; 568/751) and the ADT + placebo group (72.2%; 402/557) (Figure 1A).
Similarly, at last visit in the long-term analysis (up to 58 weeks), the majority of patients receiving ADT + brexpiprazole maintained their baseline diabetes status (69.9%; 1,919/2,746) (Figure 1B). The most common shifts in the long-term analysis were non-diabetes to prediabetes (15.4%; 422/2,746) and prediabetes to non-diabetes (9.2%; 252/2,746).
HbA1c categorical shifts. At last visit in the short-term studies (up to 6 weeks), 90.6% of patients (585/646) in the non-diabetes subgroup remained in their baseline HbA1c category (ie, non-diabetic HbA1c), with no notable differences between treatment groups. Similarly, 76.7% of patients (283/369) in the prediabetes subgroup remained in their baseline HbA1c category (ie, prediabetic or non-diabetic HbA1c), and 16.0% (59/369) shifted from prediabetic HbA1c to non-diabetic HbA1c, with no notable differences between treatment groups. Other possible shifts each occurred in < 10% of patients.
At last visit in the long-term analysis (up to 58 weeks), in the non-diabetes subgroup, 66.7% of patients (394/591) remained in their baseline HbA1c category (ie, non-diabetic HbA1c), 33.2% (196/591) shifted from non-diabetic HbA1c to prediabetic HbA1c, and 0.2% (1/591) shifted from non-diabetic HbA1c to diabetic HbA1c. In the prediabetes subgroup, 67.3% of patients (602/895) remained in their baseline HbA1c category, 20.4% (183/895) shifted from prediabetic HbA1c to non-diabetic HbA1c, 6.4% (57/895) shifted from non-diabetic HbA1c to prediabetic HbA1c, 5.8% (52/895) shifted from prediabetic HbA1c to diabetic HbA1c, and 0.1% (1/895) shifted from non-diabetic HbA1c to diabetic HbA1c.
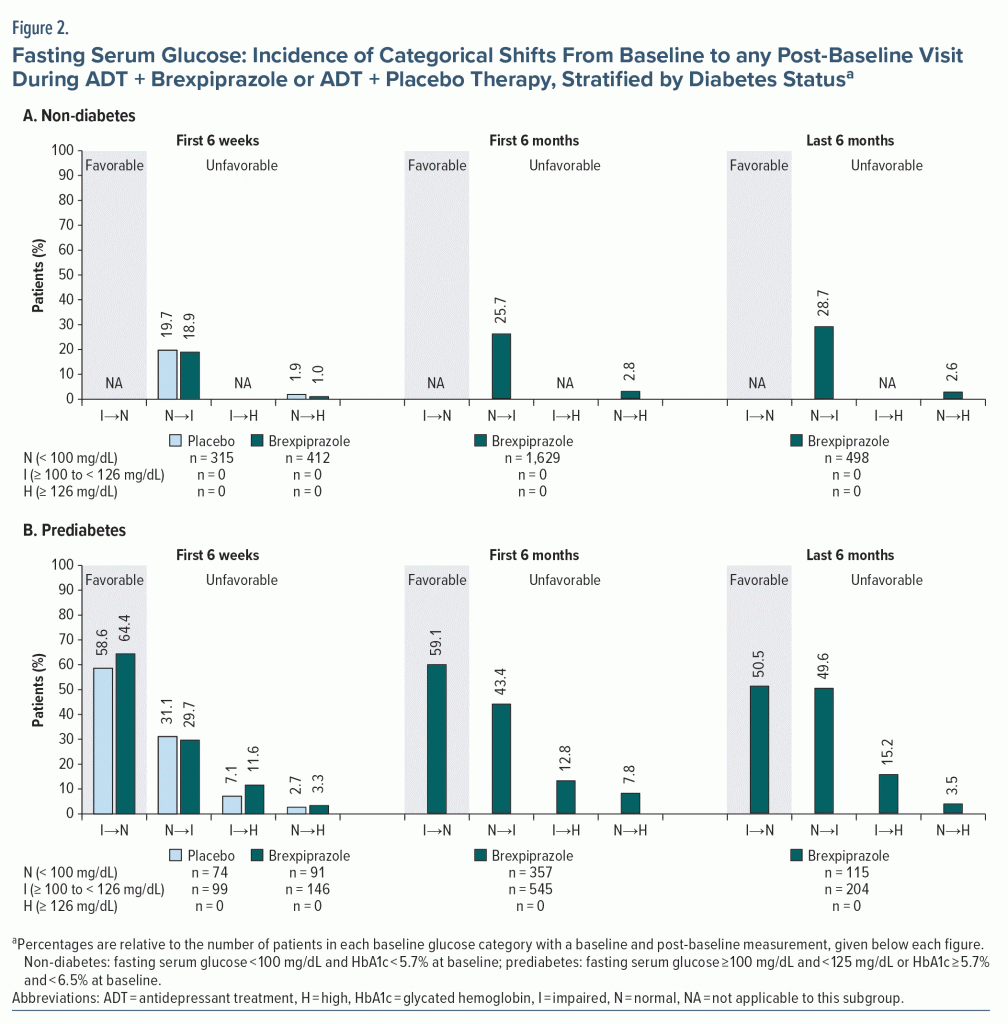
Fasting glucose categorical shifts. There were no notable differences between treatment groups in the incidence of favorable and unfavorable shifts in fasting glucose (Figure 2). By definition, only unfavorable shifts were possible for patients who did not have diabetes. Among patients with prediabetes, the proportion who shifted from impaired to normal glucose was similar to or greater than the proportion who shifted from normal to impaired glucose.
Fasting glucose mean changes. Mean changes in glucose from baseline to week 6 and week 52 (up to 58 weeks of adjunctive brexpiprazole treatment) were small in the non-diabetes and prediabetes subgroups (all < 4 mg/dL) (Supplementary Table 1).
Serum Lipid Changes
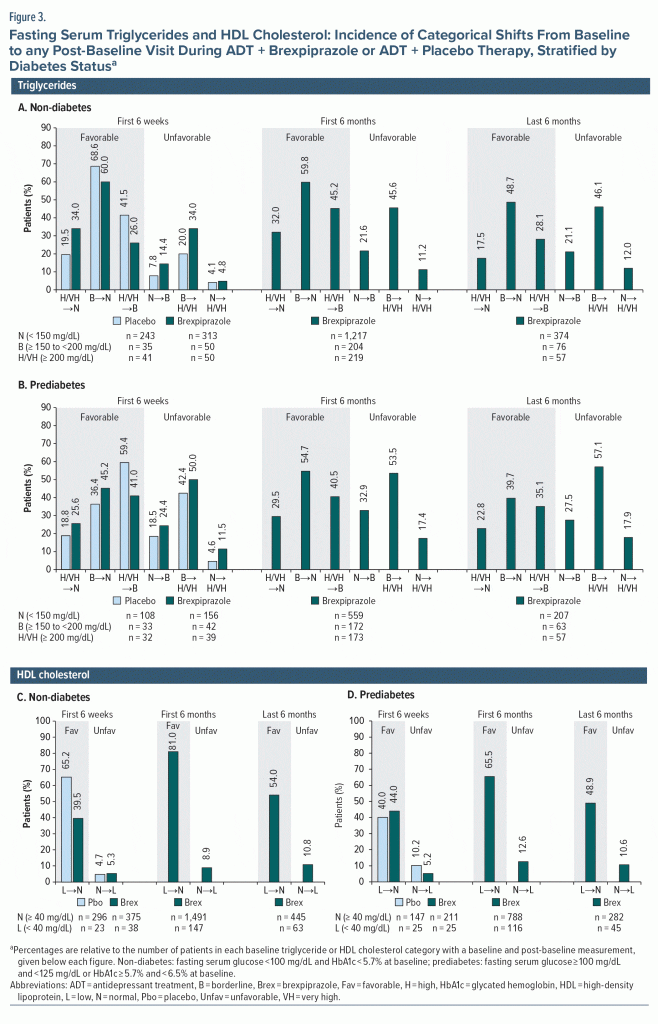
Fasting triglyceride and cholesterol categorical shifts. There were no notable differences between the treatment groups, or between the non-diabetes and prediabetes subgroups, in the incidence of favorable and unfavorable shifts in triglycerides (Figure 3A and Figure 3B), HDL cholesterol (Figure 3C and Figure 3D), total cholesterol (Supplementary Figure 1), or LDL cholesterol (Supplementary Figure 2), except where influenced by low patient numbers in a subgroup.
For most serum lipid parameters, the overall proportion of patients with favorable shifts was similar to or greater than the proportion with unfavorable shifts. An exception was serum LDL cholesterol, in which 57.4% (non-diabetes subgroup) and 65.8% (prediabetes subgroup) of patients with normal LDL cholesterol at baseline shifted to borderline LDL cholesterol over the last 6 months.
Fasting triglyceride and cholesterol mean changes. Mean changes from baseline in serum lipid parameters after 6 weeks of ADT + brexpiprazole or ADT + placebo therapy were small in the non-diabetes and prediabetes subgroups (all < 5 mg/dL) (Supplementary Table 1).
Mean changes in serum lipid parameters from baseline to week 52 (up to 58 weeks of adjunctive brexpiprazole treatment) were generally small (all < 6 mg/dL) and similar between the subgroups, except for triglycerides: non-diabetes subgroup, 18.3 mg/dL; prediabetes subgroup, 12.9 mg/dL (Supplementary Table 1).
HOMA-IR Changes
In the short-term analysis, mean (SD) HOMA-IR at baseline was 1.1 (0.1) in the non-diabetes subgroups (ADT + brexpiprazole, n = 425; ADT + placebo, n = 310) and 1.4 (0.2) in the prediabetes subgroups (ADT + brexpiprazole, n = 243; ADT + placebo, n = 177). Mean (SD) HOMA-IR scores did not change from baseline to last visit.
In the long-term analysis, in the non-diabetes subgroup, mean (SD) HOMA-IR was 1.1 (0.1) at baseline (n = 1,516), 1.1 (0.1) at week 26 (n = 952), and 1.1 (0.1) at week 52 (n = 338). In the prediabetes subgroup, mean (SD) HOMA-IR was 1.4 (0.2) at baseline (n = 868), 1.4 (0.2) at week 26 (n = 654), and 1.4 (0.2) at week 52 (n = 312).
Weight Changes
Considering body weight, mean (SD) increases from baseline to last visit of the short-term studies in the non-diabetes subgroups were 1.5 (2.2) kg (ADT + brexpiprazole, n = 476) and 0.3 (1.7) kg (ADT + placebo, n = 353); corresponding values in the prediabetes subgroups were 1.5 (2.2) kg (n = 260) and 0.5 (1.8) kg (n = 194), respectively. The proportion of patients with ≥ 7% weight increase from baseline to any post-baseline visit was low (< 5% of patients in each subgroup), and very few patients (< 1%) had ≥ 10% weight increase or ≥ 7% weight decrease from baseline to any post-baseline visit.
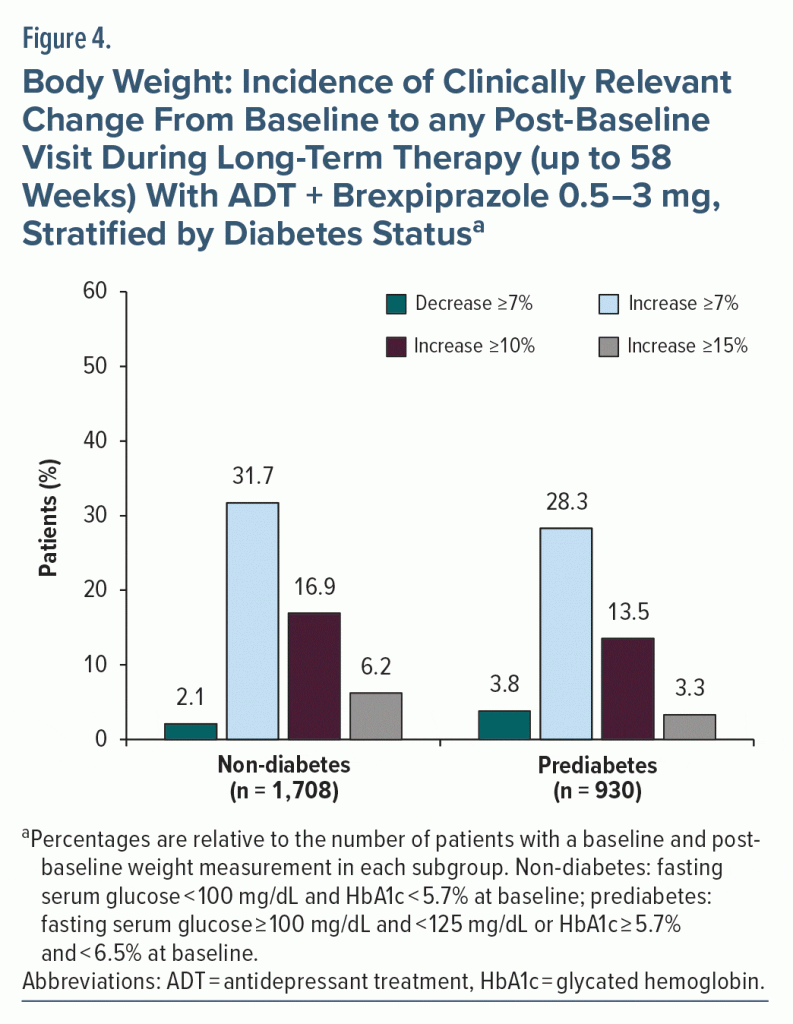
With long-term treatment, the mean (SD) body weight increase from baseline to week 26 (up to 32 weeks of adjunctive brexpiprazole treatment) was 3.1 (4.3) kg in the non-diabetes subgroup (n = 1,185) and 2.9 (4.3) kg in the prediabetes subgroup (n = 687). At week 52 (up to 58 weeks of adjunctive brexpiprazole treatment), mean weight increase was 4.1 (5.9) kg in the non-diabetes subgroup (n = 432) and 3.4 (5.3) kg in the prediabetes subgroup (n = 296). The proportion of patients with clinically relevant weight change from baseline was comparable between subgroups (Figure 4).
Efficacy
Over 6 weeks, in the non-diabetes subgroup, MADRS total score improved by a least-squares (LS) mean (SE) of −9.4 (0.5) points in the ADT + brexpiprazole 2–3 mg group and −6.6 (0.5) points in the ADT + placebo group, with an LS mean difference of −2.89 points (95% CI, −4.10 to −1.67; P < .0001). In the prediabetes subgroup, improvements were −8.6 (0.7) points in the ADT + brexpiprazole 2–3 mg group and −6.6 (0.6) points in the ADT + placebo group, with an LS mean difference of −2.01 (95% CI, −3.78 to −0.24; P = .026).
DISCUSSION
In this analysis of over 2,000 patients with MDD from several clinical trials, adjunctive brexpiprazole had a small effect on metabolic parameters and a moderate effect on weight, regardless of diabetes status (prediabetes or non-diabetes).
Patients with prediabetes were identified using baseline serum glucose and HbA1c levels, as defined by the American Diabetes Association.30 At baseline, a large proportion of the sample had prediabetes (34%), putting them at high risk of developing diabetes.30 Over 6 weeks, the majority of patients maintained their baseline diabetes status, regardless of whether they received ADT + brexpiprazole or ADT + placebo. Similarly, over the long term (≤ 58 weeks of ADT + brexpiprazole therapy), most patients (69.9%) maintained their baseline diabetes status, and the proportions with favorable and unfavorable shifts were similar, indicating that treatment did not adversely affect their risk. HbA1c, which indicates average glucose over the previous 8–12 weeks,34 followed a similar pattern of categorical shifts to overall diabetes status, which is unsurprising given that diabetic status is defined based on HbA1c in addition to blood glucose concentration.
Considering fasting glucose levels among patients with prediabetes, the incidence of a favorable shift from impaired to normal glucose was greater than the corresponding unfavorable shift, differences from adjunctive placebo were small, and mean changes in fasting glucose were minimal, suggesting that there was no adverse effect of treatment with adjunctive brexpiprazole. Minimal effect of treatment on glucose was also observed in the non-diabetes group. Increased triglycerides and decreased HDL cholesterol, characteristic of metabolic syndrome and diabetes,27,28 were observed at baseline among patients with prediabetes. In most cases, during ADT + brexpiprazole therapy, the overall proportion of patients with favorable shifts was similar to or greater than the proportion with unfavorable shifts, regardless of diabetes status. Mean changes in triglycerides were < 20 mg/dL, and mean changes in HDL cholesterol were small in all subgroups.
HOMA-IR over the long term indicated no mean change in insulin resistance in the non-diabetes subgroup (1.1) or the prediabetes subgroup (1.4). In longitudinal studies, a HOMA-IR cutoff of approximately 2 has been suggested to identify type 2 diabetes.35,36
Other adjunctive atypical antipsychotics used in MDD show varying risks for abnormal metabolic parameters in clinical trials, with quetiapine and olanzapine-fluoxetine combination having the highest risk.12,37 In general, antipsychotics are thought to increase the risk of diabetes via weight gain and/or a direct effect on insulin resistance or secretion.16 Across diagnoses, real-world data suggest that duration of antipsychotic treatment could be a relevant factor for the occurrence of diabetes.38 In a study of adolescents and young adults with no history of diabetes who were exposed to second-generation antipsychotics (amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone), the highest risk of developing diabetes was among patients who received a total of ≥ 365 daily doses.39 Thus, a strength of the present study is that it assessed up to 58 weeks of data.
With regard to weight, adjunctive brexpiprazole was associated with moderate weight gain that was comparable between the non-diabetes and prediabetes subgroups. Following a mean increase in weight of approximately 1.5 kg in the short-term studies, comparable to that reported in clinical trials of adjunctive aripiprazole, quetiapine, and risperidone,12,40 mean weight increased by another 1.5 kg (approximately) to week 26 and then showed changes ≤ 1 kg over the final 6 months of the study. Previously, adjunctive brexpiprazole and adjunctive aripiprazole have shown a similar effect on weight in long-term clinical trials in MDD.40 Across diagnoses, a study of long-term, real-world, outpatient data suggested that the weight gain associated with brexpiprazole may be lower than with olanzapine, comparable to cariprazine and iloperidone, and higher than with asenapine and lurasidone.41 A second study of long-term, real-world, outpatient data that did not include brexpiprazole found that olanzapine was associated with a larger weight increase than quetiapine and risperidone, which had comparable weight increases.42
Adjunctive brexpiprazole was efficacious on clinician-rated depressive symptoms over 6 weeks among patients with prediabetes and patients without diabetes, and the magnitude of effect was similar to that previously reported for adjunctive brexpiprazole.19 Thus, adjunctive brexpiprazole remains a viable treatment option for patients with MDD, inadequate response to ADTs, and prediabetes, thereby helping to meet the clinical need for an efficacious antipsychotic with a favorable metabolic profile. Long-term continuation of successful MDD treatment is often recommended to prevent relapse and to treat residual symptoms.43 The optimal duration of efficacious adjunctive atypical antipsychotic therapy in MDD has not been established, and clinicians should monitor each patient’s weight and metabolic status as well as their depression status over time.44
Limitations of this post hoc analysis include that, due to the lack of an adjunctive placebo arm in the long-term analysis, it was not possible to separate the effects of ADT and brexpiprazole on metabolic parameters and weight. Of note, ADT use is itself associated with an increased risk of diabetes.8,9 A small proportion of patients were taking concomitant drugs for metabolic conditions, which may have impacted the metabolic outcomes. With regard to the generalizability of the results to real-world populations, clinical trial data are limited by their study inclusion and exclusion criteria. In particular, most patients with diabetes were excluded from the present trials, meaning that there was insufficient power to assess metabolic parameters in patients with diabetes. However, the inclusion of patients with other minor or well-controlled medical conditions increased the generalizability of the results. Finally, the included patients had a high mean BMI (28–32 kg/m2), which affects generalizability to populations associated with lower BMIs.
In conclusion, among patients with baseline prediabetes, adjunctive brexpiprazole was associated with small changes in metabolic parameters and moderate weight gain during short- and long-term treatment, which are unlikely to be clinically meaningful. Results were comparable between subgroups with and without prediabetes, suggesting that adjunctive brexpiprazole had a limited impact on the metabolic profile of patients with MDD, regardless of diabetes status (prediabetes or non-diabetes).
Article Information
Published Online: August 28, 2023. https://doi.org/10.4088/JCP.23m14786
© 2023 Physicians Postgraduate Press, Inc.
Submitted: January 6, 2023; accepted June 26, 2023.
To Cite: Newcomer JW, Meehan SR, Chen D, et al. Changes in metabolic parameters and body weight in patients with prediabetes treated with adjunctive brexpiprazole for major depressive disorder: pooled analysis of short- and long-term clinical studies. J Clin Psychiatry. 2023;84(5):23m14786.
Author Affiliations: Thriving Mind South Florida, Miami, Florida (Newcomer); Department of Psychiatry, Washington University School of Medicine, St. Louis, Missouri (Newcomer); H. Lundbeck A/S, Valby, Denmark (Meehan); Otsuka Pharmaceutical Development & Commercialization Inc., Princeton, New Jersey (Chen, Brubaker, Weiss). Dr Weiss is now retired.
Corresponding Author: Stine R. Meehan, PhD, Ottiliavej 9, 2500 Valby, Denmark ([email protected]).
Relevant Financial Relationships: Dr Newcomer has received grant support from the National Institutes of Health and the Substance Abuse and Mental Health Services Administration and has served as a consultant for Alkermes, Inc.; Merck; Otsuka; and Sunovion and on a Data Safety Monitoring Board for Amgen. Dr Meehan is a full-time employee of H. Lundbeck A/S. Drs Chen and Brubaker are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Dr Weiss was a full-time employee of Otsuka Pharmaceutical Development & Commercialization Inc. at the time that this work was conducted.
Funding/Support: This work was supported by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, NJ, USA) and H. Lundbeck A/S (Valby, Denmark).
Role of the Funders/Sponsors: Authors affiliated with the sponsors were involved in the design of the study, the analysis and interpretation of data, and the writing and reviewing of this article.
Acknowledgments: Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge—a Prime Global Agency (Knutsford, UK), and funded by Otsuka Pharmaceutical Development & Commercialization Inc. and H. Lundbeck A/S.
Previous Presentation: Poster presented at the American Society of Clinical Psychopharmacology (ASCP) annual meeting, virtual conference; June 1–4, 2021.
Supplementary Material: Available at Psychiatrist.com.
Clinical Points
- Patients with prediabetes and major depressive disorder treated with adjunctive brexpiprazole may experience small changes in metabolic parameters and moderate weight gain over the short and long term, which are unlikely to be clinically meaningful.
- Adjunctive brexpiprazole can improve depressive symptoms in patients with prediabetes and major depressive disorder.
References (44)

- Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121(11 suppl 2):S8–S15. PubMed CrossRef
- Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. PubMed CrossRef
- Marazziti D, Rutigliano G, Baroni S, et al. Metabolic syndrome and major depression. CNS Spectr. 2014;19(4):293–304. PubMed CrossRef
- Berge LI, Riise T. Comorbidity between type 2 diabetes and depression in the adult population: directions of the association and its possible pathophysiological mechanisms. Int J Endocrinol. 2015;2015:164760. PubMed CrossRef
- Moradi Y, Albatineh AN, Mahmoodi H, et al. The relationship between depression and risk of metabolic syndrome: a meta-analysis of observational studies. Clin Diabetes Endocrinol. 2021;7(1):4. PubMed CrossRef
- Bonnet F, Irving K, Terra JL, et al. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178(2):339–344. PubMed CrossRef
- Bonnet F, Irving K, Terra JL, et al. Depressive symptoms are associated with unhealthy lifestyles in hypertensive patients with the metabolic syndrome. J Hypertens. 2005;23(3):611–617. PubMed CrossRef
- Barnard K, Peveler RC, Holt RIG. Antidepressant medication as a risk factor for type 2 diabetes and impaired glucose regulation: systematic review. Diabetes Care. 2013;36(10):3337–3345. PubMed CrossRef
- Salvi V, Grua I, Cerveri G, et al. The risk of new-onset diabetes in antidepressant users – a systematic review and meta-analysis. PLoS One. 2017;12(7):e0182088. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34–40. PubMed CrossRef
- Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed CrossRef
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1–93. PubMed CrossRef
- Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13(1):27–35. PubMed CrossRef
- Manu P, Correll CU, van Winkel R, et al. Prediabetes in patients treated with antipsychotic drugs. J Clin Psychiatry. 2012;73(4):460–466. PubMed CrossRef
- Holt RIG. Association between antipsychotic medication use and diabetes. Curr Diab Rep. 2019;19(10):96. PubMed CrossRef
- Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589–604. PubMed CrossRef
- Han C, Wang SM, Kato M, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013;13(7):851–870. PubMed CrossRef
- Thase ME, Zhang P, Weiss C, et al. Efficacy and safety of brexpiprazole as adjunctive treatment in major depressive disorder: overview of four short-term studies. Expert Opin Pharmacother. 2019;20(15):1907–1916. PubMed CrossRef
- Newcomer JW, Eriksson H, Zhang P, et al. Changes in metabolic parameters and body weight in patients with major depressive disorder treated with adjunctive brexpiprazole: pooled analysis of phase 3 clinical studies. J Clin Psychiatry. 2019;80(6):18m12680. PubMed CrossRef
- Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224–1231. PubMed CrossRef
- Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9):1232–1240. PubMed CrossRef
- Hobart M, Skuban A, Zhang P, et al. A randomized, placebo-controlled study of the efficacy and safety of fixed-dose brexpiprazole 2 mg/d as adjunctive treatment of adults with major depressive disorder. J Clin Psychiatry. 2018;79(4):17m12058. PubMed CrossRef
- Chandler GM, Iosifescu DV, Pollack MH, et al. Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322–325. PubMed CrossRef
- Hobart M, Zhang P, Skuban A, et al. A long-term, open-label study to evaluate the safety and tolerability of brexpiprazole as adjunctive therapy in adults with major depressive disorder. J Clin Psychopharmacol. 2019;39(3):203–209. PubMed CrossRef
- Hobart M, Skuban A, Zhang P, et al. Efficacy and safety of flexibly dosed brexpiprazole for the adjunctive treatment of major depressive disorder: a randomized, active-referenced, placebo-controlled study. Curr Med Res Opin. 2018;34(4):633–642. PubMed CrossRef
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):15019. PubMed CrossRef
- Schofield JD, Liu Y, Rao-Balakrishna P, et al. Diabetes dyslipidemia. Diabetes Ther. 2016;7(2):203–219. PubMed CrossRef
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(suppl 1):S14–S31. PubMed CrossRef
- National Cholesterol Education Program. ATP III Guidelines At-A-Glance Quick Desk Reference. Bethesda, MD: National Institutes of Health (NIH), National Heart, Lung, and Blood Institute; 2001;01-3305.
- Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47. PubMed CrossRef
- Rexulti (brexpiprazole) tablets, for oral use [Prescribing information]. Otsuka Pharmaceutical Co, Ltd. https://www.otsuka-us.com/media/static/Rexulti-PI.pdf. 2023. Accessed May 26, 2023.
- Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50(11):2239–2244. PubMed CrossRef
- Ghasemi A, Tohidi M, Derakhshan A, et al. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran lipid and glucose study. Acta Diabetol. 2015;52(5):905–915. PubMed CrossRef
- Lee CH, Shih AZL, Woo YC, et al. Optimal cut-offs of homeostasis model assessment of insulin resistance (HOMA-IR) to identify dysglycemia and type 2 diabetes mellitus: a 15-year prospective study in Chinese. PLoS One. 2016;11(9):e0163424. PubMed CrossRef
- Brunner E, Tohen M, Osuntokun O, et al. Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine monotherapy following successful combination therapy of treatment-resistant major depressive disorder. Neuropsychopharmacology. 2014;39(11):2549–2559. PubMed CrossRef
- Bernardo M, Rico-Villademoros F, García-Rizo C, et al. Real-world data on the adverse metabolic effects of second-generation antipsychotics and their potential determinants in adult patients: a systematic review of population-based studies. Adv Ther. 2021;38(5):2491–2512. PubMed CrossRef
- Tu TH, Huang KL, Bai YM, et al. Exposure to second-generation antipsychotics and risk of type 2 diabetes mellitus in adolescents and young adults: a nationwide study in Taiwan. J Clin Psychiatry. 2019;80(2):18m12284. PubMed CrossRef
- Weiss C, Weiller E, Baker RA, et al. The effects of brexpiprazole and aripiprazole on body weight as monotherapy in patients with schizophrenia and as adjunctive treatment in patients with major depressive disorder: an analysis of short-term and long-term studies. Int Clin Psychopharmacol. 2018;33(5):255–260. PubMed CrossRef
- Greger J, Aladeen T, Lewandowski E, et al. Comparison of the metabolic characteristics of newer second generation antipsychotics: brexpiprazole, lurasidone, asenapine, cariprazine, and iloperidone with olanzapine as a comparator. J Clin Psychopharmacol. 2021;41(1):5–12. PubMed CrossRef
- Bazo-Alvarez JC, Morris TP, Carpenter JR, et al. Effects of long-term antipsychotics treatment on body weight: a population-based cohort study. J Psychopharmacol. 2020;34(1):79–85. PubMed CrossRef
- Bauer M, Pfennig A, Severus E, et al. Task Force on Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013;14(5):334–385. PubMed CrossRef
- Thase ME. Adjunctive therapy with second-generation antipsychotics: the new standard for treatment-resistant depression? Focus (Am Psychiatr Publ). 2016;14(2):180–183. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite