Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: While olanzapine is frequently associated with glucoregulatory abnormalities, the underlying pathophysiologic mechanisms remain unclear. Because olanzapine’s superior therapeutic profile frequently renders switching to more metabolically safe antipsychotics a clinically undesirable option, understanding how olanzapine is involved in metabolic disturbances may be important for preventing negative health consequences and for improving treatment adherence.
Oral glucose tolerance test (oGTT) is the "gold standard" for diagnosing diabetes, reliably determining insulin sensitivity, and is a better predictor of morbidity and mortality than fasting plasma glucose (FPG).
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Clinical Correlates of Oral Glucose Tolerance Test Performance in Olanzapine-Treated Patients with Schizophrenia or Schizoaffective Disorder
To the Editor: While olanzapine is frequently associated with glucoregulatory abnormalities, the underlying pathophysiologic mechanisms remain unclear.1 Because olanzapine’s superior therapeutic profile frequently renders switching to more metabolically safe antipsychotics a clinically undesirable option,2 understanding how olanzapine is involved in metabolic disturbances may be important for preventing negative health consequences and for improving treatment adherence.
Oral glucose tolerance test (oGTT) is the "gold standard" for diagnosing diabetes, reliably determining insulin sensitivity, and is a better predictor of morbidity and mortality than fasting plasma glucose (FPG).3,4 There is a relative paucity of olanzapine oGTT studies, none of which have addressed the role of treatment duration or insulin sensitivity. The present study aimed to fill this gap. To that end, we employed the Matsuda index, which analyzes dynamic glucose/insulin interactions during oGTT (encompassing both hepatic and peripheral insulin sensitivities) and has been demonstrated to be superior to other commonly used indices for determining glucose intolerance such as homeostasis model assessment (HOMA), which only assesses peripheral insulin resistance.5,6
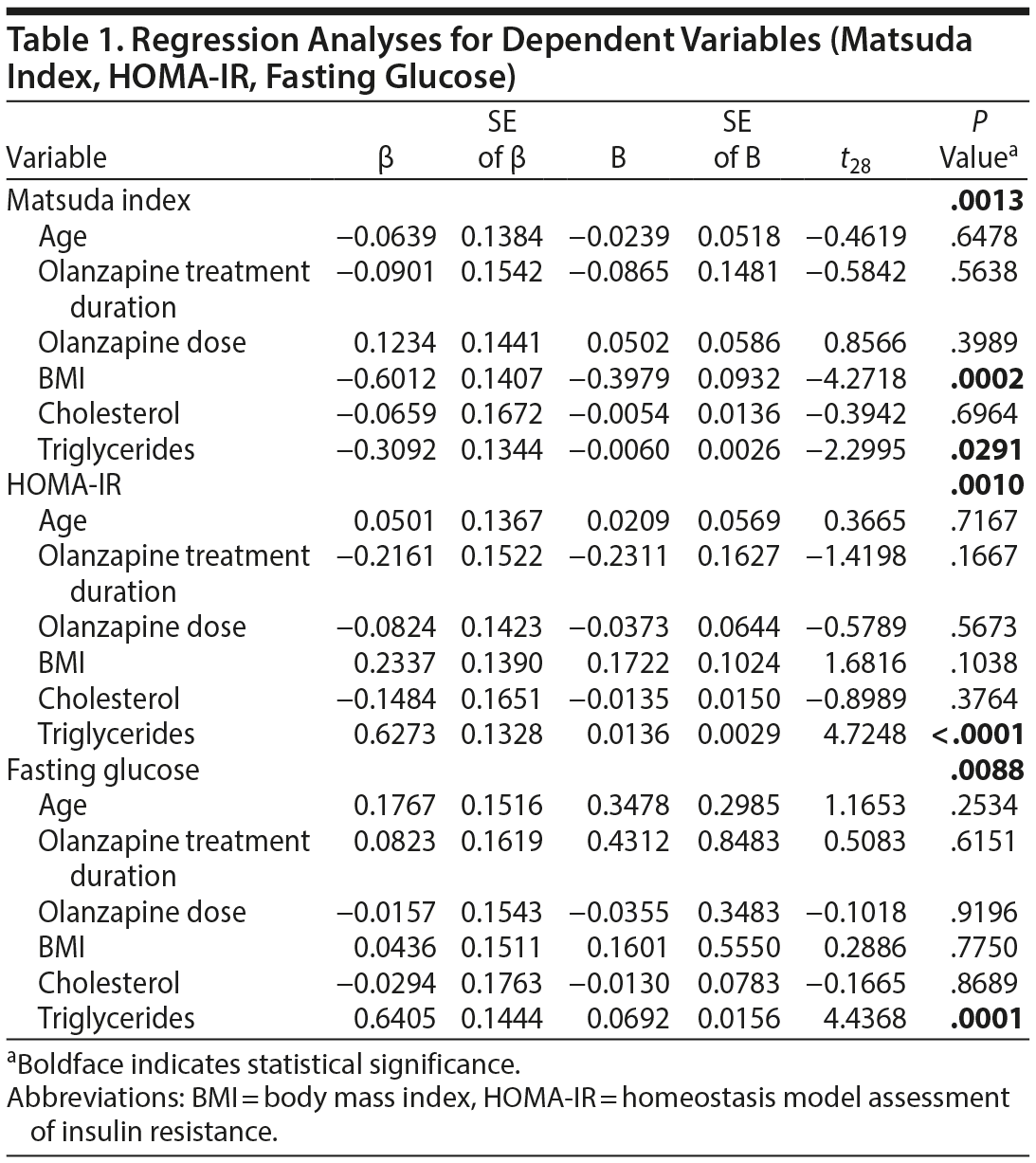
Method. Two-hour oGTT was performed in olanzapine-treated nondiabetic patients with a Structured Clinical Interview for DSM-IV Axis I Disorders diagnosis of schizophrenia or schizoaffective disorder (N = 35) from June 2008-February 2010. Detailed selection methodology and clinical trial data are reported elsewhere.7 Separate multiple linear regression analyses were performed for FPG (to determine hepatic glucose production), Matsuda index, HOMA-IR (insulin resistance), and HOMA-β (β-cell function)5,6 as dependent variables, employing a model in which independent variables were age, olanzapine dose, treatment duration, body mass index (BMI), fasting plasma cholesterol, and triglycerides. Post hoc correlative analyses were conducted using the Pearson product-moment correlation coefficient. All analyses were 2-tailed with α < .05 for statistical significance.
Results. Seven previously undiagnosed patients fulfilled American Diabetes Association diagnostic criteria3 based on oGTT (≥ 200 mg/dL), but only 1 would have been identified by FPG alone (≥ 126 mg/dL). Multiple regression analyses (Table 1) revealed that Matsuda index significantly correlated with BMI (corresponding Pearson correlation: r33 = 0.62, P = .00007) and triglycerides (r33 = 0.41, P = .0146), but not with age, dose, duration, or cholesterol. Using the same independent variables in multiple regression analyses, HOMA-IR and FPG glucose each correlated with triglycerides only (r33 = 0.65, P = .00002; and r33 = 0.62, P = .00005, respectively), while HOMA-β had no significant correlations.
In addition to the alarming rates of undiagnosed diabetes in olanzapine-treated patients, our data suggest that both triglycerides and BMI may be implicated in olanzapine-related glucoregulatory abnormalities. The lack of correlation with treatment duration or dose may suggest preexisting metabolic alterations in schizophrenia spectrum patients, at least partially independent of antipsychotic effects8,9 (eg, conditions predisposing schizophrenics to develop diabetes), and/or metabolic alterations arising early in the course of treatment10,11 (eg, insulin receptor damage/loss). Our finding that 20% of participants had undiagnosed diabetes suggests that oGTT is advantageous for clinically monitoring antipsychotic-treated patients. This is consistent with findings that about 70% of antipsychotic-treated patients are not adequately screened for diabetes.12
Although more costly and time-consuming than FPG, oGTT measures glucose and insulin sensitivity related to food intake, which is more realistic (ie, diabetics spend little time in a fasting state). Our findings suggest that all antipsychotic prescribers (psychiatric and primary care alike) should screen/monitor patients for diabetes, possibly with oGTT (or at least BMI and triglycerides)—especially those with obesity or hypertriglyceridemia.
References
1. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? a literature review. J Clin Psychiatry. 2009;70(7):1041-1050. PubMed doi:10.4088/JCP.08r04392
2. Wani RA, Dar MA, Margoob MA, et al. Diabetes mellitus and impaired glucose tolerance in patients with schizophrenia, before and after antipsychotic treatment. J Neurosci Rural Pract. 2015;6(1):17-22. PubMed doi:10.4103/0976-3147.143182
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S94.
4. Salmasi AM, Dancy M. The glucose tolerance test, but not HbA(1c), remains the gold standard in identifying unrecognized diabetes mellitus and impaired glucose tolerance in hypertensive subjects. Angiology. 2005;56(5):571-579. PubMed doi:10.1177/000331970505600508
5. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. PubMed doi:10.2337/diacare.22.9.1462
6. Pisprasert V, Ingram KH, Lopez-Davila MF, et al. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845-853. PubMed doi:10.2337/dc12-0840
7. Taveira TH, Wu WC, Tschibelu E, et al. The effect of naltrexone on body fat mass in olanzapine-treated schizophrenic or schizoaffective patients: a randomized double-blind placebo-controlled pilot study. J Psychopharmacol. 2014;28(4):395-400. PubMed doi:10.1177/0269881113509904
8. Elman I, Borsook D, Lukas SE. Food intake and reward mechanisms in patients with schizophrenia: implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31(10):2091-2120. PubMed doi:10.1038/sj.npp.1301051
9. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry suppl. 2004;47(47):S67-S71. PubMed doi:10.1192/bjp.184.47.s67
10. Sacher J, Mossaheb N, Spindelegger C, et al. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology. 2008;33(7):1633-1641. PubMed doi:10.1038/sj.npp.1301541
11. Hahn MK, Wolever TM, Arenovich T, et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol. 2013;33(6):740-746. PubMed doi:10.1097/JCP.0b013e31829e8333
12. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med. 2015;175(12):1977-1979. PubMed doi:10.1001/jamainternmed.2015.6098
aDepartment of Psychiatry, Wright State University Boonshoft School of Medicine, Dayton, Ohio
bWright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio
cDepartment of Medicine, Wright State University Boonshoft School of Medicine, Dayton, Ohio
dVA Medical Center, Dayton, Ohio
eDepartment of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania
Potential conflicts of interest: None.
Funding/support: The study was funded via Eli Lilly Neuroscience Investigator-Initiated mechanism.
Role of the sponsor: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of manuscript.
Acknowledgment: The authors gratefully acknowledge Evelyne Tschibelu, BS, for her help with the conduct of this study as a research assistant at McLean Hospital, Belmont, Massachusetts. Ms Tschibelu reports no potential conflict of interest.
J Clin Psychiatry 2016;77(12):e1650-e1651
dx.doi.org/10.4088/JCP.16l10705
© Copyright 2016 Physicians Postgraduate Press, Inc.
This PDF is free for all visitors!
Save
Cite