Abstract
Today, clinicians have an array of antipsychotic medications to choose from in treating patients with schizophrenia, as well as a range of programs and services designed to improve cognition and real-world functioning. Yet, perhaps only a third of patients can successfully live in the community long-term and remain in remission. Treatment failure or a relapse after what appeared to be significant improvement in a patient’s psychotic symptoms and functioning also affects families, employers, payers, policy makers, and huge swathes of the health care system. In short, shortcomings in the treatment of schizophrenia have a significant impact on society as a whole, an impact that can be measured not only in terms of health and well-being but in dollars and cents. Studies evaluating the efficacy and cost-efficacy of antipsychotic medications and other forms of intervention are abundant, but it is uncertain how much of this valuable information reaches the average clinician and can then be applied in daily interactions with patients. This InfoPack aims to synthesize some of the important findings on new antipsychotics by explaining how to use the data from head-to-head comparisons and meta-analyses to evaluate different agents and choose the best one for the patient. It also reviews the evidence on cognitive remediation and antipsychotics in improving cognition and functional capacity. In particular, several short-term and long-term studies of the atypical antipsychotic lurasidone are discussed, including findings associated with cognition and improved functional capacity, the side effect profile, relapse prevention, and the cost savings that may be achieved by reducing the direct costs of care through an evidence-based selection of medication.
J Clin Psychiatry InfoPack 2014;4(April):1–13
doi:10.4088/JCP.13050ip1
© Copyright 2014 Physicians Postgraduate Press, Inc.
Faculty
John M. Kane, MD, Chair
Chair and Professor of Psychiatry, Zucker Hillside Hospital, Glen Oaks, New York
Christoph U. Correll, MD
Professor of Psychiatry and Molecular Medicine, Hofstra North Shore-LIJ School of Medicine, Hempstead; Medical Director, Recognition and Prevention Program (RAP), The Zucker Hillside Hospital, Glen Oaks, New York
Philip D. Harvey, PhD
Leonard M. Miller Professor of Psychiatry and Behavioral Sciences,
University of Miami Miller School of Medicine, Miami, Florida
Mark Olfson, MD, MPH
Professor of Psychiatry, Columbia University Medical Center; Research Psychiatrist II,
New York State Psychiatric Institute, New York, New York
Faculty Disclosure
Dr Kane has been a consultant for Alkermes, Bristol-Myers Squibb, Eli Lilly, Forest, Genentech, Lundbeck, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Otsuka, Reviva, and Roche; has received honoraria from Alkermes, Bristol-Myers Squibb, Eli Lilly, Forest, Genentech, Lundbeck, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Otsuka, Reviva, and Roche; has been on the speakers/advisory boards for Bristol-Myers Squibb, Genentech, and Otsuka; and has been a stock shareholder in MedAvante.
Dr Correll has been a consultant for Bristol-Myers Squibb, Eli Lilly, Gerson Lehrman Group, Intra-Cellular Therapies, Lundbeck, MedAvante, Pfizer, ProPhase, Otsuka, Sunovion, and Vanda; has received grant/research support from Bristol-Myers Squibb, Janssen, Johnson & Johnson, Novo Nordisk A/S, and Otsuka; has received honoraria from Medscape; has been on the speaker/advisory boards of Alexza, Bristol-Myers Squibb, Eli Lilly, Genentech, Intra-Cellular Therapies, Lundbeck, Merck, Otsuka, Roche, and Sunovion; and has been a data safety monitoring board member for Eli Lilly, Cephalon, Janssen, Lundbeck, Pfizer, Takeda, and Teva.
Dr Harvey is a consultant for AbbVie, Boehringer Ingelheim, EnVivo, Forest, Genentech, Otsuka, Sunovion, and Takeda.
Dr Olfson is an employee of Columbia University, New York State Institute, Research Foundation for Mental Hygiene and has received grant/research support from the Agency for Health Care Policy & Research, the American Foundation for Suicide Prevention, and the National Institutes of Health.
Review Process
The faculty for this InfoPack discussed the content in a series of peer-review planning teleconferences, the chair and faculty reviewed the InfoPack for accuracy, and a member of the Journal’s Editorial Board who is without conflict of interest reviewed the InfoPack to determine whether the material is evidence-based and objective.
Acknowledgment
This InfoPack is based on a series of discussions by 4 experts on the treatment of schizophrenia held in December 2013 and January 2014. This evidence-based, peer-reviewed InfoPack was prepared by Healthcare Global Village, Inc. Financial support for the preparation and dissemination of this InfoPack was provided by Sunovion Pharmaceuticals, Inc. The authors acknowledge Nancy Groves, BA, MS, Healthcare Global Village, Inc, for editorial assistance in development of the manuscripts. The opinions expressed herein are those of the authors and do not necessarily reflect the views of Healthcare Global Village, Inc, the publisher, the American Society of Clinical Psychopharmacology, or the commercial supporter.
Treatment of Cognition With Pharmacologic and Cognitive Enhancement Strategies
Philip D. Harvey, PhD
Significant cognitive impairment is an intrinsic part of schizophrenia. It affects at least 70% of patients at all phases of the disease and can even predate the illness.1 This impairment has profound, disabling consequences that limit an individual’s ability to adequately function at home, in society, and in the workplace.
Cognitive impairment is unlikely to spontaneously improve, disallowing patients to smoothly and successfully resume real-world functioning. Thus, reduction of cognitive impairment has been a primary goal of schizophrenia treatment for years, and sophisticated intervention strategies, using both drug treatment and cognitive remediation, have been devised to help patients regain as much functional capacity as possible.2
However, more effective forms of intervention are sorely needed. Despite considerable efforts to reduce the number of institutionalized patients and reintegrate them into society, as well as numerous advances in antipsychotic medication that are very successful in treating the psychotic symptoms of schizophrenia, the percentage of patients able to live successfully in the community and experience stable remission of their systems has remained relatively flat, at around 30%, since 1895.3
Many studies have found minimal cognitive enhancing effects from atypical antipsychotic medications, but these results may have been biased by substantial methodological limitations, as my colleagues and I observed in a study last year.4 Alternatively, other studies, using systematic procedures to evaluate the cognitive enhancing effects of atypical antipsychotic agents, have shown long-term functional benefits.5
Reporting on the PEARL 3 study, a multicenter, randomized, 6-week, double-blind study followed by a double-blind extension study that continued up to 1 year,4 my colleagues and I found that the atypical antipsychotic lurasidone 160 mg/d was superior to placebo, a lower dose of lurasidone, and another atypical antipsychotic, quetiapine XR (200–800 mg/d), on the composite neurocognitive score in an evaluable sample of schizophrenia patients whose test scores met prespecified validity criteria.
This study4 was designed to avoid the flaws of some earlier studies of the effects of pharmacologic intervention on neurocognitive performance in schizophrenia. The initial treatment period included both active pharmacologic and placebo controls; subjects were randomized to the 2 fixed doses of lurasidone or quetiapine XR (the active control) or placebo. We examined the placebo group to evaluate the potential contribution of practice effects to improvement in cognition. Since all patients in the study were exposed to testing, all had the opportunity to benefit from practice. Therefore, the differences that were subsequently found between treatment groups could not have been attributed to differential practice effects.
The study enrolled 486 subjects and was conducted at centers in the United States, Russia, India, Ukraine, Bosnia, and Colombia. The subjects had a primary diagnosis of schizophrenia and had been recently hospitalized for an acute exacerbation of psychiatric symptoms.
Cognitive performance was assessed with the CogState computerized cognitive battery, administered at pretreatment baseline, week 6 (the end of the acute study), and weeks 19 and 32 during the extension phase. Validity criteria were set and later used to designate a subset of subjects who could adequately perform the testing procedure. The criteria included a less than 25% error rate on 5 domains: processing speed, attention/vigilance, visual learning, working memory, and social cognition. The other requirement was completion of all 3 rounds of the International Shopping List Task and all 5 rounds of the Groton Maze Learning Test. The primary outcome was the composite Z-score, calculated as an average of the 7 standardized Z-scores for the 7 cognitive domains.
Trained raters also administered the University of California San Diego Performance-Based Skills Assessment-Brief Version (UPSA-B) as a measure of functional capacity at study baseline and 6, 19, and 32 weeks.
Briefly, the results showed that in the acute phase of the study, there were no significant differences from baseline to the week 6 endpoint in composite Z-score in the full analysis sample between lurasidone 80 mg/d (P = .674) or 160 mg/d (P = .513) when compared with placebo (Figure 1). There was also no significant difference between lurasidone 80 mg/d (P = .985) or 160 mg/d (P = .827) compared with quetiapine XR.
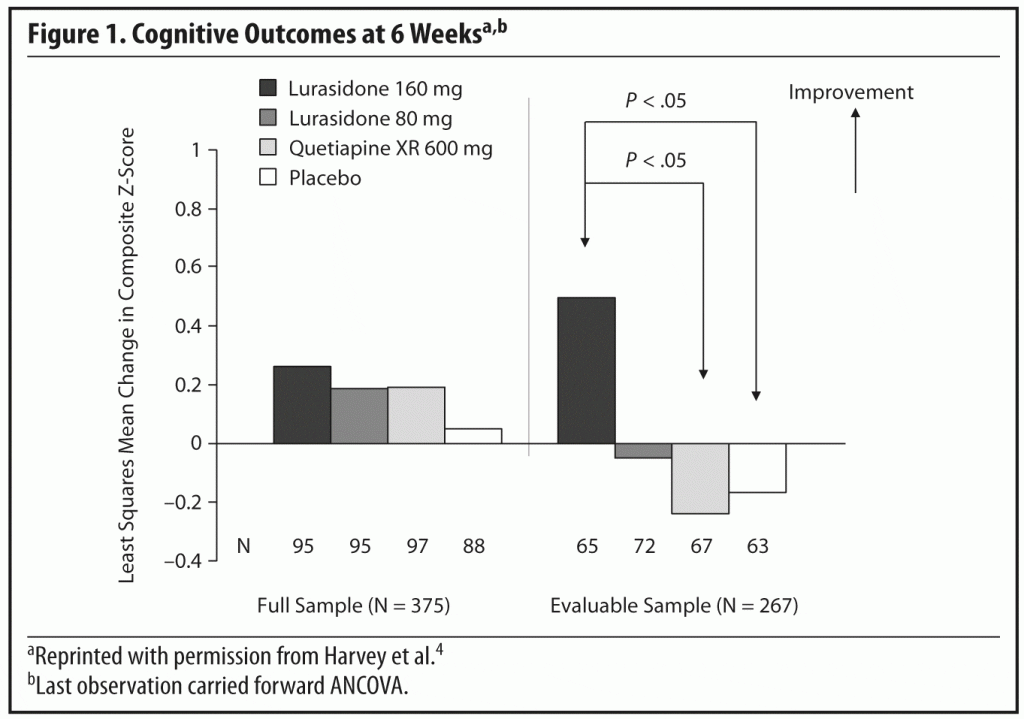
The outcome differed in the evaluable analysis sample. In this cohort, the change from baseline to week 6 in the composite Z-score was significant for the lurasidone 160 mg/d group compared to the placebo group (P = .038, Cohen d = 0.37). It was also significant compared to the quetiapine XR group (P = .018, Cohen d = 0.41). Changes for the group randomized to the lower dose of lurasidone were not significant when compared with placebo or quetiapine XR.
This summary of the findings points to an important aspect of evaluating cognitive performance and the effects of treatment. Some acutely ill patients are too disorganized to complete the neurocognitive assessments. In the acute phase of the study, 7,753 cognitive assessment tasks were performed on 481 subjects; 1,355 (17.5%) of these tasks failed the prespecified evaluability criteria. Only 77% of the subjects had composite Z-scores at both baseline and week 6 in the full sample, and only 55% in the evaluable analysis sample.
The issue of data quality clearly merits further study. The high rate of testing failure seen in this study4 and others may be due to a combination of acute illness in the subjects and to problems with the specific computerized assessment strategy.
NEUROCOGNITIVE ASSESSMENT
Stepping aside for now from the details of the study findings concerning lurasidone’s potential to improve cognitive performance, let’s look at the tools used for conducting research on cognitive function.
The MATRICS Consensus Cognitive Battery (MCCB) is a neuropsychological assessment battery that measures the following cognitive domains (available at www.matricsinc.org):
- Speed of processing
- Attention and vigilance
- Working memory
- Verbal learning
- Visual learning
- Reasoning and problem solving
- Social cognition
It was created as a standard tool for assessing the level of cognitive functioning in schizophrenia patients and changes over short time intervals. The MCCB was designed as a primary outcome measure in cognitive enhancement research. In clinical trials, clinically stable patients are enrolled so that their scores on the various domains can be compared with those of patients who have been matched for characteristics such as age and education.
Coprimary measures in many studies include the UPSA,6 a measure of everyday living skills, and 2 modified versions, the UPSA-B and UPSA-II. The original UPSA assesses 5 domains:
- Comprehension/planning
- Finance
- Communication
- Mobility
- Household chores
The UPSA-B7 is an abbreviated form that includes only 2 of the 5 subscales, finance and communication. It can be administered in a shorter time and in a wider variety of settings. The UPSA-II contains a medication management domain.
Another important measure is the Schizophrenia Cognition Rating Scale8 (SCoRS), an 18-item interview-based measure of cognitive functioning in which both the patient and an informant are questioned. The interviewer determines a global score at each visit, and changes are measured against these scores at each follow-up. In a 2006 study of the then new SCoRS, Keefe et al8 found that its global ratings were strongly correlated with cognitive performance and real-world functioning.
In the Keefe et al study,8 60 patients with schizophrenia were assessed with the SCoRS and 3 other tools for validation: the Brief Assessment of Cognition in Schizophrenia (BACS), the Independent Living Skills Inventory (ILSI), and the UPSA. The SCoRS interview global rating was significantly correlated with the BACS composite score, the UPSA total score, and the ILSI total score.8 Since the SCoRS and the UPSA themselves are highly correlated, they are conceivably interchangeable measures of everyday functioning (r = 0.53), and either could be used with a longer performance-based assessment.
COGNITIVE REMEDIATION THERAPY
The availability of these assessment tools is essential in evaluating the outcomes of cognitive remediation therapy in patients with schizophrenia. In 2011, Wykes et al2 published a meta-analysis of cognitive remediation to determine the effects of treatment.
This study, which included 39 separate reports of 40 studies enrolling 2,104 participants, demonstrated a small to moderate effect of cognitive rehabilitation on cognitive outcomes at posttreatment and follow-up assessment. This effect was not influenced by study methodology.2
Several critical findings for cognition and functioning can be drawn from this work. First, the functional benefit of cognitive remediation is better when paired with other psychiatric rehabilitation programs. Second, a strategic cognitive remediation intervention—one that teaches problem solving and coordination of component cognitive skills—is more effective than drill and practice interventions. Better outcomes with the strategic intervention apparently are derived from transfer of training. Third, there are no symptomatic effects; thus, cognitive remediation therapy is best viewed as a complement to antipsychotic therapy rather than a substitute.
Another study, published by Bowie et al in 2012,5 also demonstrated that while cognitive remediation alone may produce durable improvements in cognition, the benefits to the patient are likely to be enhanced when the remediation is provided in the context of other psychosocial treatment (AV 1). The authors found that improved everyday functioning, which is the true measure of effectiveness, is more likely when cognitive remediation is combined with functional skills training. In this study, 107 outpatients with schizophrenia were randomized to receive cognitive remediation, functional adaptation skills training, or combined treatment (cognitive remediation followed by the skills training). Patients underwent 24 weeks of treatment and had a durability assessment 12 weeks after the end of active treatment.
AV 1. Outcomes of the Study: End of Treatmenta,b
aBased on data from Bowie et al.5
bCohen d was calculated using raw score change for the completers sample between the baseline assessment and the end-of-treatment assessment.
Abbreviations: BACS = Brief Assessment of Cognition in Schizophrenia, RW = real world, UPSA = University of California San Diego (UCSD) Performance-Based Skills Assessment.
The investigators found that cognitive remediation produced robust improvements in cognition while functional skills training did not. Social competence improved in subjects who had received functional skills training or the combined treatment but not in those who had received only cognitive remediation. The greatest and most durable improvements in functional competence were found among the patients randomized to the combined treatment. The data also showed that cognitive remediation therapy led to statistically significant improvements in real-world behavior such as community or household activities and work skills when it was combined with functional skills training; alone, cognitive remediation had a limited effect on real-world behavior.
Cognitive training can also be enlisted to improve the competitive work outcomes in people with schizophrenia. Evidence supports the effectiveness of supported employment, yet not all who participate in these programs find jobs or are able to remain employed for an extended time if they do find work. Addressing the problem of illness-related impairments that may hinder employment prospects, McGurk et al9 developed the Thinking Skills for Work Program, integrating cognitive training into supported employment services.
Patients with severe mental illness and a history of job failure were randomized to either supported employment alone or supported employment with cognitive training. Patients randomized to the Thinking Skills program participated in approximately 24 hours of computer-based cognitive exercises delivered over 12 weeks. When McGurk and colleagues9 looked at 2- to 3-year employment and hospitalization outcomes, they found that participants in the Thinking Skills for Work Program (n = 23) worked more jobs than patients in the supported employment alone group (n = 21). Also, significantly more patients in the Thinking Skills program than in the supported employment alone program worked (P < .001), and they also held more jobs (P < .001), worked more hours over the follow-up period (P < .001), and earned 10-fold more wages—$5,320.19 versus $530.18 (P < .001).9
What these findings suggest, in my view, is that even a relatively brief cognitive remediation intervention can exert a substantial long-term benefit on real-world functioning.
It must be noted, though, that since cognitive functioning and symptoms were not evaluated at the 2- to 3-year follow-up, McGurk and colleagues9 concluded that they could not be certain whether improved cognitive functioning or symptoms or other factors contributed to the superior vocational outcomes in the group receiving supported employment with cognitive training.
Does the number of hours of cognitive training in a program have stronger effects on cognitive functioning? McGurk and colleagues10 also explored this hypothesis in a 2007 meta-analysis. They found that they could not adequately answer this question because only 6 of the 26 randomized controlled trials they reviewed had conducted follow-up assessments an average of 8 months after completion of the program. Discussing this point, the authors suggested that a limited amount of cognitive remediation, such as 5 to 15 hours, might be sufficient to improve cognitive function. They also speculated that the amount of cognitive remediation might be more closely related to the retention of improvements than to immediate gains.10
Additional data10 from a variety of studies support the hypothesis of a correlation between time and cognitive functioning improvement. Patients who received 50 or more training sessions with computerized cognitive remediation showed substantial improvement (0.86 standard deviation). Of note, improvements in processing speed, global cognition, and working memory also led to a functional benefit despite the absence of a skills training intervention.
Realistically, most patients could not participate in an intervention that required training twice a week for a year, but it appears that those who could make this long-term commitment might benefit greatly. It has also been shown that people with schizophrenia can treat themselves at home with a laptop computer with high levels of adherence and considerable cognitive benefits.11
PHARMACOLOGIC COGNITIVE ENHANCEMENT
The record for pharmacologic cognitive enhancement has been mixed in the past, but this therapeutic approach remains an important goal. One promising compound under investigation is EVP-6124, a selective α-7 partial agonist developed by EnVivo Pharmaceuticals that is currently in phase 3 studies. Unpublished phase 2B data (D. Hilt, MD, EnVivo) from SCoRS visits with informant present show that a 1.0-mg dose of EVP-6124 achieved a fairly significant improvement over placebo in 77 days (P < .003; effect size = 0.51).
An important detail from this study is that, based on MCCB scores, younger patients received more substantial benefits. This finding reinforces the urgency of treating patients with schizophrenia as early as possible in the course of their disease, which may be based on either less progression of illness or less burden of comorbidities such as cigarette smoking.
LURASIDONE AND COGNITION
Returning to studies of lurasidone (as discussed earlier, this compound was superior to placebo in a 6-week study and also to an active comparator, quetiapine XR, in an extension study4), this agent demonstrated improvements on SCoRS when compared to ziprasidone.12 Although differences between drugs in the effect were not statistically significant (P < .057), lurasidone patients improved significantly from baseline while the ziprasidone patients did not. It is also relevant to point out that the effect was equivalent to that of EVP-6124, a targeted cognitive enhancement agent.
In the PEARL 3 study,4 cognitive outcomes at 6 weeks in the full analysis sample showed no separation between the 2 active compounds and placebo and only a small effect size, but it bears repeating that 45% of the patients, who were clinically unstable, had very poor test scores on the computerized assessment and were unable to provide valid cognitive test data.
Looking at the treatment effects of the UPSA-B, the least squares mean change in total score at week 6 was significantly better for the lurasidone 80 mg (6.5, standard error [SE] = 1.2; P = .036), lurasidone 160 mg (7.3, SE = 1.2; P = .011), and quetiapine XR groups (7.2, SE = 1.2; P < .001) compared with the placebo group (4.3, SE = 1.3). These early gains in functional capacity continued through week 32 of the extension study. The improvement was 7.6 points at week 6 and 10.3 points at week 32 in the lurasidone group. In the quetiapine group, the improvements were 6.8 points at week 6 and 12.4 points at week 32. The differences between lurasidone and quetiapine XR treatment groups were not significant at weeks 19 or 32.
Analysis of the relationship between cognitive change and UPSA-B change/composite Z-scores demonstrated significant cross-sectional association at baseline in both the full and evaluable samples (P < .001). The longitudinal association between changes in functional capacity and changes in cognitive performance was also significant in the full and evaluable samples (P < .001 and P = .022, respectively). This relationship increased over time (P < .042) and was similar across all the treatment groups (all P values > .206).
Turning to the associations between cognitive change and Positive and Negative Syndrome Scale (PANSS) change,4 we found a significant correlation between changes in neurocognitive composite Z-score and in PANSS total score over time (P < .001). The association was similar in PANSS positive (P = .0010) and PANSS negative (P < .001) subscales. There was a significant change in cognitive performance at week 32 favoring lurasidone over quetiapine in both the full and evaluable samples (after controlling for changes in the PANSS total, positive, and negative subscales over time).
MEDIATING FACTORS
In a separate set of nonprimary analyses of cognition of PEARL 3 data, Loebel et al13 conducted a placebo-controlled study comparing the effects of lurasidone 80 mg/d and 160 mg/d and quetiapine XR 600 mg/d on sleepiness, using the Epworth Sleepiness Scale (ESS). They found that daytime sleepiness improved in the lurasidone and placebo groups but worsened in the quetiapine XR group compared to placebo (P = .001) and to either group of lurasidone (both P = .01).
Importantly, the study also found that worsening in sleepiness was associated with worsening of cognitive performance. The quetiapine group, whose sleepiness had worsened, also had a decline in cognitive performance; the lurasidone group, with reduced sleepiness, had improved performance. Analysis of items on the ESS showed an association between an increase in ESS item 6 score (propensity to doze when talking to someone) and worsening of cognitive performance in the quetiapine group compared to the lurasidone 160-mg/d group (P = .006, US sites; P = .015, all subjects). Change in the ESS total score was not a significant predictor of change in the composite performance score for either lurasidone group or quetiapine XR.13
Quetiapine XR and both doses of lurasidone were associated with improvements in functional capacity when assessed with the UPSA-B. The improvements were comparable and significant. However, at study sites in the United States, daytime sleepiness associated with quetiapine XR was a mediator of change in functional capacity; there was worsening in the UPSA-B total score (P = .003) for the difference in slopes between quetiapine and placebo.
CONCLUSION
Improvement in cognition is a crucial component in the treatment of patients with schizophrenia. Research in this field is ongoing as investigators explore ways to improve both cognitive remediation and pharmacologic interventions, all with the aim of achieving better outcomes.
Results of the PEARL 3 study have demonstrated that the atypical antipsychotic lurasidone is beneficial in improving cognitive functioning in schizophrenia. Lurasidone is the first antipsychotic compound proven to change both cognition and functional capacity in a placebo-controlled study. It cannot be overemphasized that the study design included a placebo arm to eliminate any possibility that gains in cognition stemmed from practice effects rather than the drug itself.
What this study shows us is that lurasidone may enable patients to attain a dose-related improvement in cognition accompanied by improvements in functional capacity. Further, the side effects profile demonstrates that lurasidone does not lead to worsening in cognitive performance through the induction of sleepiness.
Short-Term and Long-Term Antipsychotic Treatment of Patients With Schizophrenia: Comparison of Treatment Options
Christoph U. Correll, MD, and John M. Kane, MD
Schizophrenia is still all too often a devastating illness. Despite progress on many fronts, including the development of antipsychotic drugs that have helped many patients better achieve their goals, much is yet to be learned about the etiology and pathophysiology of the disease, predictive biomarkers, novel mechanism medications that go beyond the modulation of dopamine, and opportunities for primary and secondary prevention.14 New antipsychotics are likely to be the cornerstone of improved treatment, just as they are the basis of treatment today. The “ideal” antipsychotics of the future would be aimed at a long list of persistent gaps in the care of patients with schizophrenia.14
At the top of the list is the unfortunate fact that prevention remains a distant, although not an impossible, goal. Other priorities include developing treatments tailored to the different phases of schizophrenia as well as therapies for symptom domains that respond poorly to current medications, such as negative symptoms and cognitive dysfunction. Recovery is a constant challenge even when patients’ symptoms have diminished. And in schizophrenia as well as other acute and chronic conditions, patients’ lack of adherence to their medication regimens and other forms of therapy is an ongoing problem.14,15
Variability in adverse effects and tolerability of medication from one patient to another is also of concern. Similarly, our best efforts at managing schizophrenia often result in poor or no response, with a limited number of options to turn to for better outcomes. It is hoped that ongoing research into investigational medications with novel mechanisms of action ultimately will guide clinicians in combining different agents to achieve all of the desired effects of treatment, since it is highly unlikely that a single agent could safely and effectively do so.14
We await the arrival of personalized care based on factors such as genetic polymorphisms or the related gene products so that targeted, individualized treatment can become a clinical reality. Moreover, the discovery of disease- and subgroup-specific markers, involving, for example, metabolomic profiles or inflammatory and/or oxidative stress patterns, may help target specific outcomes in patient subgroups in whom biologic target engagement is leveraged for improved outcomes.14 Some of these gaps will be resolved and goals attained sooner than others.
Recovery is the ultimate treatment objective and one that requires functional measures as part of a more broad-based effort to optimize management of patients with schizophrenia.16 Through medication, psychotherapy, and psychoeducation, the treatment outlook has improved, yet recovery is a goal achieved in a minority of cases. According to a meta-analysis of 50 studies, published in 2013 by Jääskeläinen et al,17 the median number of individuals with schizophrenia who recover is 13.5% or 1 in 7, while the mean is 16.4%. These data indicate that despite major changes in treatment options, the proportion of patients achieving recovery, defined as at least 2 years of no more than mild symptoms with or without evidence of good social and educational/vocational functioning, has not increased in recent decades.
Disappointingly, outcomes did not seem to differ whether or not patients were followed after a chronic course or after their first episode. However, most studies did not include a structured or formalized psychosocial treatment package in addition to the pharmacologic treatment, and nonadherence was not specifically addressed. For example, through the early provision of alternative medication formulations, such as long-acting injectables, the interruptions of medication ingestion might be less common, even early in the illness.
ACUTE EFFICACY OF ANTIPSYCHOTICS
To assess the efficacy, safety, tolerability, and overall effectiveness of the antipsychotics used in the treatment of schizophrenia, it is necessary to wisely interpret a massive amount of available data. The meta-analysis is a valuable framework for pooling or integrating data from studies conducted at different times using different methodologies and assessments, yet it is still able to make valid and clinically informative comparisons.18
The most common type of meta-analysis is the pooling of head-to-head comparison studies. The advantage of this approach is that the methodology and sampling of participants are similar in each study that compares 2 or more treatments. However, direct comparisons are not always available, so the arm-based multiple-treatments or network meta-analysis has been used instead to overcome this limitation. In this approach, the result of 1 comparison, such as drug A to drug C, is taken as indirect evidence for the relative efficacy between drug A and drug B by bringing in a comparison between drug B and drug C. With drug C (or placebo) as the common comparator, the differences or similarities between drug A and drug B are estimated indirectly.
This method has the advantage of allowing a ranking among medications that have been only sparsely or never compared, but it also has potential disadvantages. Differences in study methodology, population, or treatment characteristics, such as dosing, can create biases.18 In addition, several treatment meta-analyses of different psychiatric drugs have each shown older medications to rank somewhat higher in efficacy than newer ones.18 However, it is difficult to interpret these findings because, at the same time, research procedures and populations have changed, so that there has also been a consistent trend toward larger placebo response rates in more recent studies, and active comparisons with first-generation antipsychotics have employed more appropriate doses, leading to fewer early dropouts and diminishing drug differences for newer antipsychotics.18 In the absence of sufficient numbers of high-quality, head-to-head studies, the multiple-treatments meta-analysis is helpful but should be interpreted with caution when used to inform guidelines or clinical decision making.
A multiple-treatments meta-analysis of the efficacy and tolerability of 15 antipsychotic drugs and placebo conducted by Leucht and colleagues19 illustrates not only how this form of comparison can be effectively used but also its potential pitfalls (AV 2). The authors identified 212 suitable trials with data for 43,049 participants and analyzed both recently approved and older drugs. A hierarchical ranking based on directly and indirectly obtained effect sizes resulted in a mean range of effect sizes of −0.33 to −0.88. Clozapine was significantly more effective than all the other antipsychotics, followed by amisulpride, olanzapine, and risperidone, forming a group that was significantly more effective than all the others in the analysis except paliperidone and zotepine. However, the effect size differences were negligible to small (−0.11 to −0.33). With the exception of chlorpromazine, the more recently approved agents ranked at the bottom of the hierarchy obtained mostly through indirect comparison.
AV 2. Acute Treatment Network Meta-analysis: Positive and Negative Syndrome Scale Total Scoreb
aReprinted with permission from Leucht et al.19 Error bars represent SMD +/- 95% Cis. bOnly superior to lurasidone and iloperidone. cOnly superior to iloperidone.
*Significant separation from placebo. **Significant separation from placebo and agents with one asterisk. ***Significant separation from placebo and all other agents.
As mentioned previously, 1 possible explanation for these findings is an inflation over time in the placebo effect, reducing the effect size against the major comparator that each newly introduced antipsychotic has, placebo. In the case of this particular, carefully executed, multiple-treatments meta-analysis,19 the authors tried to compensate for this effect in subanalyses. Interestingly, the same authors had also performed a head-to-head meta-analysis in 200920 of antipsychotic medications in schizophrenia available at the time; in that study, clozapine did not separate from olanzapine, quetiapine, risperidone, or ziprasidone. The different rankings in the 2 analyses may have been due to the inflation of the effect size difference for clozapine, which was often compared against first-generation antipsychotics at a time when those were used in very high doses and in studies where more patients who were refractory may have been included. These factors may have caused the differential effect of clozapine to more strongly come to light.
The lessons to be learned are (1) head-to-head studies remain important and should be used to assess the validity of the rankings resulting from a multiple-treatments meta-analysis, and (2) a meta-analysis that used study means instead of primary patient data is not a sufficiently sharp tool to ascertain which aspects of the studies might account for the similarities or differences among agents. Finally, due to its observational nature, a meta-analysis should be regarded as a hypothesis-generating rather than hypothesis-testing tool.
6-WEEK PLACEBO-CONTROLLED PEARL 3 STUDY RESULTS
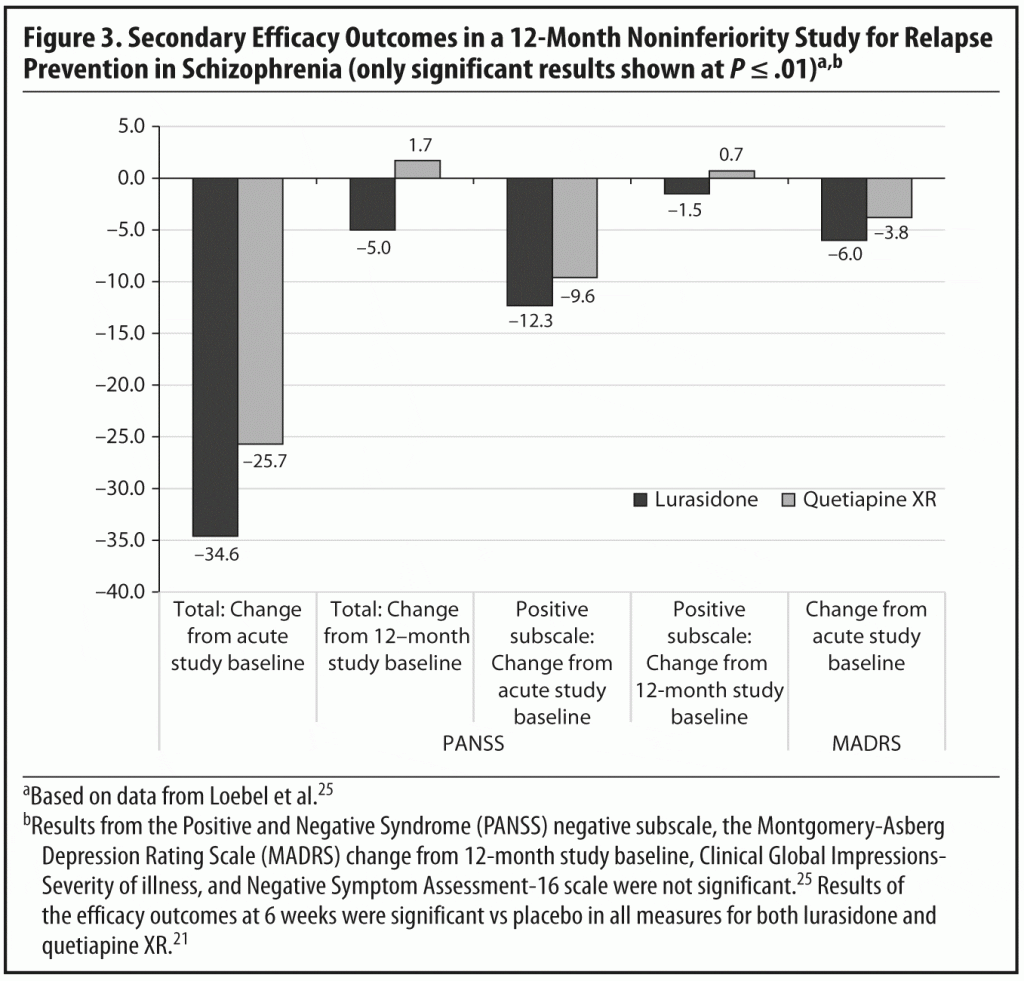
As discussed in the first section of this document, the PEARL 3 trial was a head-to-head, randomized, double-blind, placebo- and active-controlled study of fixed dosages of lurasidone 80 mg/d and 160 mg/d against placebo, with quetiapine XR 600 mg/d included for assay sensitivity. In the 6-week acute phase,21 both doses of lurasidone and quetiapine XR were significantly superior to placebo beginning on day 4, without statistically significant differences between either dose of lurasidone and quetiapine XR on the PANSS total score, PANSS positive and negative subscale scores, and Clinical Global Impressions-Severity of illness score. Treatment with both doses of lurasidone and quetiapine XR was also associated with significantly greater improvement in depressive symptoms as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) compared with placebo. Change in baseline to week 6 in quality of well-being and in medication satisfaction were also significant.
Based on the side effect profile of lurasidone and quetiapine XR in PEARL 3, lurasidone was associated with higher prolactin levels and a greater proportion of patients reporting extrapyramidal symptoms (EPS) and akathisia than quetiapine or placebo, but rating scale–based EPS and akathisia scores did not differ significantly from placebo. Conversely, quetiapine was associated with significant increases in sedation, weight gain (≥ 7%), and some metabolic parameters versus placebo, while lurasidone was not.
Lurasidone was associated with a dose-related increase in prolactin levels; the effect of the lower dose was comparable to placebo, while there was a greater median increase compared with placebo among patients treated with the higher dose (+3.0 vs −0.8 ng/mL). This difference was not clinically relevant.
Slightly more than half of the patients in all cohorts experienced at least 1 adverse event, but most were rated mild to moderate. The discontinuation rate due to EPS was 0.8% for all 3 active groups. Discontinuation due to akathisia was 1.6% for lurasidone 80 mg/d and 0.8% for lurasidone 160 mg/d; there were no discontinuations in either the quetiapine XR or placebo group.21
The dosing strategy in this study differed from previous trials of lurasidone in administering the study medication in the evening, and this may account for the lower rate of adverse events, especially of restlessness/akathisia.21 While the 80-mg/d dose of lurasidone was associated with a small but significant increase in weight and body mass index (BMI) compared with placebo, changes in weight gain, BMI, and waist circumference were similar in the lurasidone 160-mg/d and placebo groups. A clinically significant weight gain was reported by 4% of patients in the lurasidone 80-mg/d and 160-mg/d groups, 3% of the placebo group, and 15% of the quetiapine XR group, translating into a number needed to harm (NNH) compared to placebo of 100 for lurasidone 80 mg/d and of 9 for quetiapine XR 600 mg/d.
The analysis of metabolic parameters showed that changes in lipid levels were comparable for both lurasidone dose groups and the placebo group. However, there was a significant median increase (P < .05) in levels of cholesterol, low-density lipoproteins, and triglycerides in the quetiapine XR group compared to placebo.21
The efficacy and tolerability findings from the PEARL 3 study summarized above can be viewed in the context of the Leucht et al meta-analysis19 of antipsychotics, which included both lurasidone and quetiapine. Effect sizes including the indirect estimate methodology were −0.33 and −0.44 for lurasidone and quetiapine, respectively, with −0.44 being in the middle of the ranking across all antipsychotics, almost identical to haloperidol (−0.45). A comparison of extrapyramidal adverse effects in the meta-analysis showed that only clozapine had fewer such effects than placebo. Others that did not cause significantly more extrapyramidal side effects than placebo included sertindole, olanzapine, quetiapine, aripiprazole, iloperidone, amisulpride, and asenapine. Lurasidone was in a group of 5 (including also zotepine, chlorpromazine, risperidone, and paliperidone) that produced significantly more extrapyramidal side effects than several others in the analysis, yet the most extrapyramidal adverse effects were observed with haloperidol.19
In the meta-analysis of prolactin, aripiprazole, quetiapine, asenapine, chlorpromazine, and iloperidone did not cause a significant increase compared to placebo; paliperidone and risperidone were associated with a significantly greater increase than all other drugs. Quetiapine was relatively neutral, and lurasidone was 1 of several drugs that showed a mild increase.
The meta-analytic results for sedation showed that amisulpride, paliperidone, sertindole, and iloperidone were not significantly more sedating than placebo; a small increase was observed with aripiprazole, lurasidone, risperidone, and haloperidol, and larger increases were seen with olanzapine, quetiapine, chlorpromazine, and clozapine.
While the results of acute phase studies are encouraging, another highly important test of an antipsychotic agent is in whether or how long the gain can be maintained.
MAINTENANCE TREATMENT AND RELAPSE PREVENTION
With more than a dozen antipsychotics on the market, the issue of which is preferable for the treatment of schizophrenia is a relevant clinical question. However, this question will most likely have to be answered by both reviewing long-term efficacy and safety data and considering the individual history and status of a given patient. Debate over the choice of antipsychotic drugs also encompasses first-generation versus second-generation drugs, due to not only the question of relative clinical effectiveness but also that of cost-effectiveness, since the newer agents are more expensive in most cases.19 In addition, the efficacy of various agents versus placebo in maintenance treatment and relapse prevention must be considered. Leucht and colleagues,22 in a meta-analysis of 116 reports from 65 trials including data for 6,493 patients, concluded that antipsychotic maintenance treatment substantially reduced relapse rates for up to 2 years of follow-up. While the difference between drug and placebo seemed to decrease in size with time, this finding is most likely related to increasing non-adherence with time, suggested also by the fact that long-acting injectable antipsychotics had somewhat larger effect sizes versus placebo for relapse prevention than oral antipsychotics.22
Looking specifically at the relapse prevention effects of first-generation antipsychotics compared to second-generation drugs, Kishimoto et al23 conducted a meta-analysis of 23 studies with 4,504 patients. The authors found that none of the individual second-generation agents outperformed the first-generation drugs in terms of the study-defined endpoint relapse rate except in 2 single studies. However, as a group, the newer agents prevented relapse significantly better than the older drugs, translating into a number needed to treat (NNT) of 17. Additionally, they were also superior in reducing relapse at 3, 6, and 12 months and treatment failure and in preventing hospitalization.23
Glick and colleagues24 also explored antipsychotic maintenance efficacy in schizophrenia, focusing on a restricted data set of midlength (3 months to less than 1 year) and long-term (12 months or longer) studies. They compared the efficacy of first-generation drugs and specific second-generation antipsychotic drugs versus olanzapine or placebo. Their data showed that olanzapine was more effective than risperidone and that both were better than all of the other agents in the analysis except clozapine. However, the authors emphasized that there were intraclass differences in both the first- and second-generation groups as to efficacy or side effects and urged clinicians to individualize treatment based on many factors, including cardiometabolic risk and cost, rather than wholly on efficacy differences.24
12-MONTH, ACTIVE-CONTROLLED PEARL 3 STUDY RESULTS
In the maintenance phase study by Loebel and colleagues,25 outpatients who had completed the 6-week acute phase of the PEARL 3 study were enrolled in a double-blind, parallel-group extension trial using a noninferiority design to evaluate the relapse prevention efficacy of 12 months of flexible-dose treatment with lurasidone (40–160 mg/d) compared with quetiapine XR (200–800 mg/d). The study population consisted of 292 (83%) of the 353 subjects who completed the initial 6-week trial; 151 individuals continued on lurasidone and 85 on quetiapine XR treatment, while 56 who had been treated with placebo were started double-blind on lurasidone treatment.
At the end of the 12 months, a similar proportion of patients who had been taking lurasidone since the beginning of the acute study and patients originally randomized to placebo and then switched to lurasidone completed the trial—51.7% and 51.8%, respectively, compared to 38.8% in the quetiapine XR group. These retention rates are quite good for a 12-month study, and the difference in dropout rates translated into a NNT of 8 for patients to stay in treatment with lurasidone compared to quetiapine XR.25
Results for the primary endpoint, time-to-relapse, showed that lurasidone demonstrated noninferiority to quetiapine XR in relapse prevention. The Kaplan-Meier estimates of the probability of relapse at 12 months were 23.7% for the lurasidone group and 33.6% for the quetiapine XR group; this was consistent with noninferiority. While this difference was not statistically significant (hazard ratio = 0.728; 95% confidence interval, 0.140–1.295), ie, a 27.2% relative relapse risk reduction, the absolute risk reduction of 9.9% translates into a clinically relevant NNT of 10.1.25
On another important measure for patients’ well-being and health care cost, probability of hospitalization at 12 months, the Kaplan-Meier estimate was significantly lower for lurasidone than for quetiapine XR (9.8% vs 23.1%, P < .05), translating into a NNT of 8 (Figure 2A and 2B). Among subjects who relapsed, the rate of hospitalization was higher for those who had been treated with quetiapine XR (61.9%) than those treated with lurasidone (34.5%; P < .05; NNT = 4). This finding suggests that the psychotic relapses may have been more severe in patients randomized to quetiapine XR.25
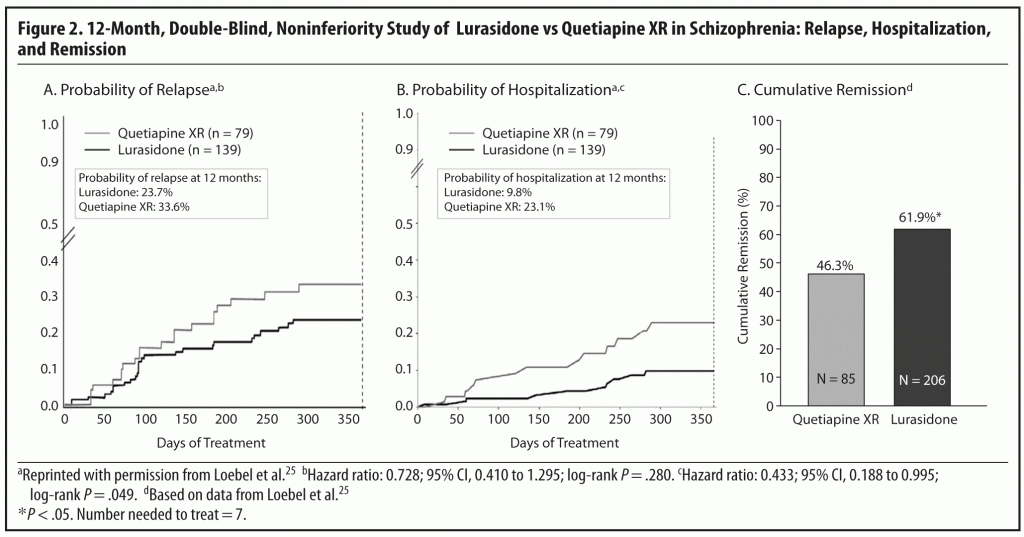
Endpoint improvement on the MADRS also favored lurasidone. The mean change from acute study baseline was −6.0 (P < .05) for lurasidone and −3.8 for quetiapine XR. From 12-month study baseline to the endpoint, the respective changes in scores were +0.1 and +1.3.25 The findings on the MADRS suggesting antidepressant benefits with lurasidone over quetiapine XR in a cohort of schizophrenia patients not selected for depressive symptoms are important, as quetiapine is known to have relevant antidepressant efficacy in and of itself,26,27 and as up to one-third of schizophrenia patients in clinical care are also prescribed antidepressants.28,29
There was little change in body weight, BMI, and waist circumference in either the lurasidone group or the placebo-lurasidone group, but there was a clinically significant increase in weight from the acute study baseline at months 6 and 12 in the lurasidone group (13.6% and 11.5%, respectively) and in the placebo-lurasidone group (10.3% and 13.8%) versus the quetiapine XR group (27.5% and 15.2%, respectively).25
CONCLUSION
A number of different antipsychotics are available for the treatment of schizophrenia, and their choice depends on patient, medication, and treatment system factors. Efficacy and tolerability aspects require consideration. Although the long-term portion of the PEARL 3 study was powered for noninferiority and it was assumed that a study drug and active comparator in the same class would show the same efficacy, lurasidone was shown to be superior to quetiapine XR in the rates of remission and probability of hospitalization. Two further aspects of this study require consideration. One is that it is unclear exactly why lurasidone showed benefits versus quetiapine XR, such as lower probability of hospitalization and a higher proportion of subjects achieving remission. Possible answers include lurasidone’s receptor-binding profile, greater likelihood of adherence, or a reduction in troubling side effects such as sedation. Further studies may offer more definitive information.
The second concerns potential issues with the trial design. Dosing in both active antipsychotic groups seemed to have been appropriate, but patients at the 12-month extension study baseline were not re-randomized. All quetiapine XR patients followed into the maintenance phase had tolerated and responded to quetiapine XR, which could have favored quetiapine XR. By contrast, a subgroup of patients who had been taking placebo was started on lurasidone (n = 56 out of 207). While this could have disfavored lurasidone, as response status and tolerability with lurasidone had not been established, improvement in symptoms may have been larger in this subgroup, and patients stabilizing sufficiently on placebo during an acute 6-week treatment phase may also have been less likely to relapse or be hospitalized subsequently when treated with an antipsychotic.
Finally, it is important to note that antipsychotic medication can undeniably improve symptoms and reduce relapses and rehospitalizations, but medication treatment alone does not always translate into better quality of life and functionality. In their meta-analysis, Leucht and colleagues22 found that despite superior relapse prevention effects, rates of employment did not differ between patients taking antipsychotics or placebo. However, this finding was based on only 2 studies. Medications are unquestionably the mainstay of treatment for schizophrenia, but they should be prescribed in combination with psychotherapeutic interventions, psychoeducation, supportive employment, and other psychosocial treatments in order to maximize chances for improved outcomes in all relevant domains.
Cost and Clinical Implications of Treating Schizophrenia
Mark Olfson, MD, MPH
Schizophrenia occurs in about 1% of the population, placing 1 person in every 100 at risk of developing the disease sometime during their lifetime.30 Due to its early onset and the high likelihood of persistent symptoms despite optimal treatment,31 schizophrenia is a leading source of disability worldwide. For the affected individual, schizophrenia is among the most disabling of all medical conditions. The World Health Organization, for example, ranks schizophrenia as more disabling than amputation of both legs, severe stroke, end-stage renal disease requiring dialysis, severe Parkinson’s disease, or terminal cancer.32
Schizophrenia can have a devastating effect on personal, social, family, and work functioning. In addition, and most importantly, schizophrenia markedly shortens life expectancy. Individuals with schizophrenia are at risk for complex cascades of unhealthy lifestyle behaviors and environmental exposures that increase their chance of developing several potentially fatal medical diseases.33 Nicotine abuse and dependence,34,35 alcohol36,37 and other substance use disorders,38 physical inactivity,34,39 obesity,40,41 and unprotected sexual behavior42 are all common in schizophrenia. People with schizophrenia are also at greatly elevated risk of suicide.43 As a result of these risks, the average life expectancy for an individual with schizophrenia is 10 to 25 years shorter than for a person without the disorder.44,45
The economic burden of schizophrenia arises not only from premature mortality but also from losses in productivity, negative effects on family members, direct health care costs, and expenditures for other public services such as homeless shelters and prisons. People with schizophrenia and other severe mental illnesses also often require a range of non-medical services including income support, vocational training, and housing assistance to help them manage their daily lives. A 2002 estimate of the overall cost of schizophrenia in the United States was $63 billion.46 Factoring in the increase in the medical component of the Consumer Price Index over the past 12 years yields a rough estimate of the current total annual national cost of nearly $100 billion.47 Further evidence of the high cost of schizophrenia comes from a ranking of the 10 most expensive brain disorders in Europe. Psychotic disorders, of which schizophrenia is the largest component, topped the list despite being far less prevalent than mood or anxiety disorders.48
The distribution of costs of schizophrenia appears to vary by country. In the United States, direct health-related costs are thought to account for only about one-third (36%) of the total economic burden of schizophrenia.46 In Canada and England, the percentage of total economic costs of schizophrenia that are attributable to direct health care costs is somewhat lower: 29%49 and 30%,50 respectively. This is partly due to lower rates of incarceration and homelessness as well as nationalized health systems in Canada and England. These differences suggest that proportionately greater societal savings may be achieved by a more efficient allocation of direct service expenditures for schizophrenia treatment.
FINANCING CARE FOR SCHIZOPHRENIA
In the United States, the financing of mental health services differs from that for general medical disorders. Most importantly, public sources play a much larger role in schizophrenia and other severe mental disorders than in other disorders. The single largest source of financing for the treatment of schizophrenia in the United States is the federal/state Medicaid program. According to 1 population-based survey that excluded institutionalized individuals, 67% of adults with schizophrenia reported they had Medicaid, 46% had Medicare, 87% had either Medicaid or Medicare, and 26% had both. Smaller numbers reported receiving health coverage from the Veterans Health Administration (8%), other public insurance (6%), or private insurance (15%) or had no health insurance (7%). By contrast, the general population is considerably less likely to have Medicare or Medicaid (26%) but much more likely to have private insurance (71%) (AV 3).51 To place these differences in a broader context, it has been estimated that public financing accounts for approximately 61% of the $135 billion dollars in annual behavioral health expenditures, but only 46% of the roughly $2 trillion dollars in annual expenditures for all health services.52
AV 3. Health Care Coverage of Schizophreniaa
aReprinted with permission from Khaykin et al,51 based on Medical Expenditures Panel Survey civilian population data.
Because of their particularly high health care costs, people with schizophrenia who receive both Medicare and Medicaid benefits are of particular interest to policy makers. This group includes Medicaid recipients who qualify for Medicare because of older age or disability and qualify for Medicaid because of disability or poverty.53 In this population, outpatient services have been found to be the largest single expenditure in younger individuals (ages 19–44: 42%; ages 45–64: 39%), constituting about two-fifths of the annual per capita expenditure. Among older individuals with schizophrenia who are in the Medicare and Medicaid programs, the greatest proportions of expenditure are for nursing home services (ages 65–74: 42%; age 75+: 68%). In general, however, Medicare has a substantially smaller role in financing behavioral health care (7%) than it does in financing overall health services (18%).52
COSTS OF SCHIZOPHRENIA: FOCUS ON HIGH-RISK GROUPS
A significant body of research indicates that health care costs are not evenly distributed across covered populations, but rather they are concentrated in a relatively small percentage of high-cost patients.54 When evaluating the health care costs of schizophrenia, it is informative therefore to consider patient groups, such as those with Medicare and Medicaid, who are at elevated risk of high health care costs. Two other groups of patients with schizophrenia who tend to have particularly high health care costs are patients who have recently relapsed and patients who are early in the course of their disease.
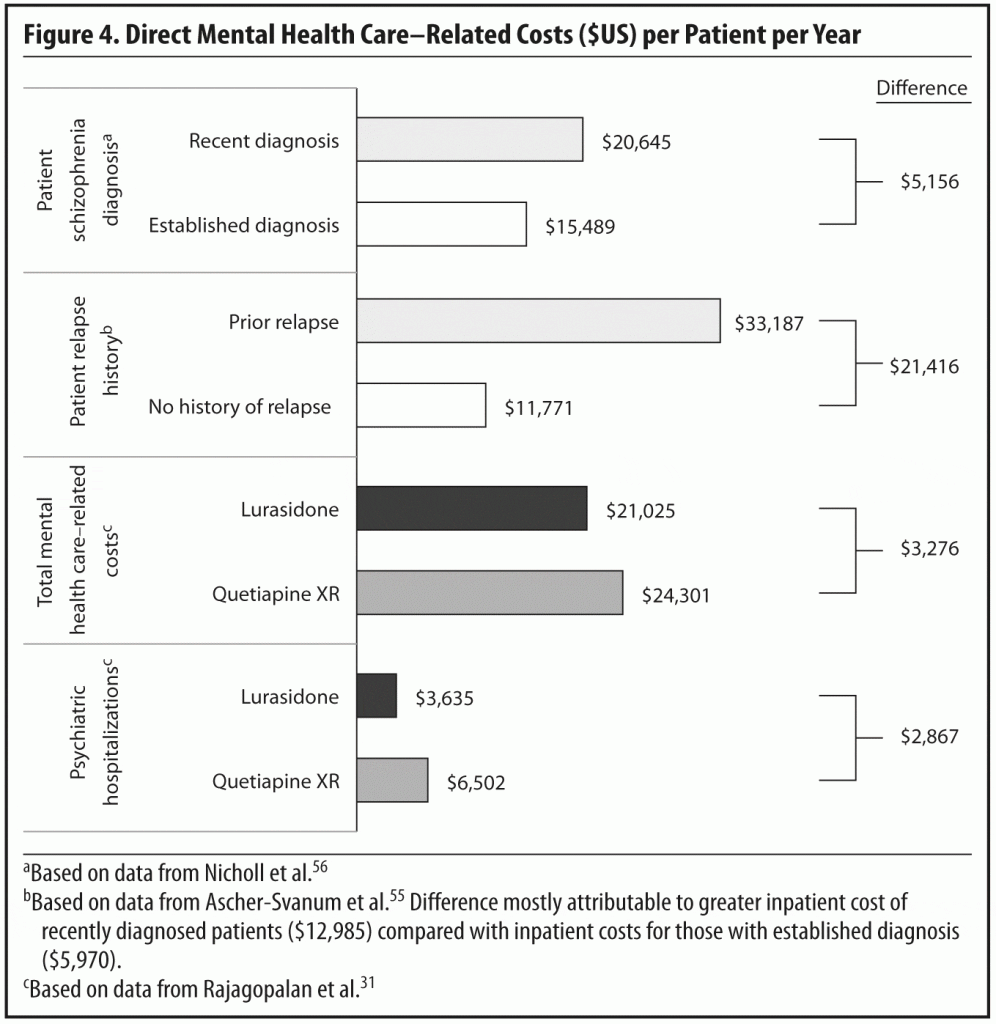
Clinical relapse is distressing and disruptive for patients and their families and places patients at high risk of future increased health care costs. A prospective, observational, non-intervention study55 compared propensity-matched schizophrenia patients with and without relapse in the previous 6 months. Relapse was defined as having a psychiatric hospital admission, emergency service visit, a crisis bed episode, or a medically injurious episode of deliberate self-harm. Over the following year, patients with prior relapse incurred direct mental health care costs nearly 3 times greater than those who had no history of relapse ($33,187 ± $47,616 vs $11,771 ± $10,611, P < .01) (Figure 4).55 The patients with a history of relapse had significantly higher costs for psychiatric hospital admissions, emergency services, medication management, day treatment, individual therapy, and assertive community treatment/case management services.
ASSESSMENT OF EARLY INTERVENTION
A recent economic evaluation of an early intervention program for first-episode schizophrenia illustrates the challenges of consolidating early improvements into long-term clinical gains and economic savings.57 The evaluation was a single-blind, randomized, controlled clinical study57 comparing an intensive early intervention program with standard treatment in community health centers at 2 sites in Denmark. A total of 547 patients received the interventions for 2 years, of which 301 were followed for 5 years. The early intervention program included an enriched assertive community treatment, psychoeducational family treatment, social skills training, and low-dose medication. After 2 years, patients were transitioned over a 2-month period to standard treatment.58 The control group received the standard, routine care offered by local community mental health centers.
At 2 years, the experimental group had significantly better global functioning than the control group (mean GAF scores: 55.2 vs 51.1, P = .03).57 The median number of psychiatric bed days of the control group was also significantly higher than that of the intervention group (median: 52 days vs 25 days, P = .04). Not surprisingly, the associated health care cost savings from reduced inpatient costs was largely offset by a significantly larger number of outpatient visits for the intervention than the control group (mean: 85 visits vs 25 visits).57 The experimental group was also significantly more likely than the control group to be living alone or with children at the end of the 2-year intervention period.
At the 5-year follow-up,58 3 years after completion of the intensive early intervention program, the significant group difference in global functioning was no longer evident. The economic evaluation at this point further revealed a modest and statistically nonsignificant trend toward lower mean total costs per person (intervention group: €123,638; control group: €148,651, P < .11).58 In years 4 and 5, however, the total health care costs were significantly lower for the experimental than the control group.
In sum, a 2-year intensive early intervention program achieved significant functional improvements over usual care during the period of intervention. However, these improvements were not sustained at long-term post-intervention follow-up. Although the model program succeeded in significantly reducing dependence on inpatient treatment, these cost savings were largely offset by increased use and expenditures for outpatient mental health services. It is possible that a longer period of intensive early treatment is necessary to improve longer term course of illness.
ROLE OF MEDICATION IN COST REDUCTION
Antipsychotic medication is a potentially important modifiable aspect of clinical care that may have cost implications for the treatment of schizophrenia. In a previous section of this document, Drs Correll and Kane discuss the findings of a 12-month, double-blind, noninferiority study of the effectiveness of lurasidone versus quetiapine XR for relapse prevention in schizophrenia.25 Efficacy analysis revealed that the estimated probability of relapse at 12 months was 23.7% for patients in the lurasidone group and 33.6% for the quetiapine group. The hazard ratio (95% CI) for probability of relapse was 0.728 (0.410–1.295).
The study results were used to estimate the mental health care costs of treating patients with lurasidone or quetiapine XR over a 1-year period as well as the mental health costs per patient per month (see Figure 4).31 Using relapse-related hospital rates for all subjects, mean per patient total mental health care–related costs for lurasidone ($21,025) were found to be significantly lower than the corresponding costs for quetiapine XR ($24,301). On a cost per patient per month basis, this difference translates to $1,752 for lurasidone-treated patients compared to $2,025 for quetiapine XR–treated patients. Similar results were reported in a secondary analysis restricted to patients who were responders in a 6-week, double-blind, randomized, placebo-controlled study.21 This study and the 12-month relapse prevention study25 were the sources of the relapse rates and relapse-related hospitalization rates for lurasidone and quetiapine XR used as inputs in the economic model.
More than half of the mental health care–related costs were associated with medications and psychiatric hospital admissions.31 A breakdown of the components of care indicated that differences in the rate of psychiatric hospitalization between lurasidone and quetiapine XR patient groups largely accounted for the difference in costs; the estimated annual per-patient cost of inpatient treatment for the quetiapine XR group ($6,502) exceeded that of the lurasidone group ($3,635).31 In all other categories (medication, day treatment, emergency services, psychosocial group therapy, medication management, outpatient individual therapy, and assertive community treatment/case management), the costs of the 2 study groups were similar.
CONCLUSIONS
Schizophrenia exacts a heavy burden on affected individuals, their families, and communities. These burdens have economic as well as social, health, and personal dimensions. There are several ways in which clinicians, clinical administrators, and policymakers might work to achieve improved outcomes for people with schizophrenia. One clinically sensitive approach involves focusing on patients who are at high risk of symptom exacerbations, such as those who have recently experienced a relapse or are early in the course of their disease. Concentrating on optimizing antipsychotic medication selection may be an important component of a broader strategy to improve patient outcomes. In developing strategies to improve the effectiveness and efficiency of care for schizophrenia, it is also reasonable to focus on interventions that have demonstrated promise in reducing the risk of relapse and the consequent clinical need for costly and disruptive inpatient psychiatric admissions.
Drug names: aripiprazole (Abilify), asenapine (Saphris), clozapine (Clozaril, FazaClo, and others), haloperidol (Haldol and others), iloperidone (Fanapt), lurasidone (Latuda), olanzapine (Zyprexa), paliperidone (Invega), quetiapine (Seroquel), risperidone (Risperdal and others), ziprasidone (Geodon).
REFERENCES
1. Harvey PD. Cognitive impairment in schizophrenia: profile, course, and neurobiological determinants. In: Schlaepfer TE, Nemeroff CB, eds. Neurobiology of Psychiatric Disorders. Amsterdam, The Netherlands: Elsevier; 2012:433–445. In: Aminoff MJ, Boller F, Swaab DF, series eds. Handbook of Clinical Neurology; vol 106.
2. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–485. PubMed doi:10.1176/appi.ajp.2010.10060855
3. Hegarty JD, Baldessarini RJ, Tohen M, et al. One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry. 1994;151(10):1409–1416. PubMed
4. Harvey PD, Siu CO, Hsu J, et al. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: a short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur Neuropsychopharmacol. 2013;23(11):1373–1382. PubMed doi:10.1016/j.euroneuro.2013.08.003
5. Bowie CR, McGurk SR, Mausbach B, et al. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169(7):710–718. PubMed doi:10.1176/appi.ajp.2012.11091337
6. Patterson TL, Goldman S, McKibbin CL, et al. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27:235–245. PubMed doi:10.1093/oxfordjournals.schbul.a006870
7. Mausbach BT, Harvey PD, Goldman SR, et al. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr Bull. 2007;33:1364–1372. PubMed doi:10.1093/schbul/sbm014
8. Keefe RSE, Poe M, Walker TM, et al. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163:426–432. PubMed doi:10.1176/appi.ajp.163.3.426
9. McGurk SR, Mueser KT, Feldman K, et al. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am J Psychiatry. 2007;164(3):437–441. PubMed doi:10.1176/appi.ajp.164.3.437
10. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. PubMed doi:10.1176/appi.ajp.2007.07060906
11. Fisher M, Loewy R, Carter C, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia [published online ahead of print January 20, 2014]. Schizophr Bull. PubMed doi:10.1093/schbul/sbt232
12. Harvey PD, Ogasa M, Cucchiaro J, et al. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs ziprasidone. Schizophr Res. 2011;127(1–3):188–194. PubMed doi:10.1016/j.schres.2011.01.004
13. Loebel AD, Siu CO, Cucchiaro JB, et al. Atypical antipsychotics and daytime sleepiness. CNS Spectr. In press.
14. Correll CU. What are we looking for in new antipsychotics? J Clin Psychiatry. 2011;72(suppl 1):9–13. PubMed doi:10.4088/JCP.10075su1.02
15. Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71(9):1115–1124. PubMed doi:10.4088/JCP.10r06264yel
16. Correll CU, Kishimoto T, Nielsen J, et al. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. 2011;33(12):B16–B39. PubMed doi:10.1016/j.clinthera.2011.11.016
17. Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296–1306. PubMed doi:10.1093/schbul/sbs130
18. Correll CU, De Hert M. Antipsychotics for acute schizophrenia: making choices. Lancet. 2013;382(9896):919–920. PubMed doi:10.1016/S0140-6736(13)61032-6
19. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. PubMed doi:10.1016/S0140-6736(13)60733-3
20. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–163. PubMed doi:10.1176/appi.ajp.2008.08030368
21. Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–109. PubMed doi:10.1016/j.schres.2013.01.009
22. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. PubMed doi:10.1016/S0140-6736(12)60239-6
23. Kishimoto T, Agarwal V, Kishi T, et al. Relapse prevention in schizophrenia: a systematic review and meta-analysis of second-generation antipsychotics versus first-generation antipsychotics. Mol Psychiatry. 2013;18(1):53–66. PubMed doi:10.1038/mp.2011.143
24. Glick ID, Correll CU, Altamura AC, et al. Mid-term and long-term efficacy and effectiveness of antipsychotic medications for schizophrenia: a data-driven, personalized clinical approach. J Clin Psychiatry. 2011;72(12):1616–1627. PubMed doi:10.4088/JCP.11r06927
25. Loebel A, Cucchiaro J, Xu J, et al. Effectiveness of lurasidone vs quetiapine XR for relapse prevention in schizophrenia: a 12-month, double-blind, noninferiority study. Schizophr Res. 2013;147(1):95–102. PubMed doi:10.1016/j.schres.2013.03.013
26. Spielmans GI, Berman MI, Linardatos E, et al. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. PubMed doi:10.1371/journal.pmed.1001403
27. Selle V, Schalkwijk S, Vázquez GH, et al. Treatments for Acute Bipolar Depression: Meta-analyses of Placebo-controlled, Monotherapy Trials of Anticonvulsants, Lithium and Antipsychotics. Pharmacopsychiatry. 2014 [Epub ahead of print]. PubMed
28. Rybakowski JK, Vansteelandt K, Szafranski T, et al; EUFEST Study Group. Treatment of depression in first episode of schizophrenia: results from EUFEST. Eur Neuropsychopharmacol. 2012;22(12):875–882. PubMed doi:10.1016/j.euroneuro.2012.04.001
29. Himelhoch S, Slade E, Kreyenbuhl J, et al. Antidepressant prescribing patterns among VA patients with schizophrenia. Schizophr Res. 2012;136(1-3):32–35. PubMed doi:10.1016/j.schres.2012.01.008
30. Saha S, Chant D, Welham J, et al. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. PubMed doi:10.1371/journal.pmed.0020141
31. Rajagopalan K, O’Day K, Meyer K, et al. Annual cost of relapses and relapse-related hospitalizations in adults with schizophrenia: results from a 12-month, double-blind, comparative study of lurasidone vs quetiapine extended-release. J Media Econ. 2013;16(8):987–996. PubMed doi:10.3111/13696998.2013.809353
32. Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129–2143. PubMed doi:10.1016/S0140-6736(12)61680-8
33. Beary M, Hodgson R, Wildgust HJ. A critical review of major mortality risk factors for all-cause mortality in first-episode schizophrenia: clinical and research implications. J Psychopharmacol. 2012;26(suppl):52–61. PubMed doi:10.1177/0269881112440512
34. Chwastiak LA, Rosenheck RA, Kazis LE. Association of psychiatric illness and obesity, physical inactivity, and smoking among a national sample of veterans. Psychosomatics. 2011;52(3):230–236. PubMed doi:10.1016/j.psym.2010.12.009
35. Roick C, Fritz-Wieacker A, Matschinger H, et al. Health habits of patients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):268–276. PubMed doi:10.1007/s00127-007-0164-5
36. Koskinen J, Löhönen J, Koponen H, et al. Prevalence of alcohol use disorders in schizophrenia—a systematic review and meta-analysis. Acta Psychiatr Scand. 2009;120(2):85–96. PubMed doi:10.1111/j.1600-0447.2009.01385.x
37. McCreadie RG; Scottish Comorbidity Study Group. Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. Br J Psychiatry. 2002;181(4):321–325. PubMed doi:10.1192/bjp.181.4.321
38. Koskinen J, Löhönen J, Koponen H, et al. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1115–1130. PubMed doi:10.1093/schbul/sbp031
39. Lindamer LA, McKibbin C, Norman GJ, et al. Assessment of physical activity in middle-aged and older adults with schizophrenia. Schizophr Res. 2008;104(1–3):294–301. PubMed doi:10.1016/j.schres.2008.04.040
40. Allison DB, Fontaine KR, Heo M, et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215–220. PubMed doi:10.4088/JCP.v60n0402
41. Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45–53. PubMed doi:10.1016/j.schres.2005.08.010
42. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted disease risk. Sex Transm Dis. 2004;31(1):8–12. PubMed doi:10.1097/01.OLQ.0000105326.57324.6F
43. Nordentoft M, Laursen TM, Agerbo E, et al. Change in suicide rates for patients with schizophrenia in Denmark, 1981–97: nested case-control study. BMJ. 2004;329(7460):261. PubMed doi:10.1136/bmj.38133.622488.63
44. Crump C, Winkleby MA, Sundquist K, et al. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am J Psychiatry. 2013;170(3):324–333. PubMed doi:10.1176/appi.ajp.2012.12050599
45. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. PubMed doi:10.1001/archpsyc.64.10.1123
46. Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66(9):1122–1129. PubMed doi:10.4088/JCP.v66n0906
47. Bureau of Labor Statistics. Databases, tables, & calculators by subject: consumer price index-all urban consumers, 12-month percent change, medical care, 2002 to 2014. http://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths. Accessed December 2013.
48. Andlin-Sobocki P, Jönsson B, Wittchen HU, et al. Cost of disorders of the brain in Europe. Eur J Neurol. 2005;12(suppl 1):1–27. PubMed doi:10.1111/j.1468-1331.2005.01202.x
49. Goeree R, Farahati F, Burke N, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin. 2005;21(12):2017–2028. PubMed doi:10.1185/030079905X75087
50. Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10(1):23–41. PubMed
51. Khaykin E, Eaton WW, Ford DE, et al. Health insurance coverage among persons with schizophrenia in the United States. Psychiatr Serv. 2010;61(8):830–834. PubMed doi:10.1176/appi.ps.61.8.830
52. Substance Abuse and Mental Health Services Administration. National Expenditures for Mental Health Services and Substance Abuse Treatment, 1986–2005. DHHS Publication No. (SMA) 10-4612. Rockville, MD: Center for Mental Health Services and Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2010.
53. Bartels SJ, Clark RE, Peacock WJ, et al. Medicare and Medicaid costs for schizophrenia patients by age cohort compared with costs for depression, dementia, and medically ill patients. Am J Geriatr Psychiatry. 2003;11(6):648–657. PubMed doi:10.1097/00019442-200311000-00009
54. Joynt KE, Gawande AA, Orav EJ, et al. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309(24):2572–2578. PubMed doi:10.1001/jama.2013.7103
55. Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2. PubMed doi:10.1186/1471-244X-10-2
56. Nicholl D, Akhras KS, Diels J, et al. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin. 2010;26(4):943–955. PubMed doi:10.1185/03007991003658956
57. Hastrup LH, Kronborg C, Bertelsen M, et al. Cost-effectiveness of early intervention in first-episode psychosis: economic evaluation of a randomised controlled trial (the OPUS study). Br J Psychiatry. 2013;202(1):35–41. PubMed doi:10.1192/bjp.bp.112.112300
58. Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry. 2008;65(7):762–771. PubMed doi:10.1001/archpsyc.65.7.762
This PDF is free for all visitors!
Save
Cite
