J Clin Psychiatry 2021;82(5):21br14028
To cite: Okusaga OO, Mitchell BG, Bernard JD, et al. Clozapine is associated with higher COVID-19 infection rate in Veterans with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2021;82(5):21br14028.
To share: https://doi.org/10.4088/JCP.21br14028
© Copyright 2021 Physicians Postgraduate Press, Inc.
aMental Health Care Line, Michael E. DeBakey VA Medical Center, Houston, Texas
bMenninger Department of Psychiatry, Baylor College of Medicine, Houston, Texas
cCenter for Innovations in Quality, Effectiveness and Safety (IQuEST), Michael E. DeBakey VA Medical Center, Houston, Texas
*Corresponding author: Olaoluwa O. Okusaga, MD, MScPHR, Michael E. DeBakey Veterans Affairs Medical Center, 2002 Holcombe Blvd, 580/116 MHCL rTMS, Houston, TX 77030 ([email protected]).
Clozapine, the only antipsychotic medication approved for treatment-resistant schizophrenia,1 was recently reported to be associated with increased risk of COVID-19 infection in patients with schizophrenia spectrum disorders.2 Additionally, a history of military service has been associated with increased risk of testing positive for COVID-19.3 However, clozapine-related risk of COVID-19 infection in Veterans with schizophrenia or schizoaffective disorder has not been evaluated. The current study attempts to fill this knowledge gap.
Methods
This is a cross-sectional analysis of electronic health record (EHR) data of Veterans with a diagnosis of schizophrenia or schizoaffective disorder who received treatment at any United States Veterans Affairs Medical Center (VAMC) between January 1, 2020–January 1, 2021. Schizophrenia and schizoaffective disorder were combined, given the identical nature of the disorders to one another on key cognitive, social cognitive, and neural measures.4 Approval for the study was granted by the Institutional Review Board of Baylor College of Medicine and the Michael E. DeBakey VAMC Research and Development Committee.
Data were collected from the Corporate Data Warehouse (CDW) and the Veterans Affairs Informatics and Computing Infrastructure (VINCI).5 CDW incorporates data from multiple data sets (including EHR) throughout the Veterans Health Administration into 1 standard database structure to facilitate reporting and data analysis. VINCI is a partner with the CDW and hosts all data available through CDW as well as other unique data. However, if a Veteran receives care at a non-VA health care facility, then the data for that encounter (including data on clozapine or COVID-19 test results) will not automatically be captured by CDW/VINCI.
The International Classification of Diseases, Ninth and Tenth Revisions codes (ICD-9 and ICD-10) were used to identify Veterans with schizophrenia or schizoaffective disorder. COVID-19 test results are documented in the EHR as “positive” or “negative.” Although Veterans with psychiatric disorders have an option to request COVID-19 testing at no cost, they are not routinely tested if they do not report exposures or symptoms and if not admitted or undergoing diagnostic or therapeutic procedures (eg, electroconvulsive therapy).
The sample was divided into 2 groups: (1) Veterans treated with clozapine between January 1, 2020, and January 1, 2021 (clozapine group), and (2) Veterans with no record of clozapine treatment but treated with other antipsychotics (non-clozapine group). The clozapine group started taking the medication prior to January 1, 2020. The odds of testing positive for COVID-19 were compared between the clozapine and non-clozapine groups using logistic regression and adjusted analysis controlled for age, race, sex, marital status, body mass index, and Charlson-Deyo score6 (an indicator of long-term mortality). Statistical analyses were performed with IBM SPSS, Version 27 (IBM Corp, Armonk, New York).
Results
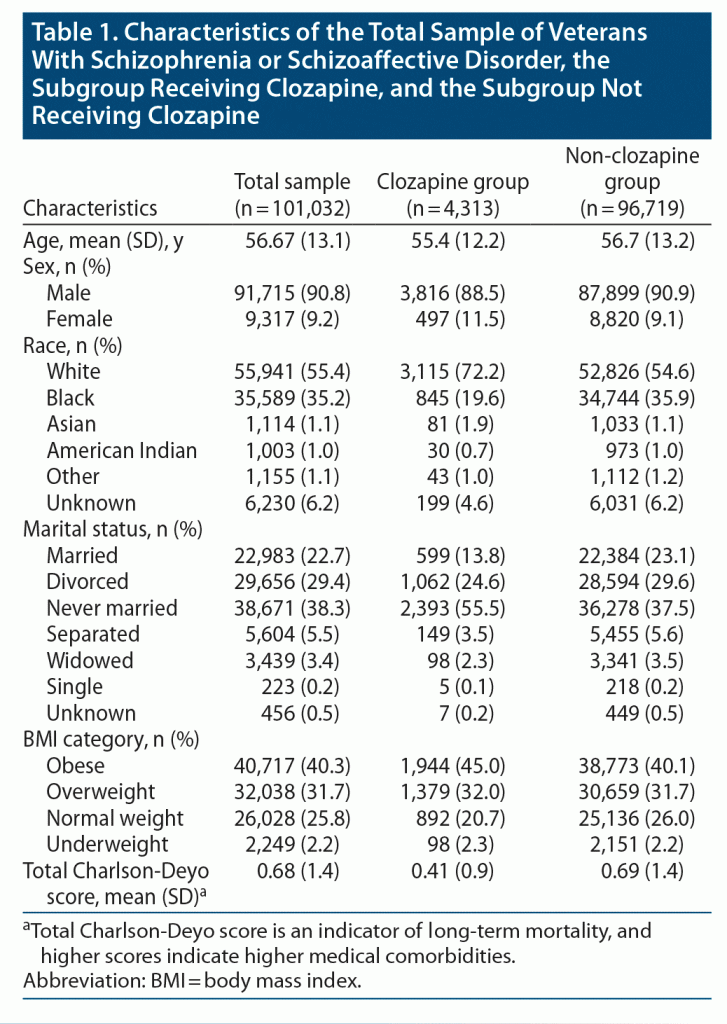
101,032 Veterans (4,313 treated with clozapine, 96,719 treated with other antipsychotics) were included in the final analyses. Table 1 depicts the demographic and clinical characteristics of the patients included in the final analyses. Forty-four (1%) Veterans in the clozapine group and 675 (0.7%) Veterans in the non-clozapine group were positive for COVID-19. The odds of testing positive for COVID-19 were higher in the clozapine group from unadjusted (odds ratio [OR] = 1.47; 95% CI, 1.08–1.99) and adjusted (OR = 1.77; 95% CI, 1.29–2.41) analyses.
Discussion
In this nationwide sample of Veterans with schizophrenia or schizoaffective disorder, clozapine treatment increased the odds of testing positive for COVID-19 by 47% in unadjusted analyses and 77% in adjusted analysis. Our results are consistent with a previous smaller study2 carried out on a non-Veteran sample. Although we do not know the specific mechanism underlying the association of clozapine with increased risk of COVID-19, speculatively, the association might be a reflection of clozapine’s effect on immune function.7 On the other hand, the observed association might not be due to clozapine, but instead treatment-resistant status itself may mediate the association, given that clozapine is most often prescribed for treatment-resistant schizophrenia/schizoaffective disorder.
Our results should be interpreted with caution due to study limitations, including the observational nature of this study, unavailability of data on smoking status, surveillance bias (the clozapine group might be seen more frequently), and our inability to adjust for inpatient status (which might be associated with increased risk of COVID-19 positivity due to increased risk of exposure and better surveillance; patients on clozapine, by virtue of being treatment resistant, are also more likely to be admitted to inpatient care). Although the patients in the clozapine group started taking clozapine prior to January 1, 2020 (ie, prior to the pandemic), since this is a cross-sectional study and we did not prospectively follow the participants, we cannot rule out the possibility that some of the Veterans on clozapine could have stopped taking the medication prior to the specified end of the study (January 1, 2021). Strengths of this study include a large sample size and the adjustment for factors known to be associated with the risk of COVID-19 infection. This study provides evidence justifying prioritization of COVID-19 vaccination in patients with schizophrenia or schizoaffective disorder who receive clozapine treatment.
Received: April 8, 2021.
Published online: August 24, 2021.
Potential conflicts of interest: The authors have no conflict of interest to report.
Funding/support: This study is not funded.
References (7)

- Martini F, Spangaro M, Buonocore M, et al. Clozapine tolerability in treatment resistant schizophrenia: exploring the role of sex. Psychiatry Res. 2021;297:113698. PubMed CrossRef
- Govind R, Fonseca de Freitas D, Pritchard M, et al. Clozapine treatment and risk of COVID-19 infection: retrospective cohort study [published online ahead of print July 27, 2020]. Br J Psychiatry. PubMed CrossRef
- Tsai J, Huang M, Elbogen E. Mental health and psychosocial characteristics associated with COVID-19 among US adults. Psychiatr Serv. 2021;72(4):444–447. PubMed CrossRef
- Hartman LI, Heinrichs RW, Mashhadi F. The continuing story of schizophrenia and schizoaffective disorder: one condition or two? Schizophr Res Cogn. 2019;16:36–42. PubMed CrossRef
- Price LE, Shea K, Gephart S. The Veterans Affairs’s Corporate Data Warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39(4):311–318. PubMed CrossRef
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. PubMed CrossRef
- Ponsford MJ, Pecoraro A, Jolles S. Clozapine-associated secondary antibody deficiency. Curr Opin Allergy Clin Immunol. 2019;19(6):553–562. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite