‘ ‹’ ‹
Click to enlarge page
Cognitive impairment is a common, often persistent, symptom of major depressive disorder (MDD) that is disproportionately represented in patients who have not returned to full psychosocial functioning. The ultimate goal of treatment in depression is full functional recovery, and assessing patients for cognitive impairment and selecting treatments that address cognitive dysfunction should lead to improved functional outcomes. Unfortunately, many clinicians use screening and assessment tools that are not suited for measuring cognitive impairment in patients with depression. The new THINC-it assessment tool is the first instrument that provides objective and subjective data on dysfunction in all the cognitive domains commonly affected by depression. In regard to treatment, several pharmacologic and nonpharmacologic interventions have been investigated as treatments for cognitive dysfunction in individuals with MDD. However very few studies of treatments for cognitive function in patients with MDD have been adequate, in terms of sample size and study methods, to guide clinical practice. The best evidence supports the moderate efficacy of some antidepressants, cognitive-behavioral therapy, and exercise.
From the Department of Family Medicine, Boston University, Massachusetts (Dr Culpepper); the Department of Mood and Anxiety Disorders, The University of British Columbia, Vancouver, Canada (Dr Lam); and the Mood Disorders Psychopharmacology Unit, University of Toronto, Ontario, Canada (Dr McIntyre).
‘ ‹’ ‹’ ‹’ ‹
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the planning teleconference series “Cognitive Impairment in Patients With Depression: Awareness, Assessment, and Management,” which was held in May and June 2017. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Takeda Pharmaceuticals U.S.A., Inc. and Lundbeck.
The teleconference was chaired by Larry Culpepper, MD, MPH, from the Department of Family Medicine, Boston University, Massachusetts. The faculty were Raymond W. Lam, MD, FRCPC, from the Department of Mood and Anxiety Disorders, The University of British Columbia, Vancouver, Canada, and Roger S. McIntyre, MD, FRCPC, from the Mood Disorders Psychopharmacology Unit, University of Toronto, Ontario, Canada.
Financial disclosure: Dr Culpepper has been a consultant for Allergan, Ironshore, Lundbeck, Shire, and Sunovion; is a stock shareholder in M3 Information; has received royalties from UpToDate and Oxford University Press; and receives payment from Physicians Postgraduate Press, Inc., for serving as Editor in Chief of The Primary Care Companion for CNS Disorders. Dr Lam has received speaker honoraria from AstraZeneca, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, Lundbeck, Lundbeck Institute, and Otsuka; has served as a consultant/advisor for Allergan, Asia-Pacific Economic Cooperation, Bristol-Myers Squibb, Canadian Depression Research and Intervention Network, Canadian Network for Mood and Anxiety Treatments, Janssen, Lundbeck, Medscape, Pfizer, and Takeda; has received research funds from BC Leading Edge Foundation, Brain Canada, Bristol-Myers Squibb, Canadian Institutes of Health Research, Canadian Depression Research and Intervention Network, Canadian Network for Mood and Anxiety Treatments, Janssen, Lundbeck, Movember Foundation, Pfizer, St Jude Medical, University Health Network Foundation, Vancouver Coastal Health Research Institute, and VGH Foundation; holds patents and copyrights for the Lam Employment Absence and Productivity Scale (LEAPS); and has received book royalties from Cambridge University Press, lnforma Press, and Oxford University Press. Dr McIntyre is a member of the advisory boards for and has received speakers fees from Lundbeck, Pfizer, AstraZeneca, Eli Lilly, Janssen-Ortho, Purdue, Johnson & Johnson, Moksha8, Sunovion, Mitsubishi, Takeda, Forest, Otsuka, Bristol-Myers Squibb, and Shire and has received research grants from Lundbeck, Janssen-Ortho, Shire, Purdue, AstraZeneca, Pfizer, Otsuka, Allergan, and Stanley Medical Research Institute.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
J Clin Psychiatry 2017;78(9):1383-1394
https://doi.org/10.4088/JCP.tk16043ah5c
© Copyright 2017 Physicians Postgraduate Press, Inc.
Major depressive disorder (MDD) is a serious, often chronic, and disabling condition that is common across cultures and age groups.1 The mean lifetime prevalence of MDD is estimated to be 14.6% in high-income countries and 11.1% in low-/middle-income countries.1 MDD is associated with psychosocial, interpersonal, and workplace disability.2 The emerging understanding is that cognitive impairment is the principal mediator of functional outcomes in those with MDD and in those with MDD and comorbid medical disorders.3-5 Assessing patients with depression for cognitive impairment and selecting treatments that address cognitive function should lead to improved functional outcomes. In this Academic Highlights, Dr Raymond W. Lam describes the burden associated with cognitive impairment in MDD. Dr Roger S. McIntyre then shares effective strategies for assessing cognitive impairment, and Dr Larry Culpepper concludes with a discussion of treatment strategies.
The Burden of Cognitive Impairment in MDD
According to the World Health Organization, depression is the leading cause of disability, affecting over 300 million people worldwide.6 The resulting financial burden of MDD is staggering; for example, lost productivity due to workplace absenteeism and presenteeism costs an estimated $246 billion annually across 8 countries (Brazil, Canada, China, Japan, Korea, Mexico, South Africa, and the United States).7,8 Dr Lam emphasized that the burden of depression is truly a global issue, since it is prevalent in both high- and low-income countries and is associated with considerable impairment.
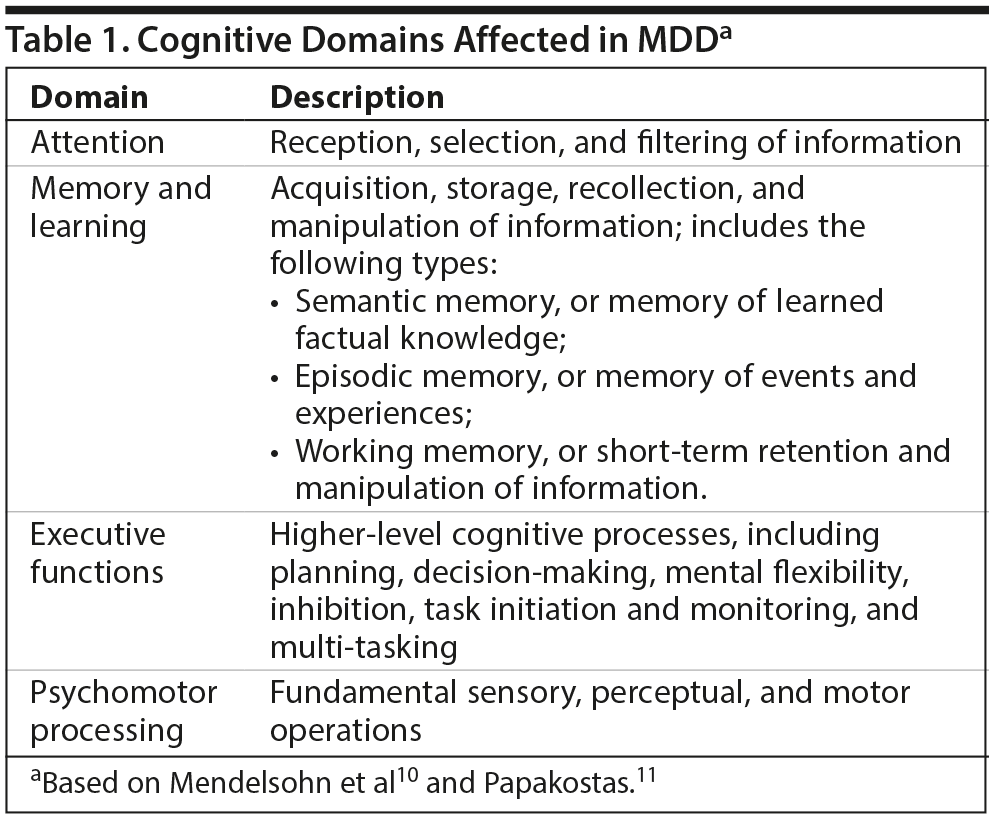
For many individuals with depression, the impairment they experience is related to the cognitive symptoms of this disorder. Although considered a mood disorder, the symptoms of MDD fall into broad clusters of emotional, physical, and cognitive symptoms.9 Cognitive functioning is a complex process that can be broken down into multiple domains, and the domains most relevant to MDD are attention, memory and learning, executive function, and psychomotor processing (Table 1).10,11 A systematic review10 of studies using the Cambridge Neuropsychological Test Automated Battery (CANTAB)12 showed moderate deficits in executive function, memory, and attention in patients with depression (n = 784) compared with healthy controls (n = 727). Researchers are now realizing the substantial impact of cognitive symptoms (eg, slowed thinking and problems with concentration, memory, and decision-making) on functioning.13,14
To illustrate the various ways in which cognitive impairment can manifest in individuals with MDD, Dr Lam offered several example case presentations. These presentations included a 62-year-old homemaker who becomes overwhelmed by the weekly grocery shopping, a 43-year-old plumber who keeps forgetting to send out his customer invoices, and a 22-year-old graduate student who has been treated for depression and is feeling better but is still unable to return to school. All of these individuals are experiencing cognitive impairment as part of their depression.
Although the experience of cognitive impairment is not required for a diagnosis of MDD, it is one of the cardinal diagnostic criteria, and as the previous examples illustrate, this symptom can affect several spheres of functioning, including employment, social life, family life, and home responsibilities.9,15 More than 90% of patients have been found to experience some level of functional impairment, with nearly 70% of these patients reporting their impairment as severe.14
The impact of different depressive symptoms on functional impairment varies, and symptoms related to cognition have been shown to be particularly debilitating. A STAR*D study report14 examined the relationship between individual depressive symptoms and the degree of functional impairment based on the Work and Social Adjustment Scale (WSAS)16 in 3,703 patients. The top 3 symptoms associated with functional impairment were sad mood, concentration problems, and fatigue. In fact, concentration was one of the most debilitating symptoms in each domain of functioning measured on the WSAS (ie, work, home management, social activities, private activities, relationships). Furthermore, Dr Lam added that he and his colleagues17 surveyed 164 patients with depression to assess the extent to which individual depressive symptoms interfered with work functioning. They discovered that cognitive difficulty contributed to clinically important interference in a substantial proportion of respondents (45% had trouble concentrating; 39% had trouble with memory). Dr McIntyre and coworkers5 reached a similar conclusion when they assessed 260 patients for the effects of perceived cognitive dysfunction and severity of depression on work productivity. A regression analysis showed that both the cognitive symptoms and the overall severity were independently related to impairment in work productivity. The magnitude of the effect of the cognitive symptoms on work productivity was greater than that of the overall depression severity.

- Recognize cognitive impairment as a barrier to functional recovery
- Assess patients with depression for cognitive impairment, both during acute episodes and after symptoms remit, using an appropriate assessment tool
- Determine each patient’s subjective perception of the burden of his or her cognitive impairment in order to prioritize treatment goals
- Evaluate both direct and indirect effects on cognition when selecting treatments
- Consider augmenting pharmacotherapy with psychotherapy, lifestyle modifications, or other interventions as appropriate to obtain optimal cognitive functioning
As Dr Lam pointed out, these widespread cognitive difficulties and the associated functional impairment should raise concern because they can negatively affect the outcomes of depression treatment. For example, one STAR*D study report18 found that some patients who had achieved MDD symptomatic remission after 12 weeks of treatment had regained normal functioning, but others still reported functional impairment, as measured by the patient-rated WSAS. Despite continued treatment during follow-up, the patients who had not regained normal functioning at remission had an odds ratio of 2.65 for depression relapse at 6 months, and 3.86 at 12 months, compared with patients who had recovered normal functioning.
According to Dr Lam, not only is functional impairment important to objective measures of improvement from MDD, it is also essential to patients’ subjective assessments of remission and recovery. A 2006 study19 of patients with MDD illustrated that similar proportions of patients prioritize the recovery of functioning and symptom resolution as the key treatment goal. Patients were asked to rate 16 factors they consider to be important in the concept of remission. Among 487 respondents, a total of 11.0% rated “Functioning well,” “Return to usual level of functioning at work, home, or school,” or “Able to fulfill usual responsibilities” as the most important factor in remission, while 10.5% said “Absence of symptoms of depression” is the most important factor. Zimmerman and colleagues20 assessed 274 outpatients receiving treatment for MDD. Approximately one-half of the patients received a score below 7 on the Hamilton Depression Rating Scale and were considered to be in remission, but on self-report, only about one-half of those patients actually considered themselves to be in remission. Self-described remitters reported significantly better quality of life and significantly less functional impairment (P < .001 for both measures) in all domains compared with those who did not consider themselves to be in remission.
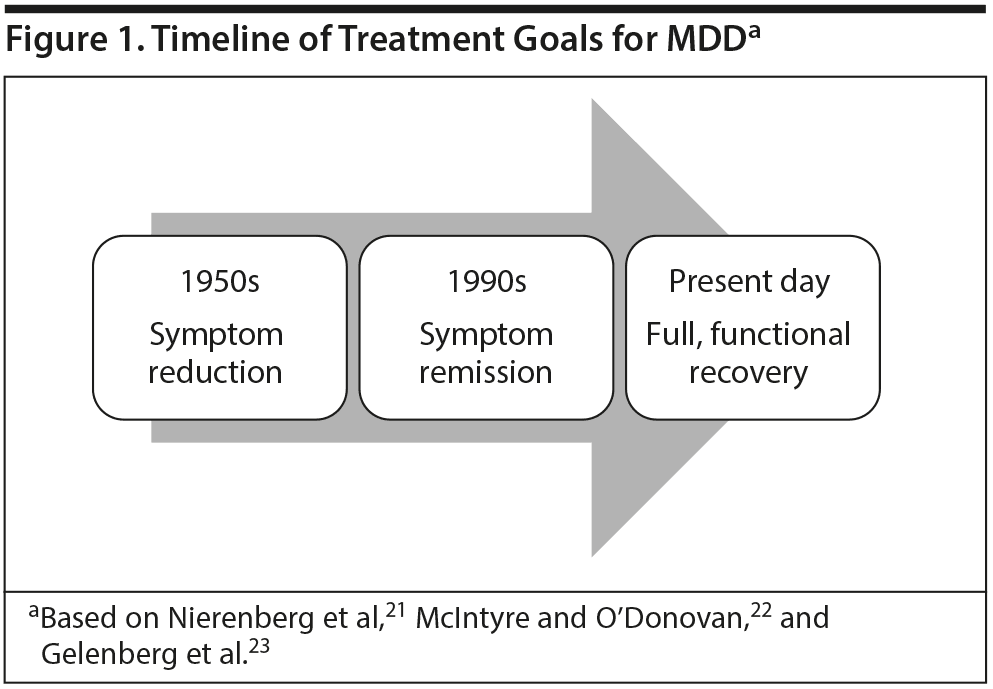
Dr Lam continued by discussing how MDD treatment goals have evolved to reflect the growing awareness of the burden created by cognitive impairment (Figure 1). When antidepressants were introduced in the 1950s through the late 1960s, a 50% or greater reduction in symptoms was considered satisfactory,21 but by the 1990s, symptom remission had become the treatment goal.22 Now, full functional recovery is the ultimate goal of treatment.23 This shift occurred because patients can continue to experience considerable cognitive deficits and fail to return to baseline functioning even after other MDD symptoms have improved. For example, the Conference Board of Canada surveyed workers returning to work after taking a depression-related leave of absence, asking them whether symptoms, including cognitive symptoms, persisted.81 Among 67 workers who reported difficulties with work-related activities upon return to the workplace, 52% had concentration problems, 42% had memory problems, 31% had difficulties making decisions, and 30% had trouble performing tasks. The Conference Board also surveyed supervisors (n = 140), and 76%-86% reported observing these cognitive difficulties in some workers who had returned from a depression-related leave of absence.
Case Practice Question
Case 1. Allison is a 36-year-old executive for a technology company. She has been depressed and isolated in her office, avoiding her coworkers. She has been procrastinating in her work, finding it difficult to make decisions, and feeling overwhelmed. Which domain of cognitive function is most likely impaired?
- Attention
- Memory and learning
- Psychomotor processing
- Executive function
Discussion of Case Practice Question
Preferred response: d. Executive function
Executive function is the cognitive domain that encompasses an individual’s mental control and ability to self-regulate. This domain includes skills such as planning, or the ability to identify and organize steps required to reach a goal; decision making, or the ability to assess probable outcomes in order to make a choice, potentially leading to a delayed reward; and response inhibition, or the ability to use higher-level executive control to suppress unnecessary or inappropriate actions.11 Allison’s procrastination suggests she may be having difficulty planning or prioritizing her work tasks, which is indicative of impaired executive functioning, as is her difficulty in making decisions.
Assessing Cognitive Impairment
Dr McIntyre began by noting that the current state of assessments for measuring cognitive impairment in MDD is far from ideal. Although MDD treatment guidelines place importance on assessing cognitive function,24 clinicians often perform these assessments with inappropriate tools, such as instruments meant for assessing depression severity or intended to be used in populations with dementia.25 El Hammi and colleagues26 administered a survey to psychiatrists practicing in several countries, including the United States, Germany, France, Spain, Hong Kong, and Australia, to determine their perceptions and evaluation of cognitive dysfunction in patients with MDD in routine clinical practice. The majority of respondents (61%) reported that they assessed cognitive dysfunction exclusively through the patient history interview. Among respondents who stated that they used assessment instruments, 29 different instruments were named, but only 6 could be considered appropriate cognitive assessment tools in MDD.
Available Assessment Tools
Many different assessment instruments are available, and according to Gelenberg et al,82 they fall into the following categories:
- Screening tools: These tools broadly establish the presence of a phenotype. Cost-effective, self-rated tools to screen for MDD include the 9-item Patient Health Questionnaire (PHQ-9),27 the 2-item Patient Health Questionnaire (PHQ-2),28 the Center for Epidemiologic Studies Depression scale (CES-D),29 and the Zung Self-Rating Depression Scale (Zung SDS).30
- Diagnostic tools: These tools aid in making a precise diagnosis of MDD. Clinician-rated diagnostic tools include the Mini-International Neuropsychiatric Interview (MINI),31 the Psychiatric Diagnostic Screening Questionnaire (PDSQ),32 and the Structured Clinical Interview for DSM-5 Disorders (SCID-5).33
- Monitoring tools: Tools in this category measure symptom severity, adverse effects, and suicidality over time. Examples are the Quick Inventory of Depressive Symptomatology (QIDS)34 and the Clinically Useful Depression Outcome Scale (CUDOS).35
Some assessment tools are used in more than 1 of the 3 categories. For example, while the PHQ-2 is solely a screening tool, the PHQ-9 is used for screening, diagnosis, and symptom monitoring. Throughout these tools, cognitive impairment is usually assessed as a core symptom of MDD. For example, the PHQ-9 asks about trouble concentrating, and the Zung SDS asks about mental clarity and decision-making. The MINI and CUDOS also include questions about concentration and decision-making. However, Dr McIntyre asserted that tools that specifically and comprehensively address cognition via self-report with both subjective and objective ratings have not been available.
To improve outcomes in MDD, the cognitive domains that should be assessed and targeted for treatment are attention, memory and learning, psychomotor processing, and all aspects of executive function.36 In addition, Dr McIntyre maintained that obtaining a complete picture of patient cognition requires both self-rated and objective measures. While several neurocognitive batteries exist, most are domain specific and are either self-rated or objective, meaning that clinicians would have to use multiple tools to comprehensively assess patient cognition. Furthermore, most cognitive assessment instruments are difficult to access, expensive, time-consuming, and not digitized to make them effectively available at the point of care.25
According to Dr McIntyre, clinicians have been lacking an effective psychometric tool that includes both self-rated and objective measures capable of comprehensively assessing the domains of cognitive function. The tool must produce consistent results across time for healthy controls, must be sensitive to the phenotype being measured (ie, cognitive impairment), and must be valid, meaning that any detected deficit can be attributed to cognitive impairment and not another confounding factor. The tool must be relatively immune to practice effects so that clinicians can be certain that patient improvements are due to cognition improvement and not patient mastery of using the measurement instrument. Finally, the tool should be available in multiple formats, be capable of cross-cultural use, and be computerized.
THINC-integrated tool
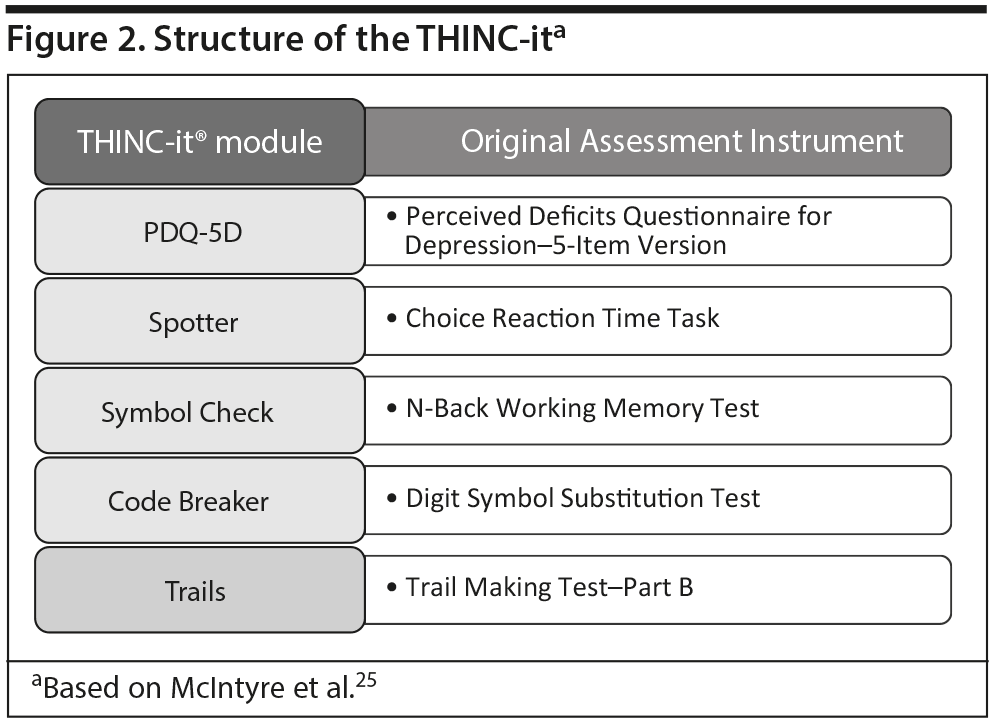
Dr McIntyre explained that, to provide clinicians with an effective and easy-to-administer tool for assessing cognitive impairment in MDD, a task force of international experts convened and developed the THINC-integrated tool (THINC-it), which is the first digital, self-administered assessment tool to include both subjective and objective measures of cognitive dysfunction in domains affected in MDD: attention, memory and learning, processing speed, and executive function.25 The THINC-it consists of 5 test modules that are variations of well-established, carefully selected assessments (Figure 2) that can be completed by patients in 10 to 15 minutes with minimal instruction. The THINC-it is available for use on desktop computers and touchscreen tablet devices, and it can be downloaded for free at https://thinc.progress.im/en/content/thinc-it-about. Plans are underway to make the tool available in multiple languages.37
Results from a recent validation study25,37 of the THINC-it were favorable. In this study,25 which was led by Dr McIntyre and colleagues, 100 patients with recurrent MDD who were experiencing a depressive episode of at least moderate severity were asked to complete the THINC-it assessment. The sensitivity and concurrent validity of the tool were assessed. The THINC-it was found to be sensitive enough not only to detect cognitive dysfunction in adults with MDD but also to quantify the magnitude of these deficits, with 44.4% of the respondents scoring 1 standard deviation or more below healthy controls (P < .001). Furthermore, the THINC-it demonstrated comparable reliability and validity to other established computerized and pen-and-paper cognitive assessments. Finally, participants completed a patient satisfaction questionnaire and reported finding the THINC-it assessment to be user-friendly, easy to complete, and to have understandable instructions. Thus, Dr McIntyre concluded that the THINC-it has the potential to be an invaluable resource for accurate, thorough cognitive assessment that is quick and easy to administer in clinical practice.
Case Practice Question
Case 2. Victor is a 39-year-old man working in the information technology sector. He has received treatment for the past 6 months for a major depressive episode, but he continues to have cognitive complaints that are interfering with his function at work. Which of the following measurement tools is best suited to evaluate Victor for cognitive dysfunction?
- THINC-it
- PDSQ
- CUDOS
- PHQ-9
Discussion of Case Practice Question
Preferred response: a. THINC-it
Because cognitive symptoms often persist once other symptoms have improved with treatment, Victor’s presentation is not unique. His cognitive symptoms must be addressed in order for him to regain full functioning at work. Effective cognitive assessments should be brief, easy to administer, and easy for the patient to complete. The assessment should also obtain both objective and subjective measures of cognitive functioning and provide actionable information. The THINC-it is the only assessment instrument in the list that meets these criteria.
Treating Cognitive Impairment
Dr Culpepper re-emphasized Dr Lam’s statement that the current expectation for successful treatment of MDD is full, functional recovery. He explained that addressing cognitive impairment is in keeping with traditional approaches to treating acute depression and managing patients with long-term, recurrent depressive illness. For treatment to be successful, cognitive dysfunction must be addressed, since impairment tends to persist after other symptoms have remitted and profoundly impact a patient’s functional abilities. Clinicians should develop an individualized, integrated management plan for cognitive impairment that includes pharmacologic and nonpharmacologic treatments.38
Pharmacotherapies
Although numerous types of pharmacologic treatments have been investigated for their effects on cognition in MDD, no drug is FDA-approved to treat cognitive impairment in MDD. Clinicians should select treatments for patients with cognitive symptoms based on the available evidence. When considering studies of depression and cognitive function, it is useful to think about whether the treatment effect on cognitive dysfunction is direct or indirect. An indirect cognitive effect of treatment is dependent on improvement in patients’ mood state, while a direct effect occurs when the treatment directly improves cognitive function, not requiring an intermediary improvement in mood symptoms. Unfortunately, very few studies of cognitive function in patients with MDD have been adequate, in terms of sample size and study methods, to guide clinical practice.39,40 A review38 found limited evidence for improvement in cognitive domains with most interventions tested, including antidepressants, psychostimulants, and experimental treatments such as the hormone erythropoietin and intranasal insulin.
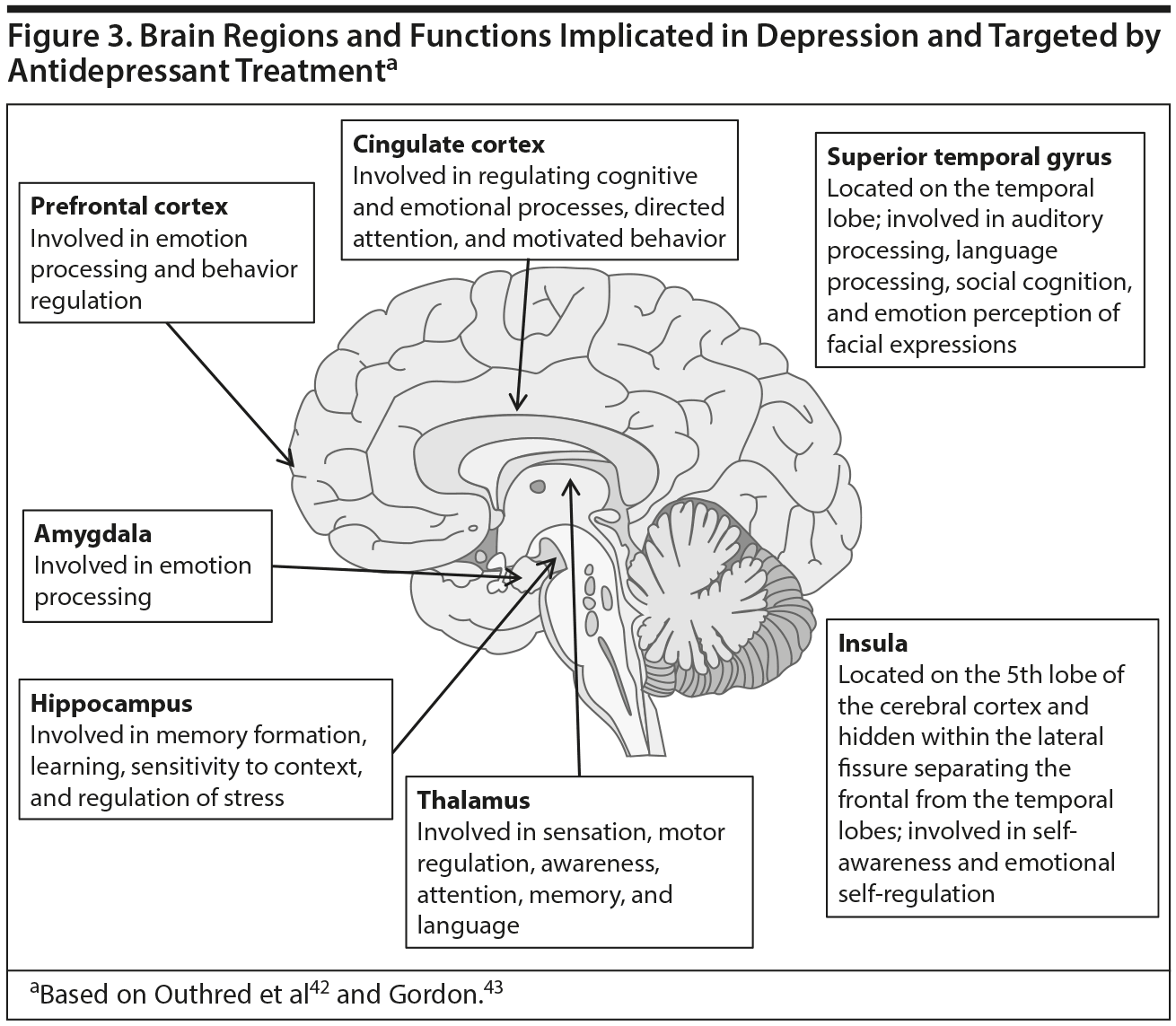
In addition to considering whether a treatment has a direct or indirect effect on cognition, continued Dr Culpepper, treatments must be understood within the context of the biological course of depression. Depression, combined with stress resulting from numerous causes, such as severe loss, adversity, or physiologic causes, leads to a series of molecular and cellular events. These events include changes in intracellular signaling, gene expression, neuronal function, and cellular architecture within brain regions that control mood and cognition.41 Affected brain regions include the amygdala, prefrontal cortex, hippocampus, thalamus, cingulate cortex, insula, and superior temporal gyrus (Figure 3).42,43 Antidepressant treatments also modify neural processes. Dr Culpepper pointed out that by considering the neural processes underlying both cognitive impairment and treatment effects, a conceptual model can be formed to guide selection of treatments. Clinicians can choose treatments that have a direct effect on cognition by targeting the processes underlying the cognitive impairments, or an indirect effect by targeting the processes underlying the mood symptoms.
Antidepressants. Different classes of antidepressants have been studied as treatments for cognitive dysfunction, and their differing mechanisms may impact their efficacy as either direct or indirect treatments for these deficits.42 For example, Outhred and colleagues42 conducted a meta-analysis of the effects of single-dose selective serotonin reuptake inhibitors (SSRIs) and noradrenergic reuptake inhibitors (NRIs) on emotion processing tasks in healthy participants, thus ensuring that they were analyzing the direct effects of study treatments. The results, as measured by functional magnetic resonance imaging (fMRI), indicated an acute effect of the antidepressants on the study participants’ emotion processing—a notable finding considering that clinical improvement with antidepressant treatment is generally not observed until 4 to 6 weeks. The results also suggest variable effects depending on the SSRI class and task. SSRIs appeared to decrease amygdala responses, reducing emotional reactivity to stimuli, whereas NRIs increased activation in the cingulate cortex and thalamus, leading to increased emotional regulation.42
These results provide a model for conceptualizing the potential effects of antidepressants, which can improve negative emotional processing earlier than they improve mood symptoms. This is relevant to the treatment of cognitive impairment because it has been hypothesized that negative emotional processing, or the tendency to display a negative bias in perception, attention, and memory, is a key feature of depression; individuals with depression tend to translate this bias into conscious thoughts, memories, and actions, often showing greater attention and memory to negative stimuli.44 Indeed, these emotion-laden functions related to negative affect are sometimes referred to as “hot” cognition, to distinguish them from “cold” cognition, or emotion-independent areas including executive function, information processing speed, learning and memory, and attention/concentration.38,45 Thus, by reducing “hot” cognitive bias, antidepressants may be able to enhance “cold” cognition by improving an individual’s ability to attend to and remember positive or neutral stimuli.44 Furthermore, Dr Culpepper noted, these findings illustrate that the treatment of cognition can be undertaken at the cellular level by using antidepressants that engage brain networks implicated in cognitive functioning.
SSRIs are thought to obtain their therapeutic effect through their ability to block the reuptake of serotonin (5-HT), thereby increasing the level of 5-HT in the synapses. Serotonergic axons innervate brain regions including the amygdala, hypothalamus, basal ganglia, thalamus, hippocampus, cingulate cortex, and prefrontal cortex, all of which are involved in both emotion and cognitive processing tasks.42 SSRIs, therefore, may be effective for alleviating cognitive dysfunction through their ability to regulate serotonin. In fact, several agents have been assessed as treatments for cognitive dysfunction in MDD.
Like citalopram, the SSRI escitalopram has been found to improve negative emotional processing through normalizing amygdala hyperactivity.46 Escitalopram has also been associated with improvements in cognitive functioning. Savaskan and colleagues47 reported significant improvement in cognitive functioning in 18 elderly patients with depression after 4 weeks of treatment with escitalopram (P = .023), and Herrera-Guzmán and colleagues48 found improvements in verbal and visual working memory, sustained attention, inhibition of automated responses, and set-shifting and planning in 36 patients aged 20 to 50 with depression. Cassano et al49 assessed cognitive function in 242 elderly patients after a year of SSRI treatment for depression. Participants had been treated with either paroxetine or fluoxetine, and at the conclusion of the study, improvements were detected in most cognitive functions tested.
Norepinephrine is associated with arousal and alertness as well as other behavioral physiological effects, and it is linked to cognition, learning, anxiety, and sleep regulation.42 Serotonin-norepinephrine reuptake inhibitors (SNRIs) block the reuptake of both norepinephrine and serotonin in the brain. Like serotonin axons, norepinephrine axons project into areas of the brain, such as the prefrontal cortex, amygdala, cingulate cortex, and thalamus, that are involved in emotion and cognitive processing tasks. Several SNRIs have been investigated for their procognitive benefits. As part of the International Study to Predict Optimized Treatment in Depression (iSPOT-D) trial, a large, randomized, open-label study50 enrolled over 1,000 patients with depression aged 18-65 years. The SNRI venlafaxine was tested, along with the SSRIs escitalopram and sertraline. The results showed no efficacy across all cognitive domains, even in patients whose MDD remitted. However, more recently, Tian and colleagues51 found improvement in executive control of attention in patients with MDD after 6 weeks of treatment with venlafaxine. The SNRI duloxetine has also shown promise for improving cognition. In older patients (aged 65-90 years), both learning and memory improved in patients taking duloxetine (N = 207) compared with those taking placebo (N = 104) for 8 weeks.52 A study48 comparing escitalopram and duloxetine for 24 weeks in younger adults with MDD (N = 73) found improvement in memory, attention, and executive functions. However, no significant differences were found between antidepressants in effects on cognition, and neither treatment improved cognition enough to match the levels of performance of the 37 nondepressed control participants.
The antidepressant bupropion is thought to inhibit both norepinephrine and dopamine and has demonstrated procognitive benefit in a number of populations, including individuals attempting to quit smoking, people with schizophrenia, and children with attention-deficit/hyperactivity disorder (ADHD).53 Herrera-Guzmán and colleagues53 conducted a small study in which 20 patients with MDD were treated with bupropion (150 mg/d) for 8 weeks. This study demonstrated that bupropion improved visual memory and mental processing in patients with MDD, but these results have not been replicated.
The multimodal antidepressant vortioxetine has a novel mechanism and is thought to work through 2 modes of action: serotonin (5-HT) reuptake inhibition and direct activity at 5-HT receptors, including 5-HT3 and 5-HT7 antagonism.54,55 One 8-week, double-blind study56 examined the effects of low-dose vortioxetine (5 mg/d) or duloxetine (60 mg/d) versus placebo in elderly patients with MDD. It found that both antidepressants improved depressive symptoms compared with placebo. Patients treated with vortioxetine, but not duloxetine, showed significant improvement in processing speed and executive function as measured by the Digit Symbol Substitution Test (DSST); both agents significantly improved learning and memory compared with placebo.56 A similar 8-week study55 in younger adults (aged 18-65 years) also showed significant cognitive improvement for vortioxetine (up to 20 mg/d), but not duloxetine (60 mg/d), versus placebo on the DSST.
A study54 comparing fixed doses of vortioxetine (10 or 20 mg/d) versus placebo in patients aged 18-65 years with MDD (N = 602) measured cognitive improvement as the primary endpoint. Both doses of vortioxetine significantly improved patients’ composite cognition score (DSST and RAVLT [Rey Auditory Verbal Learning Test]) compared with placebo (P < .0001). The efficacy of vortioxetine in areas of cognitive improvement was largely a direct effect (about one-half to two-thirds), independent of depressive symptom improvement.54 Based on path analysis, the direct and indirect effects of duloxetine on cognitive function were 48.7% and 51.3%, respectively. In contrast, 75.7% of the effect of vortioxetine on cognitive functioning (DSST performance) could be directly attributed to an independent treatment effect apart from improvements in mood symptoms (Montgomery-Asberg Depression Rating Scale score).55 Dr Culpepper remarked that this ties back to the model of pharmacologic modulation that he discussed earlier because this multimodal agent may have both a direct and an indirect effect on cognitive function.
Psychostimulants. Although psychostimulants have not been found to be effective for depressive symptoms in MDD, their pharmacodynamic profile suggests their effectiveness for treating cognitive dysfunction in these patients.38 Indeed, these agents have been examined as augmentation therapy for cognitive dysfunction.2,38 Lisdexamfetamine, a stimulant approved for the treatment of ADHD, has been considered as an agent that might improve cognition due to its ability to preferentially modulate central dopamine and norepinephrine systems, both of which are known to be critical to the maintenance of executive function mediated by the prefrontal cortex.57 Madhoo et al57 conducted a randomized, placebo-controlled trial comparing placebo with lisdexamfetamine augmentation of SSRI treatment in patients aged 18-55 years with partially or fully remitted depression and persistent executive dysfunction (N = 143) over 9 weeks of treatment. The primary endpoint was change on a self-report measure of executive function (BRIEF-A [Behavior Rating Inventory of Executive Function-Adult version]). Executive function improved significantly in the augmentation group compared with placebo (P = .0009).
Methylphenidate is another stimulant with cognitive-enhancement capabilities. However, few studies of its use for cognition have been performed in patients with depression. Lavretsky et al58 examined citalopram, methylphenidate, and their combination in geriatric patients with MDD. All treatments were associated with significantly improved depression severity and cognitive performance, with no differences among treatments on cognition.
Modafinil, an agent used to produce wakefulness in patients with sleep disorders, may have some beneficial effects on cognitive function. A proof-of-concept study59 of single-dose (200 mg) modafinil in 60 patients with remitted MDD found that memory performance, but not planning or attention, was significantly improved. However, Dr Culpepper warned that although modafinil treatment might have minor procognitive benefits, these may be outweighed by potential risks. For example, one study60 administered modafinil to a group of healthy volunteers and found no cognitive effects, but increased somatic and psychological anxiety, irritability, and aggressive moods were reported.
Other pharmacotherapies. Numerous additional pharmacotherapies from a variety of different classes have been investigated as treatment options for cognitive dysfunction in MDD. Cholinesterase inhibitors are a class of drugs that are used to enhance or maintain cognition in patients with Alzheimer’s disease.61 They derive their effect by preventing the cholinesterase enzyme from breaking down the neurotransmitter acetylcholine, thus increasing the amount and duration of acetylcholine’s action.61,62 Despite their cognitive benefits in Alzheimer’s disease, cholinesterase inhibitors approved for dementia have not been shown to improve cognitive abilities in patients with depression. In a study63 of older adults (≥ 65 years) with remitted depression (N = 130), donepezil combined with antidepressant therapy demonstrated temporary improvement in global cognition, but this was offset by an increased risk for depression relapse. A review of the literature61 did not find significant benefit for adjunctive treatment with galantamine in improving cognitive dysfunction in older adults with depression.
Another agent that has been investigated is erythropoietin (EPO), a hormone that helps produce red blood cells. EPO crosses the blood-brain barrier and has neurotrophic and neuroprotective effects.64 Using fMRI testing, a study64 including 23 healthy participants showed that single-dose EPO increased hippocampus-dependent memory during picture retrieval 7 days after administration, compared with placebo. In a placebo-controlled trial65 in patients with treatment-resistant depression (N = 40), EPO produced mood-dependent memory improvement that was maintained beyond the active-treatment phase. The effects of EPO on blood cells are direct, and its long-term use may be offset by complications in patients not requiring it for hematologic abnormalities.65 However, EPO provides a new potential mechanism to target neuroplasticity and cognition in depression.
Nonpharmacologic Treatments
Dr Culpepper further explained that in light of the failure of most pharmacotherapies to induce substantial improvements in cognitive functioning for patients with MDD, other therapeutic options should be considered. Nonpharmacologic approaches that have been investigated for cognitive impairments in MDD include psychological and behavioral therapies, neurostimulation procedures, and lifestyle interventions. These interventions have been explored both alone and in combination with pharmacologic treatment.
Psychological and behavioral treatments. Dr Culpepper began his discussion of nonpharmacologic treatments with a review of the evidence on psychotherapy for cognitive function. He explained that although considerable time has been devoted to investigating the impact of psychotherapies on cognitive function, unfortunately, he has observed that many of these studies had significant weaknesses in methods. Despite these shortcomings, a consideration of these therapies can be helpful in understanding the potential mechanisms involved that lead to resulting cognitive improvement.
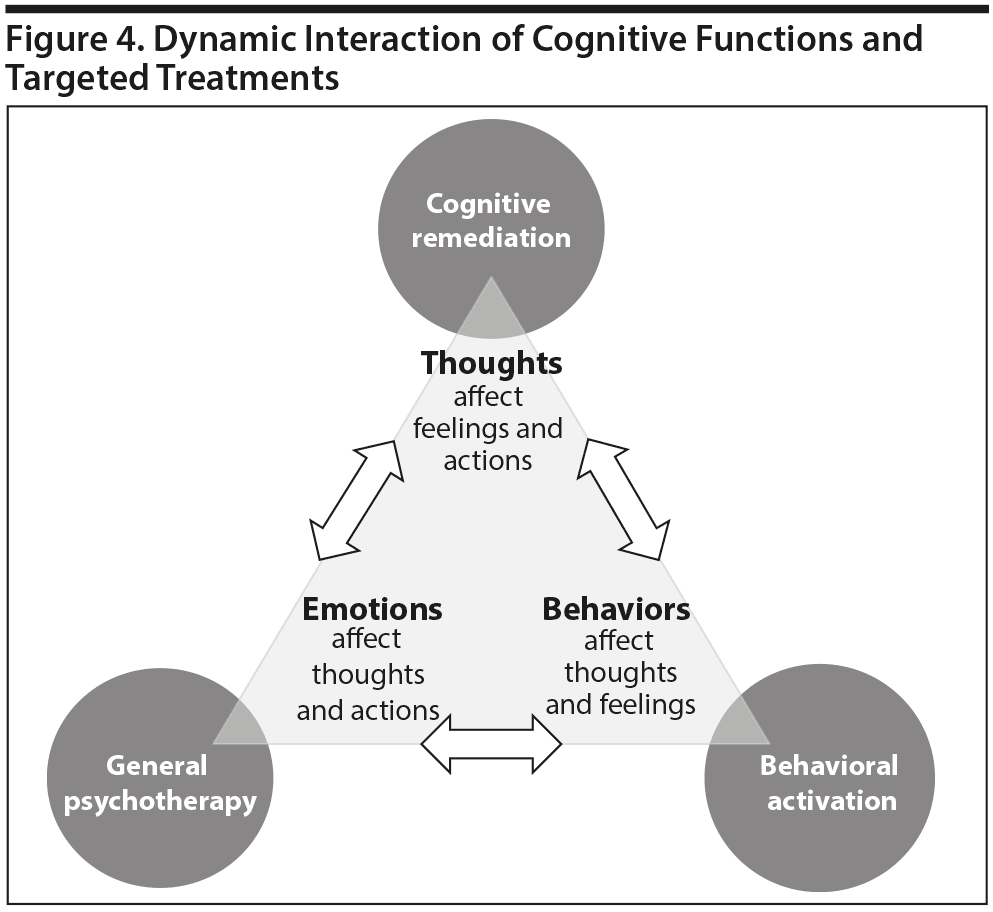
One way of conceptualizing the positive impact of psychotherapy on cognition is that, by initiating treatment focused at any of the domains involved in cognition and overall function (thoughts, feelings, and behaviors), the other domains can be affected (Figure 4). For example, cognitive remediation may address patients’ thinking and, by altering thought processes, also improve behaviors and emotions. Similarly, behavioral activation may improve behaviors and thereby lead to secondary improvement in emotions and thoughts. Furthermore, in general, psychotherapy can provide encouragement and foster positive communication strategies that have a direct positive effect on emotions, which leads to indirect benefits in thoughts and behavior.
Cognitive remediation (CR) aims to improve cognitive impairments through the use of repetitive exercises, systematic instruction, and/or structured experience targeting the impaired cognitive functions.66 Interestingly, CR has been found to lead to not only behavioral but also neurobiological changes. In a study67 of patients with schizophrenia who received CR, changes in several brain regions and circuits, including the prefrontal, parietal, and limbic areas, were detected following treatment. These changes involved both the structure and activity of the regions. Although this study was conducted in patients with schizophrenia, the same brain areas are also affected in patients with MDD.42
Elgamal and colleagues66 conducted a small study in which they enrolled 12 patients with MDD in a 10-week course of computerized CR. At study end, the patients showed improvements in attention, verbal learning and memory, psychomotor speed, and executive function. Another study68 assessed the effectiveness of CR in 10 patients with treatment-resistant depression. Although the researchers observed improvements in attention/processing speed and verbal memory, these cognitive improvements were not translated into improvements in functioning. A study by Morimoto et al69 comparing computerized CR with escitalopram in elderly patients with treatment-resistant depression (N = 11) found that CR was as effective as escitalopram in reducing depressive symptoms and more effective in improving executive function. Thus, although CR has yielded promising results for treating cognitive impairment in patients with depression,39 many studies have significant weaknesses, such as small sample sizes, short duration, or inadequate control groups and difficulty in use of repeated measures.
Behavioral activation emphasizes the impact of behavior on mood and symptoms and focuses on increasing patients’ participation in pleasant events and decreasing their tendency toward inaction and avoidance.70-72 The rationale for behavioral activation stems from the tendency of individuals with depression to withdraw from their usual activities and practice avoidance behaviors, which deprives them of situations in which they might be exposed to positive reinforcement and creates instead a self-sustaining climate of negative reinforcement.72 Behavioral activation, therefore, seeks to create opportunities for positive reinforcement through activity planning, social skills development, and time management training based on the assumption that increased engagement in pleasurable activities will be accompanied by increases in positive affect.70,72 A review72 of behavioral activation in MDD concluded that this type of behavioral therapy is as effective as other forms of psychotherapy. However its effects on cognitive impairments were not examined and will require further investigation.
Cognitive-behavioral therapy (CBT) combines cognitive and behavioral therapeutic approaches, and Dr Culpepper pointed out that this combination of strategies may be helpful for some patients because it may simultaneously provide a combined effect on thoughts, feelings, and behaviors, which can improve depression and have a positive effect on cognition. CBT has been studied extensively and has been found to be an effective treatment for MDD.73 The principle behind CBT is that targeting negative thoughts will also change emotions and behaviors.70 Through fMRI testing, CBT has been found to normalize hot cognitive impairment (emotional processing)74 and may therefore change both hot and cold cognitive processes. However, CBT is time-consuming and complex, requires systematic training, and cannot be done well by everybody. Of note, in some patients, cognitive impairments associated with depression may impede their capacity to effectively engage, at least initially, in CBT.
Neuromodulation. A review75 on the effect of ECT on cognitive function found that, while some short-term cognitive impairment occurs soon after ECT, improvement in several domains of cognitive function (eg, processing speed, memory, executive function) has been found after 15 days. Thus, ECT may be considered for some patients experiencing severe cognitive impairment associated with MDD.
Repetitive transcranial magnetic stimulation, deep-brain stimulation, and transcranial direct current stimulation are being examined as potential treatments for altering cognitive impairment,76-78 but further studies are needed.
Lifestyle modifications. Lifestyle changes have been explored as noninvasive, generally health-promoting interventions for cognitive impairment. Among these, exercise is beneficial for patients with depression38 and may improve cognition.79 Exercise may act in part through stimulation of brain-derived neurotrophic factor and cause neurogenesis in key brain areas,80 making it a reasonable option as supportive therapy in patients with cognitive impairment.
Practical Treatment Approaches Based on Available Evidence
Dr Culpepper summed up his discussion by stating that after evaluating the available evidence, depression can be seen as a disorder that involves a complex set of changes, many of which impact cognition, and these, in turn, may affect symptom severity and functional impairment. Insights into available treatment modalities have demonstrated their effects on the neural basis of cognition. On the basis of these insights, the treatment of cognitive impairment in depression should focus on effectively alleviating mood symptoms. For many patients, this will lead to improvement in cognition. For patients with significant cognitive impairment at baseline, treatment with an agent that has demonstrated efficacy, and particularly those that have direct efficacy, in cognition may be warranted, or augmentation with an adjunctive pharmacologic agent such as a psychostimulant or cognitive enhancer may be considered, or a psychological intervention may be added.
Case Practice Question
Case 3. Megan is a 29-year-old grade school teacher who is on maintenance treatment with escitalopram for an acute depressive episode she experienced 18 months ago. She reports that her mood symptoms have almost entirely resolved, but she is feeling overwhelmed at work, which has been causing her to “feel like a failure.” She is having difficulty creating her lesson plans and grading papers on time, and she says she has been avoiding her colleagues. She used to have lunch with several other teachers but has stopped because hearing them discuss the activities they have planned for their classes just causes her more stress and makes her feel even worse about her own performance as a teacher. Which nonpharmacologic treatment option would be most appropriate for addressing Megan’s current symptoms?
- Cognitive remediation
- Cognitive-behavioral therapy
- Behavioral activation
- Lifestyle modification
Discussion of Case Practice Question
Preferred response: b. Cognitive-behavioral therapy
Although Megan might be able to obtain some benefit from all of the treatments listed, she is presenting with symptoms that are being mediated through both negative thoughts (her feelings of failing at her job) and behaviors (inability to function at work and avoidance of colleagues). Because multiple domains are affected, cognitive-behavioral therapy is the best choice. This type of treatment directly affects the patient’s thoughts and actions, thereby indirectly affecting emotions.
Conclusion
Depression is the leading cause of disability worldwide. For many people with MDD, the impairment they experience is related to the cognitive symptoms of this disorder. In order for these individuals to achieve full, functional recovery, their cognitive dysfunction must be addressed. This requires evaluation with appropriate assessment tools and careful selection of pharmacologic and nonpharmacologic treatments. Clinicians must then monitor cognition and be prepared to modify or augment treatments as necessary.
Disclosure of off-label usage: While all of the pharmaceutical agents that appear in this activity have been approved by the US Food and Drug Administration (FDA), here they are discussed in consideration of their ability to improve cognition in patients with major depression. Dr Culpepper acknowledges that the improvement of cognition in depression is not an FDA-approved indication of any therapeutic agent.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138. PubMed doi:10.1146/annurev-publhealth-031912-114409
2. Lam RW, Kennedy SH, Mclntyre RS, et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654. PubMed doi:10.1177/070674371405901206
3. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527. PubMed doi:10.1002/da.22063
4. Lee Y, Smofsky A, Nykoliation P, et al. Cognitive impairment mediates workplace impairment in persons with type 2 diabetes mellitus: results from the Motivaction Study. Can J Diabetes. 2017;S1499-2671(17)30284-8. PubMed
5. McIntyre RS, Soczynska JZ, Woldeyohannes HO, et al. The impact of cognitive impairment on perceived workforce performance: results from the International Mood Disorders Collaborative Project. Compr Psychiatry. 2015;56:279-282. PubMed doi:10.1016/j.comppsych.2014.08.051
6. World Health Organization. Depression: Fact Sheet. World Health Organization website. http://www.who.int/mediacentre/factsheets/fs369/en/. Updated February 2017.
7. Evans-Lacko S, Knapp M. Global patterns of workplace productivity for people with depression: absenteeism and presenteeism costs across eight diverse countries. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1525-1537. PubMed doi:10.1007/s00127-016-1278-4
8. Gibson C. Health experts report US$246 billion cost of workplace depression across eight countries. The London School of Economics and Political Science website. http://www.lse.ac.uk/website-archive/newsAndMedia/newsArchives/2016/09/Global-workplace-depression.aspx. Updated September 2016.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Publishing; 2013.
10. Mendelsohn D, Riedel WJ, Sambeth A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev. 2009;33(6):926-952. PubMed doi:10.1016/j.neubiorev.2009.03.006
11. Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14. PubMed doi:10.4088/JCP.13r08710
12. Fray PJ, Robbins TW. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18(4):499-504. PubMed doi:10.1016/0892-0362(96)00027-X
13. Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-2040. PubMed doi:10.1017/S0033291713002535
14. Fried EI, Nesse RM. The impact of individual depressive symptoms on impairment of psychosocial functioning. PLoS One. 2014;9(2):e90311. doi:10.1371/journal.pone.0090311
15. Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24(4):267-284. PubMed doi:10.2165/11530230-000000000-00000
16. Mundt JC, Marks IM, Shear MK, et al. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry. 2002;180(5):461-464. PubMed doi:10.1192/bjp.180.5.461
17. Lam RW, Michalak EE, Bond DJ, et al. Which depressive symptoms and medication side effects are perceived by patients as interfering most with occupational functioning? Depress Res Treat. 2012;2012:630206. PubMed
18. Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression (IBI-D). J Affect Disord. 2013;151(1):59-65. PubMed doi:10.1016/j.jad.2013.05.048
19. Zimmerman M, McGlinchey JB, Posternak MA, et al. How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry. 2006;163(1):148-150. PubMed doi:10.1176/appi.ajp.163.1.148
20. Zimmerman M, Martinez JA, Attiullah N, et al. Why do some depressed outpatients who are in remission according to the Hamilton Depression Rating Scale not consider themselves to be in remission? J Clin Psychiatry. 2012;73(6):790-795. PubMed doi:10.4088/JCP.11m07203
21. Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60(4):221-225. PubMed doi:10.4088/JCP.v60n0403
22. McIntyre RS, O’ Donovan C. The human cost of not achieving full remission in depression. Can J Psychiatry. 2004;49(suppl 1):10S-16S. PubMed
23. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. Third Edition. Arlington, VA: American Psychiatric Association; 2010.
24. McIntyre RS, Suppes T, Tandon R, et al. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults With Major Depressive Disorder. J Clin Psychiatry. 2017;78(6):703-713. PubMed doi:10.4088/JCP.16cs10885
25. McIntyre RS, Best MW, Bowie CR, et al. The THINC-Integrated Tool (THINC-it) screening assessment for cognitive dysfunction: validation in patients with major depressive disorder. J Clin Psychiatry. 2017;78(7):873-881. PubMed doi:10.4088/JCP.16m11329
26. El Hammi E, Samp J, Rémuzat C, et al. Difference of perceptions and evaluation of cognitive dysfunction in major depressive disorder patients across psychiatrists internationally. Ther Adv Psychopharmacol. 2014;4(1):22-29. PubMed doi:10.1177/2045125313507946
27. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
28. Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. PubMed doi:10.1097/01.MLR.0000093487.78664.3C
29. Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1(3):385-401. doi:10.1177/014662167700100306
30. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63-70. PubMed doi:10.1001/archpsyc.1965.01720310065008
31. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
32. Zimmerman M, Mattia JI. A self-report scale to help make psychiatric diagnoses: the Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry. 2001;58(8):787-794. PubMed doi:10.1001/archpsyc.58.8.787
33. First M, Williams J, Karg R, et al. Structured Clinical Interview for DSM-5 Disorders, Clinician Version (SCID-5-CV). Arlington, VA: American Psychiatric Association; 2015.
34. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
35. Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131-140. PubMed doi:10.1016/j.comppsych.2007.10.006
36. Lee RSC, Hermens DF, Porter MA, et al. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012;140(2):113-124. PubMed doi:10.1016/j.jad.2011.10.023
37. McIntyre R. Validation of the THINC-it screening tool. Eur Neuropsychopharmacol. 2016;26(suppl 2):S759-S760. doi:10.1016/S0924-977X(16)31926-5
38. McIntyre RS, Xiao HX, Syeda K, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs. 2015;29(7):577-589. PubMed doi:10.1007/s40263-015-0263-x
39. Miskowiak KW, Ott CV, Petersen JZ, et al. Systematic review of randomized controlled trials of candidate treatments for cognitive impairment in depression and methodological challenges in the field. Eur Neuropsychopharmacol. 2016;26(12):1845-1867. PubMed doi:10.1016/j.euroneuro.2016.09.641
40. Salagre E, Solé B, Tomioka Y, et al. Treatment of neurocognitive symptoms in unipolar depression: A systematic review and future perspectives. J Affect Disord. 2017;221:205-221. PubMed doi:10.1016/j.jad.2017.06.034
41. Duric V, Duman RS. Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci. 2013;70(1):39-53. PubMed doi:10.1007/s00018-012-1020-7
42. Outhred T, Hawkshead BE, Wager TD, et al. Acute neural effects of selective serotonin reuptake inhibitors versus noradrenaline reuptake inhibitors on emotion processing: implications for differential treatment efficacy. Neurosci Biobehav Rev. 2013;37(8):1786-1800. PubMed doi:10.1016/j.neubiorev.2013.07.010
43. Gordon E. The Brain’s Anatomy. In: Integrative Neuroscience Bringing Together Biological, Psychological and Clinical Models of the Human Brain, Chapter 7. Amsterdam, the Netherlands: Harwood Academic Publishers; 2000:83-105. doi:10.1201/b16989
44. Harmer CJ, Shelley NC, Cowen PJ, et al. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161(7):1256-1263. PubMed doi:10.1176/appi.ajp.161.7.1256
45. Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18(3):139-149. PubMed doi:10.1017/S1092852913000072
46. Godlewska BR, Norbury R, Selvaraj S, et al. Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychol Med. 2012;42(12):2609-2617. PubMed doi:10.1017/S0033291712000591
47. Savaskan E, Müller SE, Böhringer A, et al. Antidepressive therapy with escitalopram improves mood, cognitive symptoms, and identity memory for angry faces in elderly depressed patients. Int J Neuropsychopharmacol. 2008;11(3):381-388. PubMed doi:10.1017/S1461145707007997
48. Herrera-Guzmán I, Herrera-Abarca JE, Gudayol-Ferré E, et al. Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Res. 2010;177(3):323-329. PubMed doi:10.1016/j.psychres.2010.03.006
49. Cassano GB, Puca F, Scapicchio PL, et al; Italian Study Group on Depression in Elderly Patients. Paroxetine and fluoxetine effects on mood and cognitive functions in depressed nondemented elderly patients. J Clin Psychiatry. 2002;63(5):396-402. PubMed doi:10.4088/JCP.v63n0504
50. Shilyansky C, Williams LM, Gyurak A, et al. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry. 2016;3(5):425-435. PubMed doi:10.1016/S2215-0366(16)00012-2
51. Tian Y, Du J, Spagna A, et al. Venlafaxine treatment reduces the deficit of executive control of attention in patients with major depressive disorder. Sci Rep. 2016;6:28028. PubMed doi:10.1038/srep28028
52. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909. PubMed doi:10.1176/ajp.2007.164.6.900
53. Herrera-Guzmán I, Gudayol-Ferré E, Lira-Mandujano J, et al. Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res. 2008;160(1):72-82. PubMed doi:10.1016/j.psychres.2007.04.012
54. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567. PubMed doi:10.1017/S1461145714000546
55. Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025-2037. PubMed doi:10.1038/npp.2015.52
56. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223. PubMed doi:10.1097/YIC.0b013e3283542457
57. Madhoo M, Keefe RSE, Roth RM, et al. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology. 2014;39(6):1388-1398. PubMed doi:10.1038/npp.2013.334
58. Lavretsky H, Reinlieb M, St Cyr N, et al. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561-569. PubMed doi:10.1176/appi.ajp.2014.14070889
59. Kaser M, Deakin JB, Michael A, et al. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(2):115-122. PubMed doi:10.1016/j.bpsc.2016.11.009
60. Randall DC, Shneerson JM, Plaha KK, et al. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol. 2003;18(3):163-173. PubMed doi:10.1002/hup.456
61. McDermott CL, Gray SL. Cholinesterase inhibitor adjunctive therapy for cognitive impairment and depressive symptoms in older adults with depression. Ann Pharmacother. 2012;46(4):599-605. PubMed doi:10.1345/aph.1Q445
62. Colović MB, Krstić DZ, Lazarević-Pašti TD, et al. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315-335. PubMed doi:10.2174/1570159X11311030006
63. Reynolds CF 3rd, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68(1):51-60. PubMed doi:10.1001/archgenpsychiatry.2010.184
64. Miskowiak K, O’ Sullivan U, Harmer CJ. Erythropoietin enhances hippocampal response during memory retrieval in humans. J Neurosci. 2007;27(11):2788-2792. PubMed doi:10.1523/JNEUROSCI.5013-06.2007
65. Miskowiak KW, Vinberg M, Christensen EM, et al. Recombinant human erythropoietin for treating treatment-resistant depression: a double-blind, randomized, placebo-controlled phase 2 trial. Neuropsychopharmacology. 2014;39(6):1399-1408. PubMed doi:10.1038/npp.2013.335
66. Elgamal S, McKinnon MC, Ramakrishnan K, et al. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: a proof of principle study. Psychol Med. 2007;37(9):1229-1238. PubMed doi:10.1017/S0033291707001110
67. Thorsen AL, Johansson K, L׸berg E-M. Neurobiology of cognitive remediation therapy for schizophrenia: a systematic review. Front Psychiatry. 2014;5:103. PubMed doi:10.3389/fpsyt.2014.00103
68. Bowie CR, Gupta M, Holshausen K, et al. Cognitive remediation for treatment-resistant depression: effects on cognition and functioning and the role of online homework. J Nerv Ment Dis. 2013;201(8):680-685. PubMed doi:10.1097/NMD.0b013e31829c5030
69. Morimoto SS, Wexler BE, Liu J, et al. Neuroplasticity-based computerized cognitive remediation for treatment-resistant geriatric depression. Nat Commun. 2014;5:4579. PubMed doi:10.1038/ncomms5579
70. Hunot V, Moore THM, Caldwell DM, et al. ‘ Third wave’ cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev. 2013;(10):CD008704. PubMed
71. McEvoy P, Law A, Bates R, et al. Using behavioural activation in the treatment of depression: a control theory perspective. J Psychiatr Ment Health Nurs. 2013;20(10):890-895. PubMed doi:10.1111/jpm.12032
72. Soucy Chartier I, Provencher MD. Behavioural activation for depression: efficacy, effectiveness and dissemination. J Affect Disord. 2013;145(3):292-299. PubMed doi:10.1016/j.jad.2012.07.023
73. Butler AC, Chapman JE, Forman EM, et al. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26(1):17-31. PubMed doi:10.1016/j.cpr.2005.07.003
74. Yoshimura S, Okamoto Y, Onoda K, et al. Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc Cogn Affect Neurosci. 2014;9(4):487-493. PubMed doi:10.1093/scan/nst009
75. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577. PubMed doi:10.1016/j.biopsych.2010.06.009
76. Pallanti S, Di Rollo A, Antonini S, et al. Low-frequency rTMS over right dorsolateral prefrontal cortex in the treatment of resistant depression: cognitive improvement is independent from clinical response, resting motor threshold is related to clinical response. Neuropsychobiology. 2012;65(4):227-235. PubMed doi:10.1159/000336999
77. Bergfeld IO, Mantione MH, Ruhé HG, et al. Cogntive functions following deep brain stimulation in depression. Brain Stimul Basic Transl Clin Res Neuromodulation. 2017;10(2):457.
78. Moreno ML, Vanderhasselt M-A, Carvalho AF, et al. Effects of acute transcranial direct current stimulation in hot and cold working memory tasks in healthy and depressed subjects. Neurosci Lett. 2015;591:126-131. PubMed doi:10.1016/j.neulet.2015.02.036
79. Imboden C, Beck J, Gerber M, et al. Aerobic exercise as add-on treatment in depressed inpatients improves cognitive domains but has no additional effect on symptom severity. Eur Neuropsychopharmacol. 2016;26:S395-S396. doi:10.1016/S0924-977X(16)31353-0
80. Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56-64. PubMed doi:10.1016/j.jpsychires.2014.10.003
81. Chenier L. Depression in the Workplace. The Conference Board of Canada. http://www.conferenceboard.ca/e-library/abstract.aspx?did=5676. Published September 10, 2013. Accessed November 29, 2017.
82. Gelenberg AJ. Using assessment tools to screen for, diagnose, and treat major depressive disorder in clinical practice. J Clin Psychiatry. 2010;71(Suppl E1):e01. Abstract PubMed