Objective: Fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine have consistently shown efficacy for posttraumatic stress disorder (PTSD) in meta-analyses of randomized controlled trials. However, no study has compared the effectiveness of these agents in routine clinical practice. We conducted a retrospective comparative effectiveness study of these 5 medications using electronic medical record data.
Methods: We identified 2,931 Department of Veterans Affairs outpatients initiating treatment for PTSD between fiscal years 2004 and 2013 who received 1 of the 5 medications at an adequate dose and duration, combined with baseline and endpoint PTSD Checklist (PCL) measurements. Patients were identified based on clinical diagnoses of PTSD (DSM-IV criteria). We weighted participants in order to balance pretreatment characteristics. We compared continuous changes on total PCL score, symptom cluster scores, and sleep items, as well as categorical changes including reliable improvement and loss of PTSD diagnosis, using weighted regression analyses. We conducted exploratory analysis to determine whether any patient characteristics or service use variables predicted loss of PTSD diagnosis.
Results: Patients improved by a mean of 5-6 points on the PCL over approximately 6 months of treatment. While half of patients had a reliable improvement of 5 points or more on the PCL, less than a fifth achieved loss of PTSD diagnosis. There were no differences between medications. The only significant (P < .001) predictor of loss of PTSD diagnosis was concurrent treatment with evidence-based psychotherapy.
Conclusions: Available evidence-based medications for PTSD are equally effective in clinical practice. Although effective, our data suggest that patients choosing medication treatment for PTSD should consider concurrent treatment with evidence-based psychotherapy in order to maximize their chances of meaningful improvement.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $5 processing fee will apply.
CME Objective
After studying this article, you should be able to:
- Talk with patients who have PTSD about their medication preferences and receiving concurrent evidence-based psychotherapy
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in September 2018 and is eligible for AMA PRA Category 1 Creditâ„¢ through October 31, 2020. The latest review of this material was September 2018.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage, has been a member of the Independent Data Safety and Monitoring Committee for Janssen, has been medical editor for the Global Organization for EPA and DHA Omega-3s newsletter, and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: Fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine have consistently shown efficacy for posttraumatic stress disorder (PTSD) in meta-analyses of randomized controlled trials. However, no study has compared the effectiveness of these agents in routine clinical practice. We conducted a retrospective comparative effectiveness study of these 5 medications using electronic medical record data.
Methods: We identified 2,931 Department of Veterans Affairs outpatients initiating treatment for PTSD between fiscal years 2004 and 2013 who received 1 of the 5 medications at an adequate dose and duration, combined with baseline and endpoint PTSD Checklist (PCL) measurements. Patients were identified based on clinical diagnoses of PTSD (DSM-IV criteria). We weighted participants in order to balance pretreatment characteristics. We compared continuous changes on total PCL score, symptom cluster scores, and sleep items, as well as categorical changes including reliable improvement and loss of PTSD diagnosis, using weighted regression analyses. We conducted exploratory analysis to determine whether any patient characteristics or service use variables predicted loss of PTSD diagnosis.
Results: Patients improved by a mean of 5-6 points on the PCL over approximately 6 months of treatment. While half of patients had a reliable improvement of 5 points or more on the PCL, less than a fifth achieved loss of PTSD diagnosis. There were no differences between medications. The only significant (P < .001) predictor of loss of PTSD diagnosis was concurrent treatment with evidence-based psychotherapy.
Conclusions: Available evidence-based medications for PTSD are equally effective in clinical practice. Although effective, our data suggest that patients choosing medication treatment for PTSD should consider concurrent treatment with evidence-based psychotherapy in order to maximize their chances of meaningful improvement.
J Clin Psychiatry 2018;79(5):18m12145
To cite: Shiner B, Leonard Westgate C, Gui J, et al. A retrospective comparative effectiveness study of medications for posttraumatic stress disorder in routine practice. J Clin Psychiatry. 2018;79(5):18m12145.
To share: https://doi.org/10.4088/JCP.18m12145
© Copyright 2018 Physicians Postgraduate Press, Inc.
aVeterans Affairs Medical Center, White River Junction, Vermont
bDepartment of Psychiatry, Geisel School of Medicine, Hanover, New Hampshire
cBiomedical Data Science, Community & Family Medicine, and The Dartmouth Institute for Health Policy & Clinical Practice, Geisel School of Medicine, Hanover, New Hampshire
dSan Francisco VA Medical Center and Department of Psychiatry, University of California San Francisco School of Medicine, San Francisco, California
eClinical Epidemiology Research Group, White River Junction, Vermont
fNational Center for PTSD, White River Junction, Vermont
gFellowships in Quality and Safety, National Center for Patient Safety, Ann Arbor, Michigan
*Corresponding author: Brian Shiner, MD, MPH, VA Medical Center, 215 North Main St, 11Q, White River Junction, VT 05009 ([email protected]).
Posttraumatic stress disorder (PTSD) is a serious condition that can follow exposure to a traumatic event, characterized by intrusive reexperiencing of the trauma in the form of flashbacks and nightmares, avoidance of trauma reminders, negative alterations in cognitions and mood, and increased arousal and reactivity.1 PTSD has a lifetime prevalence of almost 8% in the United States.2 Over 10% of veterans receiving care in the Department of Veterans Affairs (VA) health care system have PTSD, and the VA has a caseload of almost 600,000 veterans receiving PTSD treatment.3
Randomized controlled trials (RCTs) show that effective treatments for PTSD include both pharmacologic and psychotherapeutic approaches.4,5 There have been multiple meta-analyses examining the effectiveness of medications to treat PTSD, which have differed in their methods and conclusions. Watts and colleagues’ meta-analyses of all RCTs of PTSD treatment conducted through 2012 showed results consistently favoring 4 antidepressants (fluoxetine, paroxetine, sertraline, and venlafaxine), 1 anticonvulsant (topiramate), and 1 antipsychotic (risperidone) when compared directly to placebo.5 A similar review by Hoskins et al6 favored fluoxetine, paroxetine, and venlafaxine, but not sertraline, topiramate, or risperidone. A meta-analysis by Lee et al7 that included RCTs of medications for PTSD published through 2015 suggested superior efficacy for sertraline, venlafaxine, and nefazodone compared to other medications. Two studies used network meta-analysis to make indirect comparisons between medications.8 First, a 2013 Agency for Healthcare Research and Quality (AHRQ) network meta-analysis of published RCTs concluded that paroxetine and topiramate were most effective, but that fluoxetine, sertraline, and venlafaxine were also effective.4 Second, Cipriani et al9 concluded that phenelzine was superior to other medications for PTSD when considering both efficacy and dropouts. Data supporting the phenelzine finding came from 1 small RCT,10 and Cipriani et al called for further study rather than prioritization of phenelzine in clinical practice.9 Given available data, the preponderance of meta-analyses suggest fluoxetine, sertraline, paroxetine, topiramate, and venlafaxine as treatments for PTSD.
While several medications have demonstrated efficacy for PTSD in clinical trials, there have been few head-to-head comparisons and no large trials. Furthermore, while multiple medications for PTSD have shown superiority to placebo in RCTs, little is known about their effectiveness in routine clinical practice. There are several reasons to question whether medications found efficacious in highly controlled clinical studies are beneficial in typical clinical practice. First, patients with comorbidities such as substance abuse are common in the population,11 yet are routinely excluded from efficacy trials of PTSD treatments.12 Second, RCTs of psychotropic medications for PTSD typically prohibit patients from undergoing concurrent psychotherapy,5 whereas these interventions are often delivered together in practice.13 Given advancements in data, including increasing availability of patient reported outcome data in the electronic medical record (EMR)14 and the need for large numbers to support research on more personalized medicine,15 observational studies are a logical extension of comparative effectiveness research on psychotropic medications for PTSD.
We conducted a retrospective comparative effectiveness study of medications for PTSD using VA EMR data. We examined medications already determined as effective for PTSD in multiple meta-analyses, including fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine. On the basis of the AHRQ network meta-analytic results, we expected that participants receiving paroxetine and topiramate might have superior symptomatic outcomes. However, given limitations about both the applicability of RCT results to the clinical population and the relatively limited evidence for topiramate, it was not possible to make a formal prediction. We elected not to examine medications whose efficacy was supported in a single meta-analysis or single study since, in most cases, these medications have been used too infrequently in VA practice to yield reliable results.16 Lastly, we examined predictors of response to medication treatment generally as well as to each of the agents individually.

- Five medications for PTSD with consistent efficacy in meta-analyses of randomized controlled trials—including fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine—are also effective in routine clinical practice.
- It does not appear that any one of these agents is more effective than the others for PTSD, so patient preference should weigh heavily when choosing among these medications.
- Patients who elect to take one of these medications for PTSD should consider undergoing concurrent treatment with evidence-based psychotherapy delivered in an individual format, such as prolonged exposure or cognitive processing therapy.
METHODS
Data Sources
We used the VA Corporate Data Warehouse (CDW) to identify VA users with new PTSD treatment episodes from fiscal years 2004 through 2013 and obtain information on services use, clinical diagnoses, pharmacy data, and standardized measures of PTSD symptoms. This study was approved by the Veterans Institutional Review Board (IRB) of Northern New England, which is the IRB of record for the White River Junction VAMC.
Participants
Participants were drawn from a large retrospective cohort of VA users with new PTSD treatment episodes between fiscal years 2004 and 2013 that has been described elsewhere.11,17 This parent cohort included VA users who received a primary diagnosis of PTSD at 2 or more outpatient encounters, at least 1 of which occurred in a mental health setting, over the course of 90 days.18,19 Participants meeting this criterion during the prior 2 years were excluded. We examined 1 year of treatment receipt following the first encounter with a qualifying diagnosis of PTSD. The study sample was further restricted to those who had an adequate medication trial (AMT, defined below), received baseline PTSD symptom measurement at the start of treatment (up to 2 months prior and 2 weeks after the start of an AMT), and received follow-up PTSD symptom measurement (greater than 8 weeks and less than 6 months after initiating an AMT). To minimize heterogeneity and confounding, participants who received 2 or more AMTs concurrently were excluded. When patients had 2 or more AMTs sequentially, we examined only the first. Due to increasing use of standardized measurement of PTSD symptoms in clinical practice in more recent years,20,21 participants in this analysis were treated in fiscal year 2008 and later.
PTSD Symptoms
We measured PTSD symptoms using the PTSD Checklist (PCL), which is administered in clinical practice and recorded in the VA EMR. During the time period we examined, the VA used the version of the PCL corresponding to PTSD diagnostic criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).22,23 The PCL is a 17-item measure with each item rated on a 5-point Likert-type scale and with total scores ranging from 17 through 85. Minimal symptomatic criteria for PTSD using the PCL include 1 reexperiencing symptom, 3 avoidance and numbing symptoms, and 2 hyperarousal symptoms, all rated “moderately” or higher. Participants are asked to rate symptoms over the last month. Previous research in veterans shows that a change of 5 points cannot be attributed to measurement error,24 so we used a 5-point drop as our threshold for reliable improvement. The combination of a meaningful change in PTSD symptoms and no longer meeting diagnostic criteria for PTSD has been shown to be an important marker of improved quality of life.25 Therefore, we also employed no longer meeting DSM-IV criteria for PTSD as measured by the PCL, plus a clinically meaningful drop of 10 points,26 as our threshold for loss of diagnosis.
In addition to examining overall change in symptoms, we evaluated change in subscores for PTSD symptoms clusters as well as sleep difficulties using the sum of 2 items: nightmares and insomnia. Diagnostic criteria for PTSD changed in May 2013 with the publication of DSM-5.1 A key change in the criteria is replacement of the “avoidance and numbing cluster” with “avoidance” and “negative alterations in cognitions and mood” clusters. To approximate this distinction, we divided “avoidance” and “numbing” symptoms. Our symptom clusters consisted of 5 reexperiencing items, 2 avoidance items, 5 emotional numbing items, and 5 hyperarousal items.
Psychotropic Medication Receipt
We developed algorithms to measure whether participants received an AMT of sertraline, fluoxetine, paroxetine, venlafaxine, or topiramate, defined as 8 weeks of a daily dose at least as high as the dose used in the efficacy trials supporting the treatment recommendation.4,5 While the length of efficacy trials of psychotropic medications for PTSD varies, the VA practice guideline in use during the time period we examined recommended medication trials of at least 8 weeks.27 Therefore, participants receiving continuous treatment of one of the following medications daily for 8 weeks or more were considered to have received an AMT: fluoxetine 20 mg or more daily, paroxetine 20 mg or more daily, sertraline 100 mg or more daily, topiramate 100 mg or more daily, and venlafaxine 150 mg or more daily.
Independent Variables
Participant variables included demographics, military service characteristics, commonly occurring medical and mental health disorders, and baseline PCL score. Health system variables included the type of VA facility and the prescribing clinician’s service section. Service use characteristics during the year following the index PTSD diagnosis included concurrent psychotropic medication, total number of psychotherapy encounters, number of psychotherapy encounters in which participants received evidence-based psychotherapy (EBP) for PTSD, defined as prolonged exposure (PE)28 or cognitive processing therapy (CPT),29 and counts of medication management encounters, primary care encounters, and outpatient visits. We measured PE and CPT use with a natural language processing (NLP) algorithm that classifies psychotherapy notes in individual (I) and group (G) delivery formats.30 In our pilot NLP work, we attempted to identify other evidence based psychotherapies for PTSD including eye movement desensitization and reprocessing (EMDR) and Stress Inoculation Therapy.31 Despite manual review of over 7,500 notes written about patients attending PTSD clinics, we were unable to detect any examples of these therapies in routine clinical practice in VA. Therefore, their use was not included in further analysis steps.
Analysis
To understand how participants selected for this analysis differed from the rest of the parent cohort during the relevant fiscal years, we compared patient characteristics using χ2 analysis and t tests, as appropriate. We compared these same characteristics among participants who received each of the 5 medications within the smaller analytic cohort using pairwise testing with step-down Bonferroni-adjusted P values.
To account for baseline differences among participants who received each of the 5 medications, we used the RAND Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG).32 The TWANG package supports causal modeling of observational data through the estimation and evaluation of propensity scores and associated weights. In our application, the propensity score represented the probability that a particular patient would receive each medication.33 We estimated propensity scores with multinomial logistic regression using generalized booster effects,34 in which the dependent variable is an indicator for each psychotropic medication and the independent variables are an antiparsimonious specification of variables that have a plausible correlation with the outcome.33,34 Using these propensity scores, we weighted participants in order to balance the pretreatment covariate distributions.
We compared continuous and categorical outcomes among the 5 groups with regression analyses using psychotropic medication received as the sole independent variable. In general, weighted means can have greater sampling variance than unweighted means. Therefore, we used survey commands, which account for the weights, to perform the outcomes analyses when comparing the weighted groups. For continuous outcomes, we used linear regression analysis, whereby the coefficient of the variable tests the hypothesis that each of the 5 psychotropic medications has the same mean change in PTSD symptoms. For categorical outcomes, we used logistic regression analysis, whereby the coefficient of the variable tests the hypothesis that each of the 5 psychotropic medications results in the same percentage of patients achieving reliable improvement or loss of PTSD diagnosis. Finally, we conducted exploratory univariate logistic regression analyses to determine whether any independent variables predicted achievement of our categorical response criteria of loss of PTSD diagnosis by pooling all 5 groups together and using the unweighted data. Because there were 50 independent variables (see Table 2), we used a Bonferroni-corrected value of P < .001 for significance in these exploratory analyses. Analyses were performed using R, Version 3.2.0 (http://CRAN.R-project.org).
RESULTS
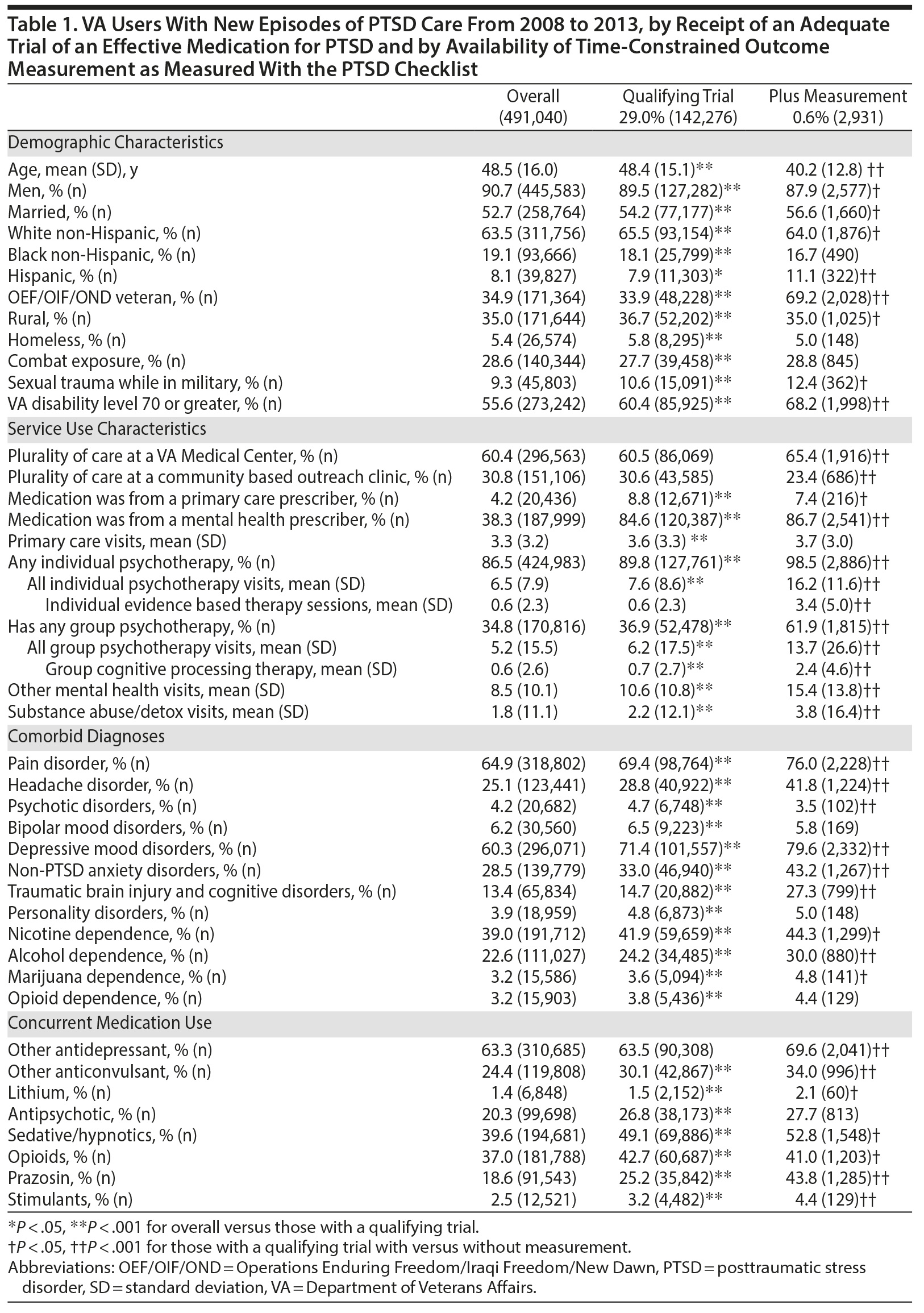
While 29.0% (142,276) of 491,040 VA users meeting our criteria for a new episode of PTSD care between fiscal years 2008 and 2013 had a qualifying medication trial, only 0.6% (2,931) also received outcome measurement within our specified time frames. The 2,931 participants included in our analyses differed from patients with AMT who did not have PCL measurement in almost every measurable way in terms of demographics, service use, comorbidity, and concurrent medication use (Table 1). Many of these differences were statistically significant but of unclear clinical relevance. In some ways, the analytic sample differed in ways that were likely clinically meaningful. Most notably, compared to the general population with PTSD, the analytic sample contained younger participants (on average 8 years younger) more likely to be Operations Enduring Freedom/Iraqi Freedom/New Dawn (OEF/OIF/OND) veterans (69.2% vs 34.9%), with higher rates of VA disability (68.2% vs 55.6%). The analytic sample also differed in important ways regarding their mental health services use: they were more likely to receive medications from a mental health clinician (86.7% vs 38.3%), had more individual psychotherapy visits (mean of 16.2 vs 6.5), and were more likely to receive group psychotherapy (61.9% vs 34.8%).
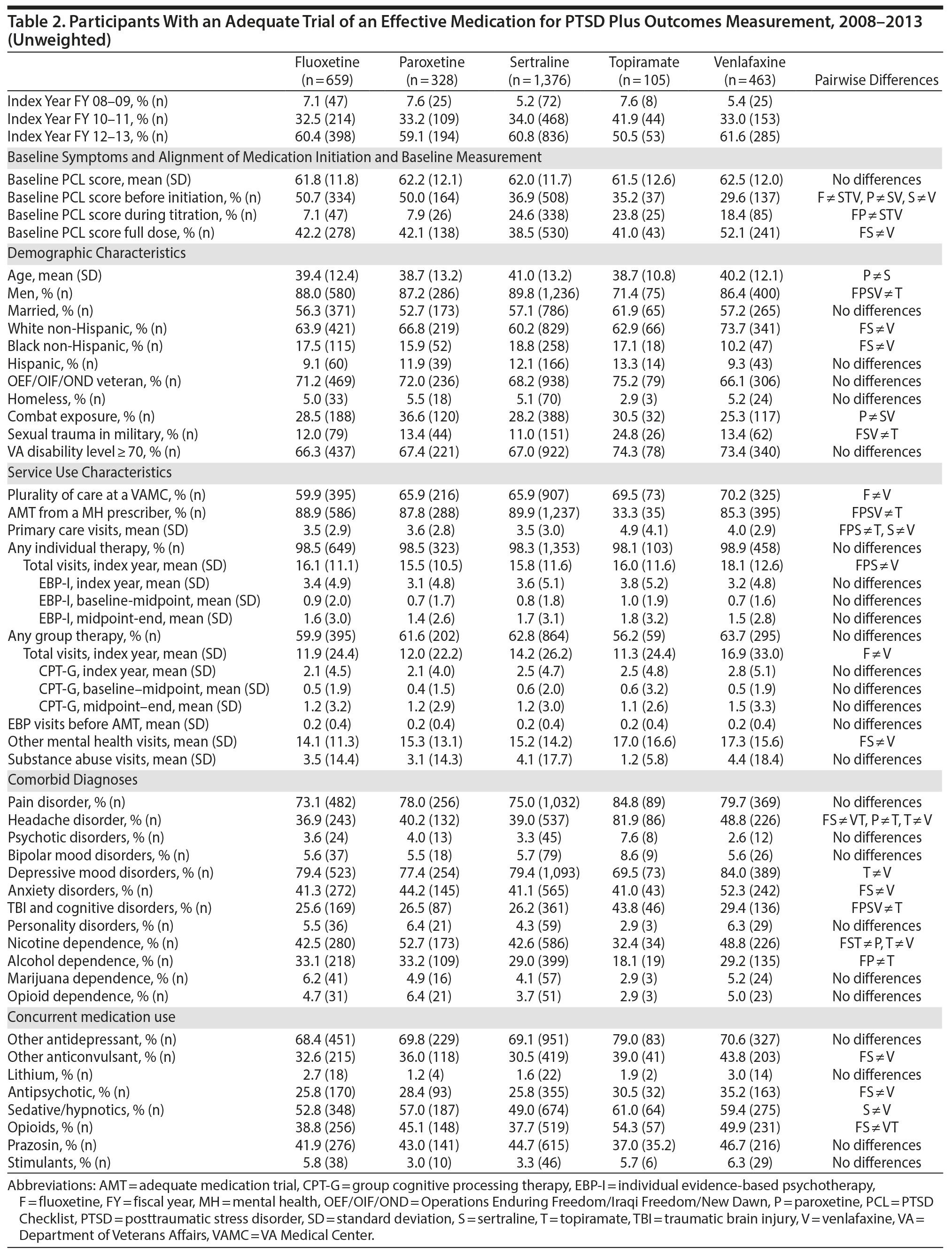
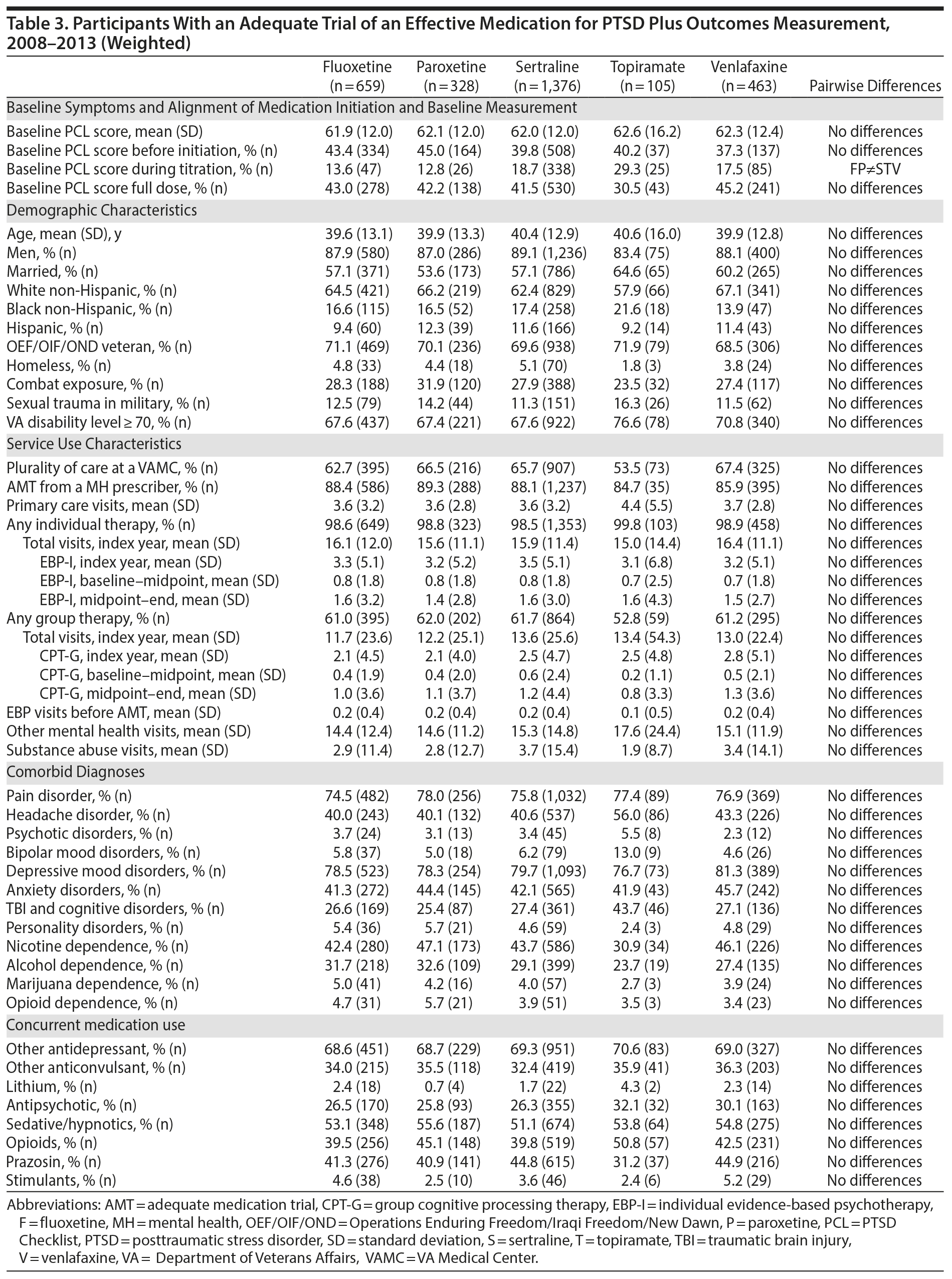
The number of participants in the analytic cohort receiving each medication ranged from 1,376 who received sertraline to 105 who received topiramate (Table 2). The number of eligible participants grew across treatment years, with the majority of participants in the analytic sample treated in fiscal years 2012 and 2013. While there were notable differences among the medication treatment groups, our weighting procedure allowed us to balance almost all covariates (Table 3). The exception was whether baseline PCL administration occurred during the medication titration period. For medications that commonly require a lengthier titration period—sertraline, topiramate, and venlafaxine—baseline PCL score was measured during the titration period more often than for fluoxetine and paroxetine, where both treatments generally start at full dose. We did not further adjust for this difference because it is more likely related to medication characteristics than to participant characteristics. The mean AMT length was 254.1 (SD = 119.5) days when continuation of treatment beyond the index year was included. Relative to the start of the AMT, participants’ baseline PCLs were administered at 9.7 (SD = 11.8) days prior to the start of the AMT and endpoint PCLs were administered at 174.7 (SD = 99.5) days after the start of the AMT. In the unweighted model, mean baseline PCL scores indicated a high burden of symptoms, ranging from 61.5 to 62.5 (Table 4).
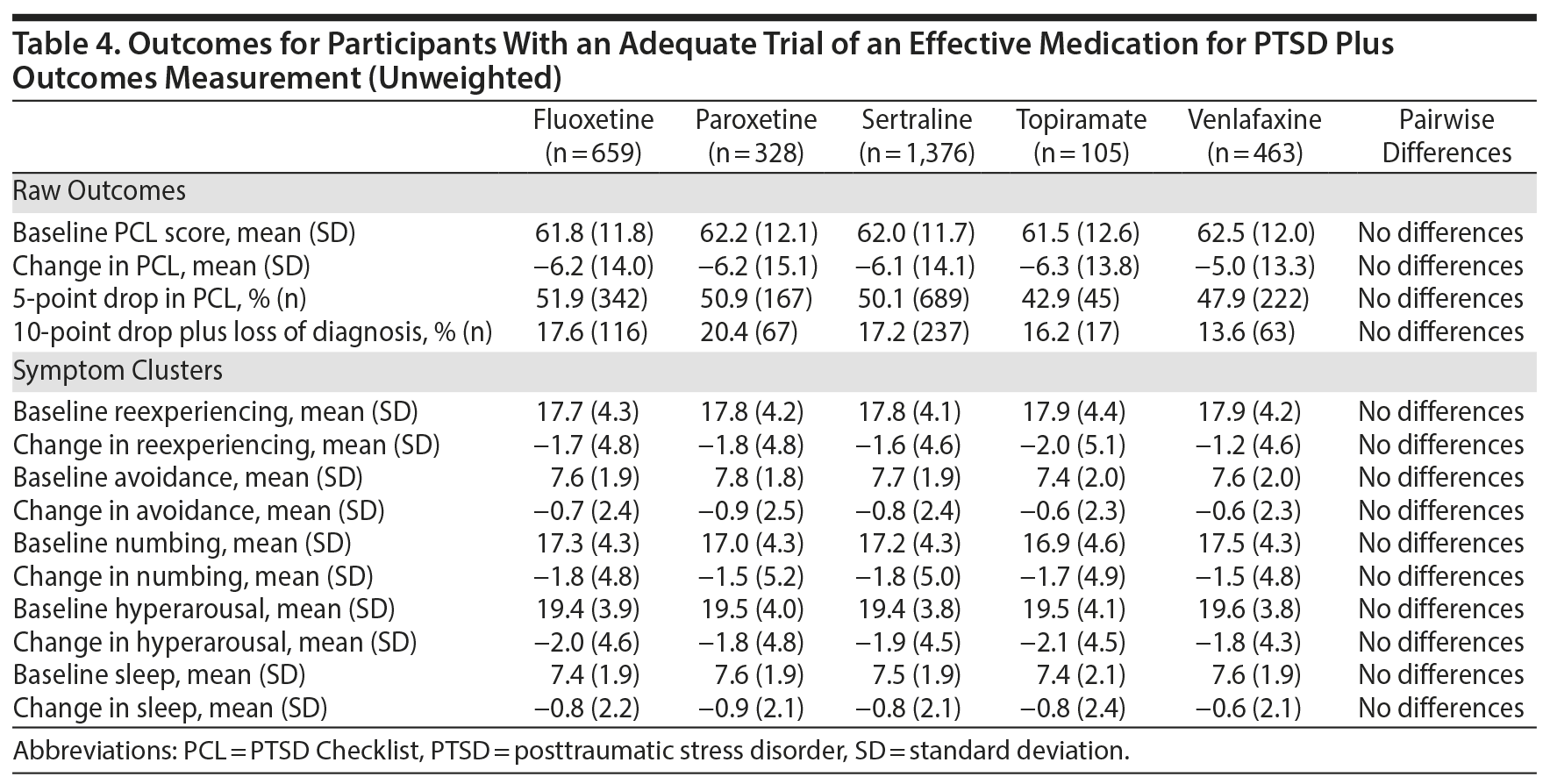
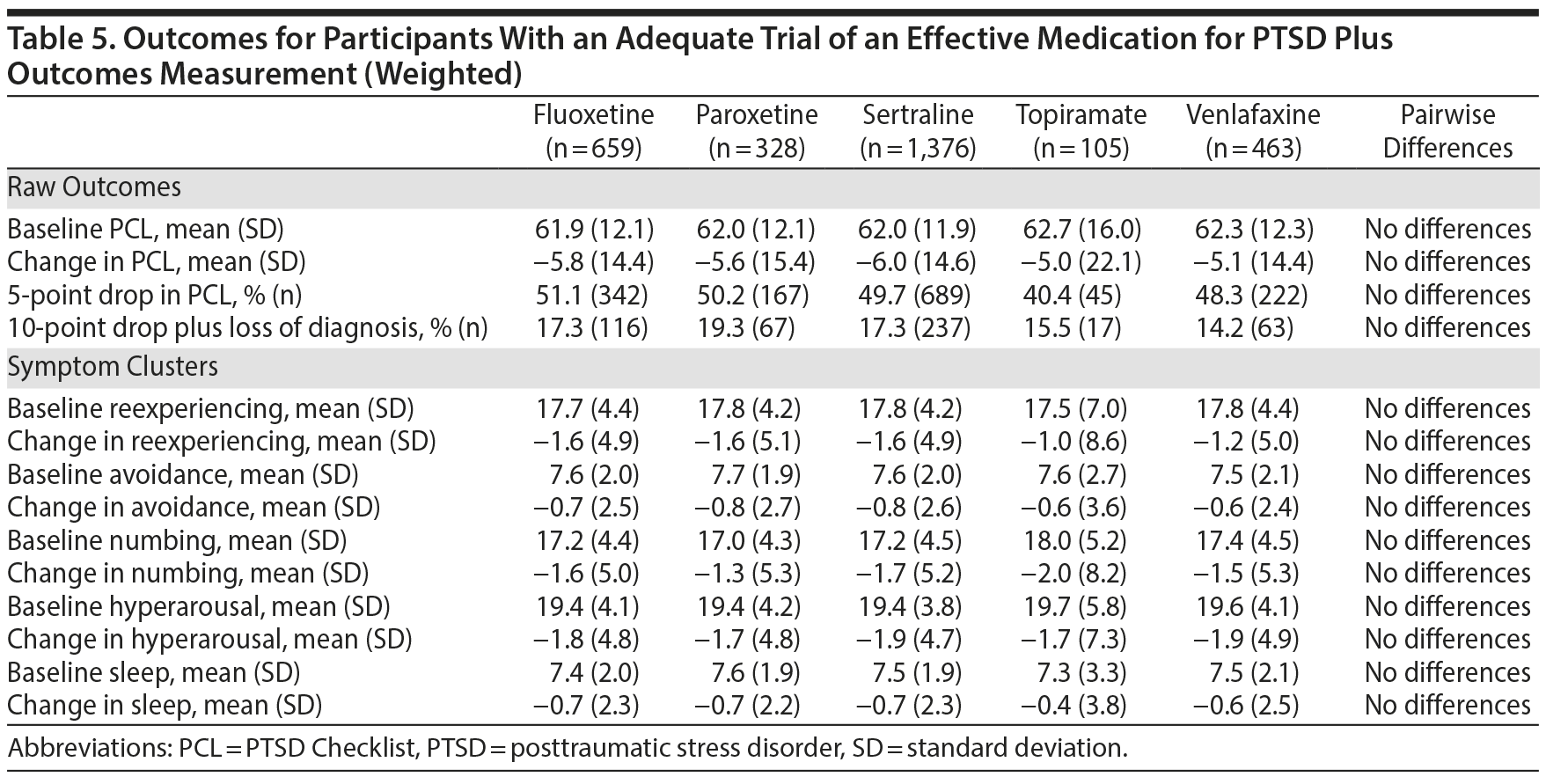
All 5 of the medications that were studied demonstrated a significant effect on PTSD symptoms. The mean improvement in PTSD symptoms measured by the PCL scores ranged from 5.0 to 6.3 points, indicating statistically reliable but modest improvements; between 41.9% and 52.9% of participants achieved an improvement of 5 points or more. As inclusion in the cohort was based on encounter-based diagnostic information (2 PTSD diagnoses within 90 days, at least 1 of which was in a mental health clinic), 12.4% (n = 363) patients did not meet PCL-based diagnostic criteria at baseline. However, there were no overall or pairwise differences among agents at baseline. Among those who met PCL-based diagnostic criteria for PTSD at baseline, between 13.6% and 20.4% of participants achieved our threshold for loss of diagnosis, an outcome associated with substantial clinical improvement. As measured by the PCL, there was a very limited range of baseline PTSD symptom clusters and sleep item scores and changes on these scores. There were no significant differences between medications in any outcome using the unweighted model. A weighted model adjusting for differences between the medication treatment groups was very similar to the unadjusted analysis, and there continued to be no differences in outcomes (Table 5), meaning that the 5 medications performed about equally in reducing PTSD symptoms, even after adjusting for differences between treatment groups.
In our exploratory univariate logistic regression models, the only significant (P < .001) patient-level predictors of loss of diagnosis were related to receipt of EBP for PTSD, including PE and CPT, delivered in an individual format (EBT-I). Across all treatment groups, the number of sessions of EBT-I during the index year of treatment predicted improvement (OR = 1.07), and the effect was greater if the sessions occurred during the AMT (OR baseline-midpoint = 1.14; OR midpoint-endpoint = 1.13). There were 4 predictors of not achieving the loss of PTSD diagnosis, including traumatic brain injury (TBI) and other cognitive disorders (OR = 0.65), male gender (OR = 0.63), OEF/OIF/OND veteran status (OR = 0.71), and non-psychotherapy mental health visits (OR = 0.98). No additional variables or patterns of variables emerged as predictors of response when we examined participants who received individual medications.
DISCUSSION
We compared the effectiveness of 5 evidence-based medications for PTSD in routine clinical practice and found that they performed similarly. During an average of 6 months of treatment, participants experienced a 5- to 6-point improvement in PCL scores. Approximately half of participants achieved a reliable improvement of 5 points or more on the PCL. Our findings are consistent with meta-analytic findings that have suggested that fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine are effective treatments for PTSD.4,5 However, less than a fifth of participants achieved our more stringent improvement criterion: loss of PTSD diagnosis. None of the medications led to superior outcomes in individual PTSD symptom clusters or sleep items.
The only independent variables that predicted loss of PTSD diagnosis were related to concurrent treatment with EBP-I for PTSD. Therefore, while fluoxetine, paroxetine, sertraline, topiramate, and venlafaxine appear to be equally effective in clinical practice, our findings do not support the idea that patient characteristics can guide the selection of the medication most likely to be effective.35 Instead, it appears that there is clinical equipoise and the choice of individual agent should be up to patients who elect to take a medication for PTSD.36 However, to maximize improvement, patients should also be encouraged to consider concurrent EBP-I. Prior analysis in the parent cohort from which this analytic sample was derived demonstrated that men and OEF/OIF/OND veterans are less likely to complete psychotherapy for PTSD.37 While studies have clearly demonstrated that patients with TBI can tolerate and benefit from evidence-based PTSD treatment,38-41 this evidence is largely derived from specialized residential settings and may not generalize to the outpatient care studied in this analysis. Thus, it is not particularly surprising that men, OEF/OIF/OND veterans, and those with a history of TBI or other cognitive disorders had poorer treatment outcomes given the importance of concurrent EBP-I. That more non-psychotherapy mental health visits is also a negative predictor of treatment response is not surprising, as these visits may indicate that participants had a greater variety of mental health treatment needs and comorbid mental health conditions.
There are several major limitations to our study. First, participants meeting inclusion criteria for our analytic cohort differed from the general VA PTSD treatment population in many ways. The patient sample in the analysis was younger, was more likely to be veterans of recent wars, and received more mental health services. Because of this, it is unclear if these findings generalize to older veterans of earlier service eras receiving less mental health services. Moreover, we have no clear understanding of whether these findings would apply to non-veterans with PTSD in general. Second, we were unable to measure all related aspects of care. As an example, we could not measure medication adherence or psychotherapy protocols that are less frequently used in the VA such as EMDR. However, the mean length of treatment was 6 months, indicating that participants typically exhausted their initial fill (which can last up to 90 days) and requested refills. Lastly, we only considered PTSD outcomes. Depression and quality of life measures were not available, but they may have enriched our exclusive focus on PTSD outcomes.
While we found that all of the medication treatments for PTSD that we studied were effective in clinical practice, their effect seemed reduced compared to that seen in the clinical trials. Such comparisons are difficult to make precisely in all cases because various studies use different measures and allowed various concurrent treatments. However, as an example, Berlant and colleagues’ open-label study of topiramate for PTSD found a mean change in PCL scores of 21 points (we found a 5-point change) and 34% of patients with loss of diagnosis (we found 16%).42 The reasons for possible reduction in effectiveness are unknown. One possibility is that drug trials have more stringent criteria for inclusion, subsequently not generalizing to the typical veteran population seen in clinic (no substance use disorders, suicidality, etc). It is also possible that VA patients are more treatment-resistant than patients enrolling in RCTs. Future work using our methods should attempt to examine patients’ treatment history longitudinally rather than cross-sectionally to address this concern.
We conclude that available evidence-based medications for PTSD are equally effective in clinical practice. Although effective, our data suggest that patients choosing medication treatment for PTSD should consider concurrent treatment with EBP-I for PTSD in order to maximize their chances of meaningful improvement.
Submitted: January 24, 2018; accepted April 23, 2018.
Published online: September 18, 2018.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, fluoxetine, topiramate, venlafaxine, risperidone, nefazodone, and phenelzine are not approved by the US Food and Drug Administration for the treatment of posttraumatic stress disorder.
Financial disclosure: Drs Shiner, Gui, Maguen, Young-Xu, Schnurr, and Watts and Ms Leonard Westgate have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: Development of the study cohort was funded by a Department of Veterans Affairs Health Services Research and Development Career Development Award (CDA11-263), VA Office of Research and Development, Washington, DC (Dr Shiner). Development of additional variables identifying the use of evidence-based psychotherapy for PTSD was funded by the Department of Defense Joint Warfighter Medical Research Program (JW140056), Congressionally Directed Medical Research Program, Fort Detrick, Maryland (Dr Maguen). Development of the analytic dataset and comparative effectiveness analyses were funded by the Department of Defense Peer Reviewed Medical Research Program (PR160203), Congressionally Directed Medical Research Program, Fort Detrick, Maryland (Dr Shiner).
Role of the sponsor: The sponsors had no role in the study design, methods, analysis, and interpretation of results or in preparation of the manuscript and the decision to submit it for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs or US Department of Defense.
Additional information: The VA Corporate Data Warehouse (CDW) contains electronic medical record data compiled from individual VA facilities and is described at http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm. Researchers with VA network access can obtain descriptions of CDW data at http://vaww.virec.research.va.gov/.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. PubMed CrossRef
3. Bernardy NC, Hoge CW, Friedman MJ, et al. VA/DoD Clinical Practice Guideline for the Management of Posttraumatic Stress Disorder and Acute Stress Disorder. Washington, DC: United States Departments of Veterans Affairs and Defense; 2017.
4. Jonas DE, Cusack K, Forneris CA, et al. Psychological and Pharmacological Treatments for Adults With Posttraumatic Stress Disorder (PTSD). Rockville, MD: Agency for Healthcare Research and Quality; 2013.
5. Watts BV, Schnurr PP, Mayo L, et al. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-e550. PubMed CrossRef
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100. PubMed CrossRef
7. Lee DJ, Schnitzlein CW, Wolf JP, et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta-analyses to determine first-line treatments. Depress Anxiety. 2016;33(9):792-806. PubMed CrossRef
8. Mavridis D, Giannatsi M, Cipriani A, et al. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40-46. PubMed CrossRef
9. Cipriani A, Williams T, Nikolakopoulou A, et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med. 2018;48(12):1975-1984. PubMed CrossRef
10. Frank JB, Kosten TR, Giller EL Jr, et al. A randomized clinical trial of phenelzine and imipramine for posttraumatic stress disorder. Am J Psychiatry. 1988;145(10):1289-1291. PubMed CrossRef
11. Shiner B, Leonard Westgate C, Bernardy NC, et al. Trends in opioid use disorder diagnoses and medication treatment among veterans with posttraumatic stress disorder. J Dual Diagn. 2017;13(3):201-212. PubMed CrossRef
12. Ronconi JM, Shiner B, Watts BV. Inclusion and exclusion criteria in randomized controlled trials of psychotherapy for PTSD. J Psychiatr Pract. 2014;20(1):25-37. PubMed CrossRef
13. Spoont MR, Murdoch M, Hodges J, et al. Treatment receipt by veterans after a PTSD diagnosis in PTSD, mental health, or general medical clinics. Psychiatr Serv. 2010;61(1):58-63. PubMed CrossRef
14. Fortney JC, Unutzer J, Wrenn G, et al. A tipping point for measurement-based care. Psychiatr Serv. 2017;68(2):179-188. PubMed CrossRef
15. Dreyer NA, Tunis SR, Berger M, et al. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood). 2010;29(10):1818-1825. PubMed CrossRef
16. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59. PubMed CrossRef
17. Shiner B, Westgate CL, Bernardy NC, et al. Anticonvulsant medication use in veterans with posttraumatic stress disorder. J Clin Psychiatry. 2017;78(5):e545-e552. PubMed CrossRef
18. Frayne SM, Miller DR, Sharkansky EJ, et al. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010;25(1):42-50. PubMed CrossRef
19. Gravely AA, Cutting A, Nugent S, et al. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self-reported PTSD Checklist scores. J Rehabil Res Dev. 2011;48(1):21-30. PubMed CrossRef
20. Maguen S, Madden E, Neylan TC, et al. Timing of mental health treatment and PTSD symptom improvement among Iraq and Afghanistan veterans. Psychiatr Serv. 2014;65(12):1414-1419. PubMed CrossRef
21. Seal KH, Maguen S, Bertenthal D, et al. Observational evidence for buprenorphine’s impact on posttraumatic stress symptoms in veterans with chronic pain and opioid use disorder. J Clin Psychiatry. 2016;77(9):1182-1188. PubMed CrossRef
22. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
23. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD Checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. PubMed CrossRef
24. Shiner B, Watts BV, Pomerantz A, et al. Sensitivity of the SF-36 to PTSD symptom change in veterans. J Trauma Stress. 2011;24(1):111-115. PubMed CrossRef
25. Schnurr PP, Lunney CA. Symptom benchmarks of improved quality of life in PTSD. Depress Anxiety. 2016;33(3):247-255. PubMed CrossRef
26. Monson CM, Gradus JL, Young-Xu Y, et al. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20(2):131-138. PubMed CrossRef
27. Friedman MJ, Lowry P, Ruzek J. VA/DoD clinical practice guidelines for the management of post-traumatic stress. US Department of Veterans Affairs website. www.healthquality.va.gov. 2010.
28. Foa E, Hembre E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences. New York, NY: Oxford University Press; 2007.
29. Resick PA, Monson CM, Chard KM. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. New York, NY: Guilford Press; 2017.
30. Maguen S, Madden E, Patterson OV, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder in a large national healthcare system. Adm Policy Ment Health. 2018;45(4):519-529. PubMed CrossRef
31. Shiner B, D’ Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. PubMed CrossRef
32. Ridgeway G, McCaffrey DF, Morral AR, et al. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial for the TWANG Package. Santa Monica, CA: RAND Corporation; 2017.
33. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1-21. PubMed CrossRef
34. McCaffrey DF, Griffin BA, Almirall D, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388-3414. PubMed CrossRef
35. Harpaz-Rotem I, Rosenheck R, Mohamed S, et al. Initiation of pharmacotherapy for post-traumatic stress disorder among veterans from Iraq and Afghanistan: a dimensional, symptom cluster approach. BJPsych Open. 2016;2(5):286-293. PubMed CrossRef
36. Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci. 2009;4(1):75. PubMed CrossRef
37. Shiner B, Leonard Westgate C, Harik JM, et al. Effect of patient-therapist gender match on psychotherapy retention among United States veterans with posttraumatic stress disorder. Adm Policy Ment Health. 2017;44(5):642-650. PubMed CrossRef
38. Chard KM, Schumm JA, McIlvain SM, et al. Exploring the efficacy of a residential treatment program incorporating cognitive processing therapy-cognitive for veterans with PTSD and traumatic brain injury. J Trauma Stress. 2011;24(3):347-351. PubMed CrossRef
39. Davis JJ, Walter KH, Chard KM, et al. Treatment adherence in cognitive processing therapy for combat-related PTSD with history of mild TBI. Rehabil Psychol. 2013;58(1):36-42. PubMed CrossRef
40. Walter KH, Dickstein BD, Barnes SM, et al. Comparing effectiveness of CPT to CPT-C among US Veterans in an interdisciplinary residential PTSD/TBI treatment program. J Trauma Stress. 2014;27(4):438-445. PubMed CrossRef
41. Walter KH, Kiefer SL, Chard KM. Relationship between posttraumatic stress disorder and postconcussive symptom improvement after completion of a posttraumatic stress disorder/traumatic brain injury residential treatment program. Rehabil Psychol. 2012;57(1):13-17. PubMed CrossRef
42. Berlant J, van Kammen DP. Open-label topiramate as primary or adjunctive therapy in chronic civilian posttraumatic stress disorder: a preliminary report. J Clin Psychiatry. 2002;63(1):15-20. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite