Background: Use of selective serotonin reuptake inhibitors (SSRIs) has been associated with an increased risk of intracranial hemorrhage. However, little is known about cerebrovascular risk in users of serotonin-norepinephrine reuptake inhibitors (SNRIs). Our aim was to determine the differential risk of cerebrovascular events between SSRIs and SNRIs.
Method: A nationwide population-based cohort study was conducted in adult patients who started taking SSRIs or SNRIs during the time period 2005 through 2009. The outcome of interest was defined by the first hospitalization diagnosis for ischemic stroke (ICD-9-CM codes 433, 434, 436) or intracranial hemorrhage (ICD-9-CM codes 430, 431, 432). We used a Cox regression model with time-varying medication use and adjusted for stroke risk factors to estimate the hazard ratios (HRs) of ischemic stroke and intracranial hemorrhage associated with SNRI use, using SSRI use as a reference.
Results: Among 582,650 SSRI and 76,920 SNRI initiators with an average follow-up period of 3.2 years, there was a nonsignificantly increased trend toward intracranial hemorrhage (adjusted HR = 1.24 [95% CI, 0.97-1.58]) in SNRI users compared to SSRI users. The risk of ischemic stroke was comparable between the 2 treatment groups (adjusted HR = 1.01 [0.90-1.12]). Similar results were obtained in sensitivity analyses, considering a dose-response relation, allowance of a 7-day grace period between study drug discontinuation and outcome occurrence, and restriction to exclusive users, who remained on the initial treatment. In the subgroup analysis, there was an increased incidence of intracranial hemorrhages in SNRI users compared to SSRI users in patients without prior depression (adjusted HR = 1.63 [1.14-2.32]).
Conclusions: Use of SNRIs is not associated with an increased risk of either ischemic stroke or intracranial hemorrhage as compared to use of SSRIs in adult patients with depression or anxiety. However, SNRIs should be used cautiously in patients without depression.

Comparison of the Effects of Serotonin-Norepinephrine Reuptake Inhibitors Versus Selective Serotonin Reuptake Inhibitors on Cerebrovascular Events

ABSTRACT
Background: Use of selective serotonin reuptake inhibitors (SSRIs) has been associated with an increased risk of intracranial hemorrhage. However, little is known about cerebrovascular risk in users of serotonin-norepinephrine reuptake inhibitors (SNRIs). Our aim was to determine the differential risk of cerebrovascular events between SSRIs and SNRIs.
Method: A nationwide population-based cohort study was conducted in adult patients who started taking SSRIs or SNRIs during the time period 2005 through 2009. The outcome of interest was defined by the first hospitalization diagnosis for ischemic stroke (ICD-9-CM codes 433, 434, 436) or intracranial hemorrhage (ICD-9-CM codes 430, 431, 432). We used a Cox regression model with time-varying medication use and adjusted for stroke risk factors to estimate the hazard ratios (HRs) of ischemic stroke and intracranial hemorrhage associated with SNRI use, using SSRI use as a reference.
Results: Among 582,650 SSRI and 76,920 SNRI initiators with an average follow-up period of 3.2 years, there was a nonsignificantly increased trend toward intracranial hemorrhage (adjusted HR = 1.24 [95% CI, 0.97-1.58]) in SNRI users compared to SSRI users. The risk of ischemic stroke was comparable between the 2 treatment groups (adjusted HR = 1.01 [0.90-1.12]). Similar results were obtained in sensitivity analyses, considering a dose-response relation, allowance of a 7-day grace period between study drug discontinuation and outcome occurrence, and restriction to exclusive users, who remained on the initial treatment. In the subgroup analysis, there was an increased incidence of intracranial hemorrhages in SNRI users compared to SSRI users in patients without prior depression (adjusted HR = 1.63 [1.14-2.32]).
Conclusions: Use of SNRIs is not associated with an increased risk of either ischemic stroke or intracranial hemorrhage as compared to use of SSRIs in adult patients with depression or anxiety. However, SNRIs should be used cautiously in patients without depression.
J Clin Psychiatry 2016;77(1):e1-e7
dx.doi.org/10.4088/JCP.14m09394
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Family Medicine, Cathay General Hospital, Taipei, Taiwan
bDepartment of Neurology, National Taiwan University Hospital, Taipei, Taiwan
cInstitute of Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan
dDepartment of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
eCardiovascular Center, National Taiwan University Hospital Yun-Lin Branch, Dou-Liou City, Yun-Lin County, Taiwan
‘ ¡These 2 authors contributed equally to this work.
*Corresponding author: Chia-Hsuin Chang, MD, ScD, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan ([email protected]).
Depression is a highly prevalent neuropsychiatric disorder with a lifetime prevalence of more than 16% in the general population.1 Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are the most frequently prescribed medications for treatment of depression and general anxiety disorder.2,3 Depression per se is associated with an increased risk of stroke,4 one of the leading causes of death and permanent disability in the world, which results in significant economic burden on society.5 Recently, the use of antidepressants, especially serotonergic drugs, has garnered attention due to their potential impacts on the risks of both ischemic stroke and intracranial hemorrhages.6,7
A recent study showed that the use of SSRIs may increase the incidence of intracranial hemorrhage.8 SNRIs, a relatively new class of antidepressants, are increasingly used to treat depression and anxiety disorders3; however, little is known about the risk of stroke. Mechanistically, both SSRIs and SNRIs block serotonin transporter, reduce the intracellular concentration of serotonin in platelets, inhibit platelet aggregation, and thus increase the risk of intracerebral hemorrhage.9 Given that SNRIs increase norepinephrine levels in neuronal synapses, resulting in elevated norepinephrine concentrations compared to SSRIs,10 it was hypothesized that these unique pharmacodynamic characteristics of SNRIs might have an impact on the sympathetic cardiovascular system and affect the cerebrovascular risk. Given that both depression and anxiety are known risk factors for stroke,4,11 comparing SNRI users with nonusers is subject to confounding by indication. Thus, we conducted a population-based cohort study to investigate the differential risks of SNRIs and SSRIs for ischemic stroke and intracranial hemorrhages in new users of these 2 medications, using SSRI users as the reference group.
METHOD
Data Source
A single-payer and compulsory national health insurance program was implemented in Taiwan in 1995, and there was 99% enrollment by 2010. The Taiwan National Health Insurance (NHI) database includes complete outpatient visits, hospital admissions, prescriptions, as well as the disease and vital statuses for 99% of the country’s population (approximately 23 million). The current analyses linked several large computerized claim datasets with the National Death Registry through the use of birth dates and the civil identification number unique to each beneficiary. Written consent from study subjects was not obtained because the NHI dataset consists of deidentified secondary data for research purposes, and the Institutional Review Board of National Taiwan University Hospital issued a formal written waiver for the need for consent.
Study Population
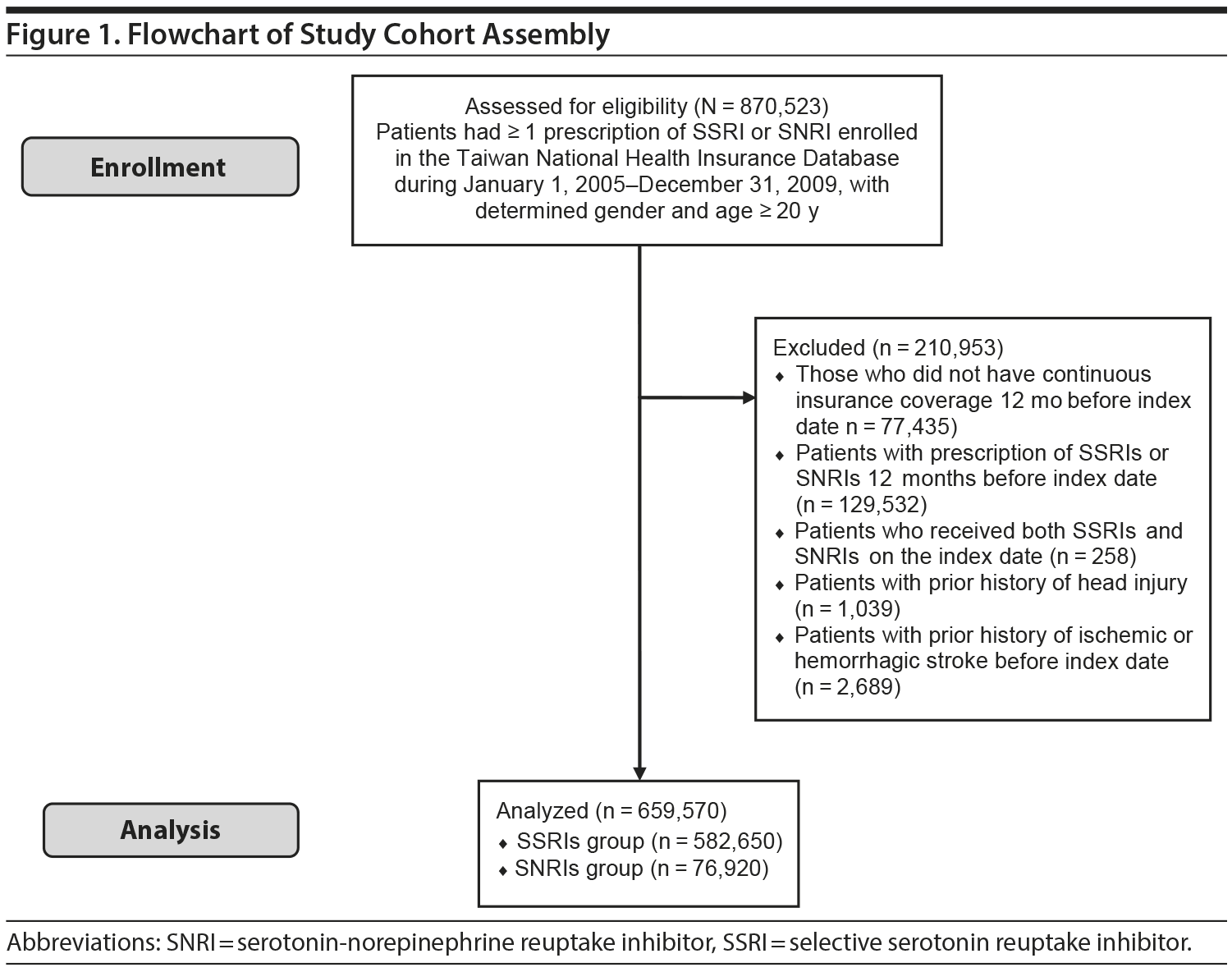
From the source population, we identified adult patients aged over 20 years who initiated treatment with an SNRI or SSRI between January 1, 2005, and December 31, 2009. Initiation was defined as not receiving treatment with any studied medication for 12 months prior to the first prescription (index date). Subjects were excluded if they (1) were without continuous insurance coverage for 12 months before the index date, (2) received both an SNRI and an SSRI on the index date, (3) had a prior history of head injury, or (4) had been diagnosed with ischemic stroke or intracranial hemorrhage before the index date.
Use of Study Drugs
The exposure drugs of interest in this study included SSRIs (paroxetine, sertraline, fluoxetine, citalopram, escitalopram, fluvoxamine) and SNRIs (venlafaxine, duloxetine, milnacipran) (the Anatomical Therapeutic Chemical [ATC] codes are provided in Supplementary eTable 1). The outpatient pharmacy prescription database was used to obtain prescribed drug types, dosages, dates of prescriptions, supply days, and total numbers of pills dispensed. We defined discontinuation of therapy if no medication refill was available. Due to possible drug switching during the follow-up period, exposure to study medications was treated as a time-varying factor in the analysis. For each patient, the mean daily dose was estimated by multiplying the cumulative number of pills dispensed by the dose prescribed, divided by the follow-up duration. Data were presented as the number of defined daily doses (DDD), which was established by an expert panel as the typical maintenance dose required when the drug is used for its main indication in an adult. The DDD values for individual drugs of SSRIs and SNRIs are 10 mg for escitalopram; 20 mg for paroxetine, fluoxetine, and citalopram; 50 mg for sertraline; 60 mg for duloxetine; and 100 mg for fluvoxamine, venlafaxine, and milnacipran.

- Recent studies have shown that use of selective serotonin reuptake inhibitors (SSRIs) may increase the incidence of intracranial hemorrhage. However, little is known about the risk of stroke for users of serotonin-norepinephrine reuptake inhibitors (SNRIs), an increasingly prescribed new class of antidepressant.
- With regard to the risk of ischemic stroke or intracranial hemorrhage, SNRIs were comparable with SSRIs in adult patients with either depression or anxiety.
Outcome Ascertainment and Follow-Up
The outcome of interest was defined by the first hospitalization diagnosis for ischemic stroke (ICD-9-CM code 433, 434, 436) or intracranial hemorrhage (ICD-9-CM code 430, 431, 432). Validation studies in Taiwan have shown that the ICD-9 diagnosis codes used to identify patients with ischemic strokes had a positive predictive value as high as 98%.12,13 Also, a systematic review of published reports suggested that algorithms to evaluate the presence of ischemic stroke and intracranial hemorrhage had high positive predictive values (80% or greater).14 In addition to ICD-9-CM codes, we also used the following criteria to increase outcome accuracy: (1) a record of computed tomography or magnetic resonance imaging of the brain during hospitalization and (2) a certificate for stroke. The Bureau of Taiwan NHI issues certificates to patients who suffer from major illnesses/injuries, including acute ischemic stroke and intracranial hemorrhage. This criterion was required in our study to increase the accuracy of the outcome definition.
Furthermore, we conducted a validation study by randomly sampling records of 226 hospitalized patients at 1 medical center with the above mentioned criteria. Their medical records were then reviewed by a physician (Y.-C.L.). The positive predictive value was 93.8% using these criteria, confirming the diagnostic accuracy of our algorithm.
Patients were followed from the index date to the earliest of the following: outcome occurrence, death, disenrollment from the NHI, or December 31, 2010.
Covariate Ascertainment and Propensity Score Adjustment
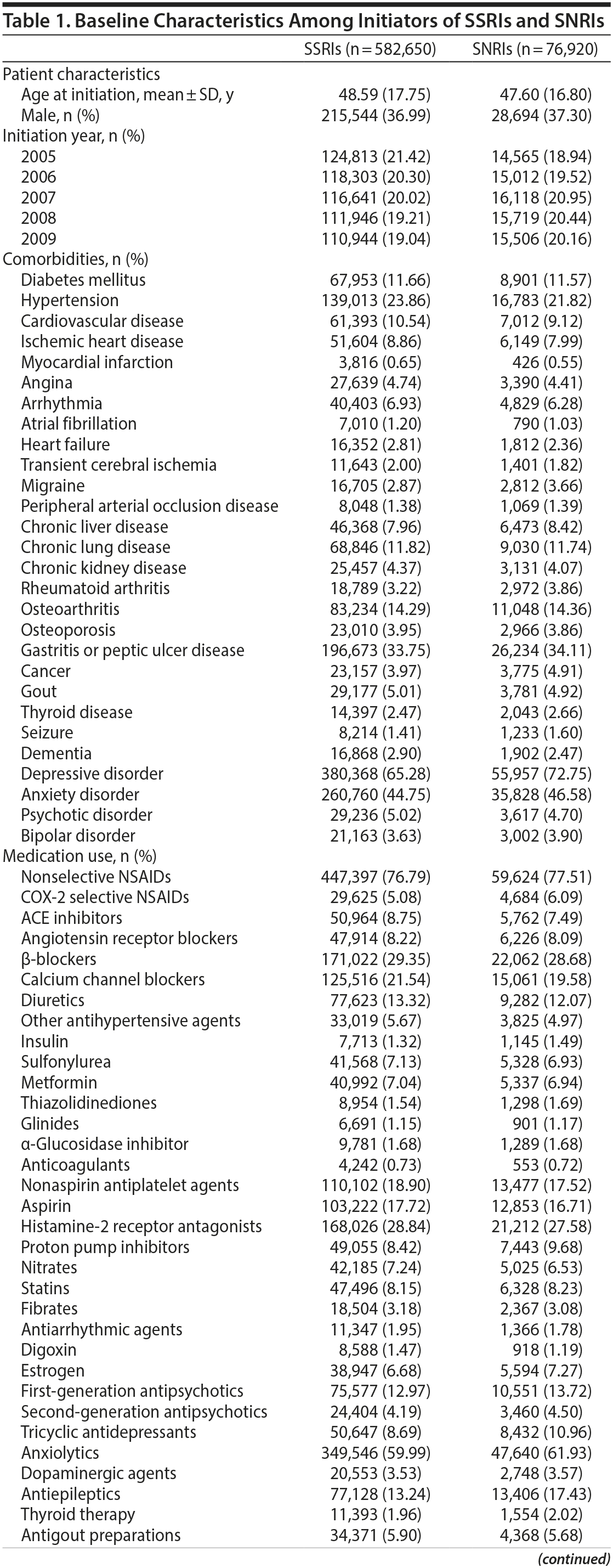
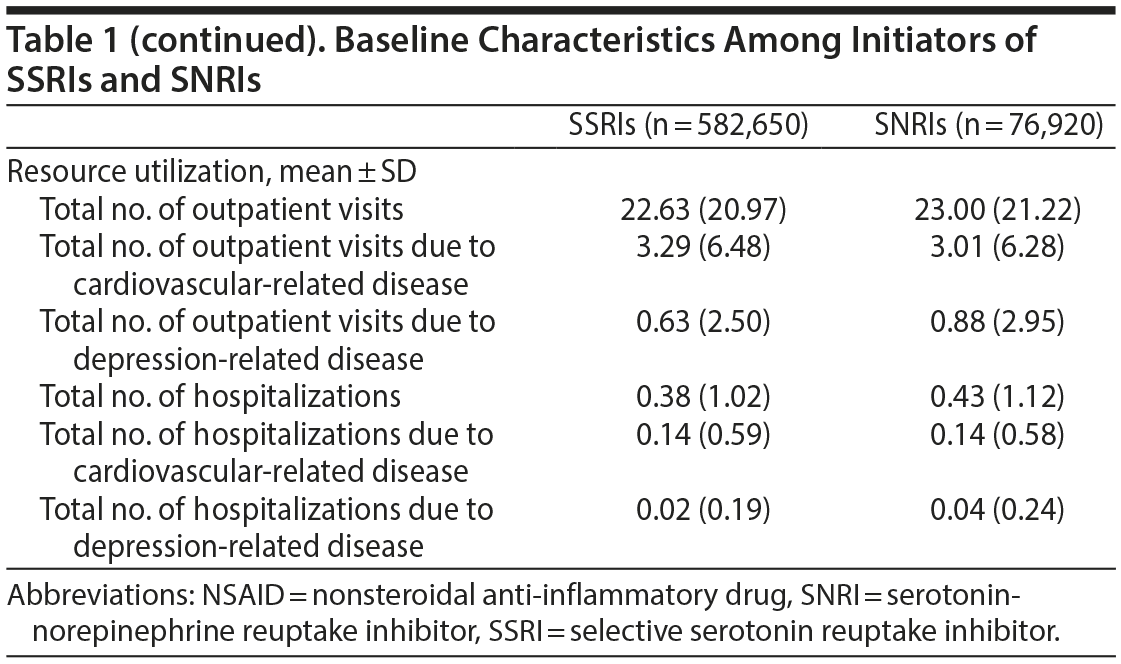
Inpatient and outpatient diagnosis files and prescription files during the 12-month period before the index date were used to ascertain patients’ demographic data (including age, sex, and resource utilization [eg, number of outpatient visits and number of hospitalizations] 12 months prior to the index date), medical history (ICD-9-CM codes provided in Supplementary eTable 1), and medications (ATC codes provided in Supplementary eTable 1). All variables including demographic data, medical and psychiatric comorbidities, medications, and medical resource utilization (Table 1) were incorporated into a nonparsimonious logistic regression model. The probability of being treated with SNRIs, that is, the propensity score, was estimated and used to adjust for baseline differences between the 2 treatment groups in the subsequent analyses.
Statistical Analysis
The descriptive data of the baseline characteristics among initiators of SNRIs and SSRIs were summarized. Person-days of follow-up were computed for all patients in the cohort for each drug use category. The crude incidence rates for ischemic stroke and intracranial hemorrhage were calculated and their 95% confidence intervals (CIs) were estimated based on a Poisson distribution.
A Cox proportional hazards regression model, stratified by baseline propensity score quintiles, was used to calculate the hazard ratios (HRs) of ischemic stroke or intracranial hemorrhage and their 95% CIs using SSRIs as the reference group. Separate models were conducted to estimate the HR of ischemic stroke and intracranial hemorrhage.
In the sensitivity analyses testing of the robustness of our results, we investigated whether effect estimates changed substantially by (1) adjusting for mean daily dosage (≥ 0.5 or < 0.5 DDD) estimated from the first SNRI or SSRI prescription; (2) allowing a grace period of 7 days between study drug discontinuation and outcome occurrence; and (3) restricting to exclusive users who remained on the initial treatment. Meanwhile, stratified analyses were performed to evaluate potential effect modifications. Participants were further stratified according to (1) age (< 60 or ≥ 60 years), (2) sex, (3) the presence/absence of hypertension, and (4) the presence/absence of a depressive disorder. Confidence intervals between subgroups were compared, and a significant interaction was suggested if they did not overlap. A 2-sided P value < .05 was considered statistically significant. All statistical analyses were performed with SAS 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
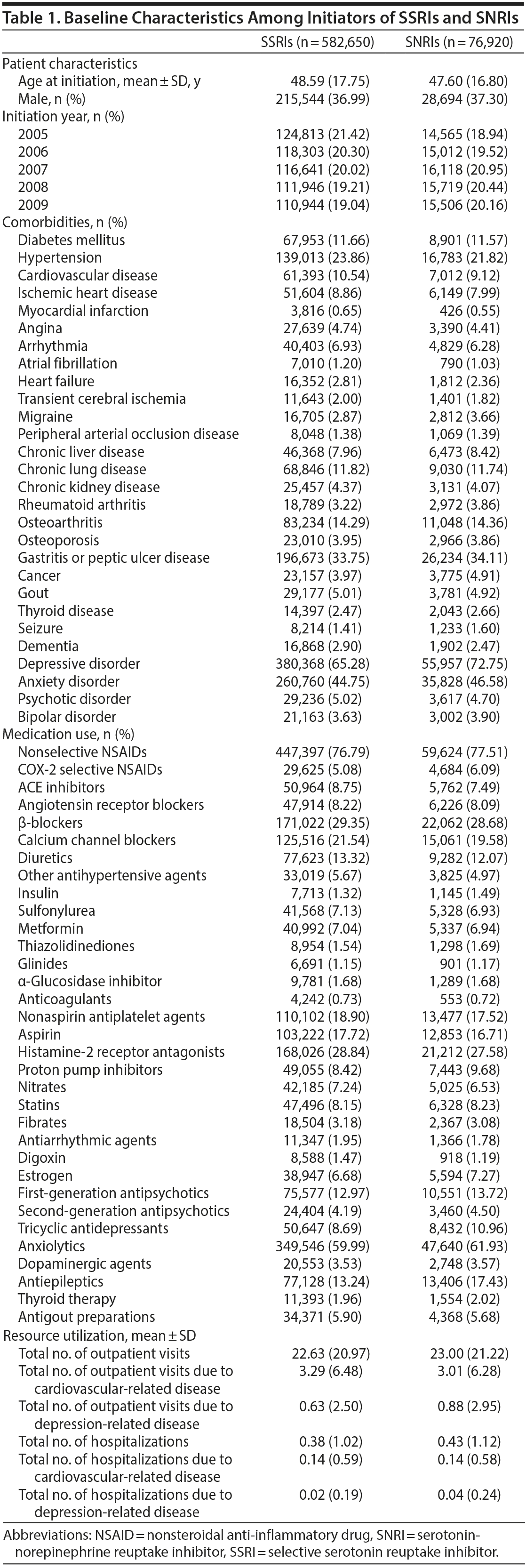
A total of 582,650 SSRI initiators and 76,920 SNRI initiators were included in the analysis (Figure 1). As shown in Table 1, initiators of both types of drugs were similar in terms of most baseline characteristics and medication use, including antiplatelet agents, anticoagulants, and nonsteroidal anti-inflammatory drugs. However, SNRI initiators had a lower proportion of hypertension but a higher percentage of depressive and anxiety disorders. Other risk factors for stroke were evenly distributed between the 2 treatment groups.
After an average follow-up period of 3.2 years, there were a total of 9,194 ischemic strokes and 1,787 intracranial hemorrhages in the SSRI group and 1,028 ischemic strokes and 208 intracranial hemorrhages in the SNRI group. In the SSRI group, the total event numbers for death, ischemic stroke, and intracranial hemorrhage were 36,111, 9,194, and 1,789 among the 582,650 users, corresponding to crude incidences (95% CIs) of 52.8 (52.3-53.4), 13.4 (13.2-13.7), and 2.61 (2.49-2.73), respectively. In the SNRI group, the total event numbers for death, ischemic stroke, and intracranial hemorrhage were 4,569, 1,028, and 208 among the 76,920 users, corresponding to crude incidences of 52.0 (50.5-53.5), 11.7 (11.0-12.4), and 2.37 (2.04-2.69), respectively. In addition, SNRI initiators were more likely to switch to SSRIs (30.8% vs 10.1%), and the mean daily dosage of SNRI users was lower than that of SSRI initiators (0.75 DDD vs 0.97 DDD).
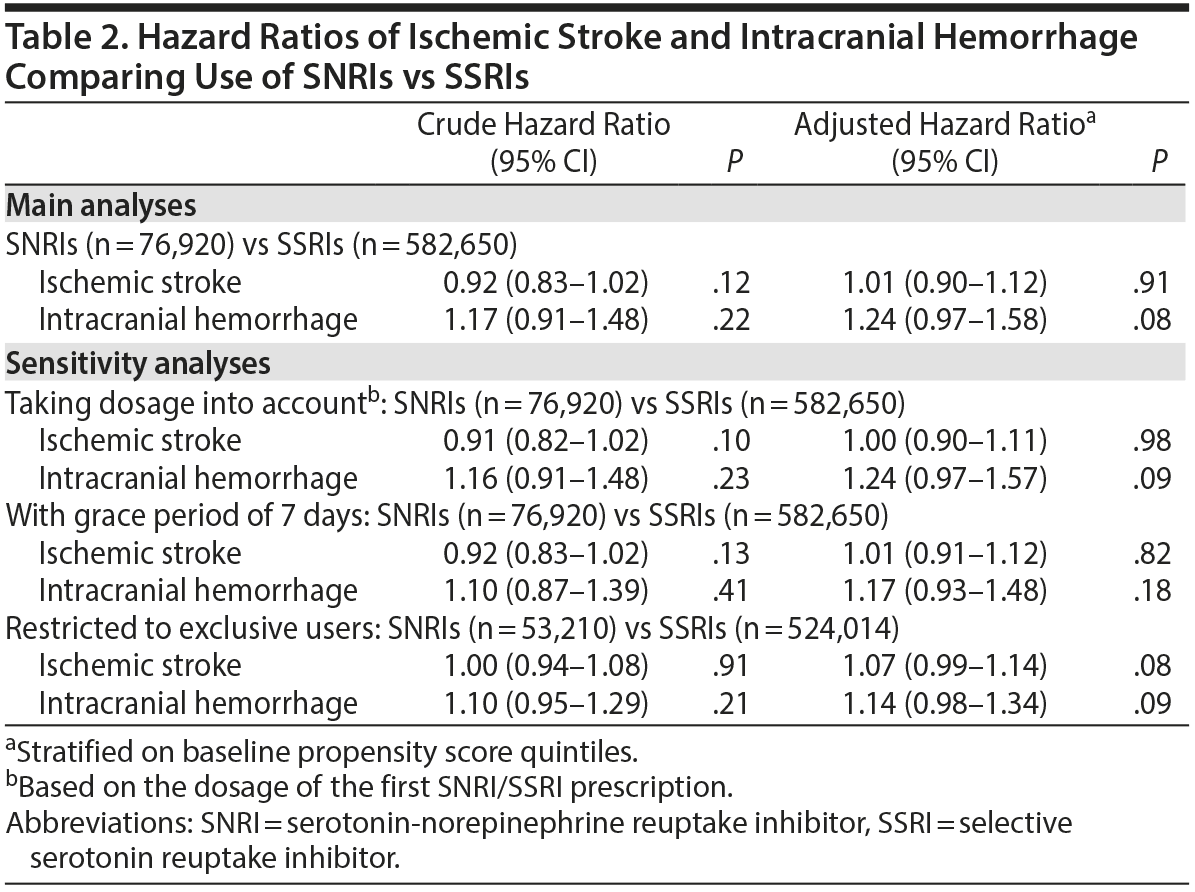
The Cox regression model with time-varying study medication use analysis showed that use of SNRIs seemed to demonstrate a trend toward increasing the occurrence of intracranial hemorrhage compared to use of SSRIs (crude HR = 1.17; 95% CI, 0.91-1.48; P = .22). Risks associated with SNRI use became more obvious after taking baseline differences into consideration (adjusted HR = 1.24; 95% CI, 0.97-1.58; P = .08). In contrast, the risk of ischemic stroke was comparable in these 2 treatment groups (adjusted HR = 1.01; 95% CI, 0.90-1.12; P = .91) (Table 2, Main Analyses). Similar results were found in the sensitivity analyses of different analytic protocols (Table 2, Sensitivity Analyses), with HRs for intracranial hemorrhage ranging from 1.14 to 1.24. Of note, SNRI treatment was also associated with a borderline modestly increased risk of ischemic stroke among exclusive users (adjusted HR = 1.07; 95% CI, 0.99-1.14; P = .08).
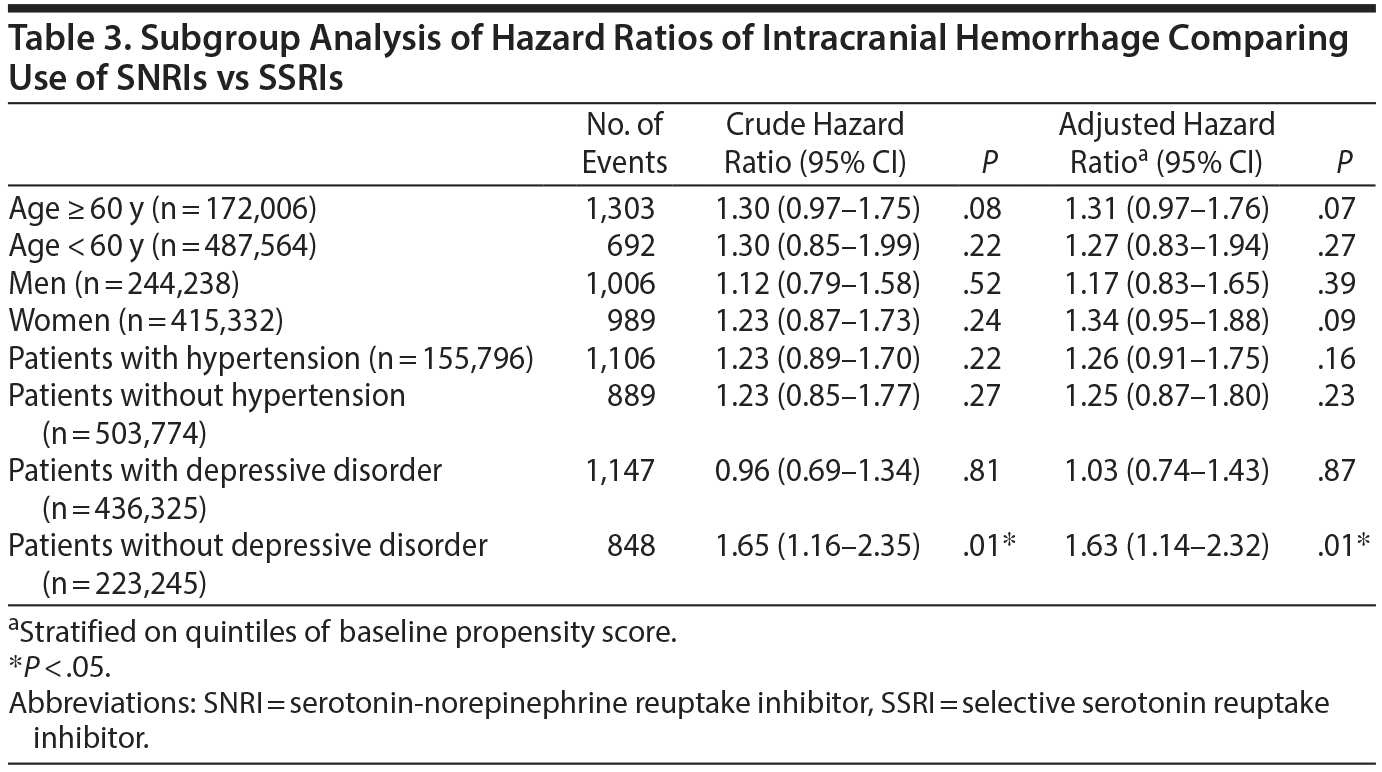
In the subgroup analysis, we found that the risk of intracranial hemorrhage was comparable between SSRI and SNRI users in patients regardless of age, sex, or comorbidities, with or without hypertension (Table 3). However, a significantly increased risk of intracranial hemorrhage was observed in SNRI users without a previous depressive disorder (adjusted HR = 1.63; 95% CI, 1.14-2.32; P = .01), although the confidence interval overlapped with that of the subgroup of patients with previous depressive disorder, suggesting no significant effect modification. In addition, a trend toward a borderline increase of intracranial hemorrhage was observed in patients 60 years or older (adjusted HR = 1.31; 95% CI, 0.97-1.76; P = .07).
DISCUSSION
Our results demonstrated that the use of SNRIs was not associated with an increased risk of either ischemic stroke or intracranial hemorrhage, compared to SSRIs, in patients with depression or anxiety. This finding was consistent in the sensitivity analyses and even for the more susceptible groups, such as patients with a past history of hypertension, men, and the elderly. However, the use of SNRIs was associated with an increased risk of intracranial hemorrhage in the subgroup of patients without depression.
It has been previously documented that the use of SSRIs was associated with an increased risk of intracerebral hemorrhage and all types of stroke.7,15 Our finding that the cerebrovascular risk was comparable between SSRI and SNRI users further suggested that serotonin might play a major role in this adverse effect. Serotonergic drugs may influence the risk of bleeding by inhibiting collagen-induced platelet aggregation and activation.16,17 Concomitant use of drugs such as antiplatelet agents, nonsteroidal anti-inflammatory drugs, and antipsychotics also increases the risk of bleeding.18
SNRIs elevate norepinephrine levels in neuronal synapses and result in increased sympathetic activities.10 Recent studies showed that use of duloxetine (one example of an SNRI) increased the frequency of tachycardia, palpitations, and hypertension.19,20 In addition, the use of SNRIs in patients with either depression or anxiety was associated with decreased vagal activity and increased blood pressure.21,22 Venlafaxine posed a significant sympathomimetic reaction on the cutaneous blood vessels through α-adrenergic receptor stimulation, which was assessed by the vasoconstrictor responses of the dorsal hand vein.23,24 Furthermore, the sequential changes in the plasma concentration of norepinephrine might differ between users of SSRIs and SNRIs.25 However, the effect of sympathetic activation of SNRIs did not seem to correlate with an increased cerebrovascular risk in our analyses. The results did not support the hypothesis that the sympathomimetic effects of SNRIs further significantly increased the risk of ischemic stroke or intracranial hemorrhage over SSRIs.
The only exception was that there was likely an increased risk of intracranial hemorrhage with the use of SNRIs as compared to SSRIs in the subgroup of patients without depression. These patients were mostly diagnosed with anxiety disorder and might include a portion of patients with fibromyalgia. A recent review including information regarding the pharmacology and pharmacokinetics of duloxetine in the management of fibromyalgia suggested that this drug must be used with caution in patients with a bleeding risk and should be avoided in patients with bleeding tendencies when used to manage fibromyalgia.26 The findings that appear to associate SNRI use with a higher risk of intracranial hemorrhage but not ischemic stroke suggest that the increased sympathetic activation in combination with decreased serotonergic effect on platelet aggregation will synergistically impose a greater impact on intracranial hemorrhage, in particular among those with lower baseline cerebrovascular risks. However, the results from a subgroup analysis might be due to type I error arising from multiple comparison analyses and should be interpreted cautiously and conservatively.
In our study, we found that the drug switch rate was higher in SNRI users than SSRI users. Regarding the adverse effects of antidepressants, adults taking SNRIs were significantly more likely to have symptoms of nausea or vomiting, irritability, insomnia, dysuria in men, and cardiovascular responses compared with adults taking SSRIs.27 We speculated that the relatively high incidence rate of the norepinephrine-related side effects of SNRIs may partly contribute to the higher switch rate. The differential switching to other study drug between 2 comparison groups may bias the risk estimates associated with SNRIs toward the null. In this study, we conducted analyses with time-varying medication use in order to take study drug switching into consideration.
The strength of our study is the enrollment of a nationally representative cohort of a large sample size. We used a new-user design and restricted our study participants to antidepressant initiators with depressive or anxiety disorders to reduce confounding by indication. Furthermore, the use of covariates including underlying diseases, medication use, and health care utilization prior to antidepressant initiation as proxies for the presence or severity of cerebrovascular and psychiatric disorders was combined into a summary propensity score for confounding adjustment.
Some limitations of the study should be considered. First, residual confounding, such as the duration or severity of depressive and anxiety disorders and cardiovascular comorbidities, blood pressure levels, obesity, smoking, and physical inactivity, may potentially exist. However, our observation of a marginal increased risk of intracranial hemorrhage associated with SNRI use may underestimate the true risk, as SNRI initiators had a lower proportion of hypertension in our study population. Second, adherence to antidepressant therapy was low, and the treatment duration was short in Taiwan. Thus, we are not able to examine the long-term cerebrovascular effect of SSRI use. Third, this study only assessed risk in patients with depressive or anxiety disorders, and our findings may not be generalizable to those without these diagnoses. Fourth, growing evidence has attributed bleeding events to SSRI use, including spontaneous bleeding in the genitourinary, respiratory, and gastrointestinal tracts.28-31 Our analysis was not designed to detect a potential detrimental effect of concomitant use of SSRIs and antiplatelet agents or anticoagulants. Thus, the additive or synergic effects on the occurrence of intracranial hemorrhage should be further examined.32 Fifth, since both SSRIs and SNRIs are, to some extent, heterogeneous groups that contain individual drugs having various effects on neurotransmitter reuptake, it would be too complicated to compare the outcome differences between individual drugs. We therefore grouped these medications into SSRIs (paroxetine, sertraline, fluoxetine, citalopram, escitalopram, fluvoxamine) and SNRIs (venlafaxine, duloxetine, milnacipran). However, this grouping might underestimate the risk of either ischemic stroke or intracranial hemorrhage on some specific drugs. Future large-scale study focusing on individual SSRIs or SNRIs is needed to clarify the cerebrovascular effects of specific class of drugs. Sixth, even though we applied a time-varying Cox regression model to study the exposure and doses of study medications in the analysis, the doses of individual class of SNRIs used by patients in the clinical practice might not be pharmacodynamically equivalent to the SSRI doses in terms of effects on serotonin or norepinephrine reuptake. Our results may not fully reflect the pharmacodynamics effects of SNRI and SSRI on the central cerebrovascular system.
In conclusion, our results showed that the use of SNRIs was not associated with an increased risk of either ischemic stroke or intracranial hemorrhage compared to the use of SSRIs in adult patients with either depression or anxiety. However, SNRIs should be cautiously used in patients without depression due to the potential risk of intracranial hemorrhage.
Submitted: July 19, 2014; accepted January 5, 2015.
Drug names: citalopram (Celexa and others), digoxin (Lanoxin and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), milnacipran (Fetzima and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Author contributions: Study concept and design: Lee YC, Lin CH, Chang CH, Lin JW. Acquisition of data: Chang CH, Lin JW. Analysis and interpretation of data: Lin CH, Lee YC, Chang CH, Lin JW. Drafting of the manuscript: Lin CH, Lee YC, Chang CH, Lin JW. Critical revision of the manuscript for important intellectual content: Lin CH, Lin MS, Lin JW. Statistical analysis: Lu Y. Obtained funding: Chang CH. Study supervision: Lin JW, Chang CH.
Potential conflicts of interest: None reported.
Funding/support: This study was supported in part by the Taiwan National Science Council (grant NSC 101-2314-B-002 -127 -MY2).
Role of the sponsor: The sponsor played no role in the study design; collection, analysis, and interpretation of data; report writing; or the decision to submit the paper for publication.
Disclaimer: The corresponding authors have full access to all data from the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained here do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Acknowledgments: The authors thank the subjects who participated in the study.
Additional information: The Taiwan National Health Insurance (NHI) Database can be found at http://nhirdnew.nhri.org.tw/en/Data_Subsets.html. The NHI Database used in this study is maintained by The Collaboration Center of Health Information Application (CCHIA), Ministry of Health and Welfare, Executive Yuan, R.O.C. CCHIA was established to promote the application of value-added health information, for the purpose of enhancing statistics-based decision support and further capacity for academic research. Utilization and publicizing of any information by the CCHIA have been in accordance with The Freedom of Government Information Law, Personal Information Protection Act, and other related regulations.
Supplementary material: See accompanying pages.
REFERENCES
1. Kessler RC, Berglund P, Demler O, et al; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095-3105. PubMed doi:10.1001/jama.289.23.3095
2. Draper B, Berman K. Tolerability of selective serotonin reuptake inhibitors: issues relevant to the elderly. Drugs Aging. 2008;25(6):501-519. PubMed doi:10.2165/00002512-200825060-00004
3. Stahl SM, Grady MM, Moret C, et al. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10(9):732-747. PubMed doi:10.1017/S1092852900019726
4. Pan A, Sun Q, Okereke OI, et al. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306(11):1241-1249. PubMed doi:10.1001/jama.2011.1282
5. Goldstein LB, Bushnell CD, Adams RJ, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research; Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517-584.PubMed
6. Lee YC, Lin CH, Lin MS, et al. Effects of selective serotonin reuptake inhibitors versus tricyclic antidepressants on cerebrovascular events: a nationwide population-based cohort study. J Clin Psychopharmacol. 2013;33(6):782-789. PubMed doi:10.1097/JCP.0b013e31829c970e
7. Shin D, Oh YH, Eom CS, et al. Use of selective serotonin reuptake inhibitors and risk of stroke: a systematic review and meta-analysis. J Neurol. 2014;261(4):686-695. PubMed doi:10.1007/s00415-014-7251-9
8. Meijer WE, Heerdink ER, Nolen WA, et al. Association of risk of abnormal bleeding with degree of serotonin reuptake inhibition by antidepressants. Arch Intern Med. 2004;164(21):2367-2370. PubMed doi:10.1001/archinte.164.21.2367
9. Andrade C, Sandarsh S, Chethan KB, et al. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71(12):1565-1575. PubMed doi:10.4088/JCP.09r05786blu
10. Licht CM, Penninx BW, de Geus EJ. Effects of antidepressants, but not psychopathology, on cardiac sympathetic control: a longitudinal study. Neuropsychopharmacology. 2012;37(11):2487-2495. PubMed doi:10.1038/npp.2012.107
11. Fiedorowicz JG, He J, Merikangas KR. The association between mood and anxiety disorders with vascular diseases and risk factors in a nationally representative sample. J Psychosom Res. 2011;70(2):145-154. PubMed doi:10.1016/j.jpsychores.2010.07.010
12. Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(3):236-242. PubMed doi:10.1002/pds.2087
13. Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29(8):1602-1604. PubMed doi:10.1161/01.STR.29.8.1602
14. Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100-128. PubMed doi:10.1002/pds.2312
15. Hackam DG, Mrkobrada M. Selective serotonin reuptake inhibitors and brain hemorrhage: a meta-analysis. Neurology. 2012;79(18):1862-1865. PubMed doi:10.1212/WNL.0b013e318271f848
16. Tseng YL, Chiang ML, Huang TF, et al. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb Res. 2010;126(6):517-523. PubMed doi:10.1016/j.thromres.2010.09.017
17. Hallbפck I, Hפgg S, Eriksson AC, et al. In vitro effects of serotonin and noradrenaline reuptake inhibitors on human platelet adhesion and coagulation. Pharmacol Rep. 2012;64(4):979-983. PubMed doi:10.1016/S1734-1140(12)70894-0
18. Maschino F, Hurault-Delarue C, Chebbane L, et al; French Association of Regional Pharmacovigilance Centers. Bleeding adverse drug reactions (ADRs) in patients exposed to antiplatelet plus serotonin reuptake inhibitor drugs: analysis of the French Spontaneous Reporting Database for a controversial ADR. Eur J Clin Pharmacol. 2012;68(11):1557-1560. PubMed doi:10.1007/s00228-012-1268-8
19. Wernicke J, Lledó A, Raskin J, et al. An evaluation of the cardiovascular safety profile of duloxetine: findings from 42 placebo-controlled studies. Drug Saf. 2007;30(5):437-455. PubMed doi:10.2165/00002018-200730050-00007
20. Koschke M, Boettger MK, Schulz S, et al. Autonomy of autonomic dysfunction in major depression. Psychosom Med. 2009;71(8):852-860. PubMed doi:10.1097/PSY.0b013e3181b8bb7a
21. Licht CM, de Geus EJ, Seldenrijk A, et al. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53(4):631-638. PubMed doi:10.1161/HYPERTENSIONAHA.108.126698
22. Licht CM, de Geus EJ, van Dyck R, et al. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol Psychiatry. 2010;68(9):861-868. PubMed doi:10.1016/j.biopsych.2010.06.032
23. Abdelmawla AH, Langley RW, Szabadi E, et al. Comparison of the effects of nadolol and bisoprolol on the isoprenaline-evoked dilatation of the dorsal hand vein in man. Br J Clin Pharmacol. 2001;51(6):583-589. PubMed doi:10.1046/j.0306-5251.2001.01404.x
24. Siepmann T, Ziemssen T, Mueck-Weymann M, et al. The effects of venlafaxine on autonomic functions in healthy volunteers. J Clin Psychopharmacol. 2007;27(6):687-691. PubMed doi:10.1097/jcp.0b013e31815a255b
25. Shores MM, Pascualy M, Lewis NL, et al. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26(4):433-439. PubMed doi:10.1016/S0306-4530(01)00002-6
26. Scholz BA, Hammonds CL, Boomershine CS. Duloxetine for the management of fibromyalgia syndrome. J Pain Res. 2009;2:99-108. PubMed
27. Anderson HD, Pace WD, Libby AM, et al. Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin Ther. 2012;34(1):113-123. PubMed doi:10.1016/j.clinthera.2011.11.024
28. Turner MS, May DB, Arthur RR, et al. Clinical impact of selective serotonin reuptake inhibitors therapy with bleeding risks. J Intern Med. 2007;261(3):205-213. PubMed doi:10.1111/j.1365-2796.2006.01720.x
29. de Abajo FJ, Garc×a-Rodr×guez LA. Risk of upper gastrointestinal tract bleeding associated with selective serotonin reuptake inhibitors and venlafaxine therapy: interaction with nonsteroidal anti-inflammatory drugs and effect of acid-suppressing agents. Arch Gen Psychiatry. 2008;65(7):795-803. PubMed doi:10.1001/archpsyc.65.7.795
30. Labos C, Dasgupta K, Nedjar H, et al. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ. 2011;183(16):1835-1843. PubMed doi:10.1503/cmaj.100912
31. Targownik LE, Bolton JM, Metge CJ, et al. Selective serotonin reuptake inhibitors are associated with a modest increase in the risk of upper gastrointestinal bleeding. Am J Gastroenterol. 2009;104(6):1475-1482. PubMed doi:10.1038/ajg.2009.128
32. Löppönen P, Tetri S, Juvela S, et al. Association between warfarin combined with serotonin-modulating antidepressants and increased case fatality in primary intracerebral hemorrhage: a population-based study. J Neurosurg. 2014;120(6):1358-1363. PubMed doi:10.3171/2013.12.JNS131898
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Helen Lavretsky, MD, MS, at [email protected], or Gary W. Small, MD, at [email protected].
Please sign in or purchase this PDF for $40.00.
Save
Cite