Antiepileptic drugs (AEDs) are prescribed for approved and off-label indications that include epilepsy, bipolar disorder, and other neurologic and psychiatric disorders. The risk of major congenital malformations (MCMs) following gestational exposure to AEDs, particularly exposure at the time of conception and early trimester exposure, has been examined in many studies. This risk has been compared with the risk in unexposed general population controls as well as in untreated controls with the same treatment indication, and this risk has been compared pairwise among the AEDs. There is consistent evidence from conventional and network meta-analyses and from prospectively collected pregnancy registry data that early gestational exposure to valproate is associated with the highest risk of MCMs among the AEDs; the risk is around 10% and is dose-dependent. Furthermore, in pairwise comparisons that attenuate confounding by indication, valproate is significantly more teratogenic than most other AEDs. Phenobarbitone, phenytoin, carbamazepine, and topiramate are the other AEDs that are consistently associated with higher MCM risk relative to unexposed control groups and relative to certain other AEDs. Besides valproate (> 650 mg/d), phenobarbitone (> 80 mg/d) and carbamazepine (> 700 mg/d) are also associated with dose-dependent risks. In the AEDs associated with elevated risks, the extent to which the risks are due to confounding by indication is unknown. Importantly, confounding by indication notwithstanding, at conventional doses lamotrigine, levetiracetam, and oxcarbazepine, and possibly zonisamide and gabapentin, as well, are associated with absolute MCM risks that are no greater than the 2%-3% MCM risk in the general population.

ABSTRACT
Antiepileptic drugs (AEDs) are prescribed for approved and off-label indications that include epilepsy, bipolar disorder, and other neurologic and psychiatric disorders. The risk of major congenital malformations (MCMs) following gestational exposure to AEDs, particularly exposure at the time of conception and early trimester exposure, has been examined in many studies. This risk has been compared with the risk in unexposed general population controls as well as in untreated controls with the same treatment indication, and this risk has been compared pairwise among the AEDs. There is consistent evidence from conventional and network meta-analyses and from prospectively collected pregnancy registry data that early gestational exposure to valproate is associated with the highest risk of MCMs among the AEDs; the risk is around 10% and is dose-dependent. Furthermore, in pairwise comparisons that attenuate confounding by indication, valproate is significantly more teratogenic than most other AEDs. Phenobarbitone, phenytoin, carbamazepine, and topiramate are the other AEDs that are consistently associated with higher MCM risk relative to unexposed control groups and relative to certain other AEDs. Besides valproate (> 650 mg/d), phenobarbitone (> 80 mg/d) and carbamazepine (> 700 mg/d) are also associated with dose-dependent risks. In the AEDs associated with elevated risks, the extent to which the risks are due to confounding by indication is unknown. Importantly, confounding by indication notwithstanding, at conventional doses lamotrigine, levetiracetam, and oxcarbazepine, and possibly zonisamide and gabapentin, as well, are associated with absolute MCM risks that are no greater than the 2%-3% MCM risk in the general population.
J Clin Psychiatry 2018;79(4):18f12449
To cite: Andrade C. Major congenital malformations associated with exposure to antiepileptic drugs during pregnancy. J Clin Psychiatry. 2018;79(4):18f12449.
To share: https://doi.org/10.4088/JCP.18f12449
© Copyright 2018 Physicians Postgraduate Press, Inc.
Antiepileptic drugs (AEDs) such as valproate, lamotrigine, carbamazepine, topiramate, gabapentin, and others are prescribed in neurology and psychiatry for a wide range of approved and off-label indications, including epilepsy, bipolar disorder, migraine, pain syndromes, alcoholism, and other disorders. Some women with severe forms of epilepsy or bipolar disorder who are receiving effective treatment with 1 or more of these medications may opt to continue with treatment during pregnancy. Other women with these and other diagnoses may opt to discontinue treatment; however, by the time they discover their pregnancy, they may already have been exposed to the medications during the critical period of organogenesis. It is therefore important to know the nature and magnitude of the risk of major congenital malformations (MCMs) associated with AED exposure during pregnancy.
A large body of literature identifies valproate as possibly the most teratogenic drug in neuropsychiatry; strong regulatory guidance discourages the use of valproate in women of childbearing potential, more especially during pregnancy.1-4 Prescriptions for the other AEDs can now be expected to increase, especially in women of childbearing age.
What are the MCM risks after gestational exposure to the other AEDs relative to those in unexposed women in the general population and women with untreated epilepsy? And what are the MCM risks of these drugs relative to each other? This article summarizes important findings from 2 recent meta-analyses and a large prospective study on the subject.
Meta-Analysis: Teratogenicity of Antiepileptic Drugs in Monotherapy
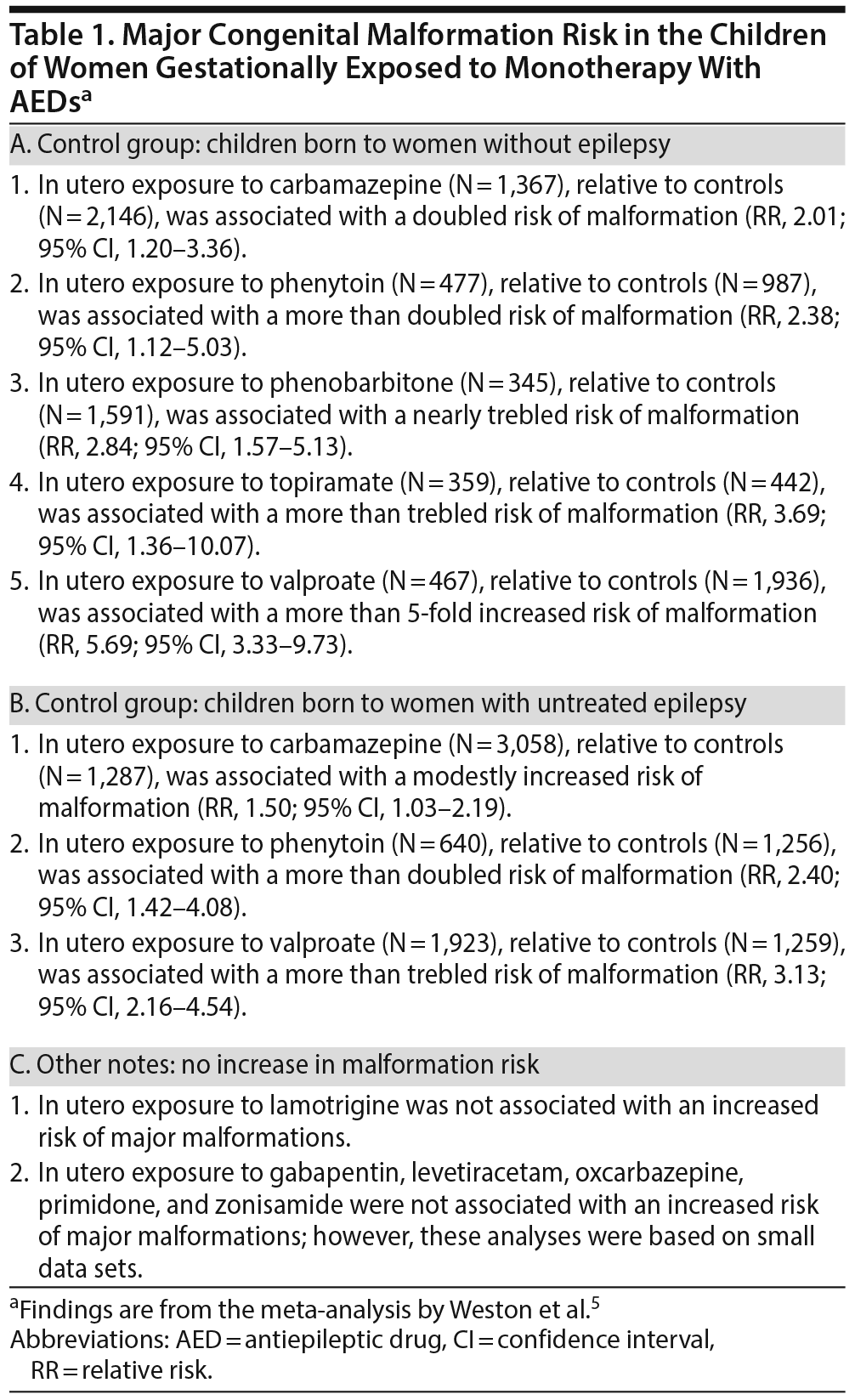
Weston et al5 described a systematic review and meta-analysis of data on MCMs in the offspring of mothers who received monotherapy with AEDs to treat epilepsy during pregnancy. The authors5 searched electronic databases and other sources and identified 50 observational studies of relevance, of which 31 contributed data for meta-analysis.
The MCM risks associated with gestational exposure to individual AEDs were compared with the risks in 2 unexposed control groups: women without epilepsy and women with untreated epilepsy; important findings are presented in Table 1. In short, in women with epilepsy, valproate, topiramate, phenytoin, phenobarbitone, and carbamazepine each significantly increased the malformation risk relative to controls without epilepsy, and valproate, phenytoin, and carbamazepine each significantly increased the malformation risk relative to controls with untreated epilepsy. Lamotrigine was not associated with an increased MCM risk. In underpowered analyses based on small data sets, gabapentin, levetiracetam, oxcarbazepine, primidone, and zonisamide were also not associated with increased risk.
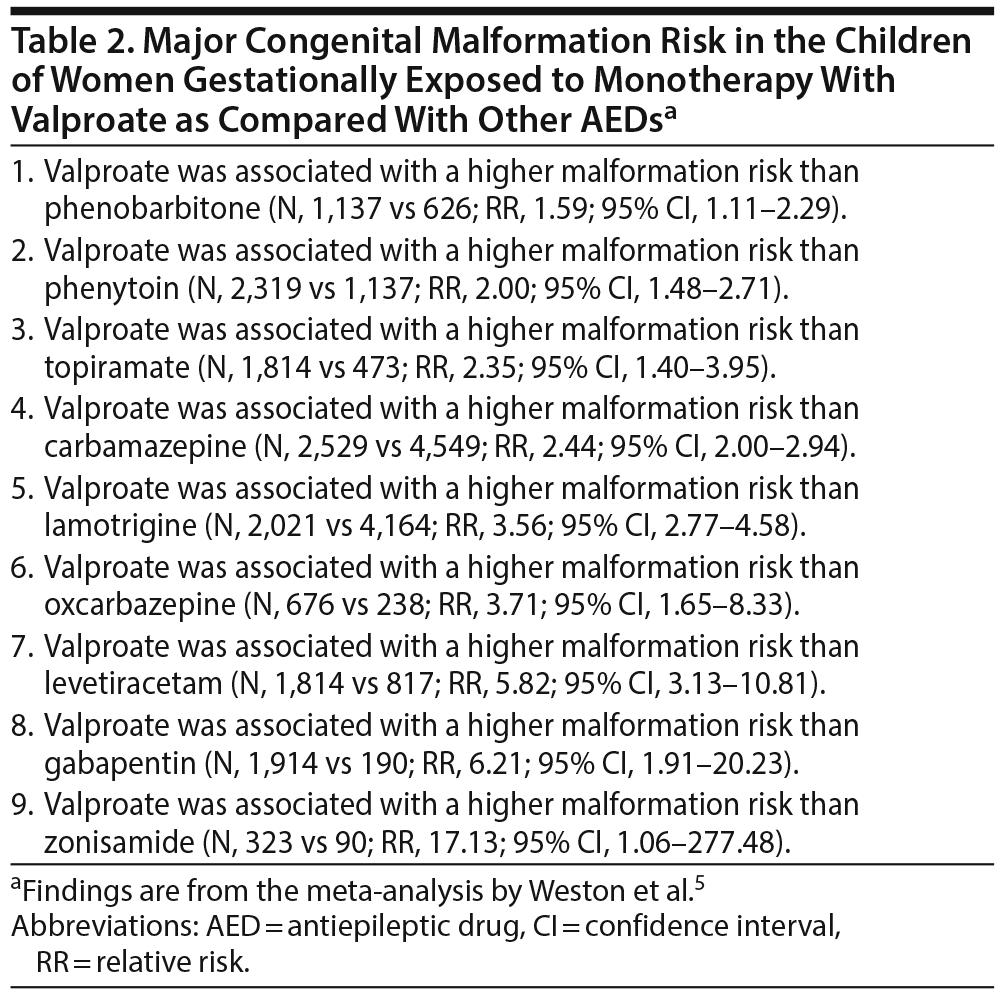
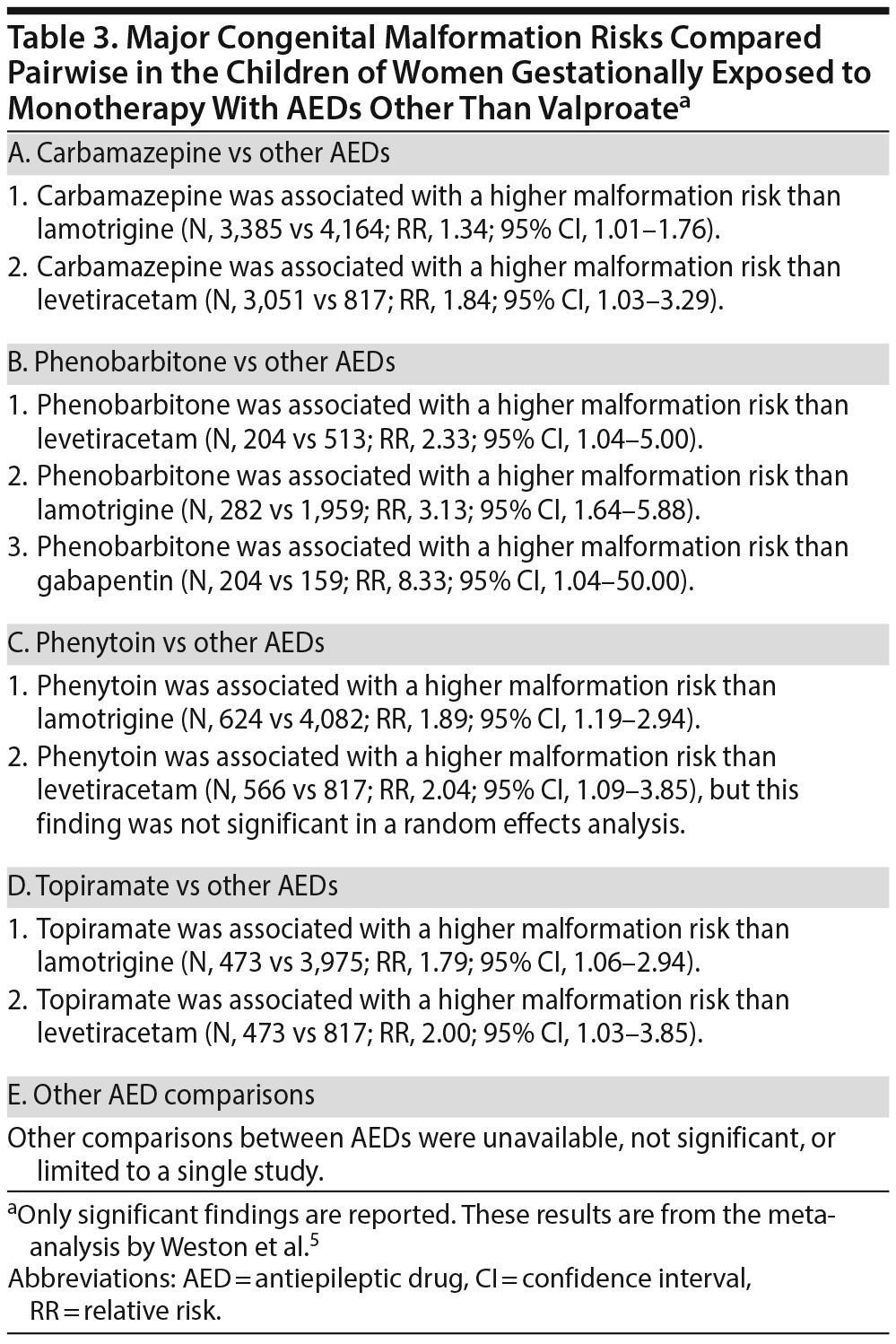
Tables 2 and 3 present the results of pairwise comparisons among the AEDs. In short, valproate was associated with a significantly higher malformation risk than 9 other AEDs: phenobarbitone, phenytoin, topiramate, carbamazepine, lamotrigine, oxcarbazepine, levetiracetam, gabapentin, and zonisamide. Carbamazepine, phenobarbitone, phenytoin, and topiramate were each associated with a higher malformation risk than lamotrigine and levetiracetam. Phenobarbitone was also associated with a higher risk than gabapentin.
Among the AEDs, valproate was associated with the highest risk of malformation (10.93%; 95% CI, 8.91-13.13). With regard to specific malformations, as compared with other AEDs, phenobarbitone was associated with a higher risk of cardiac malformations, and valproate was associated with a higher risk of neural tube, cardiac, orofacial/craniofacial, and skeletal and limb malformations. Whereas valproate exposure was associated with a dose-dependent risk of MCMs, it was not clear whether the risk was dose-dependent with the other AEDs.
Of note, because all the studies were observational, inherent biases, such as those related to confounding by indication, were inevitable. Weston et al5 considered that the biases were similar for all the AEDs and therefore did not favor or discriminate against any individual AED. This, actually, is not true for several reasons: different AEDs are prescribed for different forms and severities of epilepsy; some AEDs, depending on popular perception of safety, may be more likely to be continued or stopped during pregnancy than other AEDs; and AEDs in general (and some AEDs in particular) may be more likely to be continued during pregnancy in more severe forms of epilepsy. Therefore, the findings are best considered indicative rather than conclusive. Nevertheless, it is reasonable to accept that confounding by indication is reduced in comparisons between AEDs.
Network Meta-Analysis: Teratogenicity of Antiepileptic Drugs in Monotherapy and Polytherapy
A more recent meta-analysis6 has now become available. This is a Bayesian random effects network meta-analysis; it examined both direct and indirect comparisons between 14 AEDs. It also examined outcomes with AED polytherapy.
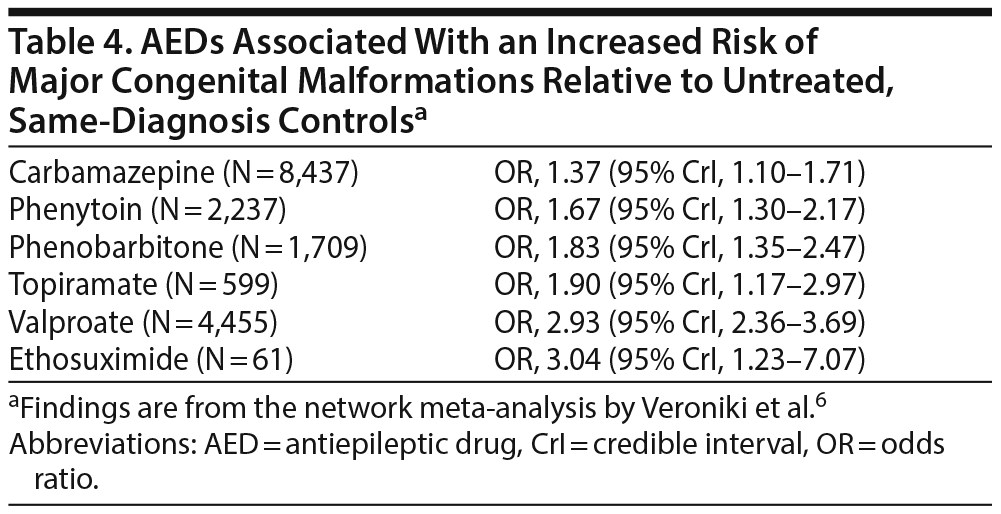
The authors6 searched electronic databases, reference lists, and other sources and identified 75 cohort studies, 2 case-control studies, and 1 randomized controlled trial that met their selection criteria; the pooled sample size was 35,016. In this meta-analysis, MCM outcomes after gestational exposure to AEDs were compared across AEDs as well as with untreated controls with the same diagnosis. The AEDs studied were carbamazepine, clobazam, clonazepam, ethosuximide, phenobarbital, phenytoin, primidone, valproate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, topiramate, and vigabatrin.
Table 4 presents the risk of MCMs after gestational exposure to AED monotherapy relative to unexposed controls with the same diagnosis. In short, carbamazepine, phenytoin, phenobarbitone, topiramate, valproate, and ethosuximide were each associated with a significant increase in risk; risks with gabapentin, lamotrigine, and levetiracetam did not reach statistical significance. The authors also examined risks associated with AED combinations; these risks were significant for some combinations but not for others. It is very likely that the analyses were underpowered for the combinations with nonsignificant risks given that many of these combinations included drugs that were found to be teratogenic in monotherapy.
The findings of the network meta-analysis were mostly consistent in subgroup and sensitivity analyses.
Prospective Findings From the EURAP Registry
The network meta-analysis6 summarized above included early data from the EURAP registry. Current data from the registry have now become available7 and are summarized here.
EURAP is an international registry that presently includes > 1,500 collaborators in 42 countries. EURAP was established in 1999. It prospectively collects data that assess risks associated with AED exposure during pregnancy. In this connection, Tomson et al7 examined data from 7,355 pregnancies in 6,393 women with epilepsy who had been exposed to AED monotherapy at the time of conception.
The mean age of the sample was 29.8 years. Recruitment occurred at a mean of 8 weeks of gestation. Most of the recruitment was from Europe (87%) and the Western Pacific region (9%). Most women had had either no (59%) or 1 (32%) previous pregnancy. The indication for AED treatment was classified as idiopathic generalized epilepsy (39%), localization-related epilepsy (50%), and undetermined or unclassifiable epilepsy (11%). Only 7% of women had generalized tonic-clonic seizures during the first trimester. Only 38% of women had appropriate folic acid intake, defined as intake for 3 months prior to conception and at a dose of at least 0.4 mg/d during the first trimester.
Follow-up data were obtained at the end of each trimester, at the time of birth, and 1 year after birth. MCMs recorded by the last (1-year postnatal) time point were examined in 7,355 offspring who had been exposed to 1 of 8 common AEDs: lamotrigine (25-1,300 mg/d; n = 2,514), carbamazepine (50-2,400 mg/d; n = 1,957), valproate (100-3,000 mg/d; n = 1,381), levetiracetam (250-4,000 mg/d; n = 599), oxcarbazepine (75-4,500 mg/d; n = 333), phenobarbitone (15-300 mg/d; n = 294), topiramate (25-500 mg/d; n = 152), and phenytoin (30-730 mg/d; n = 125). Analyses were adjusted for potential confounders and prognostic factors.
The overall prevalence of MCMs was 5.3%; cardiac malformations (1.4%) and hypospadias (0.6%) were the commonest MCMs. Strikingly, only 19.8% of the 383 MCMs had been detected antenatally; whereas 64.8% were identified within 2 months of birth, the remaining 15.4% were identified only between age 2 months and 1 year of life.
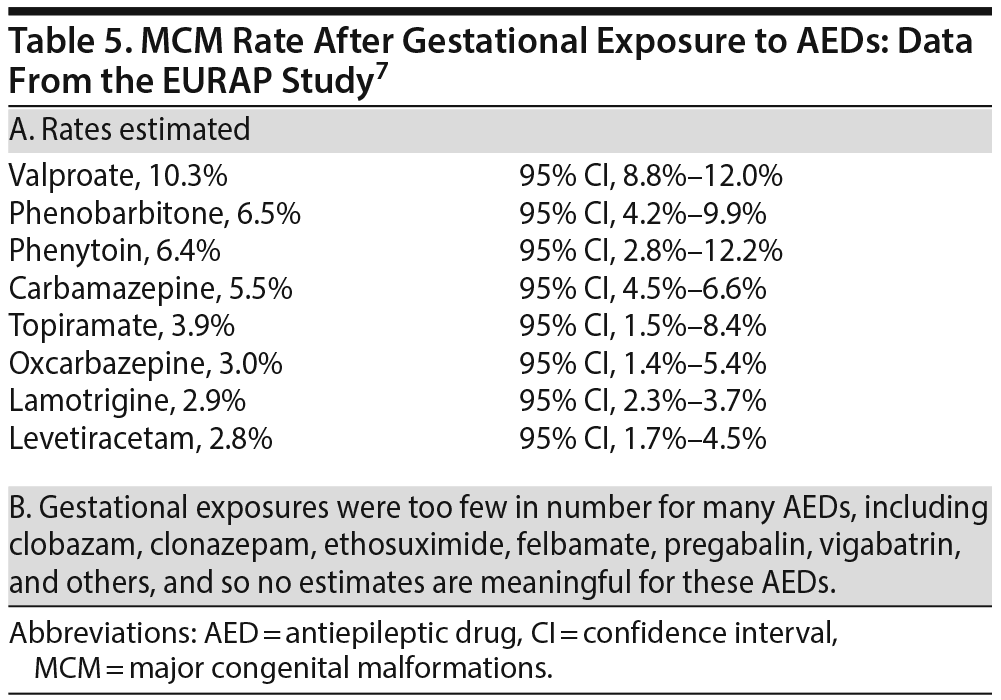
The rate of MCMs for individual drugs is presented in Table 5. Valproate was associated with the highest risk (10.3%). Other drugs associated with a > 3% risk were phenobarbitone, phenytoin, carbamazepine, and topiramate. Oxcarbazepine, lamotrigine, and levetiracetam were associated with risks in the 2%-3% range, which is widely accepted as the MCM risk in the general population.
A statistically significant dose-dependent MCM risk was observed for carbamazepine (> 700 mg/d), lamotrigine (> 325 mg/d), phenobarbitone (> 80 mg/d), and valproate (> 650 mg/d). At a valproate dose of > 1,450 mg/d, there were 29 MCMs in 115 exposed pregnancies (prevalence, 25.2%; 95% CI, 17.6%-34.2%). Note that the identification of a dose-dependent risk does not indict the drug; dose dependence in observational studies may be due to confounding by indication because higher doses are likely to be preferred in more severely ill patients.
In multivariate analyses, carbamazepine (all doses), valproate (all doses), and phenobarbitone (> 80 mg/d) were each associated with significantly higher MCM risks than lamotrigine (325 mg/d and below). Other dose-dependent risks were also identified for valproate and carbamazepine, relative to levetiracetam.
Besides the large sample size and the prospective ascertainment of data, a strength of the EURAP study7 is that it only examined data in women who had been recruited at a time when the fetal outcome was unknown; this reduces the recruitment bias. An important limitation is that, as with other studies in the field, this was an observational study in which women had not been randomized to their respective treatments.
General Summary
Evidence from meta-analyses and from prospectively collected pregnancy registry data suggests that valproate is associated with the highest risk of MCMs following early gestational exposure; the risk is around 10% and is dose-dependent. Valproate is also associated with a significantly higher risk of malformations than most other AEDs. Other AEDs associated with elevated risks relative to control groups and relative to other AEDs include phenobarbitone, phenytoin, carbamazepine, and topiramate. Besides valproate, phenobarbitone, carbamazepine, and lamotrigine are also associated with dose-dependent risks; however, the finding of MCM risk in association with high dose lamotrigine emerged from only 1 study, may have been due to confounding by indication, and requires examination in future studies. In general, at the usual therapeutic doses, lamotrigine, levetiracetam, and oxcarbazepine, and perhaps zonisamide and gabapentin, as well, appear to be associated with MCM risks that are within the range observed in the general population. In the drugs associated with elevated risk, the extent to which the risks are due to confounding by indication is unknown.
Published online: July 17, 2018.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C. Valproate in pregnancy: recent research and regulatory responses. J Clin Psychiatry. 2018;79(3):18f12351. PubMed CrossRef
2. Grover S, Avasthi A. Mood stabilizers in pregnancy and lactation. Indian J Psychiatry. 2015;57(suppl 2):S308-S323. PubMed CrossRef
3. Balon R, Riba M. Should women of childbearing potential be prescribed valproate? a call to action. J Clin Psychiatry. 2016;77(4):525-526. PubMed CrossRef
4. Gotlib D, Perelstein E, Kurlander J, et al. Guideline adherence for mentally ill reproductive-aged women on treatment with valproic acid: a retrospective chart review. J Clin Psychiatry. 2016;77(4):527-534. PubMed CrossRef
5. Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. PubMed
6. Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017;15(1):95. PubMed CrossRef
7. Tomson T, Battino D, Bonizzoni E, et al; EURAP Study Group. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018;17(6):530-538. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top