Objective: The quantification of antipsychotic levels in blood, also known as therapeutic drug monitoring (TDM), is a potentially useful tool of modern personalized therapy that can be applied to augment antipsychotic use and dosing decisions. The application of TDM for antipsychotics can be helpful in numerous challenging clinical scenarios, such as lack of therapeutic response, relapse, or adverse drug reactions (ADRs) related to antipsychotic treatment. The benefits of TDM may be particularly evident in the treatment of highly vulnerable patient subgroups, such as children, adolescents, pregnant women, and the elderly. The main aim of this article is to aid clinicians who routinely prescribe antipsychotics to successfully apply TDM in routine clinical practice in order to help optimize the efficacy and safety of those antipsychotics.
Participants: Participants were clinicians and researchers, members of the American Society of Clinical Psychopharmacology, and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (Association of Neuropsychopharmacology and Pharmacopsychiatry).
Evidence: TDM literature on antipsychotics was critically reviewed to provide a condensed clinical decision-making algorithm with therapeutic reference ranges for blood antipsychotic levels, within which patients are most likely to respond and tolerate treatment, although TDM is not equally recommended/supported for all antipsychotics.
Consensus Process: A preliminary draft was prepared and circulated to the writing group members. Consensus was achieved in all cases, and resulting recommendations focused on following areas: steady-state and sampling time, levels of recommendations, indications, therapeutic reference ranges and laboratory alert levels, practical issues, and interpretation, as well as limitations.
Conclusions: The utilization of TDM as a tool for problem solving in antipsychotic treatment offers a unique method to improve safety and efficacy. This consensus statement summarizes essential information on the routine use of TDM for antipsychotics and encourages clinicians to perform TDM with the appropriate indications as part of the clinical decision-making process.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
• Use therapeutic drug monitoring with appropriate indications as part of the clinical decision-making process to help optimize the efficacy and safety of antipsychotics
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in May 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through June 30, 2022. The latest review of this material was April 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Objective: The quantification of antipsychotic levels in blood, also known as therapeutic drug monitoring (TDM), is a potentially useful tool of modern personalized therapy that can be applied to augment antipsychotic use and dosing decisions. The application of TDM for antipsychotics can be helpful in numerous challenging clinical scenarios, such as lack of therapeutic response, relapse, or adverse drug reactions (ADRs) related to antipsychotic treatment. The benefits of TDM may be particularly evident in the treatment of highly vulnerable patient subgroups, such as children, adolescents, pregnant women, and the elderly. The main aim of this article is to aid clinicians who routinely prescribe antipsychotics to successfully apply TDM in routine clinical practice in order to help optimize the efficacy and safety of those antipsychotics.
Participants: Participants were clinicians and researchers, members of the American Society of Clinical Psychopharmacology, and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (Association of Neuropsychopharmacology and Pharmacopsychiatry).
Evidence: TDM literature on antipsychotics was critically reviewed to provide a condensed clinical decision-making algorithm with therapeutic reference ranges for blood antipsychotic levels, within which patients are most likely to respond and tolerate treatment, although TDM is not equally recommended/supported for all antipsychotics.
Consensus Process: A preliminary draft was prepared and circulated to the writing group members. Consensus was achieved in all cases, and resulting recommendations focused on following areas: steady-state and sampling time, levels of recommendations, indications, therapeutic reference ranges and laboratory alert levels, practical issues, and interpretation, as well as limitations.
Conclusions: The utilization of TDM as a tool for problem solving in antipsychotic treatment offers a unique method to improve safety and efficacy. This consensus statement summarizes essential information on the routine use of TDM for antipsychotics and encourages clinicians to perform TDM with the appropriate indications as part of the clinical decision-making process.
J Clin Psychiatry 2020;81(3):19cs13169
To cite: Schoretsanitis G, Kane JM, Correll CU, et al. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169.
To share: https://doi.org/10.4088/JCP.19cs13169
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, and Center for Psychiatric Neuroscience, Feinstein Institute for Medical Research, Manhasset, New York
bDepartment of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
cCharité Universitפtsmedizin, Department of Child and Adolescent Psychiatry, Berlin, Germany
dPsychiatry and Biobehavioral Sciences, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, California
eDepartment of Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York
fThriving Mind South Florida Behavioral Health Network, Miami, Florida; and Department of Psychiatry, Washington University School of Medicine, St Louis, Missouri
gDepartment of Psychiatry, NYU Langone Medical Center, New York, and Nathan Kline Institute, Orangeburg, New York
hDepartment of Psychiatry, University of Maryland School of Medicine, Baltimore, Maryland
iDepartment of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
jSection on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, Division of Intramural Clinical and Biological Research, National Institute on Alcohol Abuse and Alcoholism, and Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland
kAlexianer Hospital Aachen; and Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University; and JARA-Translational Brain Medicine, Aachen, Germany
lServizio Psichiatrico del Comprensorio Sanitario di Bolzano, Bolzano, Italy
mExperimental Psychiatry Unit, Department of Psychiatry and Psychotherapy, Medical University of Innsbruck, Innsbruck, and Private Practice for Psychotherapy and Court-Certified Witness, Hall in Tirol, Austria
nClinical Pharmacology, Department of Psychiatry and Psychotherapy, and Department of Pharmacology and Toxicology, University of Regensburg, Regensburg, Germany
oDepartment of Psychiatry, University of Lausanne, Prilly-Lausanne, Switzerland
pDepartment of Psychiatry and Psychotherapy and Institute of Clinical Chemistry and Laboratory Medicine, University Medical Center of Mainz, Mainz, Germany
qDepartment of Molecular Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
rMembers of the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie are listed at the end of the article.
*Corresponding author: Georgios Schoretsanitis, MD, PhD, Department of Psychiatry Research, The Zucker Hillside Hospital, Northwell Health, 75-59 263rd St, Glen Oaks, NY 11004 ([email protected]).
Measurement of drug levels in blood provides valuable insight in several areas of medicine including oncology, immunology, infectious diseases, clinical toxicology, pediatrics, and psychiatry. Specifically, in psychopharmacotherapy there is an established role for therapeutic drug monitoring (TDM) for antidepressants and mood stabilizers, such as lithium, valproate, and carbamazepine.1,2 Additionally, the quantification of antipsychotic levels in blood allows tailoring the dosage of drugs based on patients’ individual pharmacokinetic patterns.3,4 Implementation of precision medicine tools like TDM in the pharmacologic treatment of patients with severe mental disorders may help overcome suboptimal efficacy and/or tolerability related to antipsychotic treatment.5 TDM may be particularly valuable in the management of specific subgroups, such as children and adolescents, pregnant women, and the elderly, as well as patients with intellectual disabilities, patients with substance use disorders, and forensic patients.6 Regardless of patient profiles, specific indications for TDM include poor treatment response within the therapeutic dose ranges,7 evaluation of drug adherence, tolerability issues, and drug-drug interactions.5 Evidence in support of the utility of TDM as part of routine pharmacotherapy is increasingly available for the majority of the widely prescribed antipsychotic agents,8 although few trials have been performed directly comparing outcomes between TDM and treatment as usual. The aim of this consensus statement is to guide clinicians in the application of TDM as part of antipsychotic treatment to improve therapeutic outcomes. Specifically, the use of TDM is particularly meaningful in the context of prevention and treatment of several antipsychotic-related adverse drug reactions (ADRs).9-11 Clinicians further interested in TDM are encouraged to read previous work of the TDM task force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP; in English, Association of Neuropsychopharmacology and Pharmacopsychiatry),6,12,13 which provides details on the theoretical framework for the use of TDM in clinical practice enhancing the efficacy and safety of pharmacotherapy. The latest update of the AGNP consensus is publicly available at https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0043-116492.
METHODS
2.1. Participants, Evidence, and Consensus Process
Drawing on prior work by the TDM task force of the Association of Neuropsychopharmacology and Pharmacopsychiatry (AGNP),6,12,13 this group of authors reviewed the relevant literature on TDM, applying basic principles to the prescribing of antipsychotics and focusing on the practical aspects of enhanced antipsychotic prescribing via appropriate use of TDM. A preliminary draft was prepared and circulated to the members of the writing group. Revision suggestions were received, and the manuscript was revised accordingly. Consensus was achieved in all cases. Following a brief review of the theoretical framework of TDM, the resulting recommendations for clinical care are centered around the following areas: steady-state and sampling time, levels of recommendations, indications, therapeutic reference ranges and laboratory alert levels, practical issues, and interpretation, as well as limitations.

- There is an urgent need for improvement of treatment response and safety for antipsychotic treatment.
- Measuring blood levels of antipsychotics, also known as therapeutic drug monitoring (TDM), in several patient subgroups and under appropriate indications can efficiently guide clinicians in clinical routine.
- This work provides a framework for the implementation of TDM as part of antipsychotic treatment.
2.2. TDM for Antipsychotics: Theoretical Framework
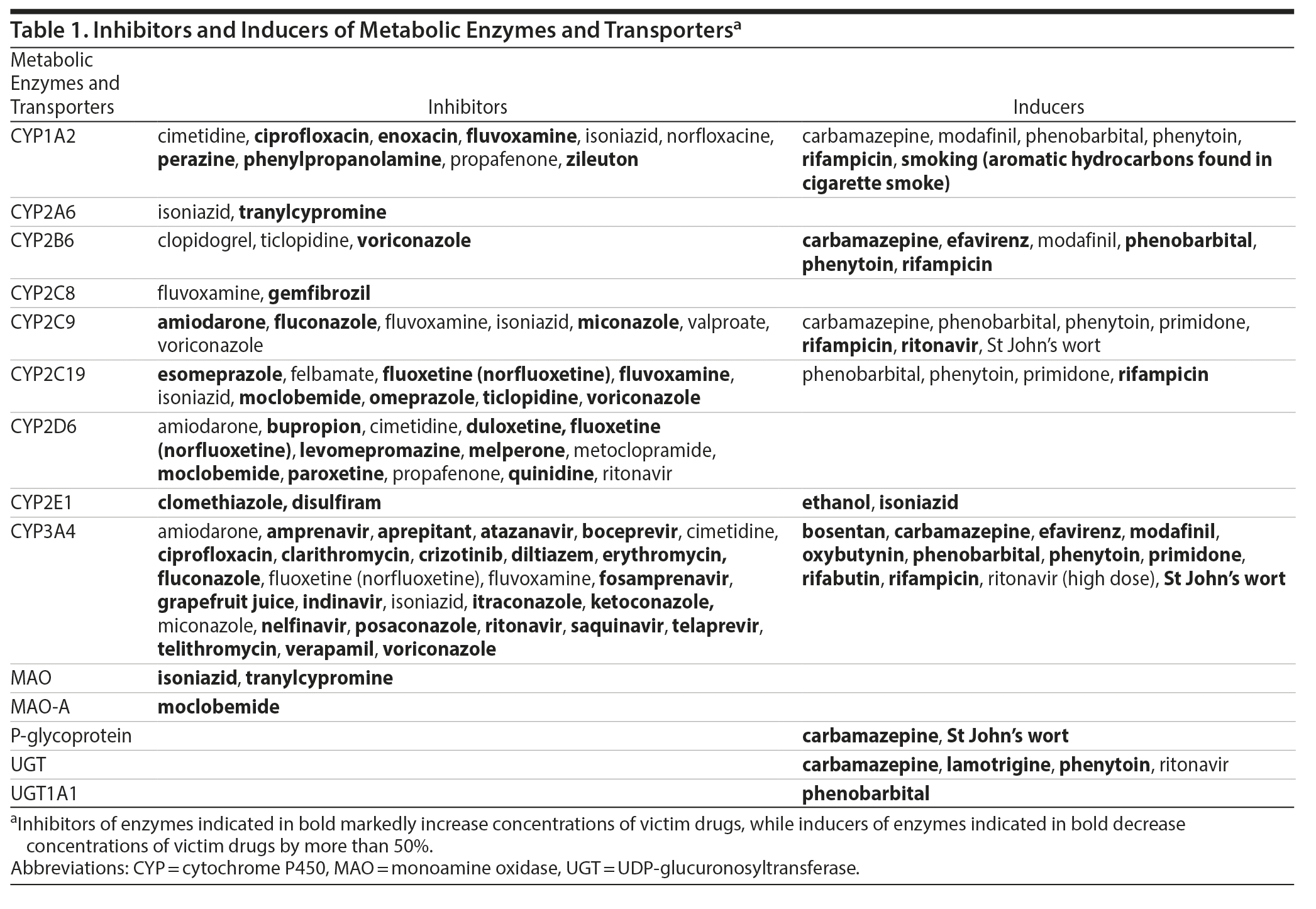
In the past decades, the development of TDM science has been mainly based upon the increasing knowledge of the pharmacokinetic/pharmacodynamic (PK/PD) target attainment. Pharmacokinetic pathways have received attention with the identification of enzymes and efflux transporters mediating the metabolism and distribution of antipsychotics. Particularly, the cytochrome P450 (CYP) isoenzyme system has been embraced as a major source of pharmacokinetic variability of medications, including antipsychotics, with 4 CYP enzymes being involved in the phase 1 metabolism of the majority of antipsychotics (CYP1A2, CYP2C19, CYP2D6, and CYP3A4).14 As regards the so-called polymorphic isoenzymes (CYP2C19, CYP2D6), their activity is crucially determined by genetic variants,15 which are increasingly tested in clinical routine with the integration of pharmacogenetic tests in clinical practice.16 Genetic variants of crucial impact also encompass genes encoding blood-brain barrier (BBB) transporters, such as the ABC transporters, including P-glycoprotein.10 While TDM can circumvent the effects of variations in metabolism related to transporters in the gut, it does not overcome variability in bioavailability related to transporters that modify passage across the BBB. Other determinants of blood antipsychotic levels include interactions with comedications from similar or different pharmacologic classes.17 Numerous medications exert inhibiting or inducing effects on drug metabolizing enzymes or transporters and can ultimately alter the metabolism and blood levels of concomitantly administered antipsychotics when affecting overlapping metabolic pathways. Therefore, the interpretation of blood antipsychotic concentrations requires information on the coprescribed medications with inducing or inhibiting properties, also known as “perpetrator” drugs. Table 1 provides a list of metabolic pathway inducers and inhibitors. Interactions at a pharmacodynamic level comprise more complicated challenges for clinicians prescribing antipsychotics and have not yet been sufficiently integrated into TDM.18
RESULTS
3.1. TDM for Antipsychotics: Steady-State and Sampling Time
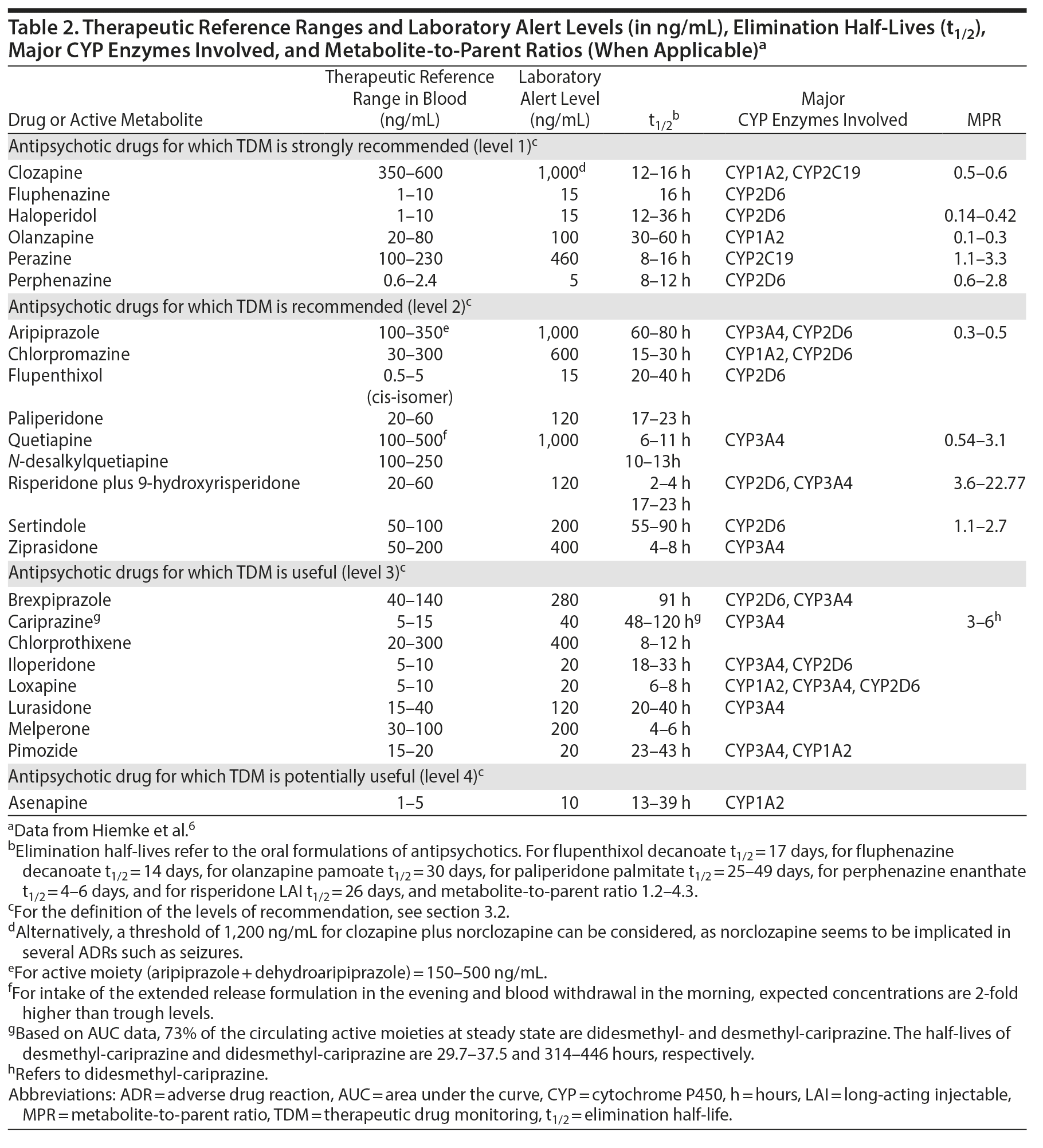
In the area of pharmacokinetics, the term steady-state concentration for a drug in blood refers to the time point when the rate of medication input is equal to the rate of medication loss. Practically, the time to reach steady state is approximately 4-5 times the elimination half-life (t1/2) of the antipsychotic (see Table 2), and, thus, steady state is reached within 1 week of maintenance dosing for the majority of antipsychotics. Exceptions are compounds with a particularly long half-life, such as aripiprazole, brexpiprazole, cariprazine (and its principal active metabolite, didesmethyl-cariprazine), and sertindole. During stable dosing, the appropriate sampling time is immediately before intake of the morning dose or, in other words, 24 hours after the last dose, if the antipsychotic is prescribed once daily in the morning. For antipsychotics prescribed to be taken in the evening, the blood draw interval is 12 hours.
The pharmacokinetics of long-acting injectable antipsychotics (LAIs) differ from their oral formulations. In LAI-treated patients, the appropriate sampling time is immediately before the next injection. Peak concentrations in blood are reached within 1-14 days after the LAI injection, and the apparent t1/2 is about 2-3 weeks for first-generation LAIs, as well as for paliperidone palmitate once-monthly.19,20 The maximal plasma concentrations for the 3-monthly paliperidone palmitate are reached between days 30 and 33, with median half-lives ranging between 84-95 and 118-139 days for deltoid and gluteal injection, respectively.6 For risperidone LAI microspheres, peak concentrations are reached after 4 weeks, and its t1/2 is 4-6 days.19 A newly available risperidone subcutaneous monthly injection displays different pharmacokinetic characteristics; single-injection data reported 2 plasma peaks, the first at 4-6 hours and the second at 10 to 14 days, with a half-life of 9-11 days.21 For aripiprazole monohydrate, LAI peak levels are reached 5-7 days after injection, with the apparent t1/2 after 400 or 300 mg aripiprazole monthly being 47 and 30 days, respectively.22 Aripiprazole lauroxil is another aripiprazole formulation that differs in terms of pharmacokinetics in that it has a substantially longer half-life ranging between 53.9 and 57.2 days, with maximum concentrations reached 20-34 days after last injection.23
3.2. TDM for Antipsychotics: Levels of Recommendations
For several antipsychotics, therapeutic reference ranges have been established based on their association with treatment response in clinical trials with sufficient reliability such that TDM can be strongly recommended and supported by data. Specificity and sensitivity vary between drugs and between trials, but the evidence from positron emission tomography has been very helpful when defining thresholds.6 For example, the reference range for clozapine has been based on 1 landmark prospective trial that randomized patients to 3 different blood level ranges of clozapine and provided rigorous data validating TDM.24 For clozapine and some other antipsychotics, including fluphenazine, haloperidol, olanzapine, perazine, and perphenazine, TDM is recommended for titration to target dose, as well as for special indications (Table 2). Specifically, blood antipsychotic concentrations within the therapeutic range are linked to favorable clinical outcomes in terms of efficacy, such as response, remission, and relapse prevention, as well as safety and tolerability. For a second group of antipsychotics, including aripiprazole, chlorpromazine, flupenthixol, paliperidone, quetiapine, risperidone, sertindole, and ziprasidone, TDM is recommended with a lower level of clinical confidence (level 2 recommendation), due to fewer data linking therapeutic blood level ranges to either benefit or harm.8,25 Nevertheless, TDM will increase the probability of response or remission in nonresponders, in that subtherapeutic blood antipsychotic concentrations may be associated with risk of poor response, whereas supratherapeutic concentrations may be related to an increased risk of intolerance or adverse effects.8,10,26-30 Evidence is less supportive for TDM application for a third group of antipsychotics, such as brexpiprazole, cariprazine, chlorprothixene, iloperidone, loxapine, lurasidone, melperone, and pimozide, where the link between blood antipsychotic concentrations and clinical effects has not been addressed yet or only in a retrospective fashion or in single case reports.31-36 For this group of antipsychotics, TDM can still potentially be useful, but more data are needed. Lastly, for asenapine, no evidence associating clinical effects and blood concentrations is available, and, thus, TDM is only considered potentially useful. It is important to emphasize that, as discussed below, some very strong indications for TDM do not necessarily depend on the definitive establishment of a therapeutic dose range. For example, in the patient experiencing a relapse of psychotic symptoms, the absence of a detectable blood level is very important information that can have a strong influence on clinical decision-making.
3.3. TDM for Antipsychotics: Indications
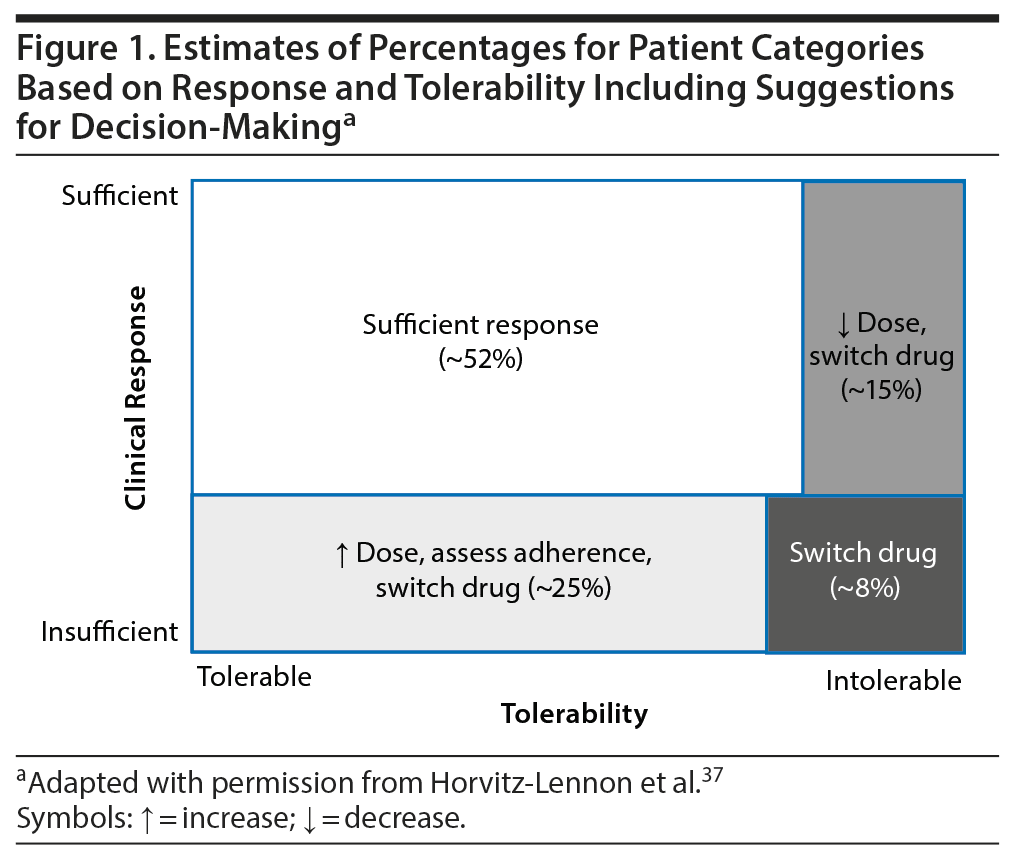
Regardless of recommendation levels for any specific antipsychotic, TDM is strongly recommended for a series of specific indications. These indications reflect challenging clinical situations for which the availability of evidence supporting TDM varies from case reports to clinical trials. However, absence of evidence is not evidence of absence. Large-scale evidence from patients with chronic schizophrenia has shown that the treatment of almost the half of the patients will dictate additional actions apart from dose titration, such as change of target dose or switching the antipsychotic.37 Instead of applying a so-called trial-and-error approach, TDM can provide valuable information for the decision-making process (Figure 1).37
TDM may be valuable in the following situations:
- Uncertain adherence to antipsychotics38
- No clinical response within established therapeutic dose ranges8
- Symptom recurrence or relapse during maintenance treatment39
- Adverse drug reactions (ADRs)9
- Combination treatment with medication(s) with inducing or inhibiting properties40
- Genetic peculiarities for the pathways involved in the metabolism of antipsychotics (the prevalence of specific genetic variants affecting drug metabolism may vary highly in different ethnic groups, eg, Caucasian versus Asian, middle Eastern vs the rest of the world)41
- Patients with abnormally high or low body weight or body mass index42
- Pregnant or lactating patients43
- Child or adolescent patients44
- Elderly patients6
- Patients with intellectual disabilities45
- Forensic patients or court mandated individuals,46 as the treatment of this patient subgroup presents special challenges that underscore the need for tracking and mitigating nonadherence
- Patients with pharmacokinetically relevant comorbidities, such as hepatic or renal dysfunction and severe cardiovascular disease (affecting hepatic and renal blood flow)47,48
- Patients with acute or chronic inflammatory conditions and infections49
- Postoperative care for patients undergoing restrictive gastrointestinal resection or bariatric surgery50
- Switching between the original preparation and generic forms of antipsychotics due to potential therapeutic equivalence differences, as well as related adherence aspects51,52
- Switching between oral antipsychotics and LAIs53
- Pharmacovigilance programs54
- Research55
3.4. TDM for Antipsychotics: Therapeutic Reference Ranges and Laboratory Alert Levels
The therapeutic reference range for antipsychotic levels in blood consists of a lower limit, below which therapeutic response is relatively unlikely, and an upper limit, above which ADRs, especially extrapyramidal symptoms or seizures, but also (depending on the nondopaminergic properties of the specific antipsychotic) other dose-dependent ADRs such as sedation, hypersalivation, dizziness/orthostasis, QTc prolongation, are more likely to occur56,57 or above which it is relatively unlikely that therapeutic response will occur. Reference ranges have been invariably obtained from studies of patients receiving antipsychotic monotherapy at presumed therapeutically effective dosages. However, clinicians need to bear in mind that ranges are an orienting, population-based tool that may not always be applicable to every patient. For example, some patients may respond despite having blood antipsychotic levels below the therapeutic reference range, while others may respond only at levels above the therapeutic reference range and tolerate those blood levels without ADRs. In fact, TDM can assist clinicians in identifying the individual’s unique therapeutic concentrations when measured regularly. For example, since only a blood level of zero clearly indicates complete nonadherence, it may indeed be useful within a measurement-based framework to obtain a blood antipsychotic level at an efficacious and well-tolerated ingested target dose in order to have a benchmark of the dose-concentration-efficacy-tolerability relationship for the individual patient. This way, partial nonadherence or variations in blood antipsychotic levels due to, among others, drug-drug interactions, intercurrent illness, or changes in liver or renal functioning can be detected and rationally addressed. Reference ranges for antipsychotics involve in most cases the parent compound, with the exception of risperidone, where active moiety concentrations are also used to determine efficacy/tolerability thresholds. Active moiety refers to the sum of parent compound and its active metabolite, as the latter is pharmacologically active (eg, active moiety = risperidone plus 9-OH-risperidone [paliperidone]).6 The literature regarding blood clozapine level monitoring is based on clozapine levels alone, even though the active metabolite norclozapine is often reported as well. However, norclozapine has only 10% activity of clozapine, and the 2 reported levels should not simply be added, but the target level of clozapine should determine the clinical decision-making for efficacy.
Further, the reference ranges derive mainly from oral antipsychotic studies. Nevertheless, they can be effectively applied for LAIs as well, although there has been some debate as to their precision in the latter context.58 From a pharmacokinetic point of view, potential deviations of LAIs from the suggested reference ranges may be more likely attributed to variations in the correctly applied, deep intramuscular injection (with lower levels when medication is wrongly placed into fat tissue) and study design issues, such as injection sites (longer t1/2 after gluteal than deltoid injections, but higher peak concentration after deltoid than gluteal injections) and sampling times.6 Lastly, therapeutic ranges apply to the primary/specific indication of the antipsychotic prescription. However, antipsychotics are increasingly prescribed for other indications apart from schizophrenia.59 Nevertheless, evidence regarding diagnosis-specific TDM is rarely available. Therefore, we are limited to providing diagnosis-specific reference ranges only for patients with schizophrenia.
Along with therapeutic reference ranges, we also provide laboratory alert levels (Table 2). Laboratory alert levels represent threshold levels above which the risk for ADRs is expected to increase. Antipsychotic levels higher than the alert levels need to be immediately reported by the laboratory to the prescribing clinicians, who may consider a dose adjustment when ADRs are present or suspected. If ADRs are not suspected or present, clinicians need to first check if the blood level was indeed obtained at 24 hours (for morning-dosed antipsychotics) or 12 hours (for evening-dosed antipsychotics) after the last dose, assure that the patient is taking the antipsychotic as prescribed, rule out accidental or intentional overdose, eliminate the possibility of white coat compliance (ie, medications ingested in anticipation of the blood draw in the context of nonadherence), rule out use of comedications (prescribed by other providers or purchased over the counter) that can alter the metabolism of the antipsychotic, and be vigilant for clinical signs indicative of an overdose/excessive blood antipsychotic levels and act accordingly.
3.5. TDM for Antipsychotics: Practical Issues and Interpretation
Table 2 provides clinically useful information, including the therapeutic reference ranges, laboratory alert levels, and elimination half-life (t1/2) of the drugs, as well as major CYP enzymes implicated and the metabolite-to-parent ratios (MPRs). The latter is calculated by dividing the concentrations of the major metabolite by the concentrations of the parent drug. The MPRs reflect the enzymatic activity implicated in the metabolism of the antipsychotics. Hence, they indirectly provide crucial information for poor or ultrarapid metabolizer status, adherence, and drug-drug interactions.60 Drug-drug interactions affecting antipsychotics (ie, antipsychotics as victim drugs) can be predicted in light of the metabolic pathways of antipsychotics, as reported in Table 2.
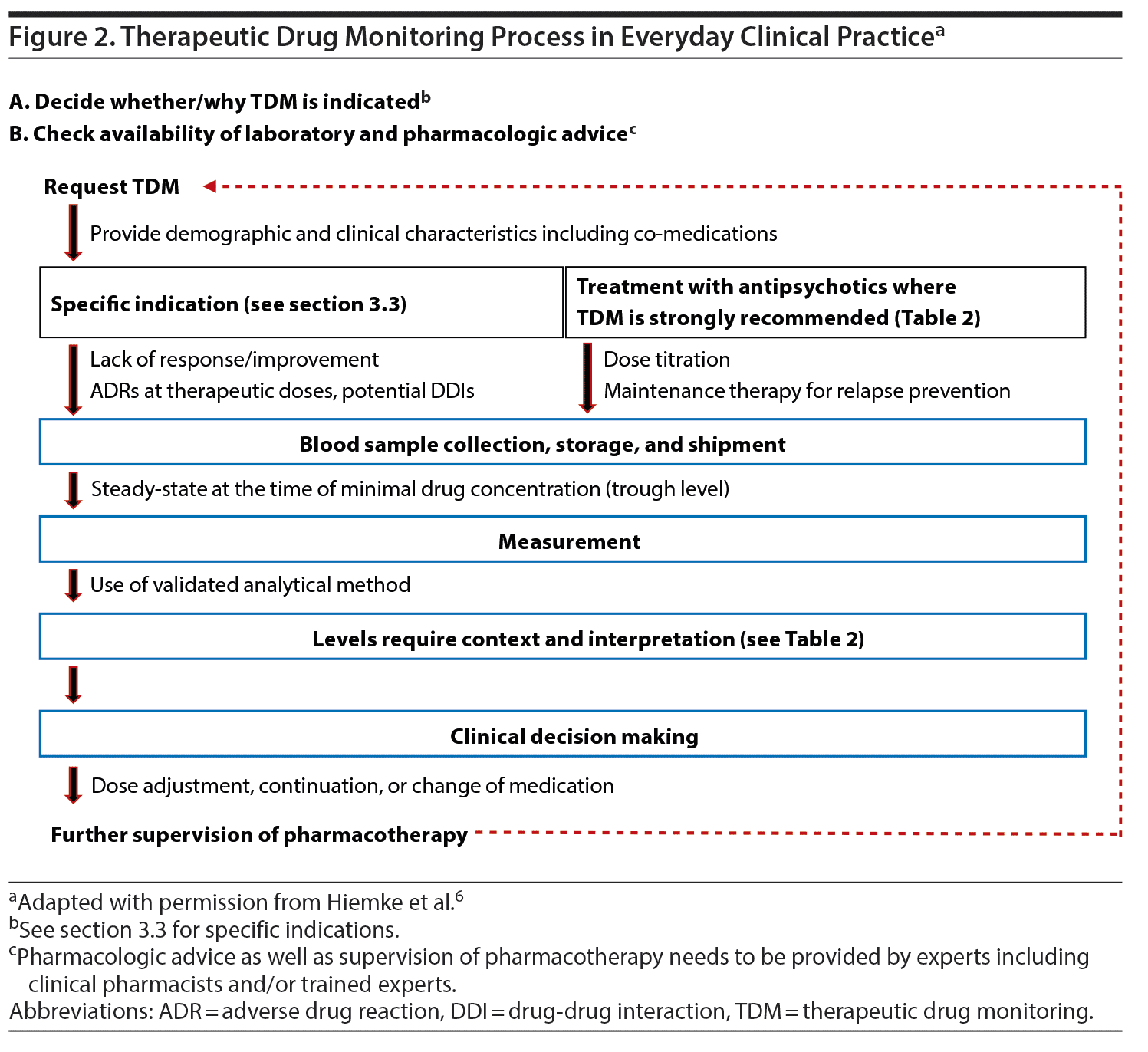
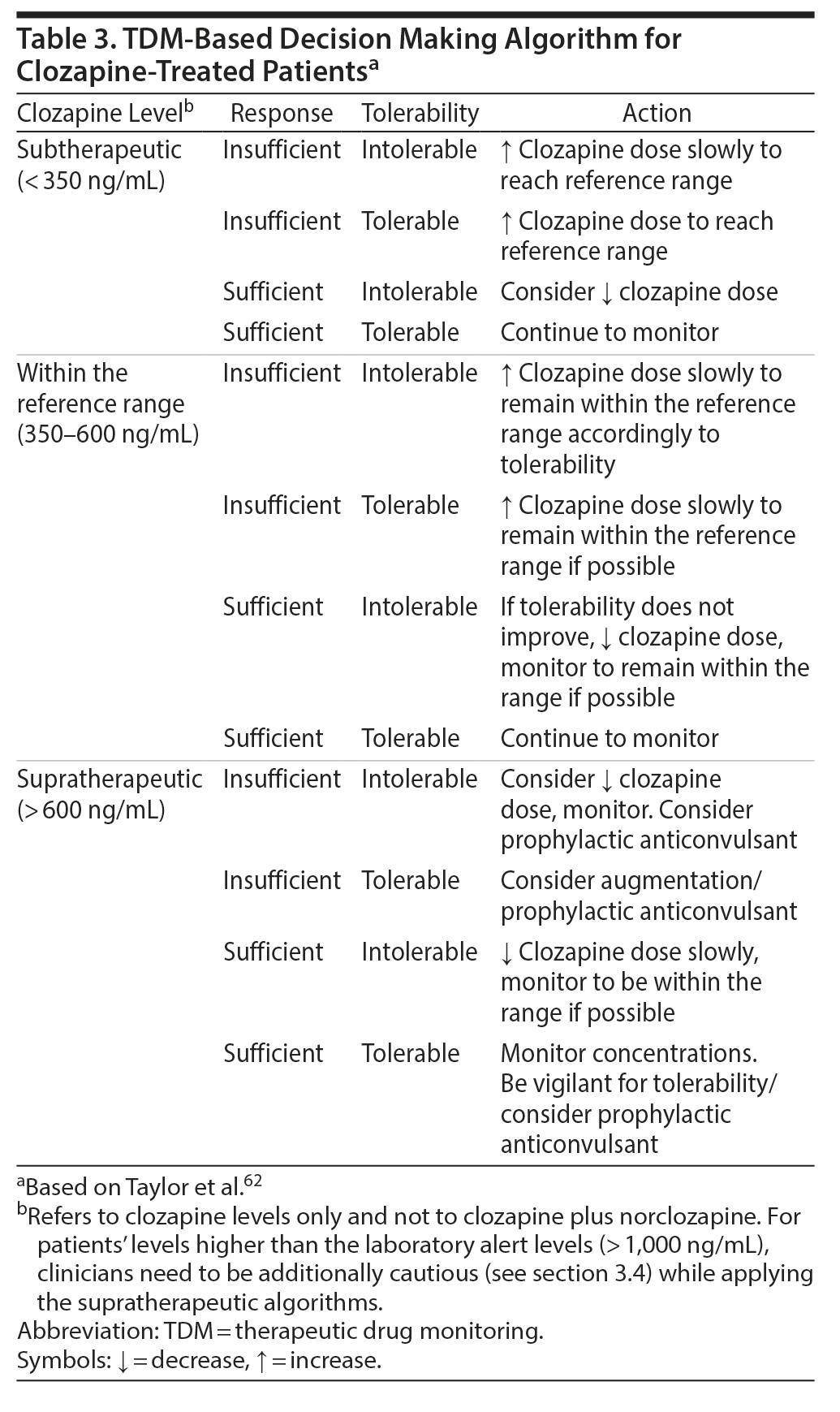
In Figure 2, we provide a schematic overview of how to apply TDM in everyday clinical practice. Regarding pharmacologic advice, recent developments in team-based care provide the opportunity to include other professionals, such as psychiatric pharmacy specialists, who can assist the psychiatrist in TDM applications.61 Electronic databases can provide additional assistance. When interpreting the antipsychotic levels, several pieces of information are required. First, comedications need to be taken into consideration, focusing on medications with antipsychotic metabolic pathways inhibiting or inducing properties. Moreover, contextual information is necessary, such as demographic and clinical characteristics, including age, sex, diagnosis, and smoking habits, as well as the indication for the TDM request. In fact, blood antipsychotic levels always need to be understood/interpreted in relationship to clinical information regarding symptom severity and response or remission, as well as ADRs. Further, the antipsychotic formulation, time since the last dose change and the time point of the blood draw must be considered. As data are more robust for clozapine, we provide a framework as an example for the clinical decision-making process previously reported elsewhere (although with a slightly lower upper clozapine level of the therapeutic range).62 It is important to note that even if rigorous clinical trial data are not available for each step of this framework, this algorithm proposes a logically structured process as an aid for the clinical practice considering response and tolerability aspects (Table 3). Additionally, patients’ case vignettes with examples of TDM that can inform clinicians on how to best utilize TDM have been published previously.6
DISCUSSION
4.1. TDM for Antipsychotics: Limitations and Future Directions
Despite the promise of TDM as a measurement-based tool to improve the effectiveness of antipsychotics in clinical care, several limitations must be acknowledged. These limitations may account for potential differences of opinion regarding the utility of TDM in clinical practice. First, as mentioned above, therapeutic blood level ranges are derived from groups of patients who agree to be studied. Their data might not generalize to usual care patients, who are more likely to have psychiatric and physical comorbidities and receive multiple comedications and who may have more severe/chronic psychiatric disorders that may possibly respond to different blood antipsychotic level ranges. In fact, although the pharmaceutical industry has routinely included PK assessments in their antipsychotic registration trials and other studies for at least the last 15 years (including studies conducted in highly controlled inpatient settings), for none of the recently approved antipsychotics have therapeutic blood antipsychotic level ranges been published. Since these data exist, our group strongly urges the pharmaceutical companies either to publish (even negative) correlational data between antipsychotic blood levels and efficacy as well as key safety/tolerability parameters or to make these data available to groups for further analysis. In fact, the scarcity of the data has resulted in pooling data from samples with patients in different treatment phases (eg, acute vs maintenance treatment),63 which may pose limitations for the precision of the estimated reference ranges. Second, blood antipsychotic level ranges available for clinically applied TDM are almost exclusively derived from patients with schizophrenia. In fact, the percentage of antipsychotic-treated patients with schizophrenia where TDM is applied is unclear; some transdiagnostic data suggest a low, however increasing, prevalence of around 4% for TDM in treatment with antipsychotics.64 Therefore, it is most likely that TDM is applied in a very small fraction of patients with schizophrenia treated with antipsychotics. Thus, more data should be collected in the many additional approved, and even off-label, indications for which antipsychotics are used. Third, TDM results are only helpful if a true 12-hour trough level is obtained and sufficient contextual data are provided to the laboratory and used for a careful interpretation of the results. Fourth, TDM is of only limited help in cases of partial antipsychotic nonadherence, unless a blood antipsychotic level is obtained at steady state of an efficacious and well-tolerated target dose to provide a dose-concentration-efficacy-tolerability relationship for the given patient that can be used as a benchmark for the interpretation of future variations in the clinical picture and blood antipsychotic levels. Apart from blood levels, the decision-making process will also need to integrate increasingly available technologies for the assessment of adherence.65,66 Fifth, as mentioned above, data are still quite limited for many, if not most, antipsychotics that could firmly establish a relationship between explicit blood level ranges for specific antipsychotics and clinical efficacy and tolerability. The scarcity of the data linking treatment outcomes and blood antipsychotic levels likely contributes to the limited use of TDM in clinical practice. Thus, clearly, more research is needed. Additionally, available data should be analyzed and published to fill this evidence gap. A typical example of urgent need for more evidence refers to the elderly, as well as children and adolescents. Ethical issues in psychopharmacology of children and adolescents discussed over recent years relate to PK/PD peculiarities and concern the safety, the indication, and the evidence-based prescription of antipsychotics in minors.67 The complexity of using TDM for antipsychotics in adolescents and children also relates to limited knowledge on therapeutic dose ranges for younger age groups and the amounts of blood volume required to perform TDM.68 However, age-dependent patterns for blood levels were reported for some antipsychotics used in adolescents, such as olanzapine69 and quetiapine,70 but not for others, such as risperidone69 and aripiprazole.71 Apart from legislative efforts to improve safety and discourage “off-label” use of antipsychotics in youth,72 additional TDM evidence is expected to guide clinicians to reduce the direct risk of under- or overdosing and the delayed risk of long-term side effects. To this aim, a Competence Network on Therapeutic Drug Monitoring in Child and Adolescent Psychiatry (http://tdm-kjp.de) was established in December 2007, including 12 departments of child and adolescent psychiatry in 3 European countries. The Network uses a multicenter TDM system including both standardized measurements of blood antipsychotic levels and the documentation of efficacy and side effects of these medications. Sixth, use of TDM depends on the availability of laboratories that analyze the blood level of any or specific antipsychotics. Parallel to the access to TDM, the availability of constantly updated reference ranges for antipsychotic levels in electronic databases will further facilitate the TDM integration in clinical practice. Finally, cost may be a considerable barrier to conducting TDM in some clinical care settings, making the collection of cost-effectiveness data mandatory in the area of TDM in general and of TDM of antipsychotics in particular. The cost-effectiveness of TDM is beyond the scope of this review. These limitations justify some skepticism regarding the rigor of the data supporting TDM use. Nevertheless, despite these limitations that are mostly addressable by more research, data indicate that TDM is an important yet underutilized measurement-based care and precision medicine tool that requires more attention and application. It is also likely that greater appreciation of the potential utility of TDM in clinical practice will lead to increased demand for the collection and publication of still needed data.
CONCLUSION
Despite the general call for personalized therapy, available relevant tools in standard psychiatric care are scarce. TDM presents a unique, evidence-based method that considers interindividual pharmacokinetic variability, which can be leveraged to enhance safety and efficacy of antipsychotic treatment. In fact, TDM has a long tradition in psychiatry, with the first attempt to establish guidelines dating back to 1985 from the American Psychiatric Association.1 Two decades later, the TDM taskforce of the AGNP published a first consensus statement on TDM in neuropsychopharmacology,13 updated twice in the past years.6,12 Nevertheless, the implementation of TDM into clinical practice has not progressed sufficiently and there are reports of TDM underutilization.73 The writing group members of this consensus statement may hold different views regarding the proven benefits of TDM for specific drugs as part of clinical routine. However, the potential of TDM as a tool for problem solving is unanimously embraced. In fact, TDM needs to be part of a clear temporal and contextual framework. Thus, it is no surprise that previous attempts to unravel pharmacokinetic correlates of clinical response several months after dose titration were of limited success.74 Likewise, blood clozapine levels measured at baseline in patients presenting with clozapine-related myocarditis at various later time points may be less accurate in estimating the risk of a clozapine-related myocarditis.75 Thus, we hope that the present collaborative work between the American Society of Clinical Psychopharmacology and the TDM Task Force of the AGNP will lead to a wider implementation, as well as stimulate further, much needed research in this area. To this end, TDM needs to receive more attention in educational programs, for example, psychopharmacology curricula for postgraduate training of specialists in psychiatry, including pharmacokinetics, metabolism, and pharmacogenetics of psychotropic drugs, and the use of TDM as a tool for optimizing psychopharmacotherapy. The authors of the Psychopharmacology section of the European Psychiatric Association created a psychopharmacology-pharmacotherapy catalog of learning objectives and a curriculum in Europe for teaching psychopharmacology during postgraduate training.76 This document contains an extensive section on TDM in psychiatry and should therefore help to encourage and implement appropriate use of this tool.74 In this consensus statement, we summarize essential information on the routine use of TDM in the antipsychotic treatment of patients with schizophrenia, including implementing and interpreting TDM results. Specifically, this joint consensus aims to encourage clinicians to perform TDM under the appropriate indications in order to inform clinical decision-making algorithms for antipsychotic prescription in patients with schizophrenia. In parallel, increasing the database on the utility of TDM for use of other psychotropic medications should also be pursued. It is hoped that with broader clinical implementation of TDM in the treatment of schizophrenia and other severe psychiatric disorders, reinforced by much needed additional research in this important area, patient outcomes can be further improved.
Submitted: November 16, 2019; accepted February 12, 2020.
Published online: May 19, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
Author contributions: Drs Hiemke, Goff, Gründer, Paulzen, Correll, Citrome, Kelly, Schoretsanitis, and Kane wrote the first article draft. All the authors critically read and revised the manuscript.
Financial disclosure: Dr Kane has been a consultant for or received honoraria from Alkermes, Dainippon Sumitomo, Eli Lilly, Forum, Allergan, Genentech, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceutica, Johnson and Johnson, LB Pharmaceuticals, Merck, Minerva, Neurocrine, Otsuka, Pierre Fabre, Reviva, Roche, Sunovion, Takeda, and Teva; has received grant support from Otsuka, Lundbeck, and Janssen; has participated in advisory boards for Alkermes, Dainippon Sumitomo, Intracellular Therapies, Lundbeck, Neurocrine, Otsuka, Pierre Fabre, Takeda, and Teva; and is a shareholder in Vanguard Research Group and LB Pharmaceuticals, Inc. Dr Correll has been a consultant and/or advisor to or has received honoraria from Alkermes, Allergan, Angelini, Boehringer-Ingelheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva; has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka; has served on a data safety monitoring board for Boehringer-Ingelheim, Lundbeck, Rovi, Supernus, and Teva; has received royalties from UpToDate and grant support from Janssen and Takeda; and is a shareholder of LB Pharma. Dr Marder has received consulting fees from Boehringer-Ingelheim, Lundbeck, Otsuka, Takeda, Teva, Roche, Genentech, Targacept, Forum, Abbvie, Allergan, and Neurocrine and has received research support from Boehringer-Ingelheim, Takeda, and Neurocrine. Dr Citrome has been a consultant in the past 12 months for Acadia, Alkermes, Allergan, Impel, Indivior, Intra-Cellular Therapies, Janssen, Lundbeck, Merck, Neurocrine, Noven, Osmotica, Otsuka, Pfizer, Shire, Sunovion, Takeda, Teva, and Vanda; has in the past 12 months also been a speaker for Acadia, Alkermes, Allergan, Janssen, Lundbeck, Merck, Neurocrine, Otsuka, Pfizer, Shire, Sunovion, Takeda, and Teva; holds stocks (small number of shares of common stock) of Bristol-Myers Squibb, Eli Lilly, J & J, Merck, and Pfizer purchased > 10 years ago; and has received royalties from Wiley (Editor-in-Chief, International Journal of Clinical Practice), UpToDate (reviewer), and Springer Healthcare (book). Dr Newcomer has served on a data safety monitoring board for Amgen and acted as a consultant for Alkermes, Otsuka, Intracellular Therapies, Auris, and Sunovion and for litigation. Dr Robinson served as a consultant for Asubio, Otsuka, and Shire and has received grants from Bristol-Myers Squibb, Janssen, and Otsuka. Dr Goff has received research support from the National Institutes of Health, Stanley Medical Research Foundation, and Avanir Pharmaceuticals and has participated in advisory boards for Avanir Pharmaceuticals and Takeda for which he accepted travel reimbursement but has not accepted honoraria. Dr Kelly has served on the advisory board for Lundbeck and as a consultant for HLS Therapeutics and Alkermes. Dr Freudenreich has served on advisory boards or as a consultant to Alkermes, Neurocrine, Janssen, Novartis, and Roche; received research grants from Avanir, Janssen, Otsuka, and Saladax; served as a content developer for Global Medical Education; and received royalties from Wolters Kluwer and UpToDate. Dr Conca has served as a consultant for Lilly, Bristol-Myers Squibb, and Pfizer and has served on the speakers’ bureau of Lilly, BMS, Astra Zeneca, Lundbeck, Italfarma, and Janssen. Dr Zernig has received speaker’s or consultancy fees or educational grants from AlcaSynn, AstraZeneca, Bio-Rad, Bristol-Myers Squibb, Eli Lilly, Lundbeck, Mundipharma, Novartis, Pfizer, and Wyeth. Dr Haen is chairman and managing director of the AGATE (www.amuep-agate.de) that supports reasonable and economic drug therapy and is shareholder of the psiac GmbH (www.psiac.de), which provides an Internet-based drug-drug interaction program. Dr Baumann has received speaker’s or consultancy fees from almost all pharmaceutical companies selling psychotropic drugs in Switzerland. Dr Hiemke has received speaker’s and consultancy fees from Janssen, Stada, and Servier and is editor of an Internet-based drug-drug interaction program (www.psiac.de). Dr Gründer has served as consultant and advisory board member during the last three years for the following companies and institutions: Allergan, Boehringer Ingelheim, Eli Lilly, IQWiG, Janssen-Cilag, Lundbeck, Otsuka, Recordati, SAGE and Takeda; has received honoraria as a speaker for Gedeon-Richter, Janssen-Cilag, Lundbeck, Neuraxpharma, Otsuka, and Recordati; has received funding for clinical trials from Boehringer Ingelheim, Lundbeck, and Saladax; and is the founder and a shareholder of Brainfoods GmbH, InMedicon GmbH, and Mind and Brain Institute GmbH. Drs Schoretsanitis, Piacentino, and Paulzen have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: No commercial organizations had any role in the completion of this work.
Group information: the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie: Niels Bergemann, MD, PhD; Hans-Willi Clement, PhD; Jürgen Deckert, MD; Gabriel Eckermann, MD; Karin Egberts, MD; Manfred Gerlach, PhD; Christine Greiner, PhD; Ursula Havemann-Reinecke, MD, PhD; Gudrun Hefner, PhD; Renate Helmer, PhD; Gerd Laux, MD; Thomas Messer, MD; Rainald Mössner, MD; Matthias J. Müller, MD, PhD; Bruno Pfuhlmann, MD; Peter Riederer, PhD; Alois Saria, MD; Bernd Schoppek, PhD; Markus Schwarz, MD; Margarete Silva Gracia, PhD; Benedikt Stegmann, PhD; Werner Steimer, MD; Manfred Uhr, MD, PhD; Sven Ulrich, PhD; Stefan Unterecker MD, Roland Waschgler, MSc; and Gabriela Zurek, PhD. The ASCP members who were involved with this consensus statement are listed as authors.
REFERENCES
1. Task Force on the Use of Laboratory Tests in Psychiatry. Tricyclic antidepressants—blood level measurements and clinical outcome: an APA Task Force report. Am J Psychiatry. 1985;142(2):155-162. PubMed CrossRef
2. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. PubMed CrossRef
3. Jönsson AK, Spigset O, Reis M. A compilation of serum concentrations of 12 antipsychotic drugs in a therapeutic drug monitoring setting. Ther Drug Monit. 2019;41(3):348-356. PubMed CrossRef
4. Ruan CJ, Zang YN, Wang CY, et al. Clozapine metabolism in East Asians and Caucasians: a pilot exploration of the prevalence of poor metabolizers and a systematic review. J Clin Psychopharmacol. 2019;39(2):135-144. PubMed CrossRef
5. Kane JM, Agid O, Baldwin ML, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80(2):18com12123. PubMed CrossRef
6. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. PubMed
7. McCutcheon R, Beck K, D’ Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46. PubMed CrossRef
8. Jukic MM, Smith RL, Haslemo T, et al. Effect of CYP2D6 genotype on exposure and efficacy of risperidone and aripiprazole: a retrospective, cohort study. Lancet Psychiatry. 2019;6(5):418-426. PubMed CrossRef
9. Varma S, Bishara D, Besag FM, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66. PubMed CrossRef
10.Schoretsanitis G, de Leon J, Diaz FJ. Prolactin levels: sex differences in the effects of risperidone, 9-hydroxyrisperidone levels, CYP2D6 and ABCB1 variants. Pharmacogenomics. 2018;19(10):815-823. PubMed CrossRef
11.Kelly DL, Richardson CM, Yu Y, et al. Plasma concentrations of high-dose olanzapine in a double-blind crossover study. Hum Psychopharmacol. 2006;21(6):393-398. PubMed CrossRef
12.Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195-235. PubMed CrossRef
13.Baumann P, Hiemke C, Ulrich S, et al; Arbeitsge-meinschaft fur neuropsychopharmakologie und pharmakopsychiatrie. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37(6):243-265. PubMed CrossRef
14.Zanger UM, Turpeinen M, Klein K, et al. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem. 2008;392(6):1093-1108. PubMed CrossRef
15.Stingl JC, Brockmöller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Mol Psychiatry. 2013;18(3):273-287. PubMed CrossRef
16.de Leon J. The future (or lack of future) of personalized prescription in psychiatry. Pharmacol Res. 2009;59(2):81-89. PubMed CrossRef
17.Bojdani E, Chen A, Buonocore S, et al. Meloxicam-desmopressin drug-drug interaction producing hyponatremia. Psychiatry Res. 2019;279:284-286. PubMed CrossRef
18.Shipkova M, Christians U. Improving therapeutic decisions: pharmacodynamic monitoring as an integral part of therapeutic drug monitoring. Ther Drug Monit. 2019;41(2):111-114. PubMed CrossRef
19.Taylor D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: a review. Br J Psychiatry suppl. 2009;52:S13-S19. PubMed CrossRef
20.Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317. PubMed CrossRef
21.Citrome L. Sustained-release risperidone via subcutaneous injection: a systematic review of RBP-7000 (PERSERIS) for the treatment of schizophrenia. Clin Schizophr Relat Psychoses. 2018;12(3):130-141. PubMed CrossRef
22.Mallikaarjun S, Kane JM, Bricmont P, et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res. 2013;150(1):281-288. PubMed CrossRef
23.Hard ML, Mills RJ, Sadler BM, et al. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617-624. PubMed CrossRef
24.VanderZwaag C, McGee M, McEvoy JP, et al. Response of patients with treatment-refractory schizophrenia to clozapine within three serum level ranges. Am J Psychiatry. 1996;153(12):1579-1584. PubMed CrossRef
25.Lv D, Zhao M, Chen L, et al. An inter-ethnic comparison study of ziprasidone plasma levels, dosage and clinical response in patients with schizophrenia. Psychiatry Investig. 2017;14(3):360-367. PubMed CrossRef
26.Mackay AV, Healey AF, Baker J. The relationship of plasma chlorpromazine to its 7-hydroxy and sulphoxide metabolites in a large population of chronic schizophrenics. Br J Clin Pharmacol. 1974;1(5):425-430. PubMed CrossRef
27.Trimble MR, Robertson MM. Flupenthixol in depression: a study of serum levels and prolactin response. J Affect Disord. 1983;5(1):81-89. PubMed CrossRef
28.Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry. 2007;68(10):1540-1545. PubMed CrossRef
29.Mauri MC, Colasanti A, Rossattini M, et al. Ziprasidone outcome and tolerability: a practical clinical trial with plasma drug levels. Pharmacopsychiatry. 2007;40(3):89-92. PubMed CrossRef
30.Olsen CK, Brennum LT, Kreilgaard M. Using pharmacokinetic-pharmacodynamic modelling as a tool for prediction of therapeutic effective plasma levels of antipsychotics. Eur J Pharmacol. 2008;584(2-3):318-327. PubMed CrossRef
31.Ishigooka J, Iwashita S, Higashi K, et al. Pharmacokinetics and safety of brexpiprazole following multiple-dose administration to Japanese patients with schizophrenia. J Clin Pharmacol. 2018;58(1):74-80. PubMed CrossRef
32.Patteet L, Morrens M, Maudens KE, et al. Therapeutic drug monitoring of common antipsychotics. Ther Drug Monit. 2012;34(6):629-651. PubMed CrossRef
33.Nakamura T, Kubota T, Iwakaji A, et al. Clinical pharmacology study of cariprazine (MP-214) in patients with schizophrenia (12-week treatment). Drug Des Devel Ther. 2016;10:327-338. PubMed CrossRef
34.Bagli M, Süverkrüp R, Quadflieg R, et al. Pharmacokinetic-pharmacodynamic modeling of tolerance to the prolactin-secreting effect of chlorprothixene after different modes of drug administration. J Pharmacol Exp Ther. 1999;291(2):547-554. PubMed
35.Molander L, Borgström L. Sedative effects and prolactin response to single oral doses of melperone. Psychopharmacology (Berl). 1983;79(2-3):142-147. PubMed CrossRef
36.Takahashi LH, Huie K, Spyker DA, et al. Effect of smoking on the pharmacokinetics of inhaled loxapine. Ther Drug Monit. 2014;36(5):618-623. PubMed CrossRef
37.Horvitz-Lennon M, Predmore Z, Mattke S. Personalizing Antipsychotic Treatment of Schizophrenia: Monitoring Plasma Levels for Improved Treatment Decisions. Santa Monica, CA: RAND Corporation; 2017. https://www.rand.org/pubs/perspectives/PE174.html.
38.Lopez LV, Shaikh A, Merson J, et al. Accuracy of clinician assessments of medication status in the emergency setting: a comparison of clinician assessment of antipsychotic usage and plasma level determination. J Clin Psychopharmacol. 2017;37(3):310-314. PubMed CrossRef
39.Melkote R, Singh A, Vermeulen A, et al. Relationship between antipsychotic blood levels and treatment failure during the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. Schizophr Res. 2018;201:324-328. PubMed CrossRef
40.Schoretsanitis G, Haen E, Gründer G, et al. Pharmacokinetic drug-drug interactions of mood stabilizers and risperidone in patients under combined treatment. J Clin Psychopharmacol. 2016;36(6):554-561. PubMed CrossRef
41.Choong E, Polari A, Kamdem RH, et al. Pharmacogenetic study on risperidone long-acting injection: influence of cytochrome P450 2D6 and pregnane X receptor on risperidone exposure and drug-induced side-effects. J Clin Psychopharmacol. 2013;33(3):289-298. PubMed CrossRef
42.Paulzen M, Haen E, Stegmann B, et al. Body mass index (BMI) but not body weight is associated with changes in the metabolism of risperidone: a pharmacokinetics-based hypothesis. Psychoneuroendocrinology. 2016;73:9-15. PubMed CrossRef
43.Schoretsanitis G, Westin AA, Deligiannidis KM, et al. Excretion of antipsychotics into the amniotic fluid, umbilical cord blood, and breast milk: a systematic critical review and combined analysis. Ther Drug Monit. 2020;42(2):245-254. PubMed CrossRef
44.Roke Y, van Harten PN, Franke B, et al. The effect of the Taq1A variant in the dopamine D2 receptor gene and common CYP2D6 alleles on prolactin levels in risperidone-treated boys. Pharmacogenet Genomics. 2013;23(9):487-493. PubMed CrossRef
45.de Leon J, Greenlee B, Barber J, et al. Practical guidelines for the use of new generation antipsychotic drugs (except clozapine) in adult individuals with intellectual disabilities. Res Dev Disabil. 2009;30(4):613-669. PubMed CrossRef
46.Meyer JM. Monitoring and improving antipsychotic adherence in outpatient forensic diversion programs [published online ahead of print May 23, 2019]. CNS Spectr. PubMed CrossRef
47.Boom S, Thyssen A, Crauwels H, et al. The influence of hepatic impairment on the pharmacokinetics of paliperidone. Int J Clin Pharmacol Ther. 2009;47(10):606-616. PubMed CrossRef
48.Coons JC, Empey P. Drug metabolism in cardiovascular disease. In: Xie W, ed. Drug Metabolism in Diseases. London, UK: Elsevier; 2017.
49.Clark SR, Warren NS, Kim G, et al. Elevated clozapine levels associated with infection: a systematic review. Schizophr Res. 2018;192:50-56. PubMed CrossRef
50.Schoretsanitis G, Kirner-Veselinovic A, Gründer G, et al. Clinically relevant changes in clozapine serum concentrations after breast reduction surgery. Aust N Z J Psychiatry. 2017;51(10):1059-1060. PubMed CrossRef
51.Carbon M, Correll CU. Rational use of generic psychotropic drugs. CNS Drugs. 2013;27(5):353-365. PubMed CrossRef
52.Blier P, Margolese HC, Wilson EA, et al. Switching medication products during the treatment of psychiatric illness. Int J Psychiatry Clin Pract. 2019;23(1):2-13. PubMed CrossRef
53.Meyer JM. Converting oral to long-acting injectable antipsychotics: a guide for the perplexed. CNS Spectr. 2017;22(S1):14-28. PubMed CrossRef
54.Sanader B, Grohmann R, Grötsch P, et al. Clozapine-induced DRESS syndrome: a case series from the AMSP Multicenter Drug Safety Surveillance Project. Pharmacopsychiatry. 2019;52(3):156-159. PubMed CrossRef
55.Bustillo M, Zabala A, Querejeta I, et al. Therapeutic drug monitoring of second-generation antipsychotics for the estimation of early drug effect in first-episode psychosis: a cross-sectional assessment. Ther Drug Monit. 2018;40(2):257-267. PubMed CrossRef
56.de Leon J, Odom-White A, Josiassen RC, et al. Serum antimuscarinic activity during clozapine treatment. J Clin Psychopharmacol. 2003;23(4):336-341. PubMed CrossRef
57.Steen NE, Aas M, Simonsen C, et al. Serum levels of second-generation antipsychotics are associated with cognitive function in psychotic disorders. World J Biol Psychiatry. 2017;18(6):471-482. PubMed CrossRef
58.Baldelli S, Clementi E, Cattaneo D. Can we rely on AGNP therapeutic targets also for LAI antipsychotics? Pharmacopsychiatry. 2018;51(6):270-271. PubMed CrossRef
59.Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA. 2011;306(12):1359-1369. PubMed CrossRef
60.Schoretsanitis G, Kane JM, Ruan CJ, et al. A comprehensive review of the clinical utility of and a combined analysis of the clozapine/norclozapine ratio in therapeutic drug monitoring for adult patients. Expert Rev Clin Pharmacol. 2019;12(7):603-621. PubMed CrossRef
61.Goldstone LW, DiPaula BA, Caballero J, et al. Improving medication-related outcomes for patients with psychiatric and neurologic disorders: Value of psychiatric pharmacists as part of the health care team. Ment Health Clin. 2015;5(1):1-28. CrossRef
62.Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. 12th ed. West Sussex, UK: Wiley Blackwell; 2015.
63.Noel C. A review of a recently published guidelines’ “strong recommendation” for therapeutic drug monitoring of olanzapine, haloperidol, perphenazine, and fluphenazine. Ment Health Clin. 2019;9(4):287-293. PubMed CrossRef
64.Wallerstedt SM, Lindh JD. Prevalence of therapeutic drug monitoring for antidepressants and antipsychotics in Stockholm, Sweden: a longitudinal analysis. Ther Drug Monit. 2015;37(4):461-465. PubMed CrossRef
65.Kane JM. Comments on Abilify MyCite. Clin Schizophr Relat Psychoses. 2018;11(4):205-206. PubMed
66.Park JYE, Li J, Howren A, et al. Mobile phone apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR Mhealth Uhealth. 2019;7(1):e11919. PubMed CrossRef
67.Koelch M, Schnoor K, Fegert JM. Ethical issues in psychopharmacology of children and adolescents. Curr Opin Psychiatry. 2008;21(6):598-605. PubMed CrossRef
68.Macleod S. Therapeutic drug monitoring in pediatrics: how do children differ? Ther Drug Monit. 2010;32(3):253-256. PubMed CrossRef
69.Aichhorn W, Marksteiner J, Walch T, et al. Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. J Child Adolesc Psychopharmacol. 2007;17(5):665-674. PubMed CrossRef
70.Gerlach M, Hünnerkopf R, Rothenhöfer S, et al. Therapeutic drug monitoring of quetiapine in adolescents with psychotic disorders. Pharmacopsychiatry. 2007;40(2):72-76. PubMed CrossRef
71.Bachmann CJ, Rieger-Gies A, Heinzel-Gutenbrunner M, et al. Large variability of aripiprazole and dehydroaripiprazole serum concentrations in adolescent patients with schizophrenia. Ther Drug Monit. 2008;30(4):462-466. PubMed
72.Zito JM, Derivan AT, Kratochvil CJ, et al. Off-label psychopharmacologic prescribing for children: history supports close clinical monitoring. Child Adolesc Psychiatry Ment Health. 2008;2(1):24. PubMed CrossRef
73.Conca A, Schmidt E, Pastore M, et al. Therapeutic drug monitoring in Italian psychiatry. Pharmacopsychiatry. 2011;44(6):259-262. PubMed CrossRef
74.Roosens L, Neels HM, Sabbe BG. Therapeutic drug monitoring of second-generation antipsychotics for the estimation of early drug effect in first-episode psychosis. Ther Drug Monit. 2019;41(2):252-253. PubMed CrossRef
75.Khan AA, Ashraf A, Baker D, et al. Clozapine and incidence of myocarditis and sudden death—long term Australian experience. Int J Cardiol. 2017;238:136-139. PubMed CrossRef
76.Baumann P, Spies M, Möller HJ, et al. A proposal for a psychopharmacology-pharmacotherapy catalogue of learning objectives and a curriculum in Europe. World J Biol Psychiatry. 2017;18(1):29-38. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K Shinn, MD, MPH, at [email protected].
This PDF is free for all visitors!
Save
Cite