Angiotensin receptor blockers (ARBs), used in the treatment of hypertension, may modulate neurotransmission in the central nervous system. One previous very small case-control study had suggested that ARBs increase the odds of suicide. In a recent, large, population-based, nested case-control study conducted in Ontario, Canada, completed suicide was associated with significantly increased odds of an ARB prescription during the 100 days preceding the suicide; in this analysis, confounding by indication was reduced by comparing ARB exposure with angiotensin-converting enzyme inhibitor exposure. The results of this new study were widely disseminated in the scientific and mass media. However, at least some of the data presented in the study appeared implausible, and at least one important calculation was incorrect. Furthermore, important subgroup analyses were not conducted. These incongruities diminish the confidence of the reader in the data and in the analyses presented in the study. A generous conclusion is that, at best, ARB use is a marker of suicide risk that merits examination in studies that include a follow-up, in which absolute risks and dose-dependent and treatment duration-dependent effects can be examined. A parsimonious conclusion is that there is no association proven between ARB exposure and suicide.

ABSTRACT
Angiotensin receptor blockers (ARBs), used in the treatment of hypertension, may modulate neurotransmission in the central nervous system. One previous very small case-control study had suggested that ARBs increase the odds of suicide. In a recent, large, population-based, nested case-control study conducted in Ontario, Canada, completed suicide was associated with significantly increased odds of an ARB prescription during the 100 days preceding the suicide; in this analysis, confounding by indication was reduced by comparing ARB exposure with angiotensin-converting enzyme inhibitor exposure. The results of this new study were widely disseminated in the scientific and mass media. However, at least some of the data presented in the study appeared implausible, and at least one important calculation was incorrect. Furthermore, important subgroup analyses were not conducted. These incongruities diminish the confidence of the reader in the data and in the analyses presented in the study. A generous conclusion is that, at best, ARB use is a marker of suicide risk that merits examination in studies that include a follow-up, in which absolute risks and dose-dependent and treatment duration-dependent effects can be examined. A parsimonious conclusion is that there is no association proven between ARB exposure and suicide.
J Clin Psychiatry 2020;81(1):20f13238
To cite: Andrade C. Angiotensin receptor blockers and the risk of suicide: an exercise in the critical examination of a case-control study. J Clin Psychiatry. 2020;81(1):20f13238.
To share: https://doi.org/10.4088/JCP.20f13238
© Copyright 2020 Physicians Postgraduate Press, Inc.
Suicide is an important public health concern. It is a potentially preventable cause of morbidity and mortality. Many drugs have been associated with risk of suicidal ideation and behavior, although causality remains to be proven; examples of such drugs include antidepressants,1,2 antiepileptic drugs,3 benzodiazepines and hypnotics,4,5 and rimonabant.6
A recent population-based, nested case-control study suggested that angiotensin receptor blockers (ARBs) are also associated with the risk of suicide.7 The results of this study were widely disseminated in the mass media, no doubt because ARBs are an important class of antihypertensive drugs and because suicide is an important adverse outcome. The present article critically examines the study and its findings with a view to help the reader understand the research design and the limitations of the study.
Why Was the Study Done?
Some studies are exploratory; that is, they are conducted without a clearly prespecified hypothesis. Some studies are fishing expeditions; that is, they mine existing data sets to “discover” which among a large number of tested associations reach statistical significance. Both types of study, and the latter, in particular, are vulnerable to Type 1 statistical errors; that is, false-positive findings that result from the large number of statistical tests conducted.
The study of Mamdani et al7 was not an investigation of the association between the use of a randomly chosen drug category with a random chosen outcome. Rather, as the authors explained, it was based on the knowledge that angiotensin 2 (A2) is a neuromodulator in the central nervous system, and that ARBs, which cross the blood-brain barrier to varying extents, block the binding of A2 to the A2 Type 1 receptor. This compensatorily up-regulates A2; the consequence is an unopposed stimulation of the A2 Type 2 receptor.
Furthermore, the authors7 noted that gene polymorphisms based on elevated A2 levels have been associated with major and minor mental illnesses. Finally, they pointed out that a small, exploratory, case-control study,8 conducted over a decade earlier, had reported that, among a number of categories of cardiovascular drugs examined, only ARBs were significantly associated with an increased odds of suicide.
What Did the Study Do?
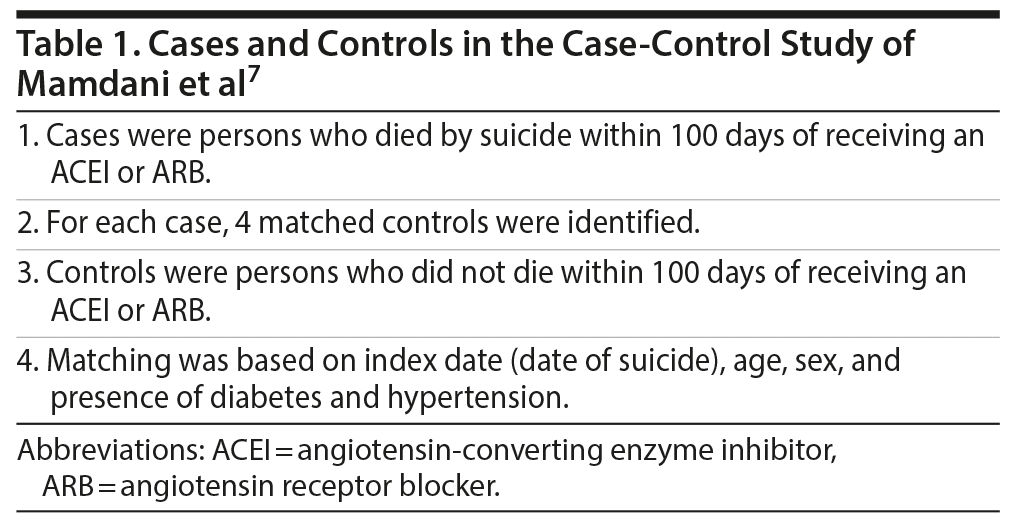
Mamdani et al7 described a population-based, nested case-control study. They drew their data from health care databases in Ontario, Canada, for the period 1995-2015. Cases comprised persons aged > 65 years who had died by suicide and who had a history of having received a prescription for an ARB or an angiotensin-converting enzyme inhibitor (ACEI), but not both, during the 100 days preceding the date of suicide (index date). Four alive controls were matched to each case. Matching was based on age, sex, and the presence of diabetes and hypertension. All controls had also received a prescription for an ARB or ACEI during the 100 days prior to the index date (Table 1).
The authors next compared the odds of suicide in persons prescribed ARBs vs those prescribed ACEIs using multivariable conditional logistic regression analysis. Thus, because both groups of drugs are prescribed for the same indication (hypertension), an important strength of the study was that the risk of confounding by indication was reduced.
This was a case-control study; subjects, obviously, were not randomized into case (suicide) and control (alive) groups. So, because it was expected that cases and controls would differ in potentially important ways, the authors statistically adjusted their analyses for important confounding variables, including demographic variables, medical conditions, psychiatric conditions, and medical and psychiatric drug prescriptions.
What Did the Study Find?
The authors7 identified 964 cases and 3,856 controls. The median age of the sample was 76 years. The sample was about 80% male. The commonest ARBs prescribed were valsartan, telmisartan, and candesartan (about 17%, each), and the commonest ACEIs prescribed were ramipril (about 39%) and enalapril (about 15%).
In general, as might have been expected, cases (suicide group) had higher medical and psychiatric morbidity than controls (alive group), were more likely to have abused alcohol, and were more likely to have had a history of deliberate self-harm. Again, as might have been expected, cases were more likely to have received psychotropic medications.
Among the 964 cases, 260 had received a prescription for an ARB and 704 had received a prescription for an ACEI. The adjusted odds of suicide were higher among those prescribed an ARB than among those prescribed an ACEI (odds ratio [OR], 1.63; 95% confidence interval [CI], 1.33-2.00). The results were almost the same in a sensitivity analysis that excluded persons with a past history of deliberate self-harm (OR, 1.60; 95% CI, 1.29-1.98). No other analyses were conducted.
Simply expressed, persons who completed suicide were more likely to have been prescribed an ARB than an ACEI during the 100 days preceding the suicide. This means that the prescription of an ARB, as compared with a prescription for an ACEI, is a crude marker of suicide risk. With the help of a little imagination, this suggests the possibility that the use of the prescribed ARB may have predisposed to the suicide through mechanisms briefly described in an earlier section of this article.
General Limitations of the Study
Implicating ARBs in the predisposition to suicide is associated with problems that are common to all case-control studies. In randomized controlled trials (RCTs), patients are randomly allocated to their respective groups, and baseline variables are therefore, usually, well balanced between groups. So the groups are closely similar on all baseline (independent) variables, including variables that might be expected to influence the study outcome (dependent variable). So if the study outcome differs significantly between groups, one might reasonably conclude that the grouping variable alone is responsible for the difference.
In case-control studies, cases are selected because they have the outcome of interest, and controls are selected because they do not have the outcome of interest. In the study of Mamdani et al,7 suicide was the outcome of interest. In case-control studies, there is no random allocation to case and control groups. So, the case and control groups usually differ on many independent variables, as indeed was observed in the study of Mamdani et al.7 So, any of these differences could explain the differences in outcome, and not just differences in the independent variable of interest (ARB vs ACEI exposure).
Investigators try to match controls with cases on at least some of the important independent variables. Most case-control studies match for index date, age, and sex. Some match for additional variables, too; this is possible when the database is very large, allowing a larger choice of subjects for closer matching. Other imbalances between groups can be “adjusted” for by including the independent variables (called confounding variables) into regression analyses. In these regressions, outcome (case vs control) is the dependent variable and the exposure of interest (ARB vs ACEI) as well as all the confounding variables (medical illnesses, psychiatric illnesses, treatments prescribed, etc) are entered as independent variables.
Such statistical adjustments can never be perfect. For example, one might be able to adjust for the presence of depression, but not for how severe or how refractory the depression was, because severity and refractoriness data are unlikely to be available in the databases from which the data are extracted. So depression is an example of an inadequately measured confound; this is important because more severe depression or more refractory depression could be expected to be more likely to predispose to suicide.
Statistical adjustment would obviously be unable to adjust for unmeasured and unknown confounds. For example, Mamdani et al7 did not have data for substance use disorders or personality disorders, which are known to influence suicide risk, and so could not adjust for these variables in their analyses. So substance use and personality disorder were unmeasured confounds. Speculatively, genetic differences between the ARB and ACEI groups could have been responsible for the differences in suicide risk between the groups. So genetic influences could have been unknown confounds.
What this means is that in nonrandomized studies, such as case-control studies, inadequately measured, unmeasured, and unknown confounds can influence the study results. Therefore, cause and effect relationships can never be established in case-control studies; they can only be hypothesized to exist, if the results are significant.
Absolute Risks
Another general problem with case-control studies is that absolute risks cannot be estimated. In the Mamdani et al7 study, for example, we know how many cases and controls had been prescribed an ARB or an ACEI in the previous 100 days. We do not know the values for the reverse scenario. That is, we do not know how many persons completed suicide if they had been prescribed an ARB or an ACEI for 100 days. Only in the latter situation is it possible to say that X% of ARB users vs Y% of ACEI users completed suicide within 100 days of use.
Absolute risks can be estimated in study designs that include follow-up. Such designs include RCTs and cohort studies.
Specific Limitations of the Study
It is puzzling that there was so little analysis in the study by Mamdani et al.7 The entire Results section comprised just 2 paragraphs, and the analysis of suicide outcomes was reported in just 4 sentences. It would have been useful to know, for example, what the ORs were in the large subgroup of persons who had psychiatric disorders and in the subgroup of persons who did not have psychiatric disorders. Whereas these analyses would have had lower statistical power than the main analysis, the analyses could have indicated whether subgroups need to be specifically studied in future research. The issue is particularly important because components of the metabolic syndrome are common in psychiatric patients, and so the safety of antihypertensive drugs classes in psychiatric patients needs study.
Finally, it is puzzling that the authors7 restricted themselves to the study of subjects aged > 65 years. Why should the odds of suicide with ARBs vs ACEIs depend on age?
Elephants in the Study
The authors7 presented a table in which they compared cases and controls on a large number of characteristics. Very curiously, among cases, anxiety or sleep disorders (n = 413), affective disorders (n = 411); psychoses, agitation, and related disorders (n = 401); and all other mental health conditions (n = 414) were each present in 41.6%-42.9% of subjects; among controls, these 4 categories were present in 568, 568, 561, and 568 subjects, respectively; that is, in 14.5%-14.7% of subjects. One would expect these 4 categories of disorders to be reasonably mutually exclusive; certainly, the last-mentioned category, that of “other” mental health conditions, should have had no overlap with the first 3 categories. So what the table implies is that in each of the case and control groups, a subgroup of patients had a very similar profile of psychiatric illness as well as of psychiatric comorbidity, with each illness/comorbidity present in identical proportions. This seems implausible; a more likely explanation is that the authors made a mistake in classifying patients, or, rather, many mistakes. If there were mistakes in these values, there would have been mistakes in the statistical adjustments. There might also have been mistakes in other extracted data and in analyses that we cannot know about.
It would have been interesting to know what the values for these percentages were in ARB and ACEI groups, separately, but the authors did not present the data. If the distribution of psychiatric disorders differed between ARB and ACEI groups, then this difference and not the ARB exposure might have explained the significant association between ARB exposure and suicide. Alternately, differences in the distribution of psychiatric disorders might suggest that ARB exposure predisposed to the development of a psychiatric condition that, in turn, predisposed to the suicide.
In another table, the authors observed that there were 260 and 704 cases in ARB and ACEI groups, respectively; and that there were 741 and 3,115 controls in the respective groups. A quick calculation tells us that the OR for these data is 1.55; yet the authors presented an (unadjusted) OR of 1.64 for these numbers. Again, if there were mistakes in what could be checked, what mistakes might there be in analyses that cannot be checked from the data presented?
General Conclusions
If the data and analyses in this study7 are trusted, at best they suggest that ARB prescription as a marker of suicide requires further study, as suggested in the next section. If the data and analyses are viewed with a high degree of concern, as suggested in the previous section, the association between ARBs and suicide is a matter that is wide open.
Further Research
The ideal research design is an RCT in which suicide rates are compared between patients who are randomized to receive ARBs or ACEIs. However, such a study would be almost impossible to conduct because suicide is a rare event, and so a very large sample would be necessary for adequate statistical power to detect a significant difference in risk.
In a more feasible research design, such as a cohort study, persons receiving ARBs and ACEIs can be identified and followed. Whereas such a study could be hard to conduct prospectively, the necessary data, including follow-up data, can be extracted from health care or insurance databases. A database that is sufficiently large could have enough of subjects exposed to each treatment (ARB or ACEI) for the comparison of a rare outcome, suicide, to have sufficient statistical power for meaningful analysis. Studies with a follow-up can provide information about absolute risks, as already discussed. Studies with a follow-up can also provide information on risks in persons who have a psychiatric disorder at the time of prescription, vs risks in persons who do not have a psychiatric disorder. In the latter group of persons, it would be important to know whether or not the development of a psychiatric disorder after drug exposure mediates the risk of suicide. Finally, in such studies, the effect of dose and duration of exposure can be examined.
Published online: January 21, 2020
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Andrade C, Bhakta SG, Singh NM. Controversy revisited: selective serotonin reuptake inhibitors in paediatric depression. World J Biol Psychiatry. 2006;7(4):251-260. PubMed CrossRef
2.Reeves RR, Ladner ME. Antidepressant-induced suicidality: an update. CNS Neurosci Ther. 2010;16(4):227-234. PubMed
3.Mula M. Do anti-epileptic drugs increase suicide in epilepsy? 10 years after the FDA alert. Expert Rev Neurother. 2018;18(3):177-178. PubMed CrossRef
4.Dodds TJ. Prescribed benzodiazepines and suicide risk: a review of the literature. Prim Care Companion CNS Disord. 2017;19(2):16r02037. PubMed CrossRef
5.McCall WV, Benca RM, Rosenquist PB, et al. Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am J Psychiatry. 2017;174(1):18-25. PubMed CrossRef
6.Topol EJ, Bousser MG, Fox KA, et al; CRESCENDO Investigators. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet. 2010;376(9740):517-523. PubMed CrossRef
7.Mamdani M, Gomes T, Greaves S, et al. Association between angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and suicide. JAMA Netw Open. 2019;2(10):e1913304. PubMed CrossRef
8.Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite