- Two single-dose laboratory studies have shown that placebo is associated with better treatment outcomes when it is stated to cost more. One study assessed motor and other outcomes in Parkinson disease patients; the other study assessed analgesia in paid, healthy volunteers to whom electric stocks were administered.
- There is no certainty that these findings can be generalized to real-world psychiatric patients who require medication for long periods. Nevertheless, the possibility remains that patients may derive less placebo-related benefit from generic medicines because of lower expectations related to lower treatment costs.
- Clinicians should therefore reassure patients that treatment costs do not necessarily have a bearing on treatment outcomes; this is especially important when generic medicines are prescribed.

ABSTRACT
Nonspecific factors have long been known in both psychotherapy and psychopharmacology. In recent years, 2 studies showed that placebo benefits were lower when the treated subjects were told that the placebo, presented as an active treatment, cost less. One of these studies had assessed motor and other outcomes in Parkinson disease patients; the other had assessed analgesia in paid, healthy volunteers to whom electric shocks were administered. The implication of the finding that lower treatment cost may diminish treatment gains is that patients who receive generic medicines may have lower expectations and may consequently derive less placebo-related benefit. This could be of concern in psychiatric disorders that are characterized by a large placebo response. Although the 2 “placebo cost” studies cannot be easily generalized to clinical and especially psychiatric contexts, clinicans should consider offering reassurance to patients receiving generic drugs that cost, per se, has no bearing on treatment-related benefit.
J Clin Psychiatry 2015;76(4):e534-e536 (doi:10.4088/JCP.15f09950).
© Copyright 2015 Physicians Postgraduate Press, Inc.
Clinical Question
Clinicians have reservations about the use of generic medications because of concerns related to the absolute content of the active drug, the bioavailability of the active drug, and the presence of impurities in the formulation,1-3 all of which can reduce efficacy of the treatment, increase adverse effects, or both. Generic drugs, however, continue to be prescribed because they carry the substantial advantage of affordability. Can this very advantage be a limitation, as well? The reason for this unusual question arises from the findings of 2 studies on the influence of treatment cost on clinical outcomes. These studies are briefly discussed.
Introduction
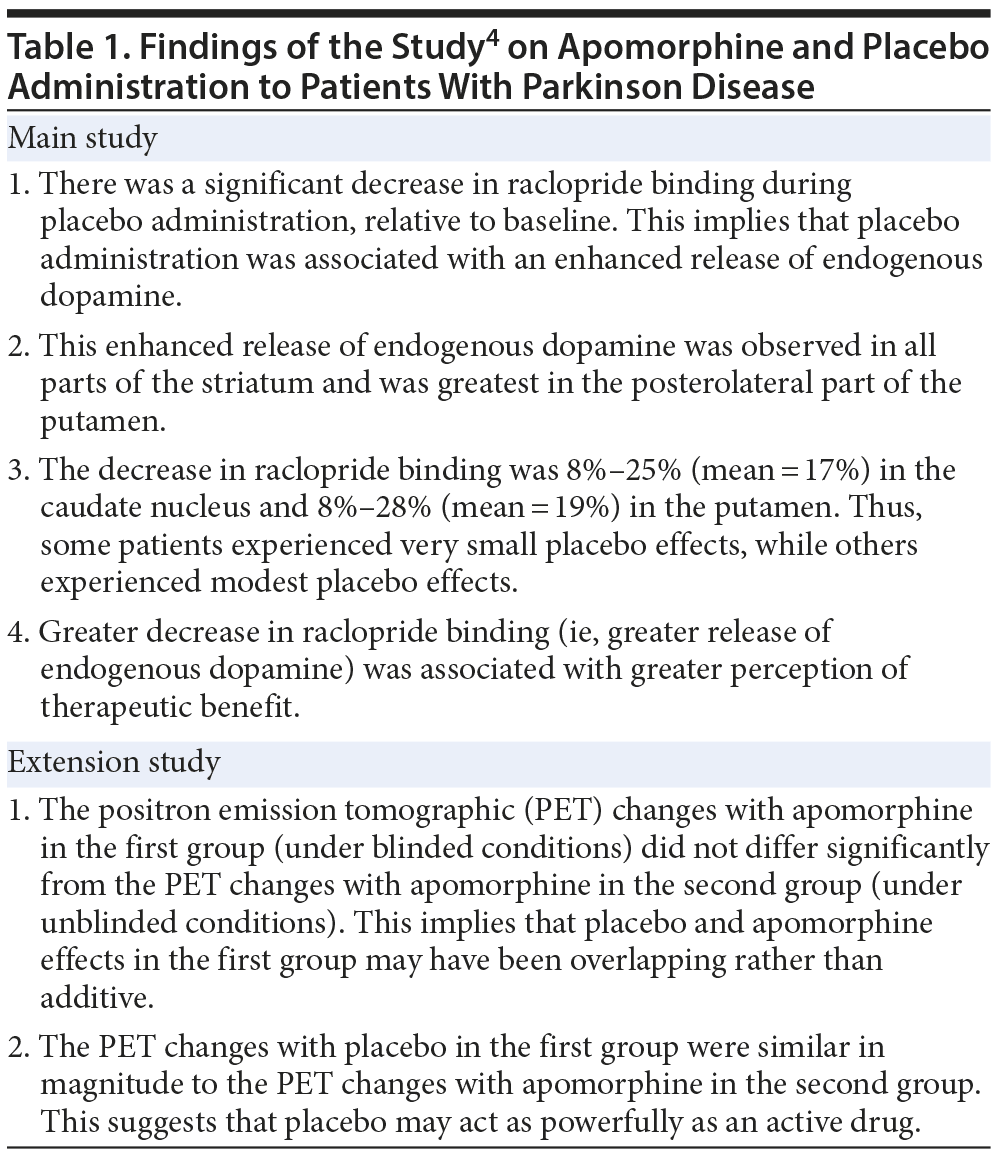
Over a decade ago, de la Fuente-Fernández et al4 presented landmark evidence for a biological mechanism of placebo in Parkinson disease. The sample comprised 6 patients with Parkinson disease. These patients were examined under 2 conditions: (1) an apomorphine versus placebo condition, in which patients did not know when they were receiving apomorphine and when they were receiving placebo, and (2) an apomorphine-only unblinded condition.
Subjects were studied using positron emission tomography (PET) and labeled raclopride. Raclopride binds to D2 and D3 dopamine receptors; greater raclopride binding, detected by PET, indicates lesser receptor occupation by endogenous dopamine. In an extension of this study, a second group of patients was studied. These patients were matched with the patients in the first group on age and severity of illness. All patients in the second group received apomorphine only, without blinding.
The findings of the study are summarized in Table 1. In brief, the study showed that, in Parkinson disease, placebo treatment under blinded conditions acts through release of dopamine in all parts of the striatum. Furthermore, the placebo effect possibly overlaps with and does not add to the effects of apomorphine.
Placebo in Parkinson Disease: The Effect of Treatment Cost
Very recently, Espay et al5 described a randomized crossover study that examined the influence of stated medication cost on the efficacy of a subcutaneously administered dopamine agonist (actually, placebo). These authors recruited 12 patients with Parkinson disease. All patients had levodopa-responsive motor fluctuations. The mean age of the sample was 62 years. The sample was 75% male. The mean duration of illness was 11 years.
These patients were assessed in an “off” state and then in an “on” state following the administration of levodopa. At the next study visit, the patients were randomized to receive 1 of 2 injections. About 4 hours later, when the effect of the first injection had worn off, they were crossed over to receive the other injection.
Patients were told that the injections were different formulations of the same dopamine agonist and that the study sought to prove that both were similar in efficacy although, for reasons related to manufacturing, one injection cost $100, and the other, $1,500. The patients did not know that both injections were actually placebo (saline). They also did not know the real objectives of the study. Unlike the patients, the assessment team was blinded to treatment allocation.
Both placebos improved treatment outcomes. Importantly, the more expensive placebo was associated with superior outcomes, but only when it preceded the less expensive placebo. Furthermore, brain activation during a functional magnetic resonance imaging task was greater with the less expensive placebo, implying the need for greater brain effort under the placebo condition of lower treatment expectations. The results are summarized in greater detail in Table 2.
Placebo and Analgesia: The Effect of Treatment Cost
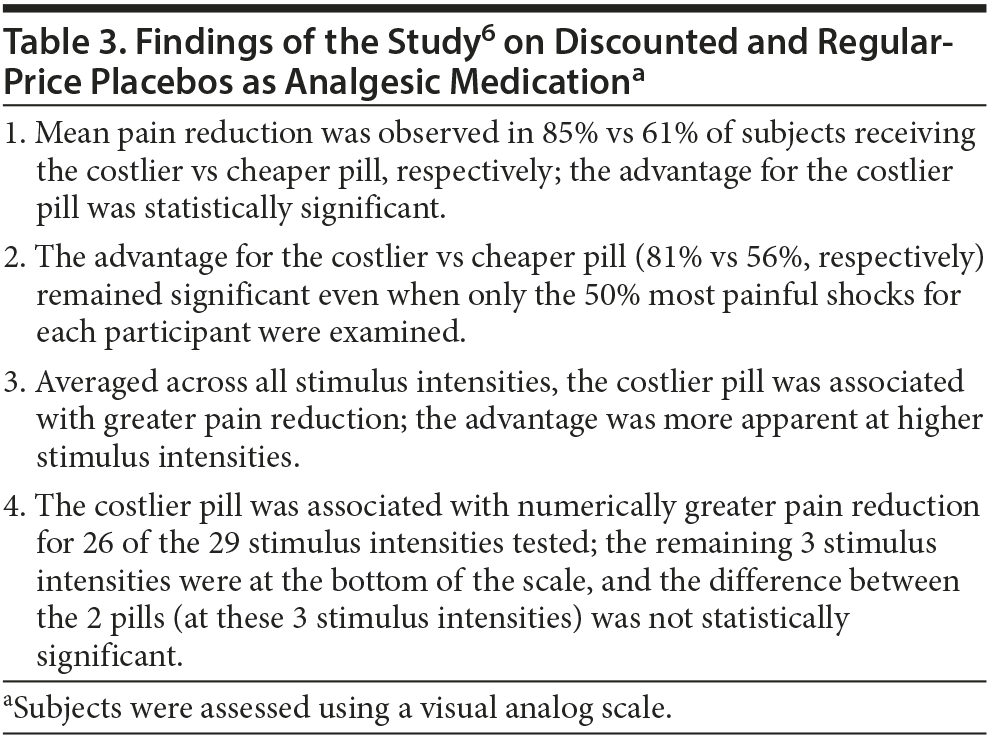
Waber et al6 recruited 82 healthy volunteers through an online advertisement. The mean age of the sample was about 30 years. The sample was 62% female. All subjects received electrical shocks to the wrist, calibrated to individual pain tolerance. All subjects also received a placebo pill with the information that the contained drug was similar to codeine but had a faster onset of action.
One half of the sample was told that the cost of treatment was $2.50 per pill. The other half was told that the cost was discounted to $0.10 per pill. Allocation to treatment group was random, and no reason was provided for the discounted cost. The subjects did not know the purpose of the study, and the study raters did not know the group to which the subjects had been randomized.
Subjects used a visual analog scale to rate pain at different intensities of electrical shock (mean = 18 shocks per subject). Important findings of the study are summarized in Table 3. In brief, analgesia was significantly greater with the regular-price pill than with the discounted pill.
Critical Appraisal and Clinical Importance
Both of the “placebo cost” studies5,6 found that placebo was more effective when its stated cost was higher. Perhaps greater expectations, generated by higher costs, mediate a greater placebo response. If this conclusion can be generalized, patients may equate lower costs with lesser treatment efficacy and thereby benefit less with generic drugs because these are cheaper than the branded originals. This concern is especially important in psychiatry because there is a strong placebo effect in many psychiatric disorders.7
Some points, however, merit consideration. The most important point is that both of the “placebo cost” studies reviewed5,6 were single-dose studies. There is no evidence to suggest that treatment cost will continue to influence treatment outcomes across weeks, months, or years of treatment, as is necessary in most psychiatric conditions. Next, both studies were conducted in the artificial setting of a laboratory rather than in the clinic, and the analgesia study6 was conducted in healthy volunteers who were paid for participation rather than in patients who had pain of clinical origin. Finally, no study has demonstrated the effect of treatment cost on clinical outcomes in psychiatric disorders, and no study has examined the effect of treatment cost using the same active drug instead of the same placebo.
Just as there are nonspecific factors in psychotherapy, there are nonspecific factors in psychopharmacology. Therefore, limitations notwithstanding, both studies5,6 remind clinicians that patient expectations are important and that treatment cost can, perhaps, influence treatment expectations and thereby treatment outcomes. Clinicians who prescribe generic drugs should therefore reassure patients that lower cost does not imply lower potency. Other concerns in the generics versus originals debate are out of the scope of this article and are not discussed here.
Parting Notes
For those who are interested, ethical issues related to the Parkinson disease “placebo cost” study5 were discussed by the authors of the study5 as well as in an accompanying editorial.8
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C. Drug impurities and adverse effects. Indian J Psychol Med. 1998;21(1):60-62.
2. Crawford P, Feely M, Guberman A, et al. Are there potential problems with generic substitution of antiepileptic drugs? a review of issues. Seizure. 2006;15(3):165-176. PubMed doi:10.1016/j.seizure.2005.12.010
3. Bagcchi S. Indian generic drugs debate heats up. Lancet. 2014;384(9951):1334. PubMed doi:10.1016/S0140-6736(14)61803-1
4. de la Fuente-Fernández R, Ruth TJ, Sossi V, et al. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164-1166. PubMed doi:10.1126/science.1060937
5. Espay AJ, Norris MM, Eliassen JC, et al. Placebo effect of medication cost in Parkinson disease: a randomized double-blind study. Neurology. 2015;84(8):794-802. PubMed doi:10.1212/WNL.0000000000001282
6. Waber RL, Shiv B, Carmon Z, et al. Commercial features of placebo and therapeutic efficacy. JAMA. 2008;299(9):1016-1017. PubMed doi:10.1001/jama.299.9.1016
7. Andrade C. There’s more to placebo-related improvement than the placebo effect alone. J Clin Psychiatry. 2012;73(10):1322-1325. PubMed doi:10.4088/JCP.12f08124
8. LeWitt PA, Kim S. The pharmacodynamics of placebo: expectation effects of price as a proxy for efficacy. Neurology. 2015;84(8):766-767. PubMed doi:10.1212/WNL.0000000000001294


 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.



