Objective: Major depressive disorder may be due to psychoneuroimmunological dysfunction, as studies have documented increased levels of a variety of inflammatory mediators in depressed subjects. Nitric oxide (NO) is marker of inflammation, and fractional exhaled NO (FeNO) is a marker of airway inflammation. Plasma NO and FeNO levels have been shown to be lower in subjects with depression in small studies. We sought to assess the association of depression with C-reactive protein (CRP) and FeNO levels in a large and representative sample of the US population.
Methods: Population-based cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES). NHANES collects health information about the US population through interviews, medical examinations, and laboratory tests. We included subjects ≥ 20 years old who participated in NHANES in 2007 to 2012, responded to the depression questions, and had CRP values or ≥ 2 reproducible FeNO measures. Depression was measured using the 9-item Patient Health Questionnaire (PHQ-9). Subjects were classified as depressed if PHQ-9 scores were ≥ 10. FeNO and CRP levels were log transformed. Unadjusted and adjusted regression analyses were conducted.
Results: A total of 14,276 subjects responded to the PHQ-9, and 7.73% had depressive symptoms. Of these subjects, 10,036 had CRP values and 12,513 had FeNO measurements. Subjects with depressive symptoms had, after adjustment, CRP levels that were 31% higher (95% confidence interval [CI], 14% to 50%) and FeNO levels that were 10.7% lower (95% CI, −2.5% to −17.1%) than in subjects with no depressive symptoms.
Conclusions: Depression is associated with high CRP levels and low FeNO levels. Of importance, this study (1) assesses the association of depression with CRP and exhaled NO levels in a large and representative sample of the US population, (2) contributes to the neuroimmunological dimension of depression, (3) confirms the association of depression with high levels of CRP, and (4) assesses, for the first time, the association of depression with peripheral NO in more than 10,000 subjects from the general population.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
See letter by Oxenkrug
Depression Is Associated With High Levels of C-Reactive Protein and Low Levels of Fractional Exhaled Nitric Oxide:
Results From the 2007-2012 National Health and Nutrition Examination Surveys

ABSTRACT
Objective: Major depressive disorder may be due to psychoneuroimmunological dysfunction, as studies have documented increased levels of a variety of inflammatory mediators in depressed subjects. Nitric oxide (NO) is marker of inflammation, and fractional exhaled NO (FeNO) is a marker of airway inflammation. Plasma NO and FeNO levels have been shown to be lower in subjects with depression in small studies. We sought to assess the association of depression with C-reactive protein (CRP) and FeNO levels in a large and representative sample of the US population.
Methods: Population-based cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES). NHANES collects health information about the US population through interviews, medical examinations, and laboratory tests. We included subjects ≥ 20 years old who participated in NHANES in 2007 to 2012, responded to the depression questions, and had CRP values or ≥ 2 reproducible FeNO measures. Depression was measured using the 9-item Patient Health Questionnaire (PHQ-9). Subjects were classified as depressed if PHQ-9 scores were ≥ 10. FeNO and CRP levels were log transformed. Unadjusted and adjusted regression analyses were conducted.
Results: A total of 14,276 subjects responded to the PHQ-9, and 7.73% had depressive symptoms. Of these subjects, 10,036 had CRP values and 12,513 had FeNO measurements. Subjects with depressive symptoms had, after adjustment, CRP levels that were 31% higher (95% confidence interval [CI], 14% to 50%) and FeNO levels that were 10.7% lower (95% CI, −2.5% to −17.1%) than in subjects with no depressive symptoms.
Conclusions: Depression is associated with high CRP levels and low FeNO levels. Of importance, this study (1) assesses the association of depression with CRP and exhaled NO levels in a large and representative sample of the US population, (2) contributes to the neuroimmunological dimension of depression, (3) confirms the association of depression with high levels of CRP, and (4) assesses, for the first time, the association of depression with peripheral NO in more than 10,000 subjects from the general population.
J Clin Psychiatry 2016;77(12):1666-1671
dx.doi.org/10.4088/JCP.15m10267
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Epidemiology, Janssen Research & Development, LLC, Titusville, New Jersey
*Corresponding author: M. Soledad Cepeda, MD, PhD, Janssen Research & Development, LLC, 1125 Trenton Harbourton Rd, Titusville, NJ 08560 ([email protected]).
Major depressive disorder may be viewed as a psychoneuroimmunological disorder1 that includes not only the traditional monoamine deficiency2 but also a persistent low-grade inflammation.3 Subjects with major depressive disorder have increased levels of a variety of inflammatory markers compared with those subjects who are not depressed,3-5 and administration of cytokines has been shown to induce behavioral changes that are consistent with the symptoms of major depressive disorder.6
High levels of C-reactive protein (CRP), a marker of inflammatory disease, have been documented in subjects with depression.3 However, this association of elevated levels of CRP with depression has been attenuated in some studies after controlling for body mass index and chronic medical conditions.3
Nitric oxide (NO), in addition to being an inflammatory mediator, is also a neurotransmitter at the neuron synapses.7 It modulates norepinephrine, serotonin, dopamine, and glutamate and thus is speculated to play a role in the pathogenesis of depression.8 Nitric oxide is also currently seen as a marker of airway inflammation and can be measured during exhalation.9 Fractional exhaled nitric oxide (FeNO) may represent both constitutive and inducible NO.10,11 Small studies suggest that subjects with depressed mood have low levels of FeNO.10,12
As the measure of exhaled NO has been standardized for clinical use, we sought to assess the association of depression with CRP and FeNO levels in a large and representative sample of the US population to shed light on the premise that neuroimmunological dysfunction could be part of the major depressive disorder pathophysiology.
METHODS
We designed and conducted a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES). NHANES is a research program that collects health information about the US population through interviews, medical examinations, and laboratory tests.13 NHANES has all the information needed for our analysis: participants respond to a validated depression screener questionnaire, provide blood samples to quantify CRP, and undergo a respiratory health examination that includes measurement of FeNO levels in the last 2 recent survey cycles, and the study findings are generalizable to the US population.
We included subjects ≥ 20 years old who participated in NHANES during the period 2007 to 2012, responded to the depression questions, and had CRP or at least 2 reproducible FeNO measures.
Depression was measured in NHANES with the Patient Health Questionnaire (PHQ-9). The PHQ-9 is a 9-item depression screening instrument that asks participants to choose 1 of 4 responses about frequency of depressive symptoms during the previous 2 weeks.14 Subjects were classified as having depressive symptoms if PHQ-9 scores were ≥ 10. Scores ≥ 10 represent moderate or severe depressive symptoms.15
CRP Measurement
All adult survey participants were eligible to provide blood samples. NHANES CRP measurements were processed at the University of Washington, Seattle, Washington. CRP levels were quantified using latex-enhanced nephelometry. Particle-enhanced assays were based on the reaction between a soluble analyte and the corresponding antigen or antibody bound to polystyrene particles. For the quantification of CRP, particles consisting of a polystyrene core and a hydrophilic shell were used to link anti-CRP antibodies covalently. A dilute solution of test sample was mixed with latex particles coated with mouse monoclonal anti-CRP antibodies. CRP present in the test sample forms an antigen antibody complex with the latex particles.16

- Assessing the association of depression with C-reactive protein and nitric oxide in a large number of subjects representative of the US population will increase the understanding of the pathophysiology of depression.
- Analysis of data from National Health and Nutrition Examination Surveys showed that depression is associated with high levels of C-reactive protein and low levels of fractional exhaled nitric oxide.
- Inflammation could play a role in the pathophysiology of depression, and depression may be seen as a psychoneuroimmunological disorder.
FeNO Measurement
Adult survey participants were eligible for FeNO testing. Participants who had current chest pain or a physical problem with forceful expiration or those who were using supplemental oxygen were excluded from participation.
FeNO was measured in NHANES using the Aerocrine NIOX MINO, a portable, hand-held NO analyzer (Aerocrine AB, Solna, Sweden) approved by the US Food and Drug Administration in 2008. This device relies on an electrochemical sensor to detect exhaled NO levels and provides measurements from 5 to 300 parts per billion (ppb) in whole numbers. The testing was conducted with participants sitting in front of a mirror so that they could see display prompts on the NO analyzer screen. Holding the device, participants were first asked to empty their lungs and then to place their mouth on the analyzer’s disposable filter mouthpiece and to fill their lungs to capacity with NO-free air. Participants were then asked to blow out all of their air at a constant pressure while exhaled NO from the bronchial tree was measured. The device automatically provides auditory and visual cues to assist in performing the test correctly. The NHANES protocol required 2 FeNO measurements that were reproducible, in accordance with testing procedures recommended by the manufacturer and similar to those published by the American Thoracic Society and European Respiratory Society.17
CRP Level Association With Depressive Symptoms
To assess the association of CRP levels with depressive symptoms, we conducted 2 types of analyses. In one, we analyzed the levels of CRP as a continuous variable, and, in the other, we categorized CRP into 4 categories of increasing CRP levels: ≤ 1.00 mg/L, 1.01-3.00 mg/L, 3.01-10.00 mg/L, and > 10.00 mg/L. This categorization has been used in previous studies of CRP and depression.3
To assess the association of depressive symptoms with CRP levels, we built linear regression models using log-transformed CRP levels as the outcome. CRP levels were log transformed to approximate normality because CRP levels were skewed. In the unadjusted analysis, the only covariate included was depressive symptoms as a binary Yes/No variable. For the adjusted analyses, we added age and body mass index (BMI) as continuous variables and gender, self-reported history of diabetes or renal disease, and a composite variable that included presence of self-reported inflammatory arthritis (eg, rheumatoid arthritis), cardiovascular conditions (coronary arteriosclerosis, acute myocardial infarction, angina, or heart failure), cancer and celiac disease, and medications to the model. Medications were grouped at level 4 of the hierarchy of the Enhanced Therapeutic Classification (ETC; http://www.fdbhealth.eu/international-drug-knowledge-classifications/). The medications included were anti-inflammatory analgesic agents, cardiovascular agents, and endocrine agents. All of these variables have been associated with CRP levels.18-20 We also included the PHQ-9 score as a continuous variable to the adjusted analysis, instead of having it as depressive symptoms Yes/No.
To assess the association of depressive symptoms with varying categories of CRP, we built a logistic regression model with depressive symptoms as the outcome variable and included the categories of CRP, with the lowest category as a reference category, in addition to the variables described above for the linear regression analysis as covariates. We report odds ratios (ORs) and 95% confidence intervals (CIs).
FeNO Level Association With Depressive Symptoms
To assess the association of depressive symptoms with FeNO levels, we built linear regression models using log-transformed FeNO levels. FeNO levels were log transformed to approximate normality because FeNO levels were skewed. In the unadjusted analysis, the only covariate included was depressive symptoms as a binary Yes/No variable. For the adjusted analyses, we added to the model: age and BMI as continuous variables and gender, race, self-reported asthma (including hay fever, chronic bronchitis), emphysema (a variable that indicated if the participant was a smoker), and medications. The medications were grouped at level 4 of the hierarchy of ETC with the exception of antidepressants that were entered as level 2 of the hierarchy. The medications included were respiratory agents, anti-inflammatory analgesic agents, and endocrine agents, which include corticosteroids. Participants were classified as smokers if they reported smoking more than 100 cigarettes in a lifetime. These variables have been known to affect FeNO levels.9,21 We also included variables reflecting behaviors immediately prior to the assessment that could acutely affect FeNO levels: self-report indicating if the participant smoked, exercised during the hour prior to the FeNO measurement, or consumed food rich in NO. Subjects were provided with the list of food items rich in NO when asked the questions.
In addition, we included the PHQ-9 score as a continuous variable to the adjusted analysis, instead of having it as depressive symptoms Yes/No.
For the linear regression analyses, we report the coefficients of a log-transformed variable and also the exponentiated coefficient. The exponentiated coefficient can be converted into a percent change in CRP or FeNO levels associated with being depressed. To help provide perspective on the magnitude of the effect of depressive symptoms, we also report the effect of asthma on FeNO levels.
Association Between FeNO Levels and CRP Levels
To assess the association between FeNO levels and CRP levels, we built a linear regression model and included age, gender, BMI, and smoking history, in addition to the FeNO and CRP levels. FeNO and CRP levels were both log transformed.
To correctly account for the complex survey design, all the analyses included the Primary Sampling Unit variable (sdmvpsu) as the stratification variable (sdmvstra) and the Mobile Examination Center (MEC) examination variable (wtmec2y) as the weight variable for the CRP. For the FeNO analyses, we multiplied the weight variable by one-third, because we included 3 survey periods. STATA SE version 12.1 (Stata Corp LP) was used to conduct the analyses.
Each of the NHANES surveys has been approved by the National Center for Health Statistics Research Ethics Review Board. NHANES releases anonymized coded survey data to the public. These are the data used in the present study.
RESULTS
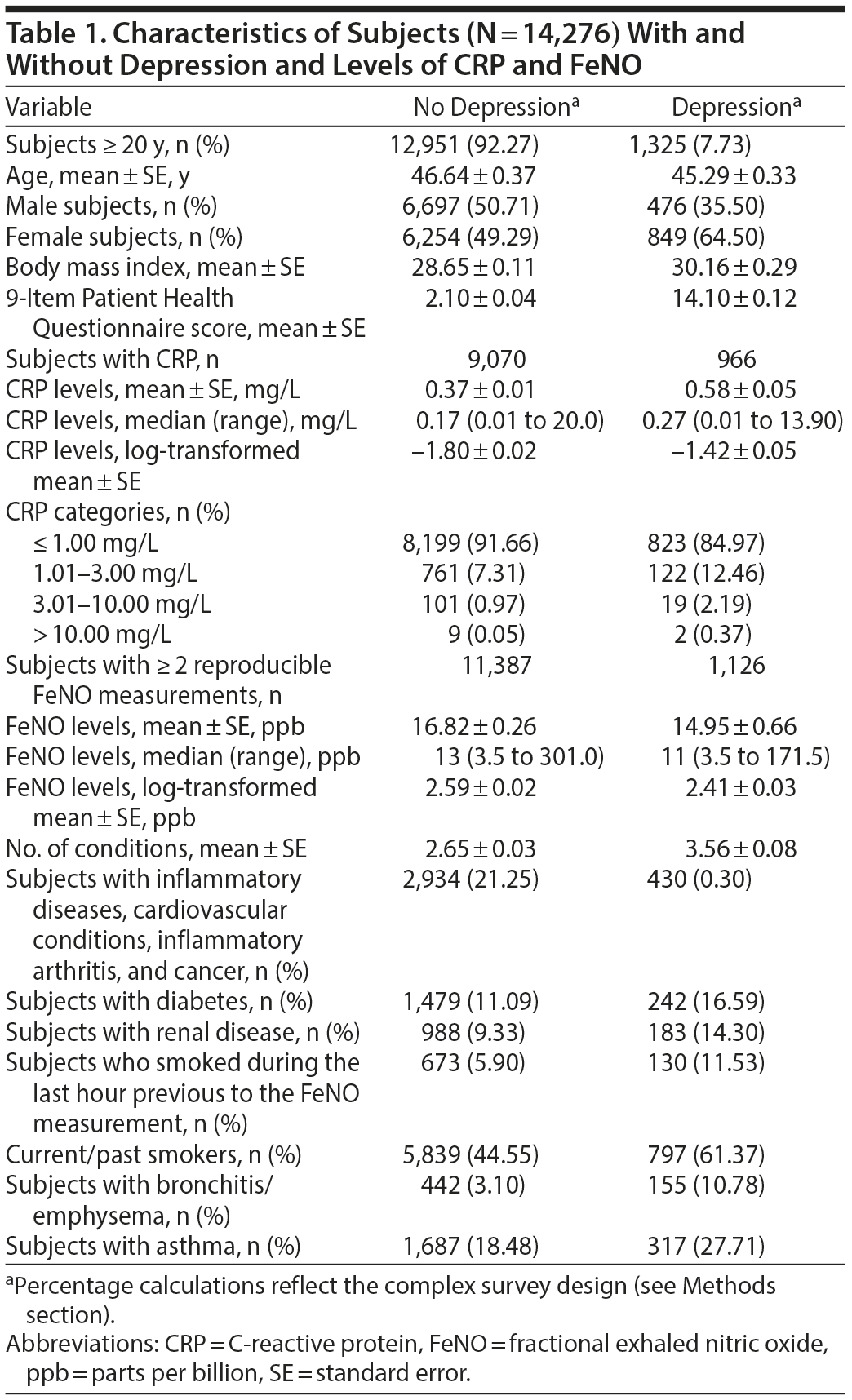
A total of 14,276 subjects responded to the PHQ-9, and 7.73% had depressive symptoms (Table 1). In terms of severity of depressive symptoms, 77.31% had scores < 5, 14.96% had scores between 5 and 9 (inclusive), 4.82% had scores between 10 and 14, 2.13% had scores between 15 and 19, and 0.78% had scores > 20.
Of the subjects classified as depressed, 46.06% were taking antidepressants. Subjects with depressive symptoms were more likely to be women and had more medical conditions than subjects with no depressive symptoms (Table 1).
CRP Levels
A total of 10,036 subjects had CRP values. CRP levels were not normally distributed. The distribution had a skewness of 7.58 and a kurtosis of 95.12. Natural logarithm transformation normalized the distribution (skewness = 0.04, kurtosis = 2.79).
Subjects with depressive symptoms were more likely to have conditions that could affect CRP levels, such as self-reported history of inflammatory conditions, diabetes, and renal diseases (Table 1).
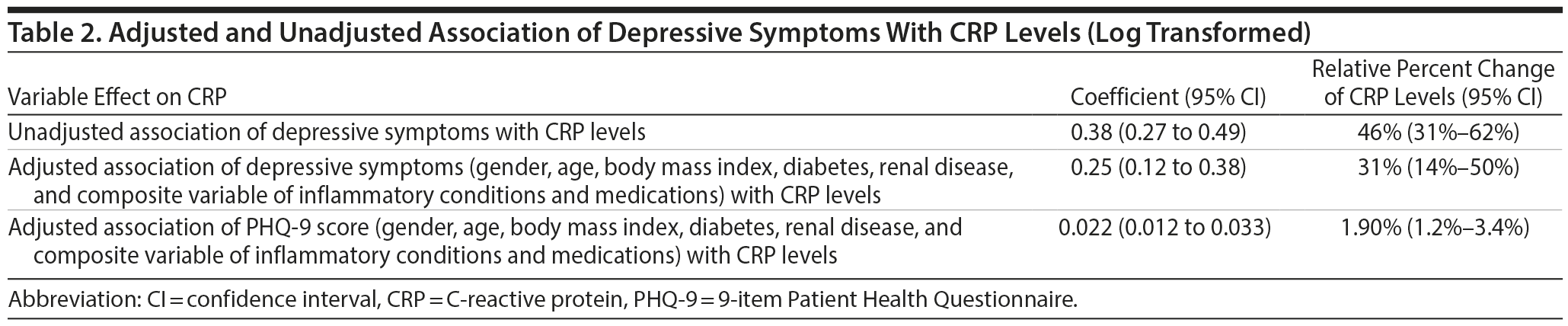
Subjects with depressive symptoms had CRP levels that were 46% higher than those of nondepressed subjects. The elevated levels persisted after adjustment, as subjects with depressive symptoms still had levels of CRP that were 31% higher (95% CI, 14% to 50%) than subjects with no depressive symptoms (Table 2). The analysis using the PHQ-9 score provided similar results, ie, there was a 1.90% increase in levels of CRP for each unit increase in PHQ-9 score (Table 2).
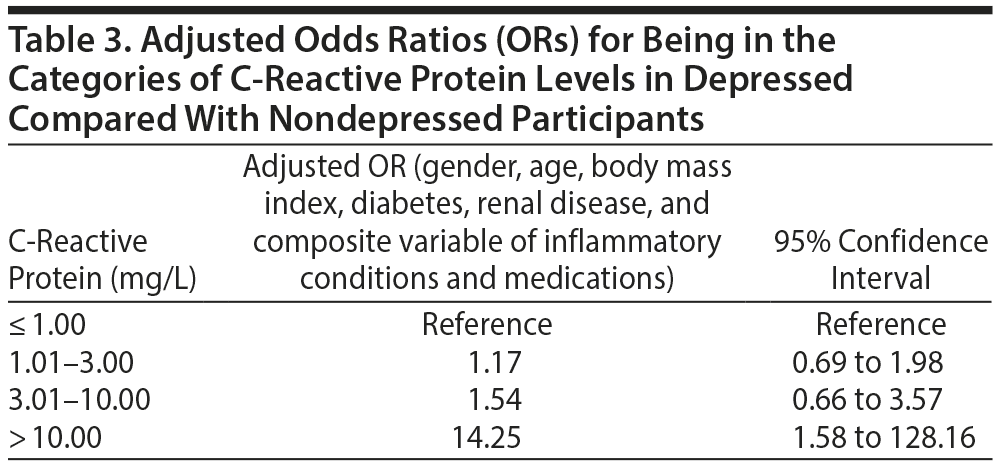
Almost all of the population (98.86%) had levels of CRP ≤ 3.0, but a larger proportion of subjects with depressive symptoms were in the high CRP categories. Subjects with depressive symptoms had much greater odds of being in the category with the highest CRP levels, > 10 mg/L. The adjusted OR for being in such a CRP category was 14.25 (95% CI, 1.58 to 128.16) (Table 3).
FeNO Levels
A total of 12,513 subjects had at least 2 reproducible FeNO measurements. FeNO levels were not normally distributed (skewness = 4.58, kurtosis = 47.54) but became normalized after natural logarithm transformation (skewness = 0.16, kurtosis = 3.32).
Subjects with depressive symptoms were more likely to smoke before the FeNO measurement, more likely to report a history of smoking, and more likely to have conditions that could affect the FeNO levels such as asthma, chronic bronchitis, and emphysema.
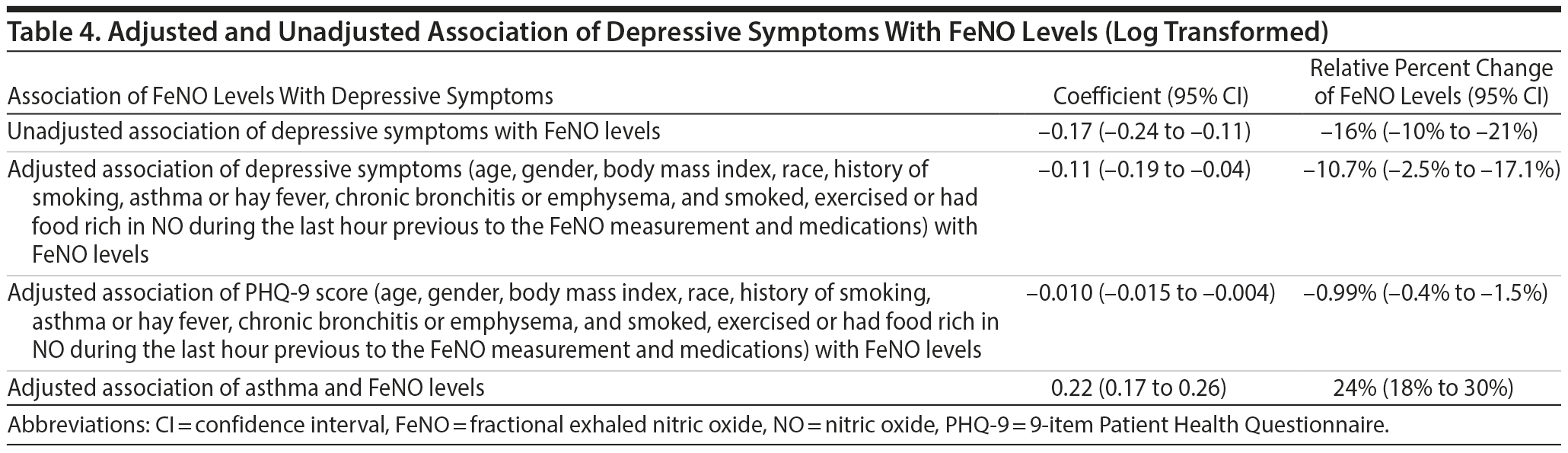
Subjects with depressive symptoms had FeNO levels that were 16% lower than those of subjects without depressive symptoms (Table 4). This relationship persisted after adjustment, as subjects with depressive symptoms continued to have lower FeNO levels (10.7% lower; 95% CI, −2.5% to −17.1%) (Table 4). Mean FeNO levels in subjects taking antidepressants were similar to FeNO levels of subjects not taking antidepressants (2.58 versus 2.61, respectively; P = .26). The analysis using the PHQ-9 score provided similar results, ie, there is a 0.99% decrease in FeNO levels for each unit increase in PHQ-9 score.
Subjects with asthma had FeNO levels that after adjustment were 24% higher than those of subjects who did not report asthma (Table 4).
Association Between FeNO Levels and CRP Levels
The negative correlation between FeNO and CRP levels was no longer statistically significant once smoking history was added in the regression model. The P value moved from .001 to a P value of .09.
DISCUSSION
We found that depressive symptoms were associated with high levels of CRP. Our findings support a body of evidence suggesting that depressive symptoms are associated with high levels of CRP and that the association remains after adjustment.18,19
We analyzed FeNO levels in more than 10,000 subjects and found that depressive symptoms were associated with low levels of FeNO. Small studies have showed that subjects with depressive mood have lower levels of FeNO.10,12 Nitric oxide in exhaled air represents 2 sources: inducible and constitutive. Elevated FeNO levels have been associated with high risk of airway inflammation,9 and the raised exhaled NO concentration observed in subjects with asthma is quite likely derived from inducible NO synthase. In the absence of airway inflammation, FeNO levels represent constitutive NO.11,22
Subjects with depression also have low levels of plasma and platelet NO.23,24 The low systemic levels of NO have been postulated to be responsible for the increased risk of cardiovascular events observed in subjects with depression,24 as NO produces vasodilatation.
The low FeNO levels and high CRP levels observed in subjects with depressive symptoms could be seen as paradoxical. The complex role of NO in inflammation, its dual role, and complex interactions can explain the findings. Nitric oxide exerts several modulating effects; it regulates humoral and cellular responses and has both anti-inflammatory and proinflammatory properties.25,26 Also, depression and chronic stress could decrease levels of NO through increases in oxidative stress or through increases in levels of arginase, an enzyme that would interfere with NO production.21
Animal studies suggest the role of NO in depression8: (1) lower levels of NO in the brain induce antidepressant-like effects, (2) NO exerts a negative control over levels of serotonin and dopamine in the brain, and (3) some antidepressants decrease central levels of NO. However, these findings are at odds with the present study findings whereby subjects with depression had lower FeNO levels than did nondepressed subjects.
The studies, including ours, that have shown an association of depression with low levels of NO have all measured NO in the periphery and not in the central nervous system, where NO modulates neurotransmission activity. The inability to make direct measurements of NO in the central nervous system makes it difficult to understand the role of NO in the pathophysiology of depression.
The magnitude of the decrease in FeNO levels in subjects with depressive symptoms is half of the magnitude of the change in FeNO levels seen in subjects with asthma,9 but it could be much smaller based on variability of the estimate. Therefore, the clinical relevance of the decrease in FeNO levels observed in subjects with depressive symptoms is unclear. Nonetheless, the study findings corroborate prior evidence that subjects with depression exhibit lower levels of NO in the periphery.
To measure depression, we used the PHQ-9; although scores ≥ 10 had an 88% sensitivity and specificity15 for major depression, depression was not formally diagnosed by a mental health professional. Errors in depression classification could lead to underestimation of the association of depression with CRP and FeNO levels. The association of depressive symptoms with high CRP levels and low FeNO levels could reflect the health status of subjects with depressive symptoms. Subjects with depressive symptoms had more medical conditions and risk factors known to be associated with elevated CRP, such as inflammatory conditions, or associated with abnormal levels of FeNO, such as being a smoker or having asthma. Although the associations remained after adjustment, the findings could still reflect the poorer health conditions of subjects with depressive symptoms. On the other hand, depression could be the driver for the occurrence of the comorbidities due to its contribution to increased neuroinflammatory and systemic inflammatory burden,27 making adjustment unnecessary.
Interesting is the finding that a large number of subjects with moderate or severe depression were not taking antidepressants. A previous report using 2005 to 2008 NHANES data found that almost 40% of subjects with severe depression reported receiving neither treatment from a mental health professional nor an antidepressant.14 As described above, our study and the latter report14 used the PHQ-9 to classify subjects instead of a formal diagnosis; thus, the subjects may not have had major depressive disorder.
The use of the NHANES data in the current study offers many advantages: first, a population that included a representative sample of the US population and assures coverage of individuals often excluded or underrepresented from studies (eg, minorities), and therefore the ability to generalize the study findings to the US population; second, the large number of subjects who responded to a validated depression screening questionnaire and had CRP and FeNO information; third, high-quality data, due to strict quality control and quality assurance protocols; fourth, the use of standardized analyses of laboratory results, which decreases variability of results; and last, transparency, as the NHANES data are made publicly available, and thus the results can be replicated.
In summary, this large population-based study found that depression is associated with high levels of CRP and low levels of FeNO. These findings corroborate the premise that inflammation could play a role in the pathophysiology of major depression and that major depression may be seen as a psychoneuroimmunological disorder. Because the study provides a snapshot, we cannot determine whether the role of inflammation is causal or reactive.
Submitted: July 27, 2015; accepted September 30, 2015.
Online first: June 21, 2016.
Potential conflicts of interest: The authors are employees of Janssen Research & Development, LLC. The authors have no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Funding/support: This study was funded by Janssen Research & Development, LLC.
Role of the sponsor: The study sponsor, as employer, was involved in the conduct of the study and the writing and submission of the manuscript.
REFERENCES
1. Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135-151. PubMed doi:10.1007/7854_2012_211
2. Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155-161. PubMed doi:10.1002/j.2051-5545.2010.tb00298.x
3. Wium-Andersen MK, טrsted DD, Nielsen SF, et al. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry. 2013;70(2):176-184. PubMed doi:10.1001/2013.jamapsychiatry.102
4. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. PubMed
5. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467-475. PubMed doi:10.1007/s11920-011-0232-0
6. Miller AH, Raison CL. Immune system contributions to the pathophysiology of depression. Focus. 2008;6(1):36-45. doi:10.1176/foc.6.1.foc36
7. Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15(6):252-259. PubMed doi:10.1007/s10787-007-0013-x
8. Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide. 2011;24(3):125-131. PubMed doi:10.1016/j.niox.2011.02.002
9. See KC, Christiani DC. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: results from the National Health and Nutrition Examination Survey 2007-2010. Chest. 2013;143(1):107-116. PubMed doi:10.1378/chest.12-0416
10. Ritz T, Trueba AF, Simon E, et al. Increases in exhaled nitric oxide after acute stress: association with measures of negative affect and depressive mood. Psychosom Med. 2014;76(9):716-725. PubMed doi:10.1097/PSY.0000000000000118
11. Lane C, Knight D, Burgess S, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59(9):757-760. PubMed doi:10.1136/thx.2003.014894
12. Trueba AF, Smith NB, Auchus RJ, et al. Academic exam stress and depressive mood are associated with reductions in exhaled nitric oxide in healthy individuals. Biol Psychol. 2013;93(1):206-212. PubMed doi:10.1016/j.biopsycho.2013.01.017
13. National Health and Nutrition Examination Survey. CDC Web site. http://www.cdc.gov/nchs/nhanes.htm. Updated 2015. Accessed July 9, 2015.
14. Shim RS, Baltrus P, Ye J, et al. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005-2008. J Am Board Fam Med. 2011;24(1):33-38. PubMed doi:10.3122/jabfm.2011.01.100121
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
16. 2009-2010 Data Documentation, Codebook, and Frequencies: C-Reactive Protein. CDC Web site. http://www.cdc.gov/nchs/nhanes/nhanes2009-2010/CRP_F.htm. Updated September 2011. Accessed March 24, 2016.
17. 2011-2012 Data Documentation, Codebook, and Frequencies: Exhaled Nitric Oxide. CDC Web site. http://wwwn.cdc.gov/nchs/nhanes/2011-2012/ENX_G.htm. Updated February 2014. Accessed March 24, 2016.
18. Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164(9):1010-1014. PubMed doi:10.1001/archinte.164.9.1010
19. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171-186. PubMed doi:10.1097/PSY.0b013e3181907c1b
20. Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24(1):33-50. PubMed doi:10.1111/j.1527-3466.2006.00033.x
21. Ritz T, Trueba AF. Airway nitric oxide and psychological processes in asthma and health: a review. Ann Allergy Asthma Immunol. 2014;112(4):302-308. PubMed doi:10.1016/j.anai.2013.11.022
22. Yates DH, Kharitonov SA, Thomas PS, et al. Endogenous nitric oxide is decreased in asthmatic patients by an inhibitor of inducible nitric oxide synthase. Am J Respir Crit Care Med. 1996;154(1):247-250. PubMed doi:10.1164/ajrccm.154.1.8680689
23. Garc×a RG, Zarruk JG, Barrera C, et al. Plasma nitrate levels and flow-mediated vasodilation in untreated major depression. Psychosom Med. 2011;73(4):344-349. PubMed doi:10.1097/PSY.0b013e31821566cf
24. Chrapko WE, Jurasz P, Radomski MW, et al. Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry. 2004;56(2):129-134. PubMed doi:10.1016/j.biopsych.2004.03.003
25. Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann Med. 1995;27(3):359-367. PubMed doi:10.3109/07853899509002589
26. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469-487. PubMed
27. Maes M, Kubera M, Obuchowiczwa E, et al. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32(1):7-24. PubMed
Save
Cite
Advertisement
GAM ID: sidebar-top