ABSTRACT
Objective: To perform a meta-analysis of hepatitis C virus (HCV) prevalence in people with serious mental illness (SMI) and to systematically review barriers to care with the contention that both individual complications and HCV community transmission can be reduced with enhanced health care strategies.
Data Sources: PubMed, Scopus, Embase, CINAHL, and Web of Science were searched for articles published in English between April 21, 1989, and July 1, 2020. The terms Hepatitis C Virus, HCV, HCV seroprevalence, and HCV prevalence were cross-referenced with serious mental illness, severe mental illness, psychiatric illness, mental illness, and psychiatric patients.
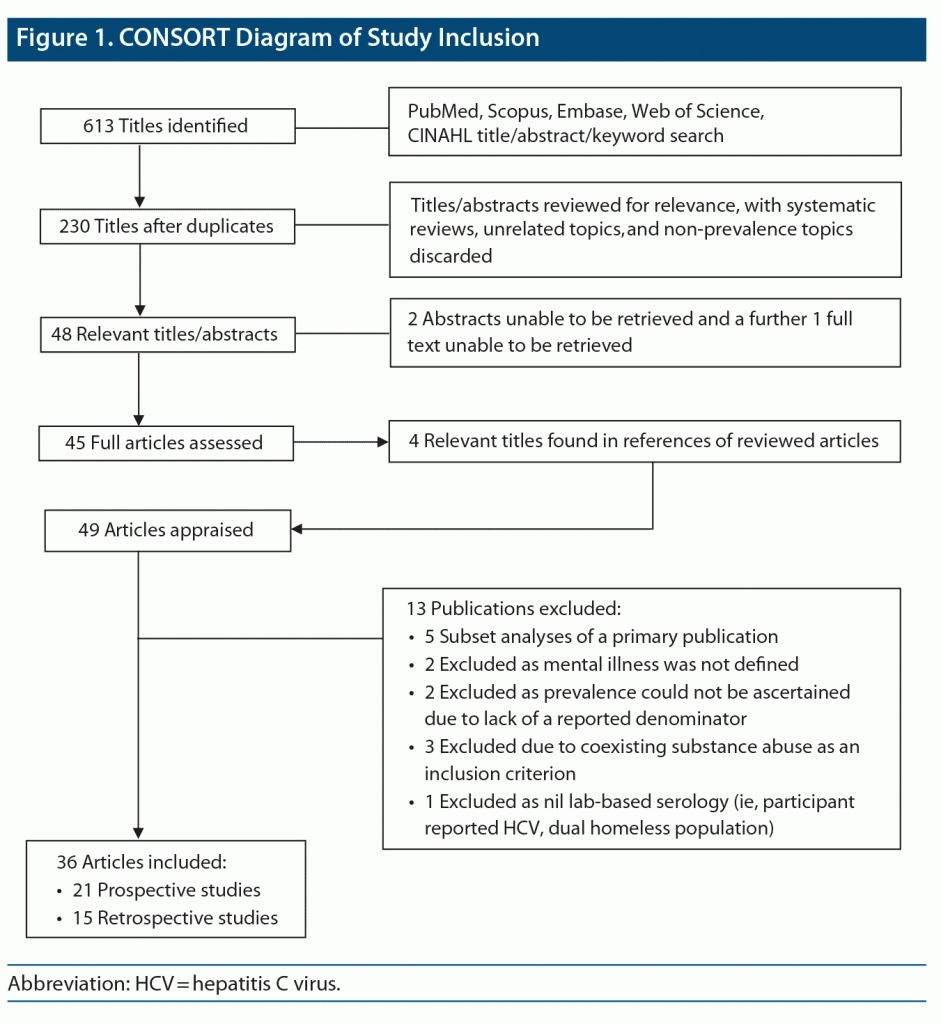
Study Selection: We identified 230 titles after removing duplicates. The final analysis included 36 publications drawn from prospective and large retrospective cohort studies that cross-sectionally screened for HCV in people with SMI ≥ 18 years of age.
Data Extraction: Pooled HCV prevalence was analyzed, with random effects modeling due to significant attributable study heterogeneity. Demographic data and HCV risk factors were subanalyzed. Qualitative and semiqualitative data relating to control cohort prevalence and the HCV care cascade were also extracted.
Results: The pooled HCV prevalence was 8.0% (95% CI, 6.0%–9.0%). Subanalysis of prospective studies (n = 9,015 individuals) demonstrated a similar prevalence, 8.0% (CI, 5.0%–11.0%), to retrospective studies (n = 289,247), 8.0% (CI, 6.0%–10.0%). HCV was 3.0- to 11.3-fold higher in people with SMI relative to controls. Semiqualitative analysis of seropositive cases showed that (1) 20.0%–58.1% did not have an identified HCV risk factor; (2) 12.5%–100% of cases were not previously known to have HCV; and (3) the majority, 57.0%–96.6%, of people with SMI were receptive to HCV screening.
Conclusions: People with SMI have high HCV seroprevalence and should be recognized as a priority group for HCV screening and health care access.
J Clin Psychiatry 2022;83(1):21r14079
To cite: Braude MR, Phan T, Dev A, et al. Determinants of hepatitis C virus prevalence in people with serious mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2022;83(1):21r14079.
To share: https://doi.org/10.4088/JCP.21r14079
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Gastroenterology and Hepatology, Monash Health, Level 3, 246 Clayton Rd, Clayton, Victoria, Australia
bSchool of Clinical Sciences, Monash University, Wellington Rd, Clayton, Victoria, Australia
*Corresponding author: Michael Rudi Braude, MSc, MBBS, Department of Gastroenterology and Hepatology, Monash Health, 246 Clayton Rd, Clayton, Victoria, Australia, 3168 ([email protected]).
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. This activity is free.
CME Objective
After studying this article, you should be able to:
- Include screening for hepatitis C virus as part of the physical health workup in people with serious mental illness
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
Release, Expiration, and Review Dates
This educational activity was published in December 2021 and is eligible for AMA PRA Category 1 Credit™ through February 29, 2024. The latest review of this material was November 2021.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past 3 years, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the Independent Data Safety and Monitoring Committee for Janssen (Johnson & Johnson) and Novartis; and has served on advisory boards for Eliem and Sage. As an employee of Massachusetts General Hospital (MGH), Dr Freeman works with the MGH National Pregnancy Registry, which receives funding from Alkermes, Aurobindo, AuroMedics, Johnson & Johnson/Janssen, Otsuka, Sage, Sunovion, Supernus, and Teva, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
Hepatitis C virus (HCV) is a high morbidity, high mortality condition that affects approximately 1% of the global population.1 Approximately 399,000 deaths occur annually due to either HCV-related cirrhosis or hepatocellular carcinoma, and HCV presently accounts for 27% of cirrhosis and 25% of hepatocellular carcinoma globally.1,2 Liver sequelae aside, HCV can impact quality of life as a consequence of neuropsychiatric and ancillary extrahepatic disease manifestations.3 However, HCV is now curable, and the prospect of global HCV eradication is achievable through the advent of highly effective, highly tolerable, oral direct-acting antiviral (DAA) therapies.4 Achieving HCV elimination is predicated upon universal health coverage, which requires equitable and financially sustainable health care.5 However, significant barriers exist, particularly in high-risk transmission populations, such as people who inject drugs (PWID). Providing DAA treatment to PWID is considered an essential strategy toward interrupting HCV transmission.6 Decentralized HCV elimination strategies in homeless, incarcerated, and opioid-substitution therapy (OST) cohorts have therefore been prioritized.7
In contrast, SMI has fundamentally been underrecognized as a priority cohort for HCV screening and management.8 HCV seroprevalence in people with SMI ranges from 3.0% (95% CI, 1.8%–5.0%) in Central and South America to 17.4% (95% CI, 13.2%–22.6%) in North America.9 Despite the high prevalence, many mental health settings do not screen for HCV or other blood-borne viruses (BBVs).8 This stems from underrecognized HCV prevalence, lack of clinician confidence, ambiguity as to whether BBV screening falls within the mental health remit, and absence of established clinical care pathways.8,10,11 People with SMI have a preponderance of HCV risk factors, including a 16%–22% estimated lifetime prevalence of injecting drug use (IDU), as well as heightened incarceration rates relative to non-SMI populations.12–15 While these risk factors may tend to overlap with other key, decentralized HCV elimination strategies, such as through OST and prison programs, SMI populations as a whole require focused strategic engagement.16,17
We performed a meta-analysis of HCV prevalence in SMI populations and analyzed for heterogeneity between prospective versus retrospective cohort study designs. We additionally systematically reviewed cross-sectional screening studies and examined data pertaining to HCV risk factors, screening consent, and barriers to care. Our contention was that tailored approaches to HCV screening and health care integration are required in order to achieve parity of care in people with SMI.
METHODS
Search Strategy and Screening
A literature search of PubMed, Scopus, Embase, CINAHL, and Web of Science was performed. Articles, including abstracts, titles, and keywords, published between April 21, 1989, and July 1, 2020, were extracted using the search terms Hepatitis C Virus, HCV, HCV seroprevalence, and HCV prevalence cross-referenced with serious mental illness, severe mental illness, psychiatric illness, mental illness, and psychiatric patients. Details of the analysis were registered on PROSPERO (registration: CRD42021237509).
Inclusion and Exclusion Criteria
Prospective observational and retrospective cross-sectional studies that assessed HCV prevalence in adults with SMI aged ≥ 18 years were systematically reviewed. HCV prevalence was determined based on International Classification of Diseases, Ninth Revision (ICD-9) coded data as well as seroprevalence data. Many of the extracted articles did not elucidate a specific definition for SMI in the recruitment strategy; however, data were included if SMI, severe psychiatric illnesses, or an enduring mental illness with functional impairment was clearly stipulated as an inclusion criterion.
We aimed to analyze HCV prevalence in general SMI populations. Data from dual substance abuse and homeless populations were therefore excluded in order to reduce ascertainment bias. Where sufficient data were available, general SMI subcohorts were specifically analyzed from mixed population settings. Studies were also excluded if (1) found to be a subanalysis of previously published data, (2) the denominator of HCV screened individuals was not cited, or (3) non-serologic methodology, such as self-report, was used for prospective HCV case identification. ICD-coded HCV, rather than serologic diagnosis, was accepted for large registry studies. A cross-sectional BBV screening strategy was required for prospective studies to minimize risk of overestimating seroprevalence due to selection bias. Retrospective and large registry–derived study prevalence data were based on the entire SMI cohort, rather than the screened cohort, again to reduce potential HCV screening selection bias.
Extraction and Data Collection
A total of 613 abstracts were identified across each of the search libraries. After removal of duplicates and non-English publications, 230 articles remained. These were reviewed by M.R.B., with 48 relevant titles/abstracts identified. There were 45 full text articles available for analysis (as 3 publications could not be sourced). An additional 4 relevant references were found in the citations from the above articles and assessed for inclusion. Of the 49 full texts, 13 publications did not meet inclusion criteria (Figure 1). This led to a total of 36 publications for analysis. Eligibility and inclusion criteria of full-text publications were verified by T.P. Key variables were recorded by M.R.B. and T.P., including study setting, recruitment strategy, participant demographics, SMI diagnosis, HCV risk factors, HCV screening modality, percentage of population screened, seroprevalence, and associations thereof. The quality of each study was recorded by M.B. and T.P. using the Quality Assessment Tool for Systematic Reviews of Observational Studies (Supplementary Table 1).18 Interuser concordance was very high, and third-party review was not required. An additional customized global assessment of study quality was performed based on 3 criteria, including study size (< 200 participants = 1, 200–500 = 1, > 500 = 2); cohort selection and reproducibility (single site with minimal participant description = 0, single site with well-described participants/recruitment or large multinational dataset = 1, multisite/national prospective study with clear selection methodology = 2); and risk factor evaluation (no assessment = 0, thorough assessment without analysis of covariables/confounders = 1, thorough risk evaluation with linear regression analysis = 2). Scores were aggregated and assigned a ranking: 0–1 = poor, 2–3 = fair, and 4–6 = good/excellent.
Analysis
Estimates of prevalence with 95% confidence intervals (CIs) were determined for each individual study. Pooled estimates of prevalence were calculated using the random effects model based on the DerSimonian and Laird method.19 The random effects model assumes that each study is estimating a different effect but that the effects come from the same underlying distribution of effects. This model fundamentally incorporates differences between studies. Heterogeneity was assessed using the I2 statistic. Subgroup analysis was performed comparing pooled prevalence between prospective and retrospective studies. A further sensitivity analysis was performed assessing prevalence based on a customized global assessment of study quality. Descriptive analysis was used for evaluation of HCV risk factors. All analyses were performed using Stata Version 16 (StataCorp, College Station, Texas).
RESULTS
Prevalence of HCV in SMI: Scale of the Problem
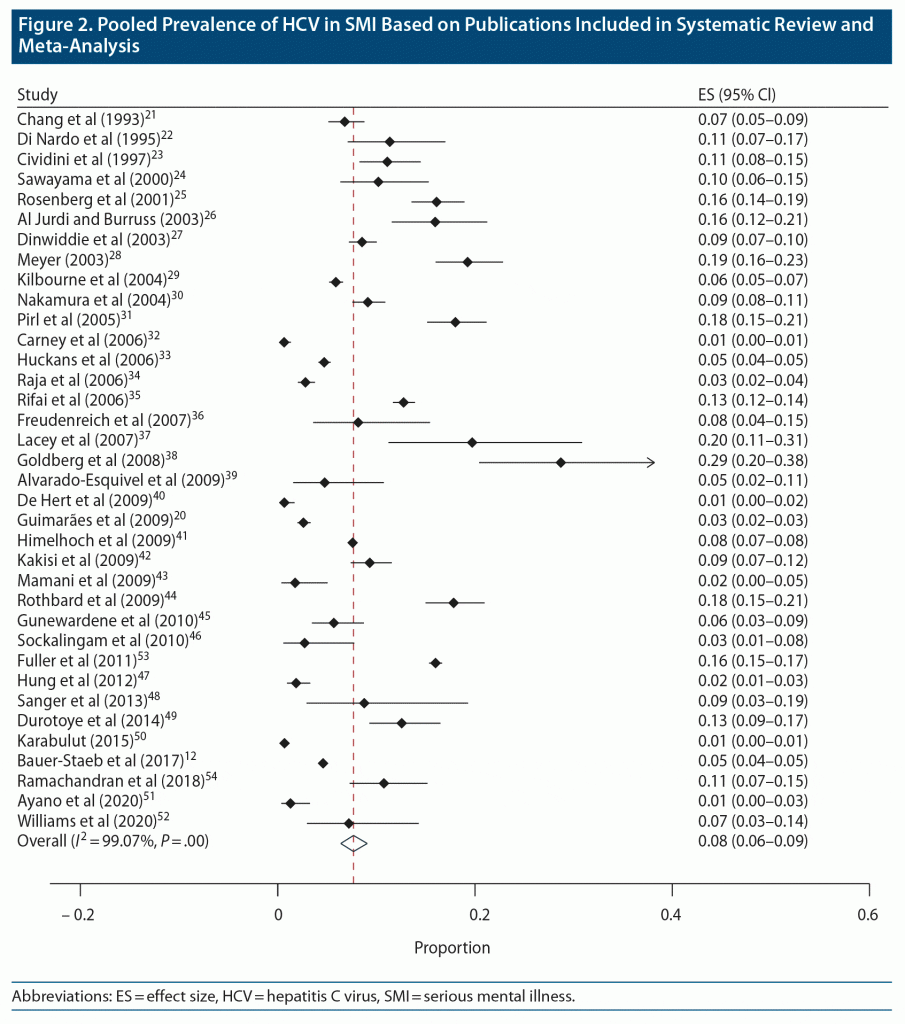
Most prospective seroprevalence data were drawn from studies that recruited consecutive inpatient or community participants from single health services, though 1 study utilized random probability selection to recruit across a large geographical region.20 Retrospective prevalence data were drawn from single-center seroprevalence studies as well as from large registry studies that used ICD coding for HCV reporting. In total, 36 studies with 298,262 individuals were included.12,20–54 This included 3 studies that were not necessarily designed to examine HCV seroprevalence but that nonetheless provided cross-sectional seroprevalence data, as well as 2 additional studies that examined HCV prevalence in the broader context of generalized medical comorbidity analysis.22,29,32,38,48 Key demographics, including country, participant age, and mental health diagnosis, are shown in Supplementary Table 2. Also shown in this table is the absolute number of people with diagnosed HCV in each study. A wide HCV prevalence range was noted (Figure 2). In prospective evaluations, a 1.3% HCV seroprevalence was seen at the lowest end of the spectrum in an Ethiopian SMI cohort.51 This was in stark contrast to 31.0% seroprevalence in a US-based cohort.38 Pooled HCV seroprevalence across all included studies was 8.0% (CI, 6.0%–9.0%) (Figure 2). Subanalysis of pooled prevalence based on study type showed an 8.0% (CI, 5.0%–11.0%) prevalence in prospective studies (n = 9,015 individuals) and an 8.0% (CI, 6.0%–10.0%) prevalence among the retrospective studies (289,247 individuals). When analyzed according to study quality using a customized global assessment of study quality tool, the pooled estimated seroprevalence for good-, fair-, and poor-quality studies was 7.0% (CI, 5.0%–9.0%), 8.0% (5.0%–11.0%), and 10.0% (1.0%–25.0%), respectively.
HCV Seroprevalence Relative to Control Populations
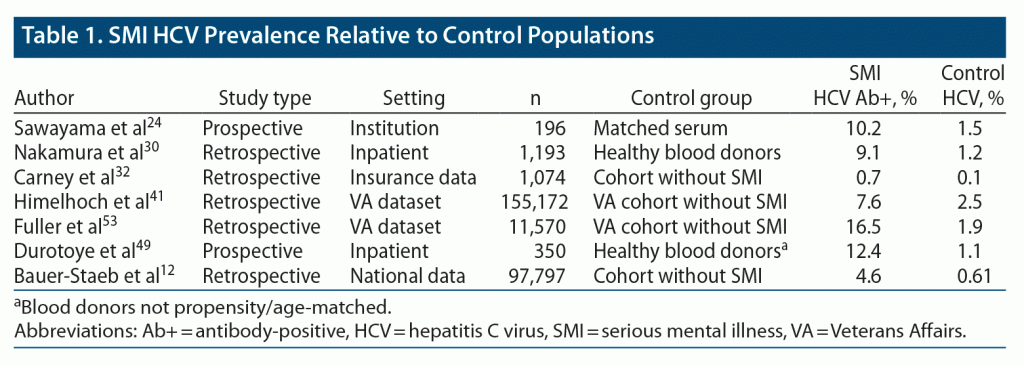
To contextualize the attributable risk of SMI to HCV, we specifically examined studies that included a non-SMI comparator cohort. Control groups were derived either from non-SMI individuals within large cohort studies or, when reported, from general population data, such as healthy blood donors. A total of 7 studies were included (Table 1).12,24,30,32,41,49,53 The studies varied in their respective data collection and comparator populations, though 4 of the studies used large data sets to compare ICD-coded HCV between people with SMI and matched controls. Across each of the studies, HCV seroprevalence was 3.0- to 11.3-fold higher in people with SMI relative to control cases. The range was tighter, 6.8- to 8.7-fold, after excluding lower and upper limit data.12,24,30,32
Risk Factors for HCV in SMI
Given the increased prevalence of HCV in people with SMI, risk factor data were appraised. Lifetime IDU was reported in 10 studies, each prospective, with a pooled size of 4,541 individuals. A wide range (0%–50%) of whole population IDU was noted across these studies (Supplementary Table 3). Studies that subanalyzed IDU among people with seropositive HCV found that 50.0%–78.6% of people with HCV had IDU as a risk factor, although it is notable that these data were drawn from US and Australian cohorts (Supplementary Table 3).22,25,37,46,54 The apparent heterogeneity in HCV seroprevalence in PWID therefore likely reflects the nature and demographic of the sample cohorts as well as the methodology of data recording and reporting. Illicit substance abuse and/or history of general substance abuse disorder was more widely reported across included studies and, for the most part, mirrored HCV seroprevalence (Supplementary Figure 1). Other reported HCV risk factors included high-risk sexual encounters, shared skin piercing equipment, transfusions, health care work exposures, and shared razor blades in institutions.24,37,39 Depression was more strongly associated with HCV compared to other primary mental health conditions based on multivariable analysis in 3 out of 4 studies that evaluated this.26,34,55,56 Quantitative analysis of risk factors relative to pooled seroprevalence was precluded by variable and lack of standardized reporting between individual studies.
Risk Factors, Prior Screening, and HCV Awareness Among Seropositive Individuals
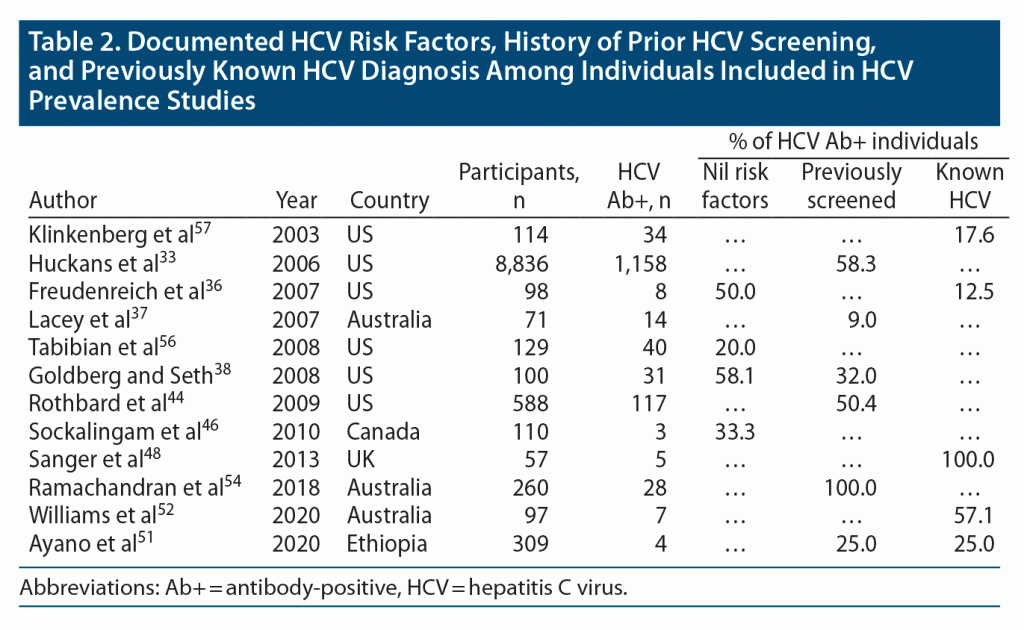
As a means of informing screening practices, we appraised the percentage of seropositive individuals from cross-sectional screening programs who had (1) documented HCV risk factors, (2) a history of prior HCV screening, or (3) previously diagnosed HCV. At least 20.0%–58.1% of seropositive individuals did not have a reported HCV risk factor in studies that comprehensively cataloged potential exposures (Table 2).36,38,46,56 Prior HCV screening in seropositive individuals varied widely (9.0%–100%) but was less than 50% in 3 of 6 studies. In 5 studies that cross-sectionally screened consecutive individuals for BBVs, 27.6% (16/58) of seropositive individuals had been previously diagnosed with HCV.36,51,52,57 The range of previously undiagnosed cases between studies was broad, likely reflecting sample size, local screening policies, and population-specific factors (Table 2).
Acceptability of BBV Screening
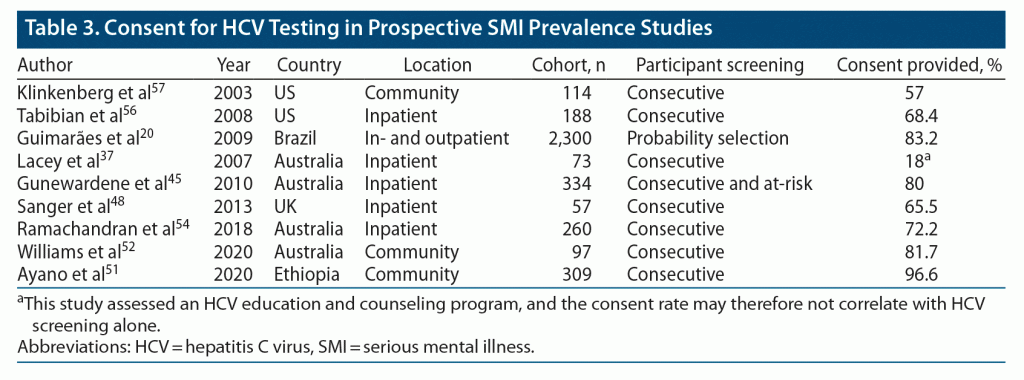
We next reviewed whether obtaining consent for HCV screening among people with SMI was a potential barrier to accurate delineation of HCV prevalence. We identified 7 prospective studies from inpatient and community settings that documented HCV consent rate and found that most people with SMI, 57.0%–96.6%, agreed to HCV screening (Table 3).20,45,48,51,52,54,56,57 There was an outlying study in which 18% of participants agreed to HCV screening. This was drawn from an HCV education and screening study and may not reflect general HCV screening acceptability.37 These data highlight that excellent screening participation rates can be achieved through comprehensive screening programs.
Liver Function Tests and HCV
Few prevalence studies analyzed liver function test derangement. Freudenreich et al36 reported an elevated alanine aminotransferase (ALT) in 25% (2/8) HCV seropositive individuals, while Hung et al47 reported 72.7% (8/11) and Dinwiddie et al27 reported 39.4% (26/66). In each study, a statistically significant increase in ALT was noted among HCV seropositive individuals compared to HCV seronegative individuals. However, these data indicate that liver function tests alone are incompletely sensitive as a screening tool for HCV screening in SMI. Beyond liver function tests, severity and staging of liver disease have not been prospectively assessed in the SMI context despite the high prevalence of HCV.
HCV Treatment Uptake and SVR in SMI
We next sought to assess linkages to care and treatment in HCV-positive people with SMI. Several studies have demonstrated excellent DAA treatment outcomes in people with psychiatric disorders.58,59 However, these data were drawn from individuals who were treated via DAA registry studies or specialist clinic services and therefore likely enriched for people with well-controlled mental health issues. Importantly, these studies did not indicate the denominator of people who did not access care following an HCV diagnosis.
Small prospective studies have shown that linking viremic individuals with SMI to HCV treatment can be complex. Among 72 individuals with viremic HCV (pooled from 3 prospective seroprevalence studies), 38 were successfully linked to care, and 9 individuals were ultimately treated, mostly with DAA therapies.36,38,54 The individuals who were successfully treated required integrated health service delivery via “nontraditional” services including support from hepatitis outreach nurses and mental health case managers. Cited barriers to care included lack of referral to specialist services and patient-related factors including refusal to attend traditional outpatient specialist care services and refusal to commence DAA therapies.36,54
Notwithstanding the potential challenges of translating HCV diagnosis to successful treatment, retrospective US Veterans Affairs data have shown that Veterans with SMI achieve similar linkage to treatment when compared to an internal Veterans Affairs control cohort (11.9% versus 13.9%).33 In a second study, 33% of Veterans who were diagnosed with HCV during an inpatient mental health admission were successfully commenced on interferon following referral to outpatient specialist services.35 This compares favorably to general population real-world interferon data, whereby a pooled rate of 19% interferon treatment initiation has been estimated.60
DISCUSSION
What Drives Increased HCV Risk in People With SMI?
In our meta-analysis, pooled HCV seroprevalence in people with SMI was 8.0%, that is, at least 8-fold higher than the estimated global prevalence.1 The overrepresentation of HCV in people with SMI appears to be increasingly driven by substance abuse, a problem deeply embedded within social disadvantage and one that may be perpetuated by cognitive issues and positive symptoms related to SMI.12–15,61,62 In terms of relative effect size, Bauer-Staeb et al12 demonstrated that the odds ratio of HCV seropositivity in a Swedish national cross-sectional analysis of people with SMI decreased from 6.18 to 1.72 after adjusting for substance abuse. Substance abuse is common among people with SMI, and in a previous systematic review of HIV risk factors in SMI, IDU was found to have a weighted mean lifetime prevalence of 21.7% (14.0%–37.0%), albeit there was a preponderance of US data.15 The relevance of this is that a history of IDU correlates with a > 40% HCV prevalence (in 61 out of 77 countries).63 Substance abuse, and in particular IDU, may explain much of the disproportionate increase in HCV seroprevalence in people with SMI and therefore requires strong consideration when screening for HCV.
Rationale for BBV Screening in SMI and Barriers to Care
We show that BBV screening delivered through population-based programs has high acceptability among people with SMI. Nonetheless, real-world data suggest that people within mental health services are underserved from an HCV screening perspective. In a cross-sectional analysis of 57,170 SMI outpatients, 4.7% were screened for HCV over a 12-month period as compared to 12.4% in a general population comparator cohort, with the strongest predictor of HCV screening in the SMI group being non-psychiatric health care utilization.64 Importantly, person-centered blood-borne virus screening delivered through collocated specialist services has been shown to significantly bolster HCV screening rates in community-managed individuals with SMI.65 However, even with positive case identification, significant attrition in follow-up and treatment is commonly encountered.65–67 Few publications in this systematic review addressed linkage to care in HCV-positive individuals; however, known barriers exist as a composite of complex patient-related, clinician, and health system factors.8,10,66 For example, issues pertaining to access and retention within traditional specialist health care models may be experienced at a patient level. This may be compounded by perceived or actual stigma.66 From a clinician perspective, legacy concerns stemming from interferon-era therapies coupled with competing mental health care priorities may hinder advocacy and referral to HCV treatment services.66,68
Looking to Other Models of Care
Mental health has lagged other high-prevalence HCV populations in terms of decentralization of screening programs,8,69 although some data exist regarding intensified support within traditional outpatient models.70 In other high-risk settings, such as the prison and opioid-substitution contexts, decentralized models of HCV care service delivery augmented by community nurse engagement, case management, and enhanced motivational support have demonstrated efficacy in engaging and improving HCV treatment access when compared to traditional health care models.17 People with SMI are often integrated within community mental health services, and a remodeling of the current care paradigm bolstered by enhanced clinician education could translate to effective BBV screening and treatment.35,70–72 These pathways could be supported through point-of-care HCV antigen assays, integration of nurse practitioners, improved clinician education, streamlined screening strategies, and horizontal integration of specialists, through either visiting sites or telehealth platforms.70,73 Notwithstanding potential barriers in the SMI setting, decentralization of traditional models of care could yield markedly improved access to highly tolerable curative therapies.58,59,74
Recommendations for Screening
With regard to the nature of BBV screening, a patient-centered, codesign screening approach would likely yield enhanced patient acceptability and engagement. Screening programs should ideally be applied cross-sectionally in order to both reduce perceived stigma and increase HCV diagnostic yield relative to individualized risk factor–based screening. Indeed, several cross-sectional screening studies included in this analysis show that HCV risk factors are not always ascertained in seropositive individuals and that many people with seropositive HCV were previously undiagnosed via traditional screening algorithms.33,36,38,44,46,54,56 Cross-sectional screening has the advantage of bypassing issues like incomplete risk factor appraisal by clinicians, patient recall bias, and/or stigma-related underreporting. Overall, a broader approach to screening would serve to change cultural attitudes toward BBV screening and improve HCV identification rates.
Study Limitations
Convenience samples included in the meta-analysis may have enriched for individuals at greater risk of HCV due to higher acuity psychosocial issues. We attempted to adjust for this in a number of ways. First, SMI cohorts drawn from dual substance abuse disorders settings were excluded. Furthermore, we specifically included retrospective studies, including those from large registry datasets, given that these data generally have a greater cross-sectional reach. Pooled seroprevalence was very similar between prospective and retrospective subcohorts despite methodological heterogeneity and remained similar in a study quality subanalysis, and therefore appears to be robust.
Notwithstanding this, marked variability in HCV seroprevalence between studies was noted. Background population HCV prevalence is likely to be a large contributing factor. For example, the general population HCV seroprevalence in Belgium is 0.57%–0.90% as compared to 1.2%–2.4% in the US.12,75 The effect size of background seroprevalence heterogeneity is likely amplified by risk factors preponderant to specific SMI cohorts. These risks may vary in frequency between regions and may even be idiosyncratic to a local cohort, such as in the case of shared razor blades as a dominant driver of HCV transmission in an institutionalized cohort of people with SMI in Japan.24 Other ascertainment biases related to study design and data acquisition may exaggerate the observed heterogeneity in HCV SMI seroprevalence. In particular, small cohort prospective data may not represent national SMI HCV seroprevalence, particularly where few representative studies exist. Regardless of absolute seroprevalence, HCV was reproducibly 3.0 to 11.3 times higher in SMI cohorts compared to controls despite heterogeneity between individual studies.12,24,30,32,33,41,51,53 These data demonstrate that SMI is associated with preponderant HCV and reinforces that people with SMI should be considered as a higher risk cohort from a BBV perspective.
CONCLUSIONS
People with SMI have high HCV seroprevalence, and a major risk factor is high-prevalence substance abuse. Given the availability of highly effective DAA therapies, tailored HCV screening and treatment programs would invariably reduce avoidable liver and extrahepatic complications at an individual level and would contribute toward global efforts toward HCV elimination. A first step is to recognize SMI as a priority group for HCV screening and to adopt broad population screening with an emphasis on a patient-centered approach. This is acknowledging that BBV screening has high acceptability among individuals with SMI and that cross-sectional screening is effective in identifying previously undiagnosed HCV. In terms of bridging existing barriers, innovative models of care that maximize opportunity for destigmatized screening and minimize complexity of follow-up will likely translate to an improved cascade of care and enhanced health outcomes.
Submitted: May 12, 2021; accepted September 10, 2021.
Published online: December 14, 2021.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration–approved labeling has been presented in this article.
Financial disclosure: Drs Braude, Phan, Dev, and Sievert have no conflicts to disclose.
Funding/support: None.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Global eradication of hepatitis C virus (HCV) is now achievable but requires intensified case identification among key populations, including people with serious mental illness (SMI).
- Cross-sectional screening for HCV has high acceptance rates among people with SMI and improves detection rates.
- HCV screening should therefore be considered as part of physical health workup and management in people with SMI.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
References (75)

- World Health Organization. Global hepatitis report, 2017. Accessed Nov 2020. https://www.who.int/publications/i/item/global-hepatitis-report-2017
- Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. PubMed CrossRef
- Dammacco F, Sansonno D, Piccoli C, et al. The lymphoid system in hepatitis C virus infection: autoimmunity, mixed cryoglobulinemia, and overt B-cell malignancy. Semin Liver Dis. 2000;20(2):143–157. PubMed CrossRef
- Dore GJ, Martinello M, Alavi M, et al. Global elimination of hepatitis C virus by 2030: why not? Nat Med. 2020;26(2):157–160. PubMed CrossRef
- World Health Assembly. Resolutions and decisions, annexes. 69th World Health Assembly: May 23–28, 2016. https://apps.who.int/iris/handle/10665/259134
- Grebely J, Dore GJ. Treatment of HCV in persons who inject drugs: treatment as prevention. Clin Liver Dis (Hoboken). 2017;9(4):77–80. PubMed CrossRef
- Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60(6):1861–1870. PubMed CrossRef
- Wainberg M, Dixon L. Ending HIV, hepatitis B, and hepatitis C: what about people with severe mental illness? Lancet Psychiatry. 2017;4(9):651–653. PubMed CrossRef
- Hughes E, Bassi S, Gilbody S, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(1):40–48. PubMed CrossRef
- De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders, 2: barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138–151. PubMed CrossRef
- Lagios K, Deane F. Psychiatrists’ knowledge and practices in screening and assessment of hepatitis C for inpatients with severe mental illness. Australas Psychiatry. 2011;19(2):156–159. PubMed CrossRef
- Bauer-Staeb C, Jörgensen L, Lewis G, et al. Prevalence and risk factors for HIV, hepatitis B, and hepatitis C in people with severe mental illness: a total population study of Sweden. Lancet Psychiatry. 2017;4(9):685–693. PubMed CrossRef
- Lagios K, Deane FP. Severe mental illness is a new risk marker for blood-borne viruses and sexually transmitted infections. Aust N Z J Public Health. 2007;31(6):562–566. PubMed CrossRef
- Sheidow AJ, McCart M, Zajac K, et al. Prevalence and impact of substance use among emerging adults with serious mental health conditions. Psychiatr Rehabil J. 2012;35(3):235–243. PubMed CrossRef
- Meade CS, Sikkema KJ. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev. 2005;25(4):433–457. PubMed CrossRef
- Fragomeli V, Weltman M. Addressing viral hepatitis in the opiate substitution setting: an integrated nursing model of care. J Gastroenterol Hepatol. 2015;30(suppl 2):6–11. PubMed CrossRef
- Papaluca T, McDonald L, Craigie A, et al. Outcomes of treatment for hepatitis C in prisoners using a nurse-led, statewide model of care. J Hepatol. 2019;70(5):839–846. PubMed CrossRef
- Quality assessment tool for observational cohort and cross-sectional studies. National Heart, Lung, and Blood Institute. 2014. Retrieved Sept 16, 2020. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. PubMed CrossRef
- Guimarães MD, Campos LN, Melo AP, et al; PESSOAS Project Network Group. Prevalence of HIV, syphilis, hepatitis B and C among adults with mental illness: a multicenter study in Brazil. Br J Psychiatry. 2009;31(1):43–47. PubMed CrossRef
- Chang TT, Lin H, Yen YS, et al. Hepatitis B and hepatitis C among institutionalized psychiatric patients in Taiwan. J Med Virol. 1993;40(2):170–173. PubMed CrossRef
- Di Nardo V, Petrosillo N, Ippolito G, et al. Prevalence and incidence of hepatitis B virus, hepatitis C virus and human immunodeficiency virus among personnel and patients of a psychiatric hospital. Eur J Epidemiol. 1995;11(2):239–242. PubMed CrossRef
- Cividini A, Pistorio A, Regazzetti A, et al. Hepatitis C virus infection among institutionalised psychiatric patients: a regression analysis of indicators of risk. J Hepatol. 1997;27(3):455–463. PubMed CrossRef
- Sawayama Y, Hayashi J, Kakuda K, et al. Hepatitis C virus infection in institutionalized psychiatric patients: possible role of transmission by razor sharing. Dig Dis Sci. 2000;45(2):351–356. PubMed CrossRef
- Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31–37. PubMed CrossRef
- Al Jurdi RK, Burruss JW. Prevalence of hepatitis C in psychiatric institutions. Psychosomatics. 2003;44(5):439–440. PubMed CrossRef
- Dinwiddie SH, Shicker L, Newman T. Prevalence of hepatitis C among psychiatric patients in the public sector. Am J Psychiatry. 2003;160(1):172–174. PubMed CrossRef
- Meyer JM. Prevalence of hepatitis A, hepatitis B, and HIV among hepatitis C-seropositive state hospital patients: results from Oregon State Hospital. J Clin Psychiatry. 2003;64(5):540–545. PubMed CrossRef
- Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6(5):368–373. PubMed CrossRef
- Nakamura Y, Koh M, Miyoshi E, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(3):591–597. PubMed CrossRef
- Pirl WF, Greer JA, Weissgarber C, et al. Screening for infectious diseases among patients in a state psychiatric hospital. Psychiatr Serv. 2005;56(12):1614–1616. PubMed CrossRef
- Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21(11):1133–1137. PubMed CrossRef
- Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorders. Psychiatr Serv. 2006;57(3):403–406. PubMed CrossRef
- Raja M, Azzoni A, Pucci D. Characteristics of HCV positive patients in an Italian urban psychiatric unit. Clin Pract Epidemiol Ment Health. 2006;2(1):26. PubMed CrossRef
- Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes among patients with psychiatric illness. Psychiatr Serv. 2006;57(4):570–572. PubMed CrossRef
- Freudenreich O, Gandhi RT, Walsh JP, et al. Hepatitis C in schizophrenia: screening experience in a community-dwelling clozapine cohort. Psychosomatics. 2007;48(5):405–411. PubMed CrossRef
- Lacey C, Ellen S, Devlin H, et al. Hepatitis C in psychiatry inpatients: testing rates, prevalence and risk behaviours. Australas Psychiatry. 2007;15(4):315–319. PubMed CrossRef
- Goldberg RW, Seth P. Hepatitis C services and individuals with serious mental illness. Community Ment Health J. 2008;44(5):381–384. PubMed CrossRef
- Alvarado-Esquivel C, Arreola-Valenzuela MA, Rodríguez-Briones A, et al. Seroprevalence of selected viral, bacterial and parasitic infections among inpatients of a public psychiatric hospital of Mexico. Rev Inst Med Trop São Paulo. 2008;50(3):161–164. PubMed CrossRef
- De Hert M, Wampers M, Van Eyck D, et al. Prevalence of HIV and hepatitis C infection among patients with schizophrenia. Schizophr Res. 2009;108(1–3):307–308. PubMed CrossRef
- Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50(1):30–37. PubMed CrossRef
- Kakisi OK, Grammatikos AA, Karageorgopoulos DE, et al. Prevalence of hepatitis B, hepatitis C, and HIV infections among patients in a psychiatric hospital in Greece. Psychiatr Serv. 2009;60(9):1269–1272. PubMed CrossRef
- Mamani M, Hashemi SH, Niayesh A, et al. Study on the frequency of hepatitis B and C infection in chronic psychiatric patients in Hamedan in 2006-2007. J Pak Med Assoc. 2009;59(8):505–507. PubMed
- Rothbard AB, Blank MB, Staab JP, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534–537. PubMed CrossRef
- Gunewardene R, Lampe L, Ilchef R. Prevalence of hepatitis C in two inpatient psychiatry populations. Australas Psychiatry. 2010;18(4):330–334. PubMed CrossRef
- Sockalingam S, Shammi C, Powell V, et al. Determining rates of hepatitis C in a clozapine treated cohort. Schizophr Res. 2010;124(1–3):86–90. PubMed CrossRef
- Hung CC, Loh W, Hu TM, et al. Prevalence of hepatitis B and hepatitis C in patients with chronic schizophrenia living in institutions. J Chin Med Assoc. 2012;75(6):275–280. PubMed CrossRef
- Sanger C, Hayward J, Patel G, et al. Acceptability and necessity of HIV and other blood-borne virus testing in a psychiatric setting. Br J Psychiatry. 2013;202(4):307–308. PubMed CrossRef
- Durotoye IA, Issa BA, Fadeyi A, et al. Sero-prevalence of hepatitis B and C among mentally ill patients attending a tertiary hospital in Nigeria. Ann Afr Med. 2014;13(4):210–216. PubMed CrossRef
- Karabulut N. Prevalence of HBV, HCV and HIV in inpatients of a mental health hospital in Turkey, 2011–2013. Iran J Public Health. 2015;44(7):1026–1028. PubMed
- Ayano G, Haile K, Tesfaye A, et al. Undiagnosed HIV, hepatitis B, and hepatitis C infections in people with severe psychiatric disorders in Ethiopia. BMC Infect Dis. 2020;20(1):180. PubMed CrossRef
- Williams J, Barclay M, Omana C, et al. Universal blood-borne virus screening in patients with severe mental illness managed in an outpatient clozapine clinic: uptake and prevalence. Australas Psychiatry. 2020;28(2):186–189. PubMed CrossRef
- Fuller BE, Rodriguez VL, Linke A, et al. Prevalence of liver disease in veterans with bipolar disorder or schizophrenia. Gen Hosp Psychiatry. 2011;33(3):232–237. PubMed CrossRef
- Ramachandran J, Budd S, Slattery H, et al. Hepatitis C virus infection in Australian psychiatric inpatients: a multicenter study of seroprevalence, risk factors and treatment experience. J Viral Hepat. 2019;26(5):609–612. PubMed CrossRef
- Himelhoch S, Goldberg R, Calmes C, et al. Screening for and prevalence of HIV and hepatitis C among an outpatient urban sample of people with serious mental illness and co-occurring substance abuse. J Community Psychol. 2011;39(2):231–239. PubMed CrossRef
- Tabibian JH, Wirshing DA, Pierre JM, et al. Hepatitis B and C among veterans on a psychiatric ward. Dig Dis Sci. 2008;53(6):1693–1698. PubMed CrossRef
- Klinkenberg WD, Caslyn RJ, Morse GA, et al. Prevalence of human immunodeficiency virus, hepatitis B, and hepatitis C among homeless persons with co-occurring severe mental illness and substance use disorders. Compr Psychiatry. 2003;44(4):293–302. PubMed CrossRef
- Back D, Belperio P, Bondin M, et al. Efficacy and safety of glecaprevir/pibrentasvir in patients with chronic HCV infection and psychiatric disorders: an integrated analysis. J Viral Hepat. 2019;26(8):951–960. PubMed CrossRef
- Gayam V, Jegede O, Tiongson B, et al. Outcomes of direct-acting antiviral treatment of psychiatric patients with comorbid hepatitis C virus infection. Dig Dis. 2020;38(3):232–239. PubMed CrossRef
North CS, Hong BA, Adewuyi SA, et al. Hepatitis C treatment and SVR: the gap between clinical trials and real-world treatment aspirations. Gen Hosp Psychiatry. 2013;35(2):122–128.PubMed CrossRef
- Draine J, Salzer MS, Culhane DP, et al. Role of social disadvantage in crime, joblessness, and homelessness among persons with serious mental illness. Psychiatr Serv. 2002;53(5):565–573. PubMed CrossRef
- Parks J, Svendsen D, Singer P, et al. Morbidity and Mortality in People with Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
- Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. PubMed CrossRef
- Trager E, Khalili M, Masson CL, et al. Hepatitis C screening rate among underserved adults with serious mental illness receiving care in California community mental health centers. Am J Public Health. 2016;106(4):740–742. PubMed CrossRef
- Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885–891. PubMed CrossRef
- Chasser Y, Kim AY, Freudenreich O. Hepatitis C treatment: clinical issues for psychiatrists in the post-interferon era. Psychosomatics. 2017;58(1):1–10. PubMed CrossRef
- McLaren M, Garber G, Cooper C. Barriers to hepatitis C virus treatment in a Canadian HIV-hepatitis C virus coinfection tertiary care clinic. Can J Gastroenterol. 2008;22(2):133–137. PubMed CrossRef
- Dixon LB, Adler DA, Berlant JL, et al. Psychiatrists and primary caring: what are our boundaries of responsibility? Psychiatr Serv. 2007;58(5):600–602. PubMed CrossRef
- Rifai MA, Gleason OC, Sabouni D. Psychiatric care of the patient with hepatitis C: a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6):PCC.09r00877whi. PubMed CrossRef
- Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101(10):2254–2262. PubMed CrossRef
- Morgan VA, Waterreus A, Jablensky A, et al. People living with psychotic illness in 2010: the second Australian national survey of psychosis. Aust N Z J Psychiatry. 2012;46(8):735–752. PubMed CrossRef
- Bonner JE, Barritt AS 4th, Fried MW, et al. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci. 2012;57(6):1469–1474. PubMed CrossRef
- Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment—Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52(3):1124–1133. PubMed CrossRef
- de Gennaro N, Diella L, Monno L, et al. Efficacy and tolerability of DAAs in HCV-monoinfected and HCV/HIV-coinfected patients with psychiatric disorders. BMC Infect Dis. 2020;20(1):196. PubMed CrossRef
- Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 suppl):S45–S57. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top