ABSTRACT
Objective: Inpatient psychiatric admissions drive the financial burden of schizophrenia, and medication adherence remains challenging. We assessed whether aripiprazole tablets with sensor (AS; system includes ingestible event-marker sensor, wearable sensor patches, and smartphone application) could reduce psychiatric hospitalizations compared with oral standard-of-care (SOC) antipsychotics.
Methods: This phase 3b, mirror-image clinical trial was conducted from April 29, 2019–August 11, 2020, in adults with schizophrenia with ≥ 1 hospitalization in the previous 48 months who had been prescribed oral SOC for the preceding 6 months (retrospective phase). All participants used AS for at least 3 months and up to 6 months. Primary endpoint was the inpatient psychiatric hospitalization rate in the modified intent-to-treat (mITT; n = 113) population during prospective months 1–3 versus retrospective phase. Proportion of days covered by medication was the secondary endpoint. Safety endpoints included adverse events related to the medication or patch and suicidality.
Results: AS significantly reduced hospitalizations during prospective months 1–3 (−9.7%) and months 1–6 (–21.3% [P ≤ .001 for all comparisons]) in the mITT population versus the corresponding retrospective phase. AS use improved confirmed medication ingestion by 26.5 percentage points in prospective months 1–3 (P ≤ .001) and reduced PANSS scores. Patches were well-tolerated, and no participant reported changes in suicide risk.
Conclusions: Compared with oral SOC, AS reduced inpatient psychiatric hospitalization rates for adults with mild-to-moderate schizophrenia. The AS system may aid medication ingestion and is associated with improvements in symptoms, potentially reducing acute-care needs among patients with schizophrenia.
Trial Registration: ClinicalTrials.gov identifier: NCT03892889
J Clin Psychiatry 2022;83(3):21m14132
To cite: Cohen EA, Skubiak T, Hadzi Boskovic D, et al. Phase 3b multicenter, prospective, open-label trial to evaluate the effects of a digital medicine system on inpatient psychiatric hospitalization rates for adults with schizophrenia. J Clin Psychiatry. 2022;83(3):21m14132.
To share: https://doi.org/10.4088/JCP.21m14132
© Copyright 2022 Physicians Postgraduate Press, Inc.
aHassman Research Institute, Marlton, New Jersey
bOtsuka Pharmaceutical Development & Commercialization, Inc., Princeton, New Jersey
cOtsuka Pharmaceutical Development & Commercialisation Europe GmbH, Hessen, Germany
dNathan S. Kline Institute for Psychiatric Research, Orangeburg, New York
*Corresponding author: J. Corey Reuteman-Fowler, PhD, 38 Hewitt Rd, Lambertville, NJ 08530 ([email protected]).
See letter by Naudet et al, and free commentary by Freudenreich
Schizophrenia is a leading cause of disability worldwide.1 In the US, there are an estimated 823,000 to > 3 million people living with schizophrenia.2–5 Inpatient costs were responsible for > $15 billion in health care costs for patients with schizophrenia in the US in 2013.6 Prior hospitalization is associated with increased risk of future hospitalization for patients with schizophrenia; in a 2017 study of patients with schizophrenia or schizoaffective disorder, 15% were readmitted within 3 months of discharge from a psychiatric hospital and 33% were readmitted within 1 year.7 Nonadherence to antipsychotic medication increases the risk of psychiatric hospitalization and relapse after discharge.8,9 The elevated risk of relapse among patients with schizophrenia contributes to monthly expenses exceeding 4 times that of a demographically matched population, with costs driven by inpatient admissions (42%), outpatient treatment (33%), and prescription drugs (25%).10
Interventions that enhance adherence and reduce hospitalization for patients with schizophrenia are greatly needed. In the present study, we investigated the effects of a digital medicine system, aripiprazole tablets with sensor (AS; Abilify MyCite, Otsuka Pharmaceutical Co., Ltd.) on inpatient psychiatric hospitalization rates. The AS system was designed to objectively confirm medication ingestion using prescription aripiprazole tablets embedded with an ingestible event-marker sensor, a wearable sensor patch, and a smartphone app to confirm tablet ingestion.11 The AS system is approved in the US to treat schizophrenia, bipolar I disorder, and major depressive disorder.12,13 After tablet ingestion, the sensor is activated by stomach fluid and signals to the adhesive sensor patch on the wearer’s torso.14 The app collects patch data via Bluetooth and transmits them to a secure digital health data server.14 Participants can access their data through the app, and health care providers and designated caregivers can access the data through separate cloud-based web portals, allowing intervention by these individuals if participants discontinue their medication.14
To date, several studies reported that users of the AS system found it to be usable, acceptable, and satisfactory; the sensor patch is wearable per user feedback with only mild safety considerations and performed well in detecting ingestions.13,15–19 The efficacy of oral aripiprazole was already established for patients with schizophrenia; thus, the US Food and Drug Administration review of the pill, patch, and smartphone app concluded the system and its components “function as intended.”20 Recent studies have evaluated the capacity of AS to detect shifts in medication ingestion patterns, characterize patient activity as markers of behavioral change, and evaluate rest quality.21–23 However, the efficacy of AS in improving clinical outcomes for patients with schizophrenia remains to be demonstrated, and many prior studies lacked a comparator arm, as noted in a 2019 systematic review by Cosgrove and colleagues.24 Thus, in the largest trial to date of patients with schizophrenia using any digital medicine system, we investigated whether AS could lower rates of psychiatric hospitalizations relative to oral standard-of-care (SOC) therapy. In this phase 3b, mirror-image study, we compared past hospitalization rates for adults with schizophrenia when they took oral SOC therapy versus when they used the AS system as part of their care plan.
METHODS
Study Design and Participants
This 6-month phase 3b, multicenter, open-label, mirror-image trial took place from April 29, 2019, to August 11, 2020, at 58 sites in the US (ClinicalTrials.gov identifier: NCT03892889) and was conducted in accordance with local laws, the International Conference on Harmonization Good Clinical Practice guidelines, and the Declaration of Helsinki. Before study initiation, written informed consent covering retrospective, screening, and prospective trial phases was obtained electronically from participants. The protocol and consent forms were approved by the relevant institutional review board (IRB) or independent ethics committee at each site. Participants were recruited via site databases of patients with schizophrenia and IRB-approved digital and print advertising.
Eligible participants were adults aged 18–65 years with schizophrenia (diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) with a Positive and Negative Syndrome Scale (PANSS)25 score between 60–90. Eligible participants had ≥ 1 inpatient psychiatric hospitalization in the preceding 48 months, had been prescribed oral SOC antipsychotics (including aripiprazole) for ≥ 6 months, owned and were able to use a smartphone, and had skin at the lower edge of the left rib cage devoid of dermatologic problems. Eligible participants were taking aripiprazole, had a history of tolerating oral aripiprazole, or, for those with unknown aripiprazole tolerance, were progressively cross-titrated to aripiprazole over ≤ 45 days. Excluded were participants currently treated with a long-acting injectable (LAI) antipsychotic, those diagnosed with a mental disorder other than schizophrenia or with comorbid psychiatric disorders, those allergic to adhesive tape or another component of the AS system, pregnant or nursing women, those who did not agree to practice 2 forms of birth control or abstinence, and participants unwilling or unable to use a smartphone.
Procedures
Study design is shown in Supplementary Figure 1. At screening, informed consent was obtained, eligibility was confirmed, participants’ antipsychotic regimen and hospitalization history were collected for the prior 6 months (“retrospective phase”), and smartphone compatibility, cellular service stability, and daily wireless network availability for the duration of the study were verified. Participants whose phones were incompatible with the AS system app were loaned a smartphone for the study.
At baseline, participants completed clinical assessments (see Study Endpoints) and were trained to use the AS system, instructed on downloading the app and using the dashboard, and given a kit containing 1 month’s supply of AS and sensor patches. Participants visited the study site monthly for prospective months 1–5 to receive study supplies and completed clinical and safety assessments digitally at months 3 and 6. Psychiatric hospitalizations and treatment (outpatient or non-inpatient) were recorded throughout the study, and accountability for study materials was performed monthly.
Participants used the AS system during initial prospective months 1–3, which included ingesting study medication (≤ 30 mg) once daily, wearing the patch, and using the smartphone app. Participants were instructed to wear patches continuously and were notified approximately weekly by the app for patch replacements. Throughout the prospective phase, participants could view their data at will via the app. Investigators and caregivers or support individuals (designated by the participant) were able to view the participant’s data through separate Web portals. Investigators could set up missed dose notifications to identify lapses. The call center provided AS technical support to participants and investigators.
After 3 months, participants continued using the AS system for prospective months 4–6 or reverted to SOC therapy based on their preferences and investigators’ discretion, in an effort to maintain person-centered care.26 All participants received identical standard follow-up care. At study termination or AS cessation, used patches and study materials were returned. Participants received a safety follow-up call 30 days (± 3 days) after discontinuing AS.
Study Endpoints
The primary endpoint was the difference in proportions of participants with ≥ 1 inpatient psychiatric hospitalization between prospective and retrospective months 1–3 in the modified intent-to-treat (mITT) population, defined as participants who completed the month 3 visit or had ≥ 80% of their study medication ingestions recorded by the AS system during prospective months 1–3. The secondary endpoint was assessing whether the AS system improved confirmed medication ingestion compared to the retrospective phase, calculated from the overall proportion of days covered (PDC) as a proxy for medication adherence27 in the ITT population (those who received ≥ 1 dose of study medication). PDC in both phases of the study were determined from the participants picking up their medication. For the retrospective phase, PDC was calculated from pharmacy records as the proportion of days a participant had medication available versus the number of days in a defined period.27 For participants who completed prospective months 1–6 using AS, PDC was determined from study drug dispensation when participants collected their medication from the study site. For participants who discontinued before 6 months, PDC during AS use was determined from study drug dispensation (ie, when participants collected their medication), whereas PDC after AS cessation was based on pharmacy records.
Key exploratory endpoints included inpatient hospitalization rates (defined as the proportion of participants with ≥ 1 inpatient psychiatric hospitalization) in the prospective and retrospective phases for months 1–6 in the mITT population (those who completed 3 months or took ≥ 80% of study medication) and in the ITT population (full study population) for months 1–3 and months 1–6. Change from baseline in clinical assessment scores was determined after 3 and 6 months of AS use via the Clinical Global Impression–Severity of Illness scale (CGI-S), the 5-dimension 5-level EuroQol (EQ-5D-5L), Patient Activation Measure–Mental Health (PAM-MH), PANSS, and Personal and Social Performance scale (PSP).25,28–31 Scores for the CGI–Improvement of Illness (CGI-I) scale were determined after 3 and 6 months of AS use.29 Treatment response was defined as ≥ 30% reduction from baseline in PANSS total score or a score of 1 (very much improved) or 2 (much improved) on the CGI-I scale.
Safety was assessed by documenting adverse events (AEs), treatment-emergent AEs (TEAEs), and serious AEs (SAEs), classified by preferred terms of the standardized Medical Dictionary for Regulatory Activities version 23.0. Reports of suicidality or suicidal ideation were assessed using the Columbia Suicide Severity Rating Scale (C-SSRS).32 Patch-related TEAEs were graded by the health care provider using the Skin Irritation Scoring System (grades 0–7),33 with grade ≥ 2 considered clinically significant.
Post hoc sensitivity analyses were conducted on hospitalization rates for months 1–6 in the ITT population. In the first analysis, participants who experienced SAEs because of schizophrenia symptoms, had post-baseline CGI-S ≥ 6, or who discontinued AS for lack of efficacy were included in the proportion of participants with hospitalization. For the second analysis, participants who met criteria from the first analysis plus those who experienced any TEAEs belonging to the system organ class of psychiatric disorders were included in the proportion of participants with hospitalization.
Statistical Analyses
The primary endpoint was assessed in the mITT population using an exact McNemar test on participants with paired hospitalization data from months 1–3 of the retrospective and prospective phases. Based on published data, the expected inpatient psychiatric hospitalization rate during retrospective months 1–3 was ~15.5%, while the expected rate during prospective months 1–3 was ~7%.34,35 We expected 13.5% of participants to have been hospitalized retrospectively but not experience a prospective hospitalization, and 5% of participants to be hospitalized prospectively who were not hospitalized retrospectively (estimates of “discordant proportions”). Based on these assumptions and a 2-sided ɑ = 0.05, 200 participants completing prospective months 1–3 would provide 80% power to detect an 8.5% difference in paired proportions of hospitalizations between prospective and retrospective phases.
Continuous variables in the ITT population were summarized by means, medians, ranges, relevant quartiles, and standard deviations (SD), with statistical significance assessed via paired t tests. The trial was stopped early at the interim analysis because of efficacy, according to a re-calculated boundary of 2-sided ɑ = 0.0025, using the alpha spending function based on the number of participants who reached the 3-month interim analysis.
RESULTS
Participants
From April 29, 2019, to August 11, 2020, 445 participants were screened and 277 were enrolled (ITT population) (Supplementary Figure 2). Most participants were male (n = 182, 65.7%) and Black or African American (n = 167, 60.3%). The mean time since diagnosis was 16.4 years (SD 12.0) (Table 1). Aripiprazole was the most common antipsychotic used previously by participants (88.1%), followed by quetiapine/quetiapine fumarate (29.2%), risperidone (20.6%), and olanzapine (11.6%) (Table 1). Other antipsychotics were used less frequently. The mITT population comprised 113 participants (mean age: 45.6 years [SD 12.8]); 9 participants discontinued before month 3 but were included in the mITT population because they had taken ≥ 80% of their study medication (Supplementary Figure 2). The mean PANSS score at baseline for the mITT population was 71.9 (SD 8.2), corresponding to mild-to-moderate schizophrenia.36
Fifty participants were discontinued before study completion when the trial was terminated early because of efficacy. Mean treatment duration was 72.9 days (SD 61.3). After completing months 1–3, 84 participants continued AS for months 4–6 and 20 participants opted to switch, with 19 participants reverting to oral SOC therapy and 1 participant choosing to begin LAI treatment.
Efficacy
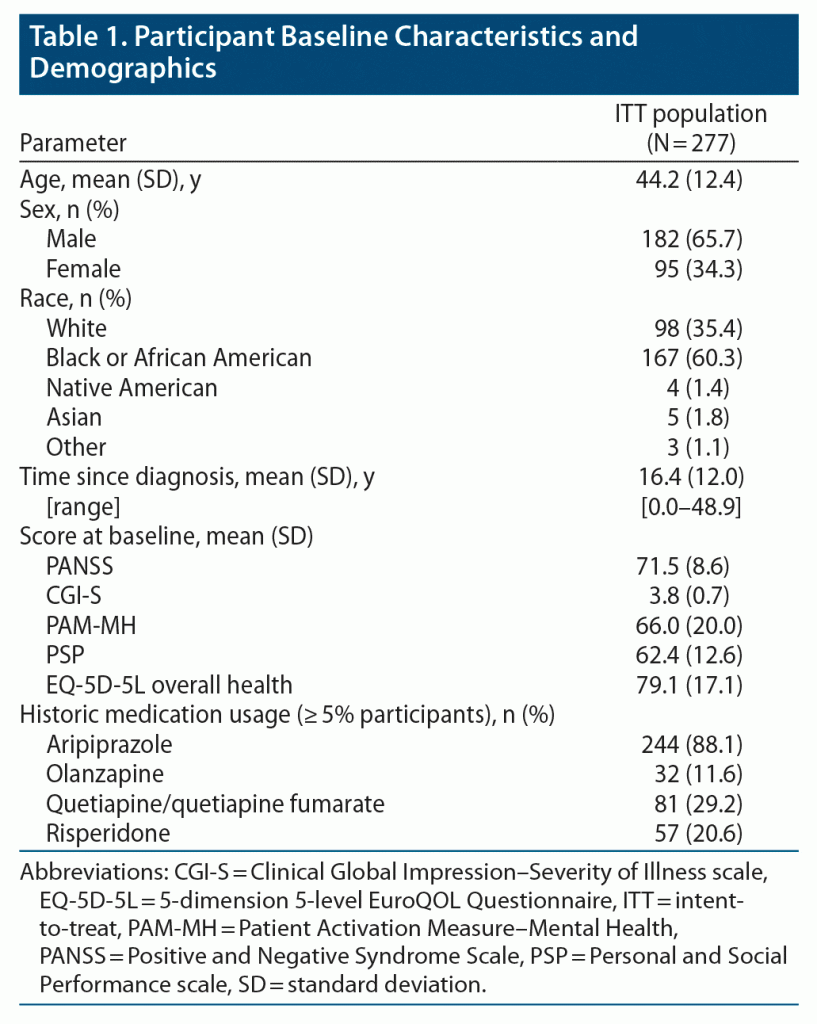
Discordant proportions were compared between the retrospective and prospective study phases, as described in Methods. No participants using the AS system were hospitalized during prospective months 1–3 in the mITT population (n = 113), while 9.7% of participants using oral SOC had been hospitalized in retrospective months 1–3 but were not hospitalized in prospective months 1–3, resulting in a difference of –9.7% (primary endpoint; P = .0010; Figure 1A). During prospective months 1–3, 1.1% of participants using AS in the ITT population (N = 277) who had not been previously hospitalized in retrospective months 1–3 were hospitalized, while 9.4% of participants using oral SOC who were not hospitalized in prospective months 1–3 were hospitalized during retrospective months 1–3, a difference of –8.3% (P < .0001; Figure 1B). No participants using AS were hospitalized during prospective months 1–6 in the mITT population (n = 86), while 19.8% of participants using oral SOC not hospitalized in prospective months 1–6 were hospitalized during retrospective months 1–6, a difference of –19.8% (P < .0001; Figure 1C). In prospective months 1–6, 1.1% of participants in the ITT population (N = 277) who used AS and who were not hospitalized retrospectively were hospitalized, while 22.4% of participants using oral SOC were hospitalized during retrospective months 1–6 but not in the prospective phase, a difference of –21.3% (P < .0001; Figure 1D).
Post hoc sensitivity analyses were conducted for months 1–6 in the ITT population. In the first analysis, 2 participants with severe illness or who experienced lack of efficacy were included in the discordant proportions of participants with prospective hospitalizations, which was 1.8% (5/277) in prospective months 1–6 versus 22.4% in retrospective months 1–6, a difference of –20.6% (P < .0001). For the second sensitivity analysis, 5 participants who experienced TEAEs specific to exacerbation of schizophrenia were included in the discordant proportions of participants with prospective hospitalizations, which was 3.6% (10/277) in prospective months 1–6 versus 22.4% in retrospective months 1–6, a difference of –18.8% (P < .0001).
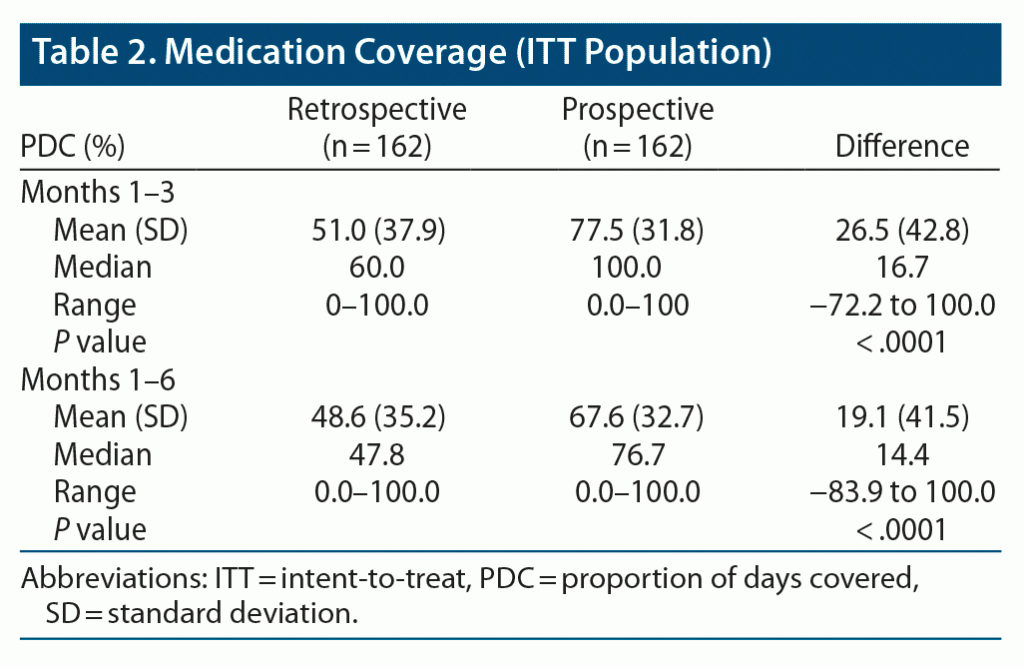
Altogether, 162 participants had PDC data available for both phases (secondary endpoint; ITT population) (Table 2). During prospective months 1–3 when participants used AS, mean PDC was 77.5% (SD 31.8) versus 51.0% (SD 37.9) during retrospective months 1–3 when participants were taking oral SOC, an improvement of 26.5 percentage points (SD 42.8; P < .0001).
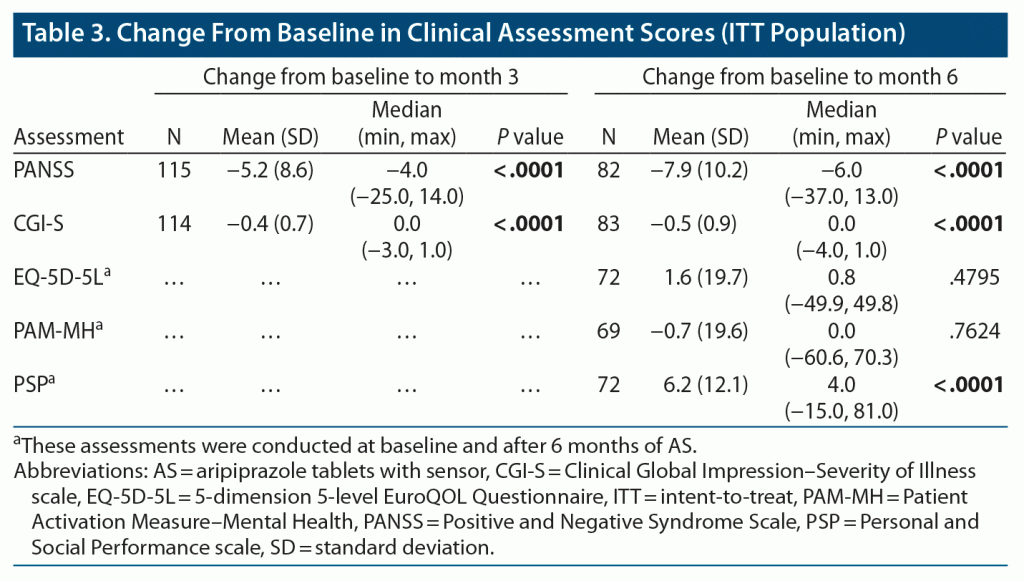
Participants underwent clinical assessments after 3 months (PANSS, CGI-S) and 6 months (PANSS, CGI-S, PSP, EQ-5D-5L, PAM-MH) of AS use (ITT population) (Table 3). At both timepoints, AS use was associated with significantly improved PANSS and CGI-S scores relative to baseline. PSP scores improved from baseline by 6 months of AS use. There was no significant change from baseline in EQ-5D-5L or PAM-MH scores. With AS use, 16.5% of the ITT population (19/115) met the definition of treatment response (≥ 30% reduction from baseline in PANSS total score or scored “very much improved” or “much improved” on CGI-I scale) by 3 months, 27.7% (23/83) by 6 months, and 16.6% (35/211) by the final study visit. The median time to discontinuation (all causes) in the ITT population was 50 days (n = 204; 50/277 [18.1%] due to the study stopping early for efficacy and 154/277 [55.6%] actual discontinuations).
Safety
Seventy participants experienced 107 AEs and 67 participants experienced 93 TEAEs (Table 4). Thirty-three participants experienced 39 TEAEs related to AS (Table 4), 21 of whom had a patch-related nonserious TEAE (medical device site reaction). Nine serious TEAEs occurred among 6 participants (2.2%) during the trial. Five serious TEAEs of schizophrenia (exacerbation of symptoms) occurred among 2 participants all were determined by site investigators to be unrelated to treatment. Other serious TEAEs occurred in 1 participant each (acute pancreatitis, drug hypersensitivity, bacterial pneumonia, and cerebrovascular accident). There were 6 clinically significant (grade ≥ 2) skin irritation events.33 Twenty-two participants who experienced a TEAE (27 events overall) discontinued treatment. The most common reasons for TEAE-related discontinuation were device site reactions (n = 8) and psychiatric disorders (n = 7) (Table 4). Mean change from baseline in C-SSRS suicidal ideation scores was considered not clinically significant. During or after AS use, no participants exhibited emergent suicidal behavior, serious suicidal ideation, or worsening of suicidal ideation. One death occurred during the study; it was caused by a cerebrovascular accident and deemed unrelated to treatment.
DISCUSSION
In this phase 3b mirror-image study, integration of the AS system into the routine care plan for participants with schizophrenia significantly reduced inpatient psychiatric hospitalizations relative to the lookback period when participants were using oral SOC therapy. This was true across study populations (mITT and ITT) and the 2 time periods assessed (months 1–3 or months 1–6) in a clinical trial setting. Confirmation of participants’ medication ingestion also improved while using AS, and symptoms of their illness improved significantly. Treatment response was achieved for approximately 1 in 5 participants after 3 months of AS use and approximately 1 in 4 after 6 months. These results suggest the AS system may improve symptoms and reduce psychiatric hospitalizations for individuals with mild-to-moderate schizophrenia.
The improvement in hospitalization rate during AS use persisted through sensitivity analyses that included participants who discontinued due to AEs or had newly emergent psychiatric AEs that might have required hospitalization. Prior hospitalization is a top risk factor for subsequent inpatient admission. Evidence has shown that patients with schizophrenia who had been hospitalized involuntarily have an elevated risk of readmission within 1 year (odds ratio: 3.25 [range: 1.19–8.85]).7 For the present study, we used published hospitalization rates to estimate expected hospitalizations34,35 and evaluated discordant proportions by comparing the proportion of participants who were hospitalized retrospectively but not prospectively to the proportion hospitalized prospectively but not retrospectively within the same study phase (eg, months 1–3).
Medication nonadherence is linked to increased hospitalizations and more frequent outpatient visits.9 In our study, in the ITT population, AS use improved medication coverage as a proxy of adherence by 26.5 percentage points in the first 3 months of use and by 19.1 percentage points after 6 months of use (P < .0001 for both) versus the retrospective phase. This improvement, combined with the 8.3% reduction in hospitalization rate among the ITT population, is consistent with Marcus et al (2008) who found that 12.3% of acute-care inpatient admissions were attributable to nonadherence.37 In the present study, mean PDC, a conservative estimate of adherence defined as the sum of nonoverlapping days of medication supply divided by the time period,38 which is widely used (eg, by Centers for Medicare & Medicaid Services plan ratings39), was similar to a previous study of AS in patients with schizophrenia, in which the mean proportion of days with good patch coverage was 64.3% (SD 20.2).18
How might the AS system affect clinical outcomes? In a recent systematic review, lack of patient insight (ie, not acknowledging their illness or the need for treatment) and negative patient attitudes were consistently associated with medication nonadherence.40 Improvements in patient insight or the therapeutic alliance were positively associated with increased medication adherence and greater improvements in clinical outcomes.41 From the provider’s perspective, awareness of medication nonadherence, from clinical case vignettes of simulated patients with serious mental illness, resulted in different treatment decisions compared with patients who were adherent to their medications.42 Future studies will elucidate whether greater insight or improvements in the therapeutic relationship via the AS system might be facilitating improvements in clinical outcomes in patients using AS.
In patients who struggle with consistently taking their medication, LAI antipsychotics can be useful to improve adherence.43 In our study, the time to discontinuation of AS for all causes was 50 days. This is comparable to a recent study in a German population where the median time to discontinuation was 50 days for patients taking oral antipsychotics (95% confidence interval [CI], 46–56) and 216 days for those on LAI antipsychotics (CI, 193–249).44 However, negative attitudes toward LAI use are relatively common among both patients and providers.43 Brissos and colleagues43 (2014) identified possible reasons for hesitance toward LAIs, including slow dose titration, difficulty adjusting the dose, pain and irritation at the injection site, feelings that LAIs were more coercive, and perceived stigma for LAI antipsychotics. In qualitative interviews of patients in early intervention teams in the UK, patients considered LAI antipsychotics easy to use and helpful in that they didn’t need to take daily medication, yet some patients associated LAIs with injection pain, fear of needles, and feelings of coercion.45 Thus, we feel the AS system is a midpoint approach between oral antipsychotics and LAIs in terms of helping patients with more regular medication ingestion. Additionally, the AS system provides passive biomarker data (eg, patient activity and rest quality) to monitor alongside trends in medication ingestion.21,23
AS use was associated with significantly improved clinician-reported outcomes (ClinROs; PANSS, CGI-S) by 3 and 6 months; PSP scores also improved by month 6 (P < .001 for all assessments). Participants who completed 3 or 6 months of AS use had similar baseline ClinROs values. The relatively high discontinuation rate did not numerically affect the change from baseline in ClinROs scores. No significant change of EQ-5D-5L or PAM-MH scores (patient-reported outcomes [PROs]) was observed, indicating no perceived change in participant quality of life or patient activation. PROs are direct reports of patients’ feelings and perceptions of function, while ClinROs are indirect measures46; hence, the timing of detected changes may not always align between PROs and ClinROs, particularly because patients may require longer duration of treatment to perceive clinical benefit. In a recent study of patients with schizophrenia taking an aripiprazole LAI, self-reported mental health–related QOL improved progressively over ≥ 2 years.47
The current study had a high overall discontinuation rate (73.6%). However, 50 participants were terminated early because of favorable interim analysis findings (18.1%); the actual rate of discontinuation was 154/277, or 55.6%. Of these, 50 (18.1%) opted to discontinue the study and 46 (16.6%) were lost to follow-up; challenges with using the technology (eg, dose not registering on the app) and needing to wear patches continually may have contributed to these discontinuations. Unfortunately, high discontinuation rates are common in trials of antipsychotic medications, ranging from 19%–75% in a 2009 meta-analysis,48 and patients’ decisions to withdraw consent are unpredictable.49 Discontinuations may have also been impacted by the digital nature of the AS system. Digital tools hold promise to address many unmet needs in patients with serious mental illness, but technology is not for everyone; pre-screening to identify participants in clinical studies and patients in clinical practice who might struggle with using digital tools will likely be critical to successfully navigating this evolving space in digital medicine.
Several limitations are notable for this study. This study, like most studies of patients with schizophrenia, had an overrepresentation of Black men relative to the general population, which may limit interpretability. Participants in this study had a mean PANSS score corresponding to mild-to-moderate disease36; the ability of AS to reduce psychiatric hospitalizations in patients with severe schizophrenia requires further study. Eligible participants were required to be taking an atypical antipsychotic, but study entry was not based on meeting an efficacy parameter for their medication regimen. The PANSS range of 60–90 suggests mild-to-moderate schizophrenia symptoms36; while some participants may not have been experiencing active psychosis at the time of study, they could still benefit from AS. The mITT population had high adherence (> 80%) during the prospective period, so additional work is needed to understand the effect of AS in patients with a history of documented nonadherence. This study was also limited by reliance on participants’ recalling locations where they had been hospitalized.
Mirror-image studies have limited capacity to identify causal relationships due to lack of a side-by-side control group50; however, this study design entails participants serving as their own internal control, to assess prospective outcomes relative to their own retrospective outcomes. As the mirror-image design necessitated using PDC as an indirect read-out of medication adherence, future studies on hospitalization rates will benefit from objective data confirming medication ingestion provided by the AS system.
Additional factors warranting caution in interpreting these data include the relatively small sample size, nonrandomized nature of the study, overrepresentation of participants who had previously taken aripiprazole, and the trial stopping early for efficacy. Clinical trials stopped early for benefit may overestimate treatment effects51; however, these overestimations are largely due to random error.52 In a study of simulated truncated randomized clinical trials, overestimation of treatment effects due to stopping early was generally small and of minimal impact in meta-analyses of pooled treatment effect.53 Finally, longer-term studies are needed to assess how greater duration of participants’ AS usage impacts hospitalization rates.
Additional research is encouraged to determine whether AS could reduce caregiver stress, as caregivers of patients with schizophrenia have perceived greater burden when caring for patients with acute psychosis, with poor medication adherence, or in psychiatric hospitals.54
CONCLUSION
To our knowledge, this is the largest trial to date of patients with serious mental illness using a digital medicine system. Compared with standard oral antipsychotics, AS use significantly reduced inpatient psychiatric hospitalization rates for adults with mild-to-moderate schizophrenia; several limitations of our study necessitate future studies to elucidate the mechanisms whereby AS helps improve clinical outcomes in patients. AS was well tolerated, with lower rates of skin irritation than in previous trials.18 The AS system is a tool that can aid patients and physicians in making informed choices to determine the course of pharmacotherapy most likely to be successful for patients. By maximizing chances of patient success and thereby reducing rates of psychiatric hospitalization, inclusion of AS in the routine care plan may help alleviate the burden on health care resources that is common in this population.
Submitted: June 16, 2021; accepted February 15, 2022.
Published online: April 11, 2022.
Author contributions: All authors contributed to the study conception and design, analysis, and interpretation of data. All authors contributed to drafting and critical revisions of the manuscript and approved the final version for submission. All authors accept accountability for the work reported herein and ensure the integrity of their contributions.
Relevant financial relationships: Mss Skubiak, Hadzi Boskovic, and Norman and Drs Reuteman-Fowler, Knights, Fang, and Peters-Strickland are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Dr Coppin-Renz is an employee of Otsuka Pharmaceutical Development and Commercialisation Europe GmbH. Dr Lindenmayer has received research grant support from Avanir, Roche, Takeda, Otsuka, Lundbeck, Intracellular, and GW. Dr Cohen has no disclosures.
Funding/support: This manuscript and study were funded by Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ.
Role of the sponsor: This research and manuscript were funded and supported by Otsuka Pharmaceutical Development & Commercialization, Inc. Several authors are employees of Otsuka and therefore the study sponsor was involved in all aspects of this study and manuscript preparation.
Previous presentations: Some of these findings were presented virtually at the Annual Meeting of the American Psychiatric Association, May 1–3, 2021, and at the Annual Meeting of the American Society of Clinical Psychopharmacology, June 1–4, 2021.
Acknowledgments: The authors thank the participants, investigators, and study personnel for their contributions to the study. Medical writing support for this manuscript was provided by Shawna Matthews, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, which was funded by Otsuka Pharmaceutical Development & Commercialization, Inc.
Ethics and consent: The trial was conducted by study investigators, coordinators, raters, and nurses, in accordance with local laws and the International Council for Harmonization Good Clinical Practice guidelines. The protocol was approved by the relevant institutional review boards. Participants were identified by unique numbers to protect their privacy; information generated by the trial was considered highly confidential and was available for remote or real-time viewing only by authorized personnel. After receiving a complete trial description, all participants signed written informed consent, which covered the retrospective, screening, and prospective phases of the trial.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Prior psychiatric hospitalization is a leading risk factor for rehospitalization in patients with schizophrenia; medication nonadherence is a contributing factor to hospitalization, as many patients struggle to take their antipsychotic medication regularly.
- Patients with schizophrenia are willing and able to use digital medicine technologies to help manage their condition; in prior studies of aripiprazole tablets with sensor (AS, composed of pills with ingestible event-marker, a wearable sensor patch, and a smartphone application]), patients found that the system fit into their daily routine and the wearable patch provided good coverage of medication ingestion.
- In the largest trial to date of patients with serious mental illness using a digital medicine system, compared with standard oral antipsychotics, AS use significantly reduced inpatient psychiatric hospitalization rates for adults with mild-to-moderate schizophrenia, increased the proportion of days covered by medication, and improved clinical symptoms.
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Psychosis section. Please contact Ann K. Shinn, MD, MPH, at [email protected].
References (54)

- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. PubMed CrossRef
- National Alliance on Mental Illness. Mental Health by the Numbers. https://www.nami.org/mhstats. Accessed December 23, 2021.
- United States Census Bureau. National population totals: 2010–2020. Annual estimates of the resident population for the United States, regions, states, the District of Columbia, and Puerto Rico: April 1, 2010 to July 1, 2020 (NST-EST2020). https://www.census.gov/programs-surveys/popest/technical-documentation/research/evaluation-estimates/2020-evaluation-estimates/2010s-totals-national.html. Accessed December 23, 2021.
- National Institute of Mental Health. Schizophrenia. NIH website. https://www.nimh.nih.gov/health/statistics/schizophrenia. 2018. Accessed December 23, 2021.
- Chen E, Bazargan-Hejazi S, Ani C, et al. Schizophrenia hospitalization in the US 2005–2014: examination of trends in demographics, length of stay, and cost. Medicine (Baltimore). 2021;100(15):e25206. PubMed CrossRef
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. PubMed CrossRef
- Hung YY, Chan HY, Pan YJ. Risk factors for readmission in schizophrenia patients following involuntary admission. PLoS One. 2017;12(10):e0186768. PubMed CrossRef
- Dilla T, Ciudad A, Alvarez M. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient Prefer Adherence. 2013;7:275–284. PubMed CrossRef
- Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. PubMed CrossRef
- Fitch K, Iwasaki K, Villa KF. Resource utilization and cost in a commercially insured population with schizophrenia. Am Health Drug Benefits. 2014;7(1):18–26. PubMed
- Knights J, Heidary Z, Peters-Strickland T, et al. Evaluating digital medicine ingestion data from seriously mentally ill patients with a Bayesian Hybrid Model. NPJ Digit Med. 2019;2(1):20. PubMed CrossRef
- Abilify MyCite (aripiprazole tablets with sensor), for oral use. Package insert. Otsuka Pharmaceutical Co, Ltd; 2017.
- Peters-Strickland T, Pestreich L, Hatch A, et al. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatr Dis Treat. 2016;12:2587–2594. PubMed CrossRef
- Fowler JC, Cope N, Knights J, et al. Hummingbird Study: a study protocol for a multicentre exploratory trial to assess the acceptance and performance of a digital medicine system in adults with schizophrenia, schizoaffective disorder or first-episode psychosis. BMJ Open. 2019;9(6):e025952. PubMed CrossRef
- Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533–e540. PubMed CrossRef
- Profit D, Rohatagi S, Zhao C, et al. Developing a digital medicine system in psychiatry: ingestion detection rate and latency period. J Clin Psychiatry. 2016;77(9):e1095–e1100. PubMed CrossRef
- Rohatagi S, Profit D, Hatch A, et al. Optimization of a digital medicine system in psychiatry. J Clin Psychiatry. 2016;77(9):e1101–e1107. PubMed CrossRef
- Fowler JC, Cope N, Knights J, et al. Hummingbird Study: results from an exploratory trial assessing the performance and acceptance of a digital medicine system in adults with schizophrenia, schizoaffective disorder, or first-episode psychosis. Neuropsychiatr Dis Treat. 2021;17:483–492. PubMed CrossRef
- Kane JM. Comments on Abilify MyCite. Clin Schizophr Relat Psychoses. 2018;11(4):205–206. PubMed CrossRef
- Lee DJ, Farchione TR, Mathis MV, et al. US Food and Drug Administration’s approval of aripiprazole tablets with sensor: our perspective. J Clin Psychiatry. 2018;79(3):18com12255. PubMed CrossRef
- Cochran JM, Heidary Z, Knights J. Characterization of activity behavior using a digital medicine system and comparison to medication ingestion in patients with serious mental illness. NPJ Digit Med. 2021;4(1):63. PubMed CrossRef
- Knights J, Heidary Z, Cochran JM. Detection of behavioral anomalies in medication adherence patterns among patients with serious mental illness engaged with a digital medicine system. JMIR Ment Health. 2020;7(9):e21378. PubMed CrossRef
- Heidary Z, Cochran JM, Peters-Strickland T, et al. A rest quality metric using a cluster-based analysis of accelerometer data and correlation with digital medicine ingestion data: algorithm development. JMIR Form Res. 2021;5(3):e17993. PubMed CrossRef
- Cosgrove L, Cristea IA, Shaughnessy AF, et al. Digital aripiprazole or digital evergreening? a systematic review of the evidence and its dissemination in the scientific literature and in the media. BMJ Evid Based Med. 2019;24(6):231–238. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Allerby K, Goulding A, Ali L, et al. Striving for a more person-centered psychosis care: results of a hospital-based multi-professional educational intervention. BMC Psychiatry. 2020;20(1):523. PubMed CrossRef
- Canfield SL, Zuckerman A, Anguiano RH, et al. Navigating the wild west of medication adherence reporting in specialty pharmacy. J Manag Care Spec Pharm. 2019;25(10):1073–1077. PubMed CrossRef
- EuroQol Research Foundation. EQ-5D-5L User Guide. Euroqol website. https://euroqol.org/publications/user-guides. 2019. Accessed December 23, 2021.
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. PubMed
- Green CA, Perrin NA, Polen MR, et al. Development of the Patient Activation Measure for Mental Health. Adm Policy Ment Health. 2010;37(4):327–333. PubMed CrossRef
- Juckel G. Personal and Social Performance Scale. In: Michalos AC. Encyclopedia of Quality of Life and Well-Being Research. Springer; 2014:4719–4724.
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- US Department of Health and Human Services, Food and Drug Administration. Assessing the Irritation and Sensitization Potential of Transdermal and Topical Delivery Systems for ANDAs Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-irritation-and-sensitization-potential-transdermal-and-topical-delivery-systems-andas. 2018.
- Chue P, Llorca P-M, Duchesne I, et al. Hospitalization rates in patients during long-term treatment with long-acting risperidone injection. J Appl Res. 2005;5(2):266–274.
- Kane JM, Zhao C, Johnson BR, et al. Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ. 2015;18(2):145–154. PubMed CrossRef
- Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. PubMed CrossRef
- Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull. 2008;34(1):173–180. PubMed CrossRef
- Patel C, Khoury AE, Huang A, et al. Healthcare resource utilization and costs among patients with schizophrenia switching from oral risperidone/paliperidone to once-monthly paliperidone palmitate: a Veterans Health Administration claims analysis. Curr Ther Res Clin Exp. 2020;92:100587. PubMed CrossRef
- Centers for Medicare & Medicaid Services. Proportion of Days Covered (PDC). https://cmit.cms.gov/CMIT_public/ViewMeasure?MeasureId=2895. Accessed March 22, 2022.
- El Abdellati K, De Picker L, Morrens M. Antipsychotic treatment failure: a systematic review on risk factors and interventions for treatment adherence in psychosis. Front Neurosci. 2020;14:531763. PubMed CrossRef
- Novick D, Montgomery W, Treuer T, et al. Relationship of insight with medication adherence and the impact on outcomes in patients with schizophrenia and bipolar disorder: results from a 1-year European outpatient observational study. BMC Psychiatry. 2015;15(1):189. PubMed CrossRef
- Shafrin J, May SG, Shrestha A, et al. Access to credible information on schizophrenia patients’ medication adherence by prescribers can change their treatment strategies: evidence from an online survey of providers. Patient Prefer Adherence. 2017;11:1071–1081. PubMed CrossRef
- Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219. PubMed CrossRef
- Mahlich J, Olbrich K, Wilk A, et al. Time to treatment discontinuation in German patients with schizophrenia: long-acting injectables versus oral antipsychotics. Clin Drug Investig. 2021;41(1):99–113. PubMed CrossRef
- Das AK, Malik A, Haddad PM. A qualitative study of the attitudes of patients in an early intervention service towards antipsychotic long-acting injections. Ther Adv Psychopharmacol. 2014;4(5):179–185. PubMed CrossRef
- Powers JH 3rd, Patrick DL, Walton MK, et al. Clinician-Reported Outcome Assessments of Treatment Benefit: Report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force. Value Health. 2017;20(1):2–14. PubMed CrossRef
- McEvoy JP, Weiden PJ, Lysaker PH, et al. Long-term effect of aripiprazole lauroxil on health-related quality of life in patients with schizophrenia. BMC Psychiatry. 2021;21(1):164. PubMed CrossRef
- Rabinowitz J, Levine SZ, Barkai O, et al. Dropout rates in randomized clinical trials of antipsychotics: a meta-analysis comparing first- and second-generation drugs and an examination of the role of trial design features. Schizophr Bull. 2009;35(4):775–788. PubMed CrossRef
- Schoemaker JH, Vingerhoets AJJM, Emsley RA. Factors associated with poor satisfaction with treatment and trial discontinuation in chronic schizophrenia. CNS Spectr. 2019;24(4):380–389. PubMed CrossRef
- Faries DE, Nyhuis AW, Ascher-Svanum H. Methodological issues in assessing changes in costs pre- and post-medication switch: a schizophrenia study example. Cost Eff Resour Alloc. 2009;7(1):11. PubMed CrossRef
- Bassler D, Montori VM, Briel M, et al. Early stopping of randomized clinical trials for overt efficacy is problematic. J Clin Epidemiol. 2008;61(3):241–246. PubMed CrossRef
- Bassler D, Montori VM, Briel M, et al. Reflections on meta-analyses involving trials stopped early for benefit: is there a problem and if so, what is it? Stat Methods Med Res. 2013;22(2):159–168. PubMed CrossRef
- Wang H, Rosner GL, Goodman SN. Quantifying over-estimation in early stopped clinical trials and the “freezing effect” on subsequent research. Clin Trials. 2016;13(6):621–631. PubMed CrossRef
- Lippi G. Schizophrenia in a member of the family: burden, expressed emotion and addressing the needs of the whole family. S Afr J Psychiatr. 2016;22(1):922. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite