Objective: Docosahexaenoic acid (DHA) might help prevent or attenuate posttraumatic stress disorder (PTSD) symptoms. We examined the efficacy and safety of DHA for preventing PTSD (DSM-IV) after severe accidental injury.
Method: From December 2008 to August 2013, we conducted a randomized, double-blind, placebo-controlled trial of 110 accident-injured patients consecutively admitted to an intensive care unit of the National Disaster Medical Center in Tokyo, Japan. All patients were taught about their psychological reactions to accidental injury for 20 minutes and were randomly assigned to receive 1,470 mg/d of DHA plus 147 mg/d of eicosapentaenoic acid (EPA; n = 53) or placebo (n = 57) for 12 weeks. The primary outcome was total score on the Clinician-Administered PTSD Scale (CAPS) at 3-month follow-up. Secondary outcomes included PTSD diagnosis (full-blown or partial PTSD). Adherence to the interventions was assessed by erythrocyte fatty acid composition.
Results: At 3 months, the CAPS total score revealed no differences between the 2 groups (10.78 in the DHA group vs 9.22 in the placebo group; n = 100; P = .572). We found that 11.1% of the DHA group and 5.5% of the placebo group developed PTSD. The erythrocyte level of DHA and EPA in the DHA group was significantly elevated compared to the placebo group (P < .01).
Conclusions: Docosahexaenoic acid supplementation was not superior to placebo for the secondary prevention of PTSD symptoms at 3 months after severe accidental injury. The efficacy of a different ratio of DHA and EPA and higher doses of omega-3 fatty acids as secondary prevention of PTSD remains to be determined.
Trial Registration: ClinicalTrials.gov Identifier: NCT00671099
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
ABSTRACT
Objective: Docosahexaenoic acid (DHA) might help prevent or attenuate posttraumatic stress disorder (PTSD) symptoms. We examined the efficacy and safety of DHA for preventing PTSD (DSM-IV) after severe accidental injury.
Method: From December 2008 to August 2013, we conducted a randomized, double-blind, placebo-controlled trial of 110 accident-injured patients consecutively admitted to an intensive care unit of the National Disaster Medical Center in Tokyo, Japan. All patients were taught about their psychological reactions to accidental injury for 20 minutes and were randomly assigned to receive 1,470 mg/d of DHA plus 147 mg/d of eicosapentaenoic acid (EPA; n = 53) or placebo (n = 57) for 12 weeks. The primary outcome was total score on the Clinician-Administered PTSD Scale (CAPS) at 3-month follow-up. Secondary outcomes included PTSD diagnosis (full-blown or partial PTSD). Adherence to the interventions was assessed by erythrocyte fatty acid composition.
Results: At 3 months, the CAPS total score revealed no differences between the 2 groups (10.78 in the DHA group vs 9.22 in the placebo group; n = 100; P = .572). We found that 11.1% of the DHA group and 5.5% of the placebo group developed PTSD. The erythrocyte level of DHA and EPA in the DHA group was significantly elevated compared to the placebo group (P < .01).
Conclusions: Docosahexaenoic acid supplementation was not superior to placebo for the secondary prevention of PTSD symptoms at 3 months after severe accidental injury. The efficacy of a different ratio of DHA and EPA and higher doses of omega-3 fatty acids as secondary prevention of PTSD remains to be determined.
Trial Registration: ClinicalTrials.gov Identifier: NCT00671099
J Clin Psychiatry 2015;76(8):e1015-e1022
dx.doi.org/10.4088/JCP.14m09260
© Copyright 2015 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, National Disaster Medical Center, Tachikawa, Tokyo, Japan
bCREST, Japan Science and Technology Agency, Tokyo, Japan
cDepartment of Public Health, Faculty of Medicine, University of Toyama, Toyama, Japan
dDivision of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan
eDepartment of Clinical Sciences, Institute of Natural Medicine, University of Toyama, Toyama, Japan
*Corresponding author: Yutaka Matsuoka, MD, PhD, Department of Psychiatry, National Disaster Medical Center, 3256 Midoricho, Tachikawa, Tokyo 190-0014, Japan ([email protected]).
Significant numbers of accident-injured individuals worldwide who are admitted to intensive care units (ICUs) or emergency departments develop posttraumatic stress disorder (PTSD), and comorbidity between PTSD and major depressive disorder is also highly prevalent.1-4 Approximately 1 in every 4 patients develops full-blown or partial PTSD after accidental injury. Posttraumatic stress disorder is associated with suicide attempts, subsequent other mental disorders, work impairment, and adverse life course.5 Developing a preventive intervention strategy for PTSD that is easy to use and disseminate is a pressing public health need.
Preclinical approaches to PTSD are examining the mechanism of memory consolidation and how this consolidation process could be interrupted to prevent the development of trauma-related disorder.6 The hippocampus is crucial for converting short-term memory into long-term memory,7 and it can process and temporarily store new memory during the transition period before labile memory is transferred to the cortex for permanent storage.8 In the pathogenesis of PTSD, fear memory becomes excessively consolidated and extinction learning does not progress.9 Kitamura et al10 found that the period of hippocampus-dependent fear memory is longer in mice with decreased hippocampal neurogenesis and shorter in mice with active hippocampal neurogenesis. The possibility arises then that the fear memory characteristic of PTSD can be controlled by properly regulating hippocampal neurogenesis.6
Omega-3 polyunsaturated fatty acids (PUFAs) are critical for the normal development and function of the mammalian brain. Basic experimental research has revealed that docosahexaenoic acid (DHA, 22:6[n-3]) promotes hippocampal neurogenesis in the adult brain.11,12 Moreover, He et al13 have shown that fat-1 transgenic mice, which have enriched levels of DHA in the brain because they can endogenously convert omega-6 to omega-3 PUFAs, exhibited increased hippocampal neurogenesis compared to wild-type mice. Although the specific mechanisms of DHA action on neurogenesis are not yet known, it is plausible that higher consumption of DHA might promote hippocampal neurogenesis. Thus, Matsuoka6 proposed that promoting adult neurogenesis by omega-3 PUFA supplementation early in the posttrauma period might facilitate the clearance of fear memory from the hippocampus and consequently minimize PTSD symptoms.
A preliminary open study14 found that PTSD symptoms at the end of the trial were significantly alleviated in injured patients who took DHA compared with historical controls. Another single-blind, randomized, parallel-group trial found that DHA was efficacious for attenuating PTSD symptoms in female rescue workers who were deployed during the acute disaster phase of the Great East Japan Earthquake.15 Therefore, the present study aimed to examine the effectiveness of DHA for preventing PTSD in injured patients admitted to the ICU immediately after accidental injury.
METHOD
Trial Design
This double-blind, parallel-group, randomized controlled trial compares an intervention group receiving DHA supplementation with a parallel control group receiving placebo supplementation. This study was registered at ClinicalTrials.gov (NCT00671099). The full protocol16 can be accessed at http://www.biomedcentral.com/1471-244X/13/8.
Participants
Participants were recruited solely from the ICU of the National Disaster Medical Center, Tokyo, Japan. The 110 injured patients were randomized to either the DHA group or the placebo group (1:1). The inclusion criteria applied to patients with accidental injury were as follows: (1) aged 18 years or older, (2) a native Japanese speaker, (3) contact with us within 240 hours after injury, and (4) physical and psychological ability to understand the scope of the present trial and to provide written consent for study participation. Exclusion criteria were the presence of the following: (1) acute brain parenchymal damage that is obviously irreparable, or subdural or subarachnoidal bleeding; (2) a score of < 24 on the Mini-Mental State Examination17; (3) a serious drinking problem; (4) a smoking habit of ≥ 40 cigarettes per day; (5) history and current suspicion of psychosis or bipolar I disorder; (6) suspicion of alcohol- or substance-related disorder or eating disorder; (7) a serious psychiatric condition such as suicidal ideation, self-harm behavior, or severe dissociation; (8) regular treatment with antiepilepsy medication, lithium, ethyl icosapentate, aspirin, or warfarin within the last 3 months; (9) regular preaccident consumption of PUFA supplements within the last 3 months; and (10) a habit of eating fish ≥ 4 times per week.

- Developing an easy prevention strategy for posttraumatic stress disorder is a pressing public health need.
- Control of fear memory might become a fundamental strategy for overcoming posttraumatic stress disorder.
- The results of this clinical trial suggest that docosahexaenoic acid has no benefit in the prevention of posttraumatic stress disorder in frequent fish eaters such as the Japanese.
Procedure and Baseline Assessment
The ethics committee of the National Disaster Medical Center approved the study protocol on February 7, 2008. Eligible patients were screened through the daily conference records for patients newly admitted to the ICU. After a complete description of the study, participants provided written informed consent to take part in this study. We started participant recruitment on December 16, 2008, and ended on June 6, 2013. Follow-up assessment was completed on August 29, 2013.
Participants filled out the Peritraumatic Distress Inventory,18,19 Connor-Davidson Resilience Scale (CD-RISC),20 and Food Frequency Questionnaire21,22 at baseline. Blood samples were obtained in the emergency room. All data were stored at the institution’s data management section.
Interventions
Active treatment involved dietary supplementation with 300 mg of blackish-brown gelatin capsules containing concentrated marine fish oil. The daily dose of 7 capsules provided 1,470 mg of DHA, 147 mg of eicosapentaenoic acid (EPA, 20:5[n-3]), and 0.3% of α-tocopherol. We based the daily dose of approximately 1,600 mg of omega-3 PUFAs on our previous trial of university students who were under stress.23 The content of the control oil was slightly modified from our previous study.24 The present control oil was a mixture of rapeseed oil (47%), soybean oil (25%), olive oil (25%), fish oil (3%), and 0.3% α-tocopherol. The fatty acid composition of this mixture was similar to the average composition of fatty acid intake in Japan. We added a small amount of not fully deodorized fish oil to the base of the control oil to give it a fishy odor and make it indistinguishable from the active oil. Safety issues were monitored at least twice a week during hospitalization and once a week after discharge. Adherence to the study medication was monitored by bottle check and self-report as well as by erythrocyte fatty acid quantification.
Measures
The primary outcome measure was the total score on the Clinician-Administered PTSD Scale (CAPS)25,26 at 3-month follow-up. Trained psychiatrists (Y.M. and D.N.), blinded to treatment assignment, conducted structured interviews to evaluate PTSD and other psychiatric morbidity at months 1 and 3. A diagnosis of full-blown PTSD required DSM-IV diagnostic criteria A (traumatic event) through F (impairment).27 Participants were deemed to have partial PTSD if they fulfilled 2 of 3 symptom criteria (B [reexperiencing], C [avoidance], or D [hyperarousal]) and also at least 1 of the criteria A-1 (stressor), E (duration), or F (impairment). Interrater reliability of PTSD diagnosis was reported previously.28
We used several secondary measures. The Impact of Event Scale-Revised (IES-R)29 provided an additional measure of PTSD severity. The Mini-International Neuropsychiatric Interview30,31 was used to assess major depressive disorder. The Montgomery-Asberg Depression Rating Scale (MADRS)32,33 provided a measure of depression severity. The Hospital Anxiety and Depression Scale (HADS)34,35 was used to measure severity of general distress. The CD-RISC20 was used to measure resilience.
At baseline (ie, the time point before ICU admission) and 3-month follow-up (end of intervention), erythrocyte fatty acid composition was quantified. The detailed laboratory procedure was described elsewhere.16 Eicosapentaenoic acid, DHA, and arachidonic acid (ARA) were used to index pretreatment to posttreatment fatty acid composition of the erythrocyte membrane as an objective measure of treatment adherence.
Sample Size
For sample size estimation, at least 49 cases per group were required given that the expected difference in CAPS score between the groups at 3 months was set at 10 (SD = 15) with an α level of .05 (2-tailed) and 90% power. The desired total number of participants was set at 140, with 70 cases per group based on our estimate that around 30% of the participants would discontinue or drop out of the study before it began. We stopped accumulating participants before the desired number was achieved due to a 10% dropout rate based on study monitoring reports of the blinded groups and because this trial became too difficult to continue for financial reasons and diminished numbers of injured patients admitted.
Randomization and Blinding
An independent statistician generated randomization lists with 3 stratification factors using a computer-generated random allocation sequence by block-randomization method. Stratification factors included sex (male or female), age (< 40 or ≥ 40 years), and sense of life threat (yes or no). The randomization list was sent to an independent pharmacist who securely kept the tables and prepared numbered supplement bottles according to the list. Both the research team and the participants were blinded to the randomization until the last participant completed the protocol and the spreadsheets of all results were finalized. Stratification by sex, age, and sense of life threat were justified in previous studies: prevalence of PTSD and major depressive disorder were higher in women than in men,36,37 and our previous cohort study of survivors of motor vehicle accidents showed that sense of life threat was a strong predictor of subsequent PTSD.4 Moreover, according to the National Health and Nutrition Survey on food group consumption by age group, the amount of meat products consumed, such as pork and beef, is higher for both sexes before age 40, but after age 40, seafood consumption increases.38 Also, the omega-3 fatty acid content in red blood cells increases with age in the Japanese population.39
All members of the research team, including all authors, physicians, nurses, research assistants, and study participants, remained blinded to the actual intervention assignments until data collection was completed and confirmed and erythrocyte fatty acids were analyzed. An allocation Excel sheet file was blinded and securely kept under passcode protection by an independent pharmacist.
Statistical Analysis
All analyses were performed on an intent-to-treat basis using data from all randomized participants. Statistical tests were performed by 2-tailed tests and an α level of .05. We calculated proportions and 95% confidence intervals (CIs) for binary data and the median (minimum-maximum range) and mean (SD) for continuous grouped data. We calculated mean differences and 95% CIs for the primary and secondary outcomes of continuous data using analysis of covariance and regression models for adjustment. We calculated risk ratios and their 95% CIs for secondary outcomes of binary data using regression models for risk adjustment.40 Randomized stratified factors and strong prognosis factors in previous reports were entered as covariates in the model-based analysis. To complement the primary analyses, we analyzed additional evaluations of secondary endpoints and subgroups. Because the analysis was exploratory, multiplicity was not controlled. All statistical analyses were performed using JMP software version 10.0 (SAS Institute Inc, Cary, North Carolina) and SAS software version 9.2 (SAS Institute).
We reported the factors affecting dropouts from this study in a prior report.41 Therefore, we determined that sex, IES-R score, injury severity score, subjective loss of consciousness, education, and interventions were potential prognostic factors for dropout, and we assumed data were missing at random according to the Rubin missing data mechanism.42 For sensitivity analysis under the missing-at-random assumption, we performed multiple imputations with those factors to impute outcomes after the motor vehicle accident for participants who dropped out. Multiple imputation for missing data was performed using PROC MI and MIANALYZE in SAS software version 9.1.3 (SAS Institute).
RESULTS
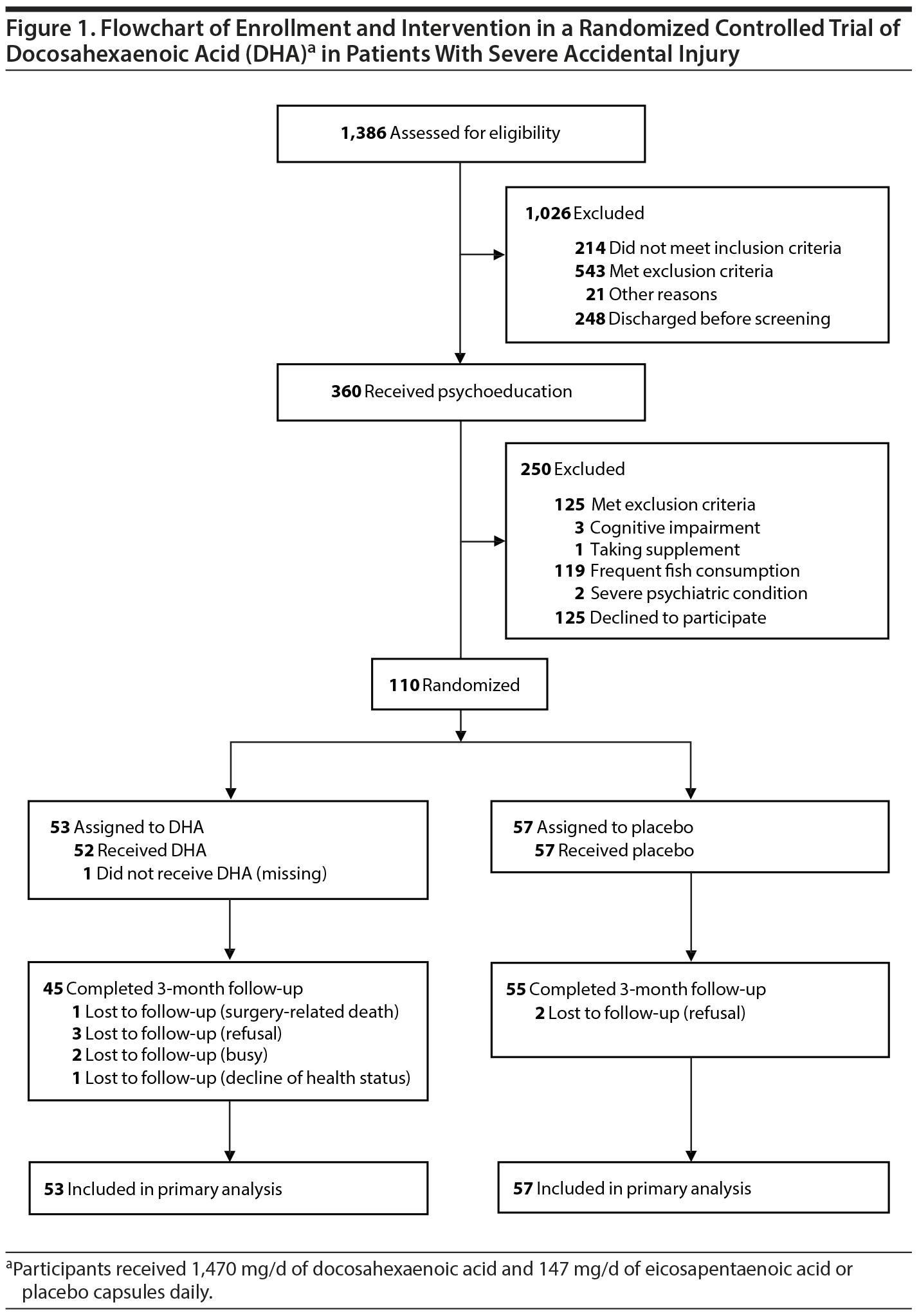
Details of the method have been published elsewhere.16 Briefly, 1,386 patients with high-energy accidental injury were assessed for first-step eligibility screening. Among them, 360 injured patients were taught about their psychological reactions to accidental injury for 20 minutes; of these, 110 met the inclusion criteria and consented to participate (Figure 1). One hundred five participants (95%) were retained at the 1-month follow-up, and 100 (91%) were retained at the 3-month follow-up (Figure 1).
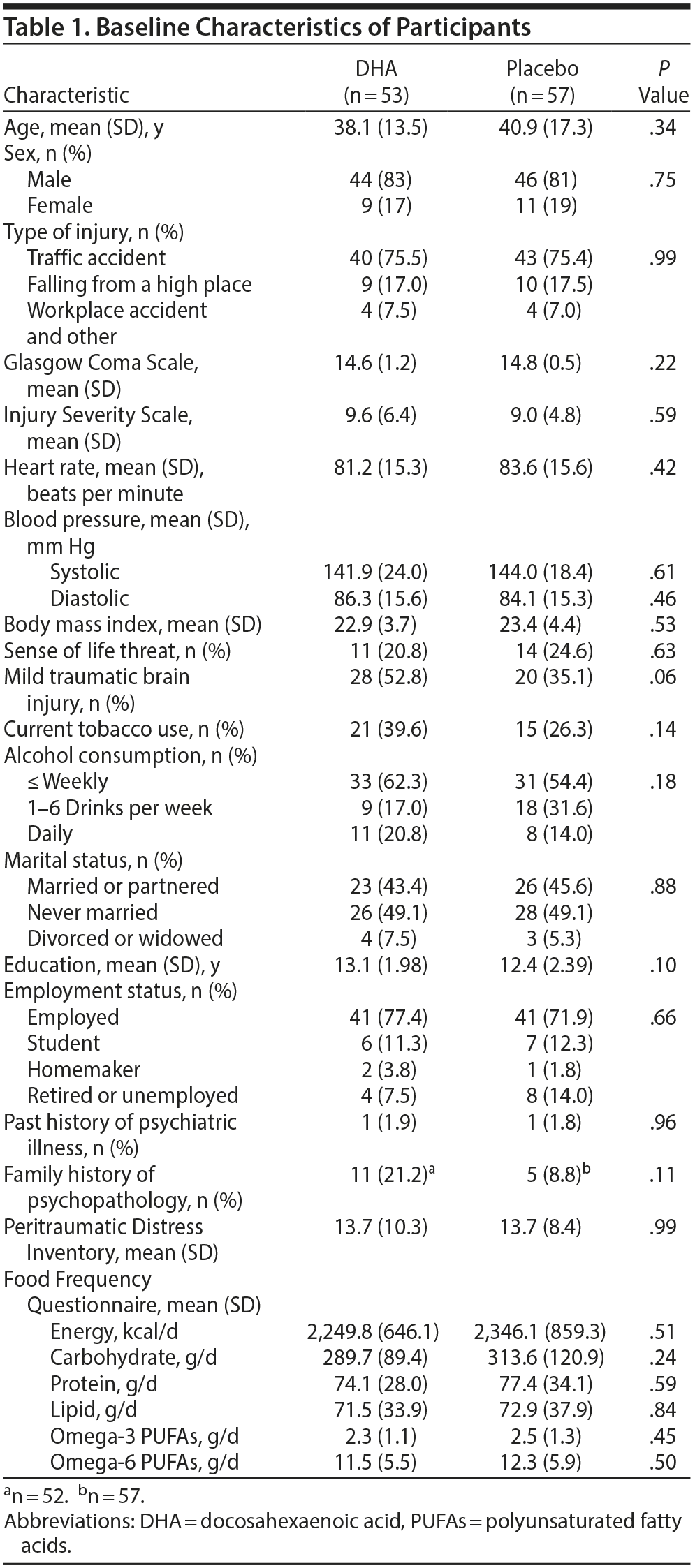
Baseline demographic, medical, psychosocial, and nutritional data are presented in Table 1. No significant differences were seen between the 2 groups. The 110 injured patients were randomly assigned to an intervention group to receive either DHA (n = 53) or placebo (n = 57) capsules. Treatment began a mean 3.4 days (SD = 2.1 days) after accidental injury. Ten participants (9%) dropped out of the study before the end of the treatment period. No clear between-group differences existed for these variables. Most participants (98%) had no past history of psychiatric illness before their accidental injury.
Primary and Secondary Outcome Measures
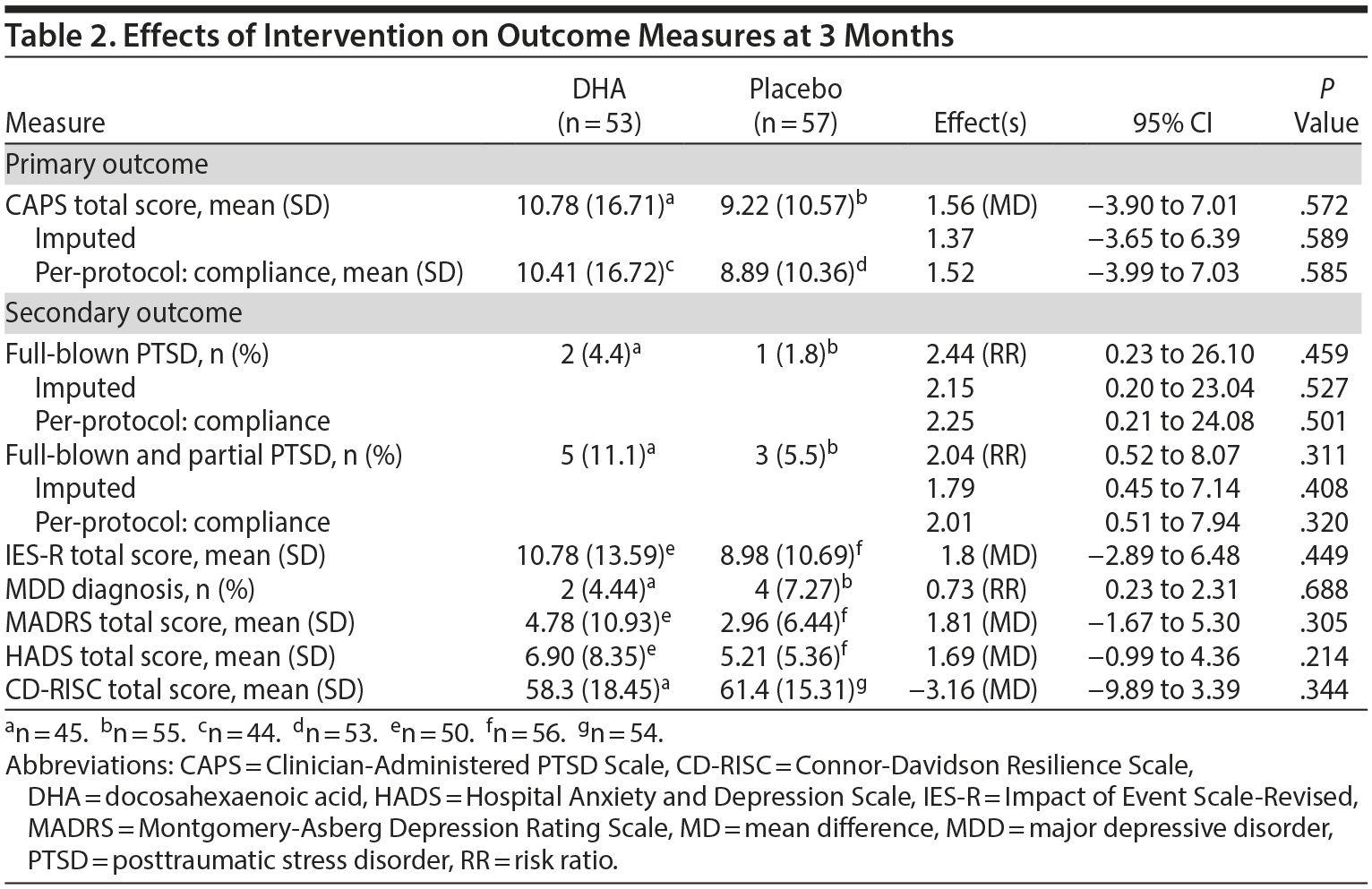
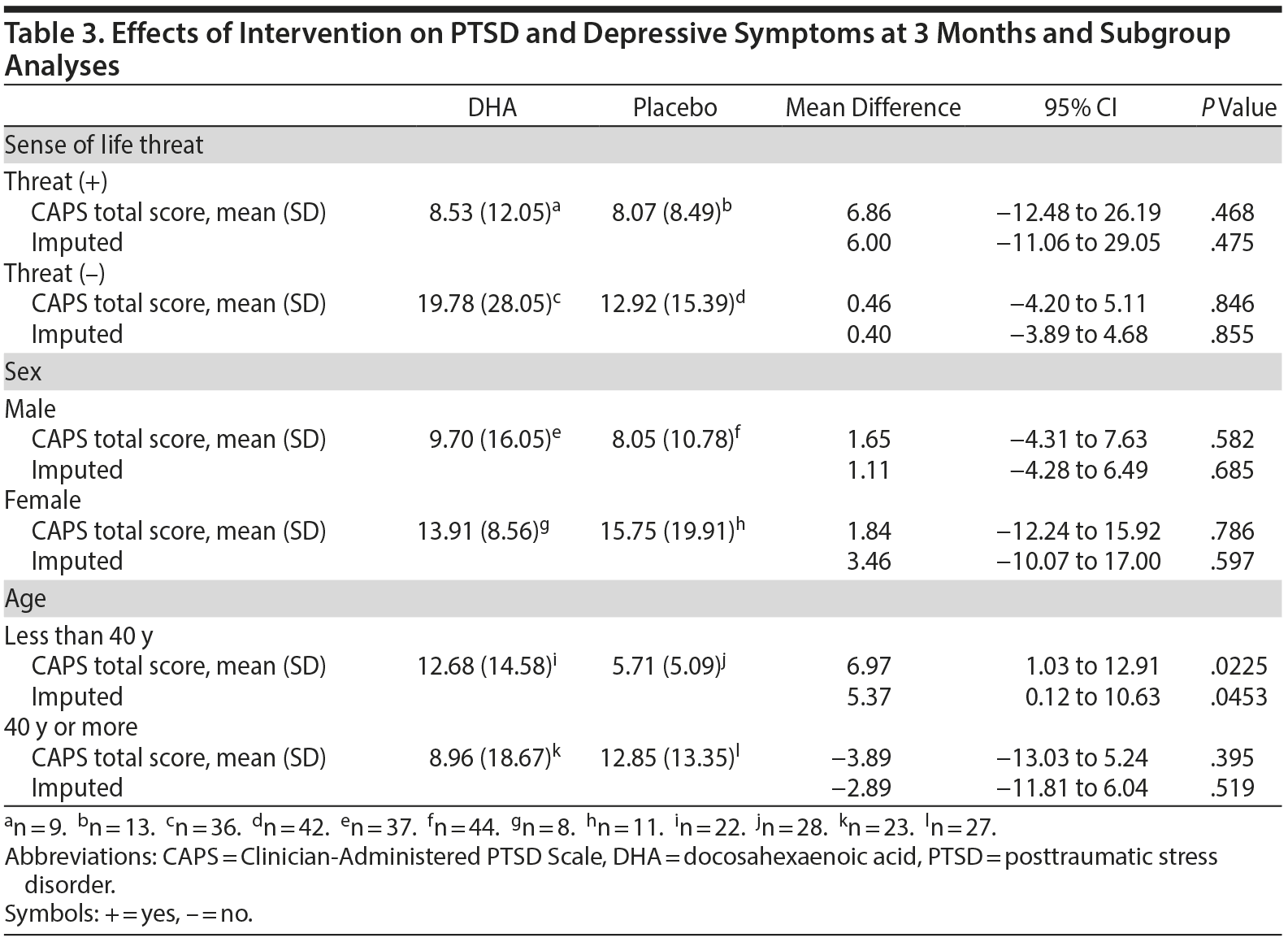
Table 2 summarizes the effect of DHA on outcome measures at 3 months. No difference existed between the 2 groups in the total CAPS score (10.78 [SD = 16.71] in the DHA group vs 9.22 [SD = 10.57] in the placebo group, mean difference = 1.56; 95% CI, −3.90 to 7.01; P = .572). Imputed and per-protocol data sets continued to show no difference between the groups. Also, no differences existed between the groups in the incidence proportion of full-blown PTSD and that of partial PTSD. No difference existed between the groups in mean total scores on the IES-R, MADRS, HADS, and CD-RISC at 3 months (Table 2). Also, no difference existed between the groups in the diagnosis of major depressive disorder (Table 2). Subgroup analyses stratified by sense of life threat and sex revealed no differences between the 2 groups. However, subgroup analysis for age < 40 years showed that the CAPS total score in the DHA group was higher than that in the placebo group at 3 months (Table 3).
Treatment Adherence and Adverse Events
The proportion of adherence with supplementation based on self-report and bottle check was 88.7% (n = 47) in the DHA group and 93.0% (n = 53) in the placebo group (P = .517). There was no difference in days of supplementation between the 2 groups (77.3 [SD = 2.43] vs 80.2 [2.34]; P = .40).
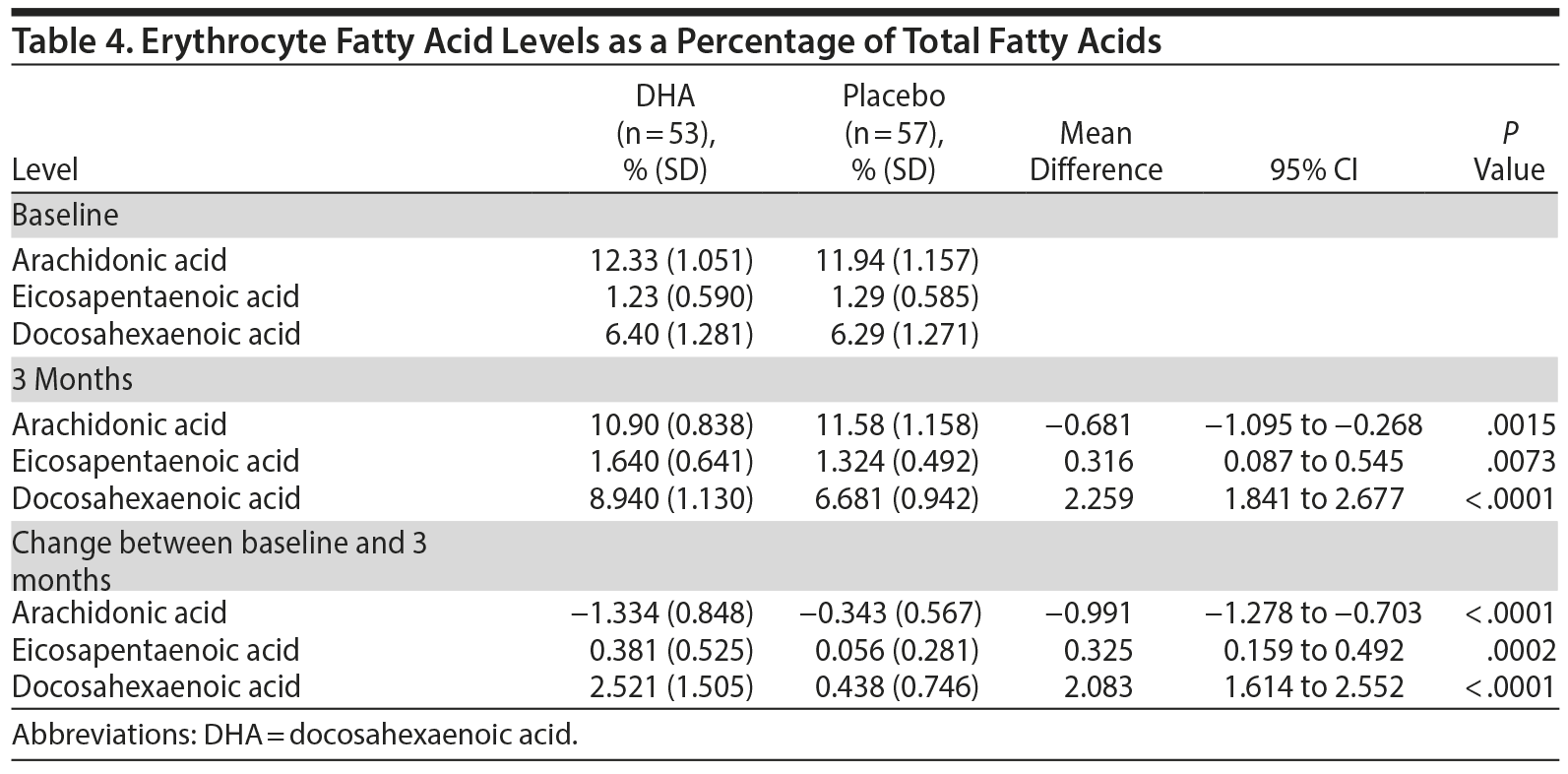
Table 4 shows mean changes in erythrocyte PUFAs from baseline to 3 months in the 2 groups. There were significant increases in EPA and DHA in the DHA group and a significant decrease in ARA in the DHA group compared with the placebo group. One participant in the placebo group was prescribed hypnotics and an antidepressant for insomnia after randomization. Almost one-third of participants complained of adverse events, with loose stool (10%) and constipation (3.6%) occurring frequently, but there were no significant differences between the 2 groups (32.0% in the DHA group vs 32.2% in the placebo group).
DISCUSSION
To our knowledge, this is the first randomized, placebo-controlled trial of traumatized patients at high risk of PTSD to test the preventive efficacy of DHA. Although DHA was well tolerated, it did not demonstrate an advantage over a placebo for secondary prevention of PTSD. The 2 groups also did not differ significantly on measures of symptoms of depression and resilience. This finding is inconsistent with our open-label pilot trial of injured patients in which DHA supplements reduced the development of PTSD symptoms.14 Although another single-blind, randomized trial of disaster rescue workers also failed to find beneficial effects of DHA, it is suggested that DHA supplementation is associated with reduced PTSD symptoms, particularly in women.15 Unfortunately, no other clinical trials have examined the effect of DHA on PTSD, so we cannot determine whether DHA has beneficial effects or not. Also, the generalizability of these negative findings to another country is uncertain.
The findings revealed that our original hypothesis was rejected. We presume that the negative findings were due to the use of DHA. A recent meta-analysis of depression found that EPA at ratios ≥ 60% of total EPA + DHA was effective against primary depression.43-45 Sublette et al45 suggested that supplements containing around 2,000 mg/d of EPA in excess of DHA were effective for depression. In addition, EPA rather than DHA has been used in prevention trials of several psychiatric disorders, including interferon-α-induced depression (3,500 mg/d of EPA)46 and psychosis (700 mg/d of EPA and 480 mg/d of DHA).47 The correct ratio and adequate dosage of omega-3 PUFAs in the prevention of PTSD remain unknown.
In our previous prospective cohort study, baseline serum levels of ARA and EPA immediately after the accident were inversely related to the risk of developing PTSD at 6 months after accidental injury, but we observed no significant association between DHA and PTSD.48 Maekawa and colleagues49 found that ARA supplementation to rat pups through mother’s breast milk via an ARA-rich diet given to the mother rats promoted the proliferation of postnatal neural stem/progenitor cells in the hippocampus. On the other hand, oral administration of DHA promoted adult neurogenesis in the hippocampus of rats that were fed a fish oil-deficient diet over 3 generations.12 It was also reported that feeding an omega-3 PUFA-rich diet to aged rats increased immature hippocampal neurons.50 These results suggest that DHA is necessary but not sufficient for regulating postnatal neural stem/progenitor cells under physiological conditions but that ARA is sufficient to affect postnatal neural stem/progenitor cells even under physiological conditions.51 Taken together, these results suggest that for patients with extremely low DHA levels, DHA might have a beneficial effect on preventing PTSD through hippocampal neurogenesis.
In the present study, around 7% of injured patients developed PTSD after accidental injury. The incidence proportion was consistent with previous observational studies in Japan.4,52,53 The severity of PTSD in injured patients was generally lower than in those who were physically assaulted. Small effects might not be detected by our modest sample size. An earlier larger epidemiologic study found that traumas most commonly associated with PTSD were combat exposure and witnessing among men and rape and sexual molestation among women.54 Had our trial been conducted with people traumatized by physical assault, our results might have been different. Although we failed to find benefits of DHA over placebo for PTSD prevention in accident-injured patients, further study would be meaningful.
The strengths of this study included the study design, use of reliable and standardized assessments, the application of an objective measure of treatment adherence, and a low attrition rate (9%). However, several caveats should be noted. First, because no other studies have examined the effect of DHA on PTSD, we estimated sample size with reference to previous data on omega-3 PUFAs for treating depression. Second, the results were obtained from only 1 teaching hospital in Tokyo. Third, because seafood consumption is prevalent in Japan, we excluded subjects who reported eating fish 4 times or more per week. However, the baseline erythrocyte omega-3 PUFA composition (about 7.6%) in the participants of the present study was still higher than that in people in Western countries. Thus, a ceiling effect might mask the results. Despite these limitations, this randomized, double-blind, placebo-controlled trial found no evidence for the superiority of DHA supplementation over placebo for the selective prevention of PTSD in patients with accidental injury. Related analyses of our patient sample are in progress to examine factors such as levels of serum brain-derived neurotrophic factor, neuropeptide Y, resolvins, and protectins as potential moderators of treatment response. Further studies are needed to determine the ratio and dosage of EPA and DHA and the indications for other traumatized and non-Japanese populations.
Submitted: May 19, 2014; accepted September 3, 2014.
Drug names: lithium (Lithobid and others), warfarin (Coumadin, Jantoven, and others).
Potential conflicts of interest: Dr Matsuoka has received research grants from the Japan Science and Technology Agency; the National Center of Neurology and Psychiatry, Japan; and the Ministry of Health, Labour and Welfare of Japan. He has been a paid speaker for Ono, Mochida, Takeda, Suntory Wellness, Otsuka, and the DHA & EPA Association. Dr Nishi has received research grants from the Japan Society for the Promotion of Science and lecture fees from Qol, the DHA & EPA Association, NTT DoCoMo, and Emotional Quotient Academy. Dr K. Hamazaki has received research support from the National Center of Neurology and Psychiatry, Japan; the Japan Society for the Promotion of Science; the Tamura Foundation for Promotion of Science and Technology; and the Ichiro Kanehara Foundation for Promotion of Medical Sciences and Medical Care. He has received consultant fees from Polyene Project and scholarship donations from Otsuka. He has been a paid speaker for the DHA & EPA Association. Mr Yonemoto has received research grants from the Japan Science and Technology Agency; the National Center of Neurology and Psychiatry, Japan; and Ministry of Health, Labour and Welfare of Japan. Dr Matsumura has received research grants from the Japan Society for the Promotion of Science and developed the iPhysioMeter app, distributed for free at the iTunes App Store (Apple, Inc). There are no further patents, products in development, or marketed products to declare. Dr Hashimoto has served as a scientific consultant to Astellas and Taisho. He has also received research support from Abbvie, Dainippon Sumitomo, Otsuka, and Taisho. Dr T. Hamazaki has received research support from the Japan Society for the Promotion of Science, the Open Research Center for Lipid Nutrition (Kinjo Gakuin University), and Nippon Suisan Kaisha, Ltd; consultancy fees from Polyene Project and Otsuka; lecture fees from Otsuka; and travel expenses from Aker BioMarine. Dr Noguchi declares that she has no competing interests in relation to this work.
Funding/support: This study was supported by CREST from the Japan Science and Technology Agency (primary investigator: Dr Matsuoka). All supplements used in the study were supplied by Kentech Co, Ltd, Toyama, Japan.
Role of the sponsor: The Japan Science and Technology Agency and Kentech Co, Ltd, had no role in the design and conduct of the study, data collection, data management, analysis, interpretation of the data, review or approval of the manuscript, and decision to submit the manuscript for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Disaster Medical Center or the Japan Science and Technology Agency.
Acknowledgments: We thank Kyoko Sakuma, MS; Keiko Sano, MS; Kana Miyazaki, MS; Sanae Morita, MS; and Naoko Okanobori, MS, at Musashino University for their day-to-day help with data collection and research coordination. We express our sincere appreciation to Satoru Kobayashi, PhD, at Josai International University and Akiko Kada, MPH, at the Clinical Research Center of Nagoya Medical Center for randomization and intervention assignment; Ms Kyoko Akutsu and Ms Yumiko Kamoshida at National Disaster Medical Center for clinical data management; and Ms Shizuko Takebe at University of Toyama for technical assistance with fatty acid analysis. None of the acknowledged individuals report any financial or other conflict of interest in relation to the subject of this work.
Additional information: Drs Matsuoka and Nishi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
1. Shalev AY, Freedman S, Peri T, et al. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155(5):630-637. PubMed doi:10.1176/ajp.155.5.630
2. Schnyder U, Moergeli H, Klaghofer R, et al. Incidence and prediction of posttraumatic stress disorder symptoms in severely injured accident victims. Am J Psychiatry. 2001;158(4):594-599. PubMed doi:10.1176/appi.ajp.158.4.594
3. O’ Donnell ML, Creamer M, Pattison P, et al. Psychiatric morbidity following injury. Am J Psychiatry. 2004;161(3):507-514. PubMed doi:10.1176/appi.ajp.161.3.507
4. Matsuoka Y, Nishi D, Nakajima S, et al. Incidence and prediction of psychiatric morbidity after a motor vehicle accident in Japan: the Tachikawa Cohort of Motor Vehicle Accident Study. Crit Care Med. 2008;36(1):74-80. PubMed doi:10.1097/01.CCM.0000291650.70816.D6
5. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4-12, discussion 13-14. PubMed
6. Matsuoka Y. Clearance of fear memory from the hippocampus through neurogenesis by omega-3 fatty acids: a novel preventive strategy for posttraumatic stress disorder? Biopsychosoc Med. 2011;5(1):3. PubMed doi:10.1186/1751-0759-5-3
7. Squire LR. Memory and Brain. Oxford, UK: Oxford University Press; 1987.
8. Feng R, Rampon C, Tang YP, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32(5):911-926. PubMed doi:10.1016/S0896-6273(01)00523-2
9. Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116-1124. PubMed doi:10.1038/nn1944
10. Kitamura T, Saitoh Y, Takashima N, et al. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139(4):814-827. PubMed doi:10.1016/j.cell.2009.10.020
11. Beltz BS, Tlusty MF, Benton JL, et al. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415(2):154-158. PubMed doi:10.1016/j.neulet.2007.01.010
12. Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139(3):991-997. PubMed doi:10.1016/j.neuroscience.2006.01.021
13. He C, Qu X, Cui L, et al. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci U S A. 2009;106(27):11370-11375. PubMed doi:10.1073/pnas.0904835106
14. Matsuoka Y, Nishi D, Yonemoto N, et al. Omega-3 fatty acids for secondary prevention of posttraumatic stress disorder after accidental injury: an open-label pilot study. J Clin Psychopharmacol. 2010;30(2):217-219. PubMed doi:10.1097/JCP.0b013e3181d48830
15. Nishi D, Koido Y, Nakaya N, et al. Fish oil for attenuating posttraumatic stress symptoms among rescue workers after the Great East Japan Earthquake: a randomized controlled trial. Psychother Psychosom. 2012;81(5):315-317. PubMed doi:10.1159/000336811
16. Matsuoka Y, Nishi D, Yonemoto N, et al. Tachikawa project for prevention of posttraumatic stress disorder with polyunsaturated fatty acid (TPOP): study protocol for a randomized controlled trial. BMC Psychiatry. 2013;13(1):8. PubMed doi:10.1186/1471-244X-13-8
17. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
18. Brunet A, Weiss DS, Metzler TJ, et al. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158(9):1480-1485. PubMed doi:10.1176/appi.ajp.158.9.1480
19. Nishi D, Matsuoka Y, Noguchi H, et al. Reliability and validity of the Japanese version of the Peritraumatic Distress Inventory. Gen Hosp Psychiatry. 2009;31(1):75-79. PubMed doi:10.1016/j.genhosppsych.2008.09.002
20. Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76-82. PubMed doi:10.1002/da.10113
21. Tsubono Y, Takamori S, Kobayashi M, et al. A data-based approach for designing a semiquantitative food frequency questionnaire for a population-based prospective study in Japan. J Epidemiol. 1996;6(1):45-53. PubMed doi:10.2188/jea.6.45
22. Tsugane S, Sasaki S, Kobayashi M, et al. Validity and reproducibility of the self-administered Food Frequency Questionnaire in the JPHC Study Cohort I: study design, conduct and participant profiles. J Epidemiol. 2003;13(suppl):S2-S12. PubMed doi:10.2188/jea.13.1sup_2
23. Hamazaki T, Sawazaki S, Itomura M, et al. The effect of docosahexaenoic acid on aggression in young adults: a placebo-controlled double-blind study. J Clin Invest. 1996;97(4):1129-1133. PubMed doi:10.1172/JCI118507
24. Sawazaki S, Hamazaki T, Yazawa K, et al. The effect of docosahexaenoic acid on plasma catecholamine concentrations and glucose tolerance during long-lasting psychological stress: a double-blind placebo-controlled study. J Nutr Sci Vitaminol (Tokyo). 1999;45(5):655-665. PubMed doi:10.3177/jnsv.45.655
25. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. PubMed doi:10.1002/jts.2490080106
26. Asukai N, Hirohata S, Kato H, et al. Psychometric properties of the Japanese-language version of the Clinician-Administered PTSD Scale for DSM-IV. [in Japanese] Jpn J Trauma Stress. 2003;1(1):47-53.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington DC: American Psychiatric Association; 2000.
28. Matsuoka Y, Nishi D, Nakajima S, et al. The Tachikawa cohort of motor vehicle accident study investigating psychological distress: design, methods and cohort profiles. Soc Psychiatry Psychiatr Epidemiol. 2009;44(4):333-340. PubMed doi:10.1007/s00127-008-0438-6
29. Weiss DS, Marmar CR. The Impact of Event Scale Revised. In: Wilson JP, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York, NY: The Guilford Press; 1997:399-411.
30. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
31. Otsubo T, Tanaka K, Koda R, et al. Reliability and validity of Japanese version of the Mini-International Neuropsychiatric Interview. Psychiatry Clin Neurosci. 2005;59(5):517-526. PubMed doi:10.1111/j.1440-1819.2005.01408.x
32. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
33. Takahashi N, Tomita K, Higuchi T, et al. The inter-rater reliability of the Japanese version of the Montgomery-Asberg Depression Rating Scale (MADRS) using a structured interview guide for MADRS (SIGMA). Hum Psychopharmacol. 2004;19(3):187-192. PubMed doi:10.1002/hup.576
34. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361-370. PubMed doi:10.1111/j.1600-0447.1983.tb09716.x
35. Kugaya A, Akechi T, Okuyama T, et al. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998;28(5):333-338. PubMed doi:10.1093/jjco/28.5.333
36. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68(5):748-766. PubMed doi:10.1037/0022-006X.68.5.748
37. Ozer EJ, Best SR, Lipsey TL, et al. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull. 2003;129(1):52-73. PubMed doi:10.1037/0033-2909.129.1.52
38. The Ministry of Health, Labour and Welfare, Japan. National Health and Nutrition Survey: 2005. http://www.mhlw.go.jp/houdou/2005/04/h0421-1b.html. Accessed February 2008.
39. Itomura M, Fujioka S, Hamazaki K, et al. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22(1):131-135. PubMed
40. McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943. PubMed doi:10.1093/aje/kwg074
41. Nishi D, Matsuoka Y, Nakajima S, et al. Are patients after severe injury who drop out of a longitudinal study at high risk of mental disorder? Compr Psychiatry. 2008;49(4):393-398. PubMed doi:10.1016/j.comppsych.2008.02.003
42. Enders CK. A primer on the use of modern missing-data methods in psychosomatic medicine research. Psychosom Med. 2006;68(3):427-436. PubMed doi:10.1097/01.psy.0000221275.75056.d8
43. Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? the effect depends upon diagnosis, supplement preparation, and severity of depression. Mol Psychiatry. 2012;17(12):1161-1163, author reply 1163-1167. PubMed doi:10.1038/mp.2012.111
44. Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17(12):1144-1149, discussion 1163-1167. PubMed doi:10.1038/mp.2012.25
45. Sublette ME, Ellis SP, Geant AL, et al. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72(12):1577-1584. PubMed doi:10.4088/JCP.10m06634
46. Su KP, Lai HC, Yang HT, et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. 2014;76(7):559-566. PubMed doi:10.1016/j.biopsych.2014.01.008
47. Amminger GP, Schפfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146-154. PubMed doi:10.1001/archgenpsychiatry.2009.192
48. Matsuoka Y, Nishi D, Hamazaki K. Serum levels of polyunsaturated fatty acids and the risk of posttraumatic stress disorder. Psychother Psychosom. 2013;82(6):408-410. PubMed doi:10.1159/000351993
49. Maekawa M, Takashima N, Matsumata M, et al. Arachidonic acid drives postnatal neurogenesis and elicits a beneficial effect on prepulse inhibition, a biological trait of psychiatric illnesses. PLoS ONE. 2009;4(4):e5085. PubMed doi:10.1371/journal.pone.0005085
50. Dyall SC, Michael GJ, Michael-Titus AT. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res. 2010;88(10):2091-2102. PubMed doi:10.1002/jnr.22390
51. Sakayori N, Kimura R, Osumi N. Impact of lipid nutrition on neural stem/progenitor cells. Stem Cells Int. 2013;2013:973508. PubMed doi:10.1155/2013/973508
52. Hamanaka S, Asukai N, Kamijo Y, et al. Acute stress disorder and posttraumatic stress disorder symptoms among patients severely injured in motor vehicle accidents in Japan. Gen Hosp Psychiatry. 2006;28(3):234-241. PubMed doi:10.1016/j.genhosppsych.2006.02.007
53. Nishi D, Noguchi H, Yonemoto N, et al. Incidence and prediction of post-traumatic stress disorder at 6 months after motor vehicle accidents in Japan. Psychosomatics. 2013;54(3):263-271. PubMed
54. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. PubMed doi:10.1001/archpsyc.1995.03950240066012
Save
Cite
Advertisement
GAM ID: sidebar-top