Posttraumatic stress disorder (PTSD) is a serious problem for many Veterans. Innovative and fast-acting therapeutic interventions are needed for those not helped by first-line treatments.1-4 Nitrous oxide, an inhaled anesthetic and noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, may be a promising new approach for rapid reduction in PTSD symptoms.5 Nitrous oxide is reported to speed the reduction of distressing intrusive memories in an experimental model of psychological trauma6 and has shown promise as a rapidly acting antidepressant.7 To our knowledge, this is the first study testing the effects of nitrous oxide in individuals with PTSD.
Method
Three veteran outpatients (ages 31, 43, and 46 years) who met PTSD criteria of at least moderate symptom severity (Clinician-Administered PTSD Scale for DSM-5 [CAPS-5] score ≥ 40)8 were recruited from the Veterans Affairs Palo Alto Health Care System between April 2018 and July 2019. Individuals were excluded if they had comorbid psychiatric or medical conditions that increased the risk of participation.
All participants completed a single 1-hour subanesthetic inhalation of 50% nitrous oxide and 50% oxygen administered by an anesthesiologist via face mask and standard semiclosed anesthetic circuit with a total gas flow rate of 4-8 L/min. A trained independent evaluator rated PTSD symptoms using CAPS-5 at baseline and 1 week after drug administration. Response was defined a priori as a CAPS-5 score reduction of 12 points at 7 days.9 We additionally explored immediate (1 hour post-inhalation [120 minutes]) and interim (1, 2, 3, and 7 days) effects of nitrous oxide inhalation on self-reported PTSD symptoms using the Impact of Events Scale—Revised (IES-R).10 To evaluate safety, participants were assessed for symptoms of dissociation, psychosis, mania, suicidality, and other side effects at the same time points.
Results
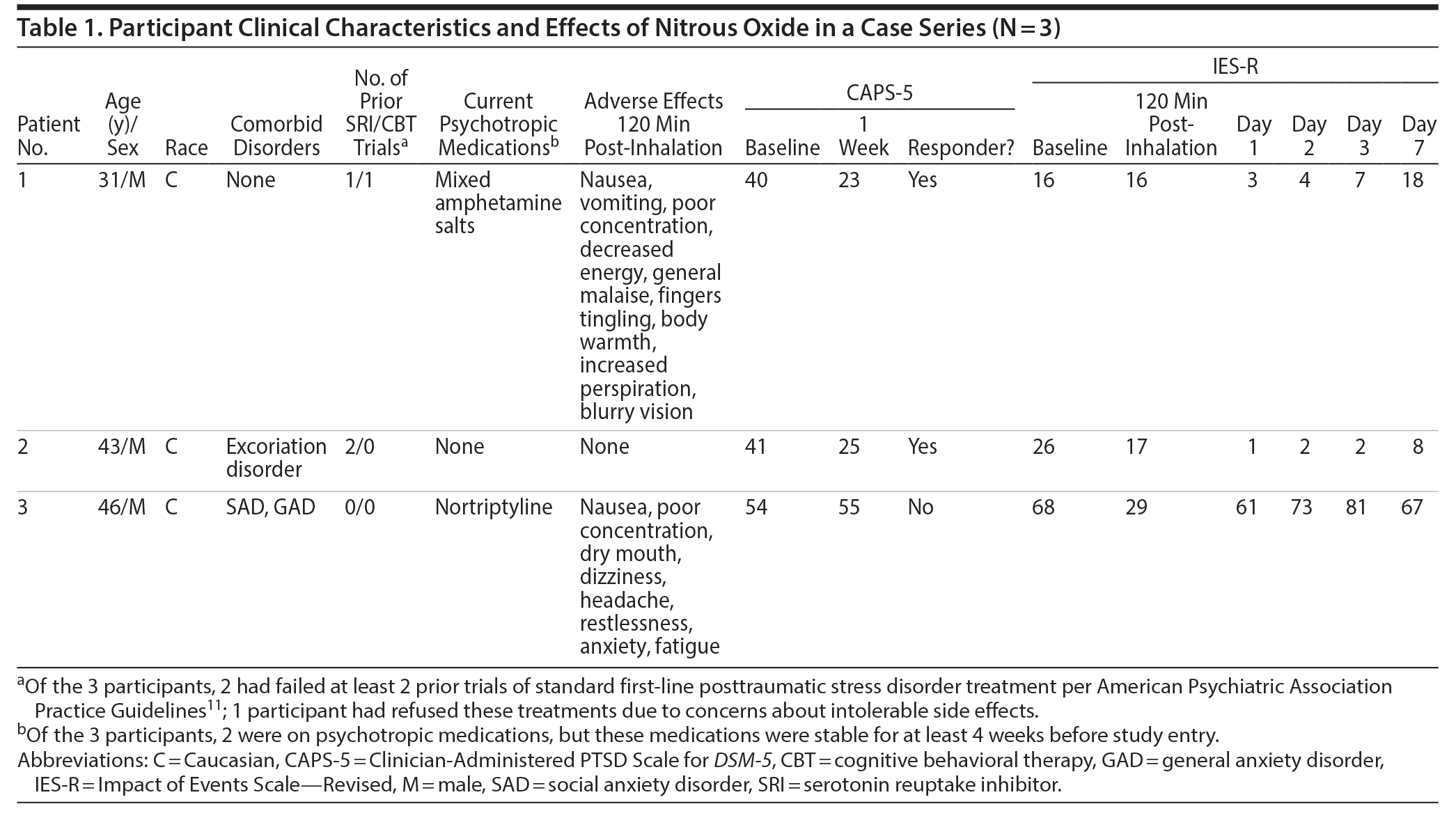
Participant clinical characteristics and effects of nitrous oxide are shown in Table 1. At baseline, all participants exceeded the criteria for clinically significant PTSD (CAPS-5 score ≥ 40); the mean CAPS-5 was 45 (SD = 6). At 1 week post-inhalation, 2 out of 3 participants met treatment response criteria by CAPS-5 with a 17-point (40 to 23) and 16-point change (41 to 25). Both responders showed near complete cessation of symptoms by IES-R by day 1; one showed sustained improvement, while the other showed gradual return of symptoms to near baseline by day 7. In contrast, the nonresponder reported early reduction in PTSD by IES-R by 120 minutes and quickly returned to baseline by day 1. There were no reports of new-onset dissociation, psychosis, mania, or suicidal ideation at any time point. Two of 3 participants reported side effects including nausea and poor concentration (see Table 1 for full list). The first participant vomited once during the inhalation yet nevertheless wished to continue with study procedures. Because of this, nitrous oxide concentration was increased slowly over 10 minutes to target dose for the 2 subsequent participants; neither vomited.
There are several limitations of this small, open-label case series, including inability to control for placebo effects and nonspecific effects of nitrous oxide. This case series suggests that adjunctive nitrous oxide can reduce self-reported PTSD symptoms within 1 day, lasting up to 1 week without causing new onset psychiatric symptoms. Future research is warranted to determine whether nitrous oxide effects are replicated in a larger sample under randomized, controlled conditions and whether the effects benefit specific PTSD domains. If our hypotheses are replicated in independent samples, it may be feasible that nitrous oxide can be safely and effectively implemented to achieve rapid symptom reduction while longer-term PTSD treatments like psychotherapy or pharmacology are allowed to take effect over a longer time course.
Published online: June 30, 2020.
Clinical trials registration: ClinicalTrials.gov identifier: NCT03375294
Potential conflicts of interest: In the last 3 years, Dr Rodriguez has served as a consultant for BlackThorn Therapeutics and Epiodyne and receives research grant support from Biohaven Pharmaceuticals and a stipend from American Psychiatric Association Publishing for her role as Deputy Editor of The American Journal of Psychiatry. Dr Clark has a consulting arrangement with Teikoku Pharma USA. The other authors report no additional financial or other relationships relevant to the subject of this report.
Funding/support: This research was supported by the VA Office of Research and Development Clinical Science Research & Development Service, US, Department of Veterans Affairs (principal investigator: Dr Rodriguez, 1I01CX001789-01A2).
Role of the sponsor: The sponsor had no role in the planning, conduct, or publication of the study.
Acknowledgments: The authors thank those individuals with PTSD who generously donated their time to participate in this research study.
aDepartment of Psychiatry and Behavioral Sciences, Stanford University, Stanford, California
bVeterans Affairs Palo Alto Health Care System, Palo Alto, California
cSan Francisco Veterans Affairs Medical Center, San Francisco, California
dDepartments of Psychiatry and Neurology, University of California San Francisco, San Francisco, California
eDepartment of Anesthesia and Critical Care, University of Chicago Medicine, Chicago, Illinois
fDepartment of Anesthesiology Perioperative and Pain Medicine, Stanford University, Stanford, California
‡Ms Varias and Dr van Roessel are co-first authors.
§Drs Clark and Rodriguez are co-last authors.
*Corresponding author: Carolyn I. Rodriguez, MD, PhD, Department of Psychiatry and Behavioral Sciences, Stanford University, 401 Quarry Rd, Stanford, CA 94305 ([email protected]).
J Clin Psychiatry 2020;81(4):20l13393
To cite: Varias A, van Roessel P, Parsiani M, et al. Does nitrous oxide help Veterans with posttraumatic stress disorder? a case series. J Clin Psychiatry. 2020;81(4):20l13383.
To share: https://doi.org/10.4088/JCP.20l13393
© Copyright 2020 Physicians Postgraduate Press, Inc.
REFERENCES
1.Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59. PubMed CrossRef
2.Feder A, Parides MK, Murrough JW, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681-688. PubMed CrossRef
3.Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657. PubMed CrossRef
4.Wilk JE, West JC, Duffy FF, et al. Use of evidence-based treatment for posttraumatic stress disorder in Army behavioral healthcare. Psychiatry. 2013;76(4):336-348. PubMed CrossRef
5.Nagele P, Zorumski CF, Conway C. Exploring nitrous oxide as treatment of mood disorders: basic concepts. J Clin Psychopharmacol. 2018;38(2):144-148. PubMed CrossRef
6.Das RK, Tamman A, Nikolova V, et al. Nitrous oxide speeds the reduction of distressing intrusive memories in an experimental model of psychological trauma. Psychol Med. 2016;46(8):1749-1759. PubMed CrossRef
7.Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18. PubMed CrossRef
8.Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395. PubMed CrossRef
9.Stefanovics EA, Rosenheck RA, Jones KM, et al. Minimal clinically important differences (MCID) in assessing outcomes of post-traumatic stress disorder. Psychiatr Q. 2018;89(1):141-155. PubMed CrossRef
10.Weiss DS, Marmar CR. Impact of Events Scale—Revised. In: Wilson J, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York, NY; Guilford; 1996:399-411.
11.Ursano RJ, Bell C, Eth S, et al. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. Washington, DC: American Psychiatric Association; 2004.
This PDF is free for all visitors!
Save
Cite